Abstract
Dysregulation of the inflammatory response is a critical component of many clinically challenging disorders such as sepsis. Inflammation is a biological process designed to lead to healing and recovery, ultimately restoring homeostasis; however, the failure to fully achieve those beneficial results can leave a patient in a dangerous persistent inflammatory state. One of the primary challenges in developing novel therapies in this area is that inflammation is comprised of a complex network of interacting pathways. Here, we discuss our approaches towards addressing this problem through computational systems biology, with a particular focus on how the presence of biological rhythms and the disruption of these rhythms in inflammation may be applied in a translational context. By leveraging the information content embedded in physiologic variability, ranging in scale from oscillations in autonomic activity driving short-term heart rate variability (HRV) to circadian rhythms in immunomodulatory hormones, there is significant potential to gain insight into the underlying physiology.
Keywords: systemic inflammation, heart rate variability, cortisol, circadian rhythms, decomplexification
Introduction
In response to a stressor, such as an injury or an infection, the body mounts an inflammatory response aimed at resolving the deleterious effects of the stressor and restoring homeostasis, such as through the healing of a wound or the elimination of a bacterial infection. Normally, inflammation successfully results in a return to homeostasis. However, when restoration of homeostasis fails, a state of prolonged inflammation can arise, often bringing its own clinical challenges along with those of the original stressor. Dysregulation of the inflammatory response contributes to the pathology of sepsis [1]. Sepsis is a major clinical challenge due to its common incidence, high cost, and high mortality rate [2]. Furthermore, there are limited treatment options for sepsis and progress on developing novel therapies has been slow. Obtaining a more fundamental understanding of the mechanisms involved in the inflammatory response may open up new opportunities for monitoring and treating patients with inflammatory disorders [3].
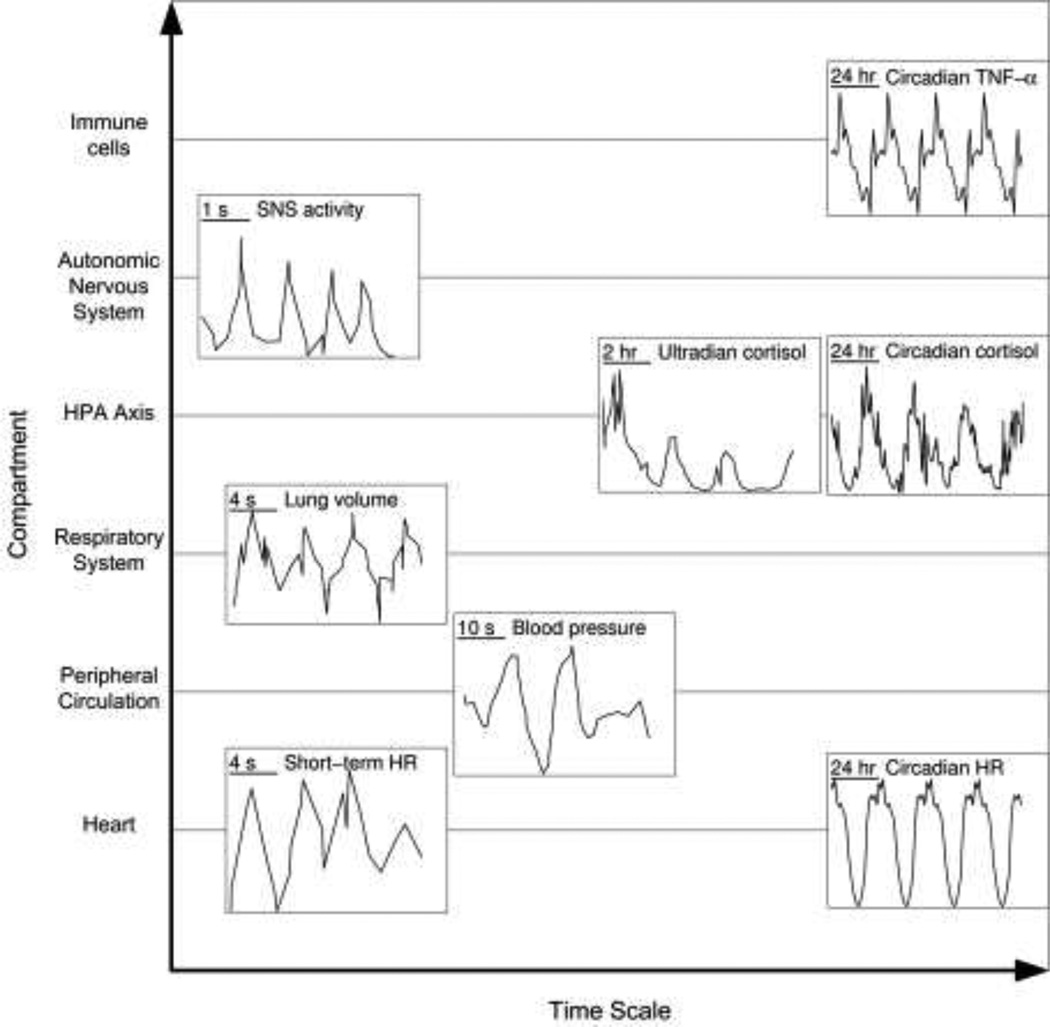
Many of the components involved in the inflammatory response contain homeostatic rhythmic variability, and advances in clinical and experimental tools make assaying the state of these rhythms increasingly plausible. Fig. 1 shows several of these homeostatic rhythms, including mediators directly involved in inflammation such as cytokines and immunomodulatory hormones, as well as oscillatory components that give rise to patterns in heart rate variability (HRV) that are disrupted in inflammation such as breathing pattern, blood pressure rhythms, and autonomic activity. Underlying every biochemical oscillator is some type of negative feedback mechanism [4]. Possibly the simplest system to consider is a protein that acts as a transcription factor by inhibiting the transcription of its own mRNA. Given appropriate time delays in transcription, translation, and translocation, as well as appropriate rate constants, this simple single-gene system oscillates [4]. Real physiological systems typically consist of much more complex networks comprised of multiple feedback loops. Even still, physiological rhythms are of clinical interest because characteristics of those patterns convey information about the underlying system that is producing them, beyond simply measuring the mean value of a signal. For instance, in some cases, the network structure giving rise to rhythms can be reverse engineered from the pattern of rhythmicity [5]. Given that negative feedback loops are critical in maintaining homeostasis and acute responsiveness, perturbations in negative feedback loops culminating in altered rhythmic patterns can reveal information about the integrity of the negative feedback system [6].
Fig. 1.
Homeostatic rhythms, at a variety of time scales, which contribute to altered physiologic variability in endotoxemia. TNF-α data: [110]. Sympathetic nervous system activity data: [111]. Circadian cortisol data: [112]. Ultradian cortisol data: [113]. Lung volume, blood pressure, and short-term HR data: [114]. Circadian HR data: [115]. TNF-α is an inflammatory cytokine that has a clear circadian pattern in response to LPS stimulation, and other cytokines also exhibit circadian patterns [110]. Autonomic signaling in inflammation contributes both to changes in cardiac function and modulation of the inflammatory response, and oscillatory activity is inherent in both sympathetic and parasympathetic branches. Cortisol is an anti-inflammatory hormone, with a large circadian rhythm riding on top of an ultradian rhythm. Blood pressure and respiratory rhythms contribute to short-term patterns in HR, which are diminished in endotoxemia.
Clinically, patterns in physiological signals represent a broad source of potential diagnostic and prognostic markers. For instance, perturbations in the rhythmicity of heart beats precede the onset of neonatal sepsis [7,8]. At a much longer time scale, circadian variations in plasma cortisol concentration, a factor that has been linked to immune dysfunction, has been associated with chronic stress resulting from depression [9], obesity [10], psychological stress [11], and cancer [12,13]. The loss of rhythmicity in these cases may reflect an underlying loss in the regulation of negative feedback control systems.
To take full advantage of the information conveyed by altered rhythmicity in a biological signal, the mechanism behind the change in rhythmicity must be known. Lacking this, variability-based metrics can still yield significant clinical uses purely based on observed correlations, which is often the case for applications of HRV analysis. More mechanistic detail would allow for understanding of what physiological processes drive changes in HRV, potentially opening the door to novel and more refined therapeutic approaches. In this context, controlled experimental and mathematical models represent critical avenues by which mechanisms giving rise to both the homeostatic generation of rhythms and the alteration of rhythms in disease can be explored. The human endotoxemia model, which consists of the injection of low doses of endotoxin (lipopolysaccharide, LPS) to healthy human volunteers, provides an experimental model of systemic inflammation that can be studied in humans in a controlled environment [14] and mathematically modeled to gain further insight [15–21]. LPS is a component of the outer membrane of Gram-negative bacteria that provokes an inflammatory response subsequent to its recognition by the innate immune system. In addition to provoking acute changes in systemic inflammatory mediators, endotoxemia also alters biological rhythms at multiple scales, most notably provoking significant changes in HRV [22]. Therefore, human endotoxemia provides the opportunity to study the relationship between inflammation and biological rhythms, which may lead to clinically important mechanistic insights due to the physiological similarities between endotoxemia and sepsis [23,14], acute respiratory distress syndrome (ARDS) [24], and trauma [25]. Responses to LPS are ultimately driven by the binding of LPS to Toll-like receptor 4 (TLR4) in immune cells, leading to the activation of inflammation-related pathways such as the NF-κB, JAK-STAT, and MAPK signaling pathways [15]. This transcriptional activity in immune cells propagates to the systemic level, provoking physiological changes such as the secretion of hormones, activation of the autonomic activity, and altered body temperature [26]. Here, we discuss how biological rhythms convey relevant information about the underlying biological state of a patient, how those rhythms are disrupted in endotoxemia, and how translational systems biology approaches can potentially leverage this information to improve clinical care.
Mathematical modeling of endotoxemia
The control of chronic inflammation is at the core of a variety of inflammation-related diseases, which lead to the search for therapies that can target inflammatory processes in a wide variety of clinical conditions. To date, clinical developments have been slow and clinical trials have produced some unintuitive results. As an example, anti-cytokine therapies have shown promise in recent years for treating rheumatoid arthritis and inflammatory bowel disease, but similar strategies have not produced positive results sepsis [27,28]. Similar challenges exist in different experimental models, where a technique that appears promising in an in vitro or animal model of a disease may fail for uncertain reasons in humans.
One of the key challenges impeding the development of more effective and novel therapies for inflammation-driven diseases is that the inflammatory response is ultimately the convolution of multiple interacting pathways, which means that the output of the system is often the unintuitive result of a complex network [29]. Model-based techniques for unraveling the complexity of inflammation are a promising approach to adequately confront and overcome these issues [30,3]. Mathematical models, in some respect, are quantitative and explicit hypotheses about the behavior of a system, with the level of detail dependent on both the structure of the model and the depth of knowledge contributing to the model. Models of inflammation have been used to study biologically relevant patterns from a broader and mathematically rigorous perspective than would otherwise be possible. Depending on the scale of a model, both spatial and temporal, insight into different components of the system can be obtained. For instance, relatively small equation-based models allow for more mathematically rigorous analysis [31–35]. By trading off simplicity for mechanistic accuracy, larger models facilitate a more realistic description of a network [36–39]. Agent-based models, which operate by simulating discrete events, allow for the consideration of spatial heterogeneity and stochasticity [40–49].
Transcriptional responses
A first step in modeling a dynamic system is the definition of the state space of the model [50]. This is not only related to domain knowledge of putative mechanisms, but it also depends on the availability of supporting experimental data. The development of experimental techniques to sample the transcriptional state of cells has resulted in a massive increase in the amount of available data. This type of high-throughput experimentation has been applied in human endotoxemia [51]. By combining gene expression data with a clustering technique designed to identify key profiles in high-dimensional timecourse data [52], we identified three essential expression motifs: (i) upregulation of pro-inflammatory signaling (P); (ii) upregulation of anti-inflammatory signaling (A); and (iii) downregulation of cellular bio-energetic processes (E) [15].
These critical transcriptional responses were then used to define variables in a physicochemical [53] model which linked LPS recognition with its transcriptional effects through the NF-κB pathway [15]. NF-κB serves as a prototypical transcription factor modulating the production of inflammatory genes in response to TLR4 activation. In total, the network defined in this model accounts for both a normal self-limited endotoxemia response as well as a chronic heightened inflammatory state that can persist in the absence of LPS.
Endogenous and exogenous hormones
The ultimate translational goals of modeling endotoxemia necessitate the consideration not only of the transcriptional-level response to LPS, but also of other endogenous and exogenous influences, such as inflammation-modulating hormones. One of the key endogenous immunomodulatory pathways is the hypothalamic-pituitary-adrenal axis (HPA) which regulates inflammation through the production and release of glucocorticoids (cortisol in humans), which leads to the inhibition of pro-inflammatory cytokine expression [54]. In addition to regulation of inflammation by cortisol, other regulatory hormones responsive to stress such as epinephrine modulate immune functions [55]. Epinephrine secretion is stimulated by sympathetic nervous system (SNS) activity, leading to immunomodulatory effects mediated by binding to receptors on immune cells. Pro-inflammatory cytokines recognized by the afferent vagus nerve stimulate central components of the stress response system [56], leading to the secretion of cortisol and epinephrine. Additionally, both cortisol and epinephrine lead to anti-inflammatory signaling, cortisol through glucocorticoid receptor-mediated signaling and epinephrine through adrenergic stimulation and elevated intracellular cAMP levels [57,58]. In addition to regulating the release of hormones, the central nervous system also more directly modulates inflammation through the cholinergic anti-inflammatory pathway, which consists of the parasympathetic nervous system releasing acetylcholine which regulates the immune response in real time [59]. Given the wide distribution of vagal efferents through peripheral tissues, the cholinergic anti-inflammatory pathway can exert a broad and significant anti-inflammatory effect.
Immunomodulatory hormones have been studied in human endotoxemia experiments where exogenous hormone infusion is given [60,57,61,62]. We applied established pharmacokinetic/pharmacodynamic models of hormone activity [63] to account for the joint endogenous and exogenous effects of both cortisol and epinephrine. By integrating indirect response models of these anti-inflammatory hormones, we were able to investigate how cellular-level transcriptional responses to inflammation are governed by systemic hormonal cues [16], such as how the response to these hormones can determine whether the system will undergo a healthy self-limited response to LPS and a return to homeostasis or whether it will instead go to a persistent chronic inflammatory state. This integrated model of both hormonal and transcriptional responses to LPS also served as a platform for further research on biological rhythms and physiologic variability in endotoxemia.
Physiological rhythms
Circadian rhythms
The presence of circadian rhythms plays a critical role in immune function in general [64] and also specifically in endotoxemia [65,66]. To begin accounting for these relationships within a model of endotoxemia, we attempted to define the relationship between circadian rhythms and inflammatory mediators [20]. By leveraging hormone pharmacokinetic/pharmacodynamic models [67] that also account for the circadian production for both cortisol and melatonin and modeling their downstream effects, we produced an extended model which reproduced homeostatic diurnal patterns in inflammatory mediators and hypothesized about the time dependence of the response to endotoxemia in the presence of circadian rhythms, illustrating the critical role that the presence of biological rhythms can have on outcome [20].
Ultradian rhythms
In addition to circadian rhythmicity operating with a 24 hour period, there are relevant biological rhythms at other frequencies as well. Cortisol’s circadian rhythms is, more fundamentally, a higher-level view of a higher frequency rhythm called an ultradian rhythm. Cortisol is secreted in roughly hourly bursts that have garnered much interest in recent years towards unraveling their physiological implications [68]. The rapid binding interaction between activated glucocorticoid receptor (GR) and DNA [69] leads to clearly pulsatile patterns transcriptional activity that track rhythmic glucocorticoid levels [70], producing broad transcriptional differences relative to a constant level of glucocorticoids that are only just beginning to be studied [71]. Along these lines, we explored how nonlinear ligand-receptor kinetics contribute to differential responses to ultradian and constant cortisol exposure in homeostasis [72]. In a follow-up study [73], we further investigated how altering the parameters governing HPA axis feedback loops leads to perturbed ultradian patterns in homeostatic rhythms, illustrating how analysis of biological rhythms can give insight into underlying mechanisms. Additionally, we tested the stress responsiveness of the HPA axis by quantifying the peak levels of glucocorticoid-responsive genes in response to acute CRH stimulation. This was based on a library of randomly generated HPA axis parameter values which produced a broad spectrum of oscillatory patterns. We found peak stress responsiveness to be proportional to the amplitude of ultradian rhythms, even when mean homeostatic gene transcript levels were the same. This again illustrates how analysis of biological rhythms can reveal critical information about the state and behavior of a physiological system.
While cytokine and hormone responses, as can be measured in the blood, are important markers of the state of inflammation and thus are critical components in a translational model of inflammation, these circulating mediators do not reveal the full inflammatory state of the host and they can be difficult or expensive to assess clinically, particularly at the sampling frequencies needed to fully capture higher frequency rhythms. This motivated our investigation into how physiological responses to endotoxemia are related to changes in HRV, which often correlates with disease severity and can be measured noninvasively [74].
Heart rate variability
Diminished HRV is a common clinical phenotype expressed by critically ill patients, and quantifying HRV can produce clinically useful information about the state of a patient. However, a quantitative understanding of the mechanisms by which underlying inflammatory processes modulate HRV is lacking, and thus most clinical applications HRV in this context are phenomenological. As human endotoxemia provides an excellent platform for studying these relationships in a controlled environment [75–77,22,78,60,62,79,80], our goal is formalize and extend these experimental observations through further experiments combined with semi-mechanistic modeling of the cardiovascular effects of endotoxemia.
Sympathetic and parasympathetic nerves converge at the sinoatrial (SA) node of the heart, and oscsillations in the release of autonomic neurotransmitters thus produce oscillatory patterns in the firing of action potentials and the beating of the heart. Circadian rhythms exert a clear pattern on HR and HRV. Homeostatic short-term (i.e. mush shorter than circadian) rhythms in HR exist largely in two frequency bands [81,82]. High frequency (HF) rhythms, ranging from 0.15–0.4 Hz, are driven largely by the respiratory sinus arrhythmia, which is transduced to the heart via the vagus nerve [83]. Low frequency (LF) rhythms, ranging from 0.04–0.15 Hz, have more uncertain physiological interpretations, but are driven in part to baroreflex-mediated fluctuations in blood pressure and respond to changes in both sympathetic and parasympathetic activity. The relationship between blood pressure and HRV is interesting in the context of inflammation, where changes in HR and autonomic activity both alter peripheral resistance and thus regulate blood pressure. This feedback loop has been implicated in driving oscillatory patterns in homeostatic HRV [84]. While the spectral power in each frequency band (LF and HF) gives some indication of autonomic activity and their ratio is commonly used to quantify sympathovagal balance, these metrics really are, at best, indirect and imprecise measures of autonomic activity [85].
Given that systems-level properties of biological oscillators can be studied through quantitative modeling [4], we developed a model of HR and HRV modulation in human endotoxemia based on the concept of multiple rhythmic signals regulating the pattern of heart beats through a continuous model of autonomic influence on the heart, accounting for changes in both rate and variability, combined with a discrete model that outputs a series of heart beats for further analysis [21]. This translation from a continuous oscillatory system output to a noisy discrete output is an essential step in modeling a fundamentally discrete process like the beating of the heart.
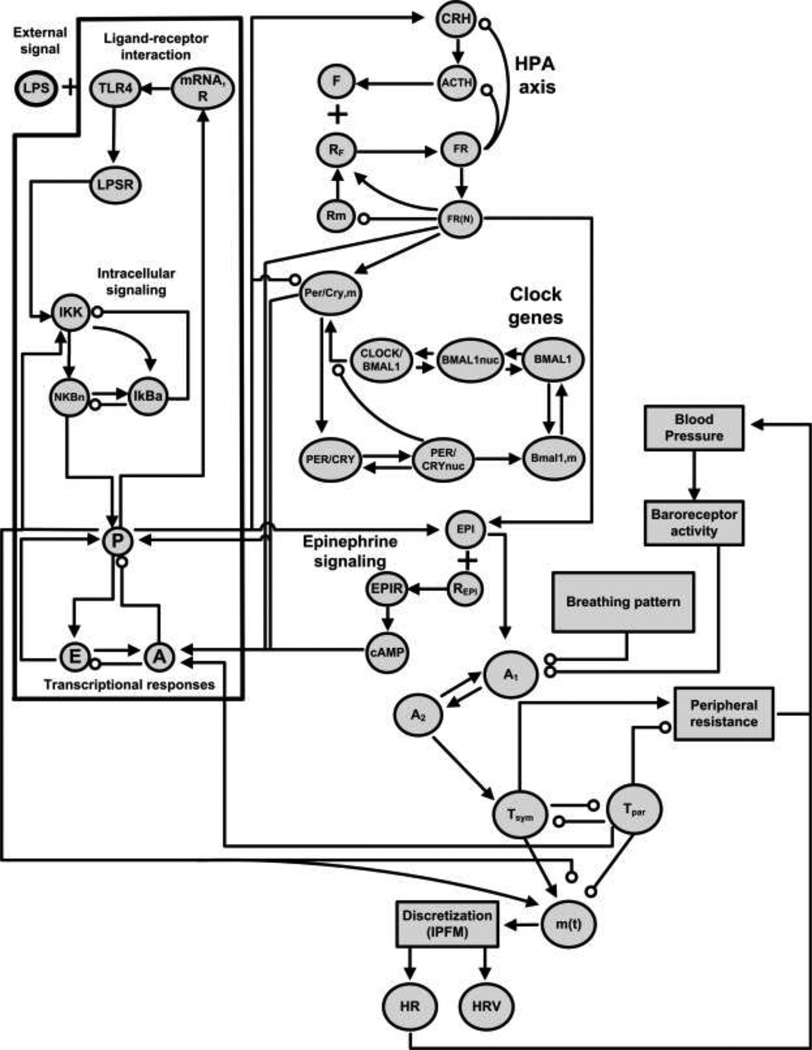
A conceptual extension to this model is shown in Fig. 2. In addition to the components described above, Fig. 2 adds several sources of relevant physiologic variability: ultradian cortisol rhythms generated by the HPA axis, peripheral clock genes in immune cells, baroreflex-mediated blood pressure oscillations, and rhythmic breathing patterns. The latter two are reflected relatively directly in short-term HRV patterns, and the sensitivity of the heart to those rhythms is governed by the state of the inflammatory response. The network structure in Fig. 2 also fully encompasses the homeostatic rhythms shown in Fig. 1. Through increasingly mechanistic modeling of HR and HRV in human endotoxemia, we are continuing to work towards understanding the biological processes linking inflammation and physiologic rhythmicity [86].
Fig. 2.
Network structure of a model containing the cellular-level responses to LPS, central secretion of immunomodulatory hormones, the production of discrete heart beats, and the modulation of HR patterns by rhythmic autonomic signals. All of the periodic components shown in Fig. 1 are reflected in this network. Most of these interactions are described in more detail in [21].
Translational applications of models of inflammation
Analysis of HRV, in particular, has intriguing translational potential due to its broad availability through noninvasive measurement and its ability to give insight into the progression and recovery from diseases involving systemic infection and inflammation. Extrinsic signals converge on the sinoatrial node of the heart and regulate the pattern of heart beats [87]. Within the context of the relationship between rhythms and negative feedback loops, the loss of HRV can then be seen as a dysregulation of physiological regulatory systems. Thus, when the loss of HRV is linked to poor clinical outcomes [88], it is not necessarily that the variability itself is important, it is what the variability tells us about the integrity of the rhythmic systems that give rise to HRV, across time scales from high frequency neural oscillations to circadian rhythms. For instance, the baroreflex maintains homeostatic blood pressure levels through a negative feedback loop. In sepsis, the baroreceptor mechanism is perturbed, producing changes in neural oscillations which can be quantified through HRV analysis [89]. In other words, some of the loss of rhyhthmicity observed in HRV in sepsis may be a reflection of dysregulated baroreflex activity. This type of homeostatic variability in physiological control systems allows for robust responses to disturbances, and studying the mechanistic origins of rhythms in HRV with the concept of negative feedback loops in mind will allow for the rational quantification of how this signal changes in response to stress.
Despite this, mechanistic links between inflammation and HRV remain unclear. Although it is difficult to generalize about how a change in HRV relates to underlying physiology [90], in specific contexts HRV has been shown to correlate with particular disease states and physiological processes. Recent studies have demonstrated potential clinical applications of predictive HRV analysis in trauma patients [91–94] and in sepsis [95,8]. Endotoxemia experiments have begun to elucidate some mechanistic details by assessing changes in HRV along with other biomarkers in response to LPS [75–77,22,78,60,62,79,80], although significant work remains in deciphering the mechanistic details.
Ultimately, the mechanistic linkages between changes in heart beat patterns and the progression of disease are poorly understood. For example, given that the sympathetic and parasympathetic branches of the autonomic nervous system innervate the sinoatrial node of the heart, changes in autonomic outflow in inflammation likely propagate to the heart via these nerves and regulate beating patterns. This creates an apparent paradox, given that HRV decreases in endotoxemia experiments, precisely the opposite that one would expect to happen given what we know about how the activation of the autonomic nervous system as a component of the inflammatory response [96,78,75]. This paradox has been explored by recent experimental work. In [96] it was shown that pathogen-mediated effects on cardiac function, which are mediated by the vagus nerve, desensitize the heart’s response to vagal signaling in mice. This is further supported by a rat study directly measuring an increase in vagal activity in endotoxemia [97]. In humans, endotoxemia was shown to produce a decrease in sympathetic activity, as assessed in the peroneal nerve [78], despite the fact that conventional wisdom holds that sympathetic activity predominates in endotoxemia due to changes in heart rate and hormone levels that are typically indicative of sympathetic activation. Although peroneal nerve activity is not necessarily the same as sympathetic activity in other tissues, namely the heart [80], it was also shown that cardiac sensitivity to drug-induced sympathetic modulation was diminished in endotoxemia [78], similar to the previous study on the vagus nerve.
Thus, there is mounting evidence that signal transduction from the autonomic nervous system to the heart is diminished in endotoxemia, producing a decrease in HRV. Furthermore, in vitro experiments suggest that more direct interactions between inflammation and cardiac tissue may be reflected in altered beating patterns which may also contribute to diminished sensitivity to the autonomic nervous system as well as increased heart rate and possibly changes in variability [98,99,75]. Even in this highly studied, highly clinically relevant system, the complexity of rhythms generated and regulated by multiple sources make it difficult to precisely determine the relevant signaling pathways. However, this complexity also means that there is a wealth of information embedded in HRV. This presents an excellent opportunity to apply computational techniques to attempt to solve this problem. It has been hypothesized that a reduction in HRV and physiologic variability in general reflect increased isolation of the heart from other organs [100], which is a concept with theoretical underpinnings provided by mathematical analysis of coupled oscillators [101]. If this is true, then reduced HRV may reflect systemic-level loss of inter-organ communication [26,102]. A mathematical model has the ability to represent the dynamic interactions between network components, taking advantage of mechanistic knowledge to unravel how intermediate components (cytokines, hormones) ultimately regulate systemic output (HRV).
While the lack of precise mechanistic understanding of the interplay between inflammation and HRV has not fully limited practical applications [8], novel opportunities and strategies will become apparent with increasing understanding of these systems. For instance, the discovery of the mechanistic link between the vagus nerve and inflammation [103] has lead to promising preliminary results on applications of vagus nerve stimulation in the treatment of inflammatory diseases [104].
While HRV may be the most promising metric of physiologic variability, in part due to the ease of noninvasive assessment, inflammation also alters rhythmic characteristics of other physiological signals. Peripheral clock genes are entrained by circadian hormone patterns [105] and there is bidirectional feedback between cytokine expression and clock gene expression [106,107]. This is particularly interesting in light of the associations between dysregulated circadian rhythmicity and disease discussed above. Furthermore, human endotoxemia at different times throughout the day suppresses and synchronizes clock gene expression in peripheral blood leukocytes [65]. This decoupling between central and peripheral circadian controls is particularly interesting given the interactions between circadian rhythms and disease [108], which prompted our recent investigation into the entrainment of peripheral clock genes by cortisol [109]. Thus, it is important to consider how feedback between the rhythmic signals in Fig. 1 is important in pathological conditions. As another example, elevated cytokine levels can lead to altered breathing patterns, which in turn can exert regulatory effects on the inflammatory response [86], raising the issue of the importance of rhythmicity in pathophysiological conditions where inflammation and respiration are simultaneously dysregulated such as obstructive sleep apnea.
These relationships between physiological rhythms, inter-organ communication, and disease collectively represent a plausible mechanism for both the diagnostic value of reduction in signal complexity and the potential clinical benefits of restoration of complexity. However, the hypothesis that variability is generally beneficial is not reflected in many current clinical practices, which typically are designed to modulate the patient’s state to a fixed point deemed healthy without regard for homeostatic variability [26]. Much of our critical care infrastructure is designed to eliminate rhythmic physiological patterns in the patient by imposing constant treatment, such as through ventilators, glucose control, and continuous feeding, although the benefits of these types of invariant clinical management practices are under study [26,102]. Furthermore, the effects of disrupted sleep patterns on circadian rhythmicity may exacerbate the implications of disease and of exogenous therapies designed to reduce physiological variability.
The past computational and experimental studies described here have certainly contributed towards unraveling the complex physiological mechanisms leading to clinically relevant alterations rhythmic signals in human endotoxemia and critical illness. As further progress is made to improve our mechanistic knowledge, we expect translational applications of these concepts to become increasingly effective and common.
Acknowledgements
PDM and IPA acknowledge support from NIH GM082974. PDM, JDS, and SEC are supported, in part, from NIH GM34695.
References
- 1.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8(10):776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Namas R, Zamora R, Namas R, An G, Doyle J, Dick TE, Jacono FJ, Androulakis IP, Nieman GF, Chang S, Billiar TR, Kellum JA, Angus DC, Vodovotz Y. Sepsis: Something old, something new, a systems view. J Crit Care. 2011;27(3):314 e311–314 e311. doi: 10.1016/j.jcrc.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novak B, Tyson JJ. Design principles of biochemical oscillators. Nat Rev Mol Cell Biol. 2008;9(12):981–991. doi: 10.1038/nrm2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pigolotti S, Krishna S, Jensen MH. Oscillation patterns in negative feedback loops. Proc Natl Acad Sci U S A. 2007;104(16):6533–6537. doi: 10.1073/pnas.0610759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29(7):823–864. doi: 10.1210/er.2008-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin MP, O'Shea TM, Bissonette EA, Harrell FE, Jr, Lake DE, Moorman JR. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res. 2003;53(6):920–926. doi: 10.1203/01.PDR.0000064904.05313.D2. [DOI] [PubMed] [Google Scholar]
- 8.Moorman JR, Carlo WA, Kattwinkel J, Schelonka RL, Porcelli PJ, Navarrete CT, Bancalari E, Aschner JL, Whit Walker M, Perez JA, Palmer C, Stukenborg GJ, Lake DE, Michael O'Shea T. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr. 2011;159(6):900 e901–906 e901. doi: 10.1016/j.jpeds.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40(2):79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- 10.Rosmond R, Dallman MF, Bjorntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83(6):1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 11.Polk DE, Cohen S, Doyle WJ, Skoner DP, Kirschbaum C. State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology. 2005;30(3):261–272. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 13.Mormont MC, Levi F. Circadian-system alterations during cancer processes: a review. Int J Cancer. 1997;70(2):241–247. doi: 10.1002/(sici)1097-0215(19970117)70:2<241::aid-ijc16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Lowry SF. Human endotoxemia: a model for mechanistic insight and therapeutic targeting. Shock. 2005;24(Suppl 1):94–100. doi: 10.1097/01.shk.0000191340.23907.a1. [DOI] [PubMed] [Google Scholar]
- 15.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Modeling endotoxin-induced systemic inflammation using an indirect response approach. Math Biosci. 2009;217(1):27–42. doi: 10.1016/j.mbs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. In silico simulation of corticosteroids effect on an NFkB- dependent physicochemical model of systemic inflammation. PLoS One. 2009;4(3):e4706. doi: 10.1371/journal.pone.0004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Multiscale model for the assessment of autonomic dysfunction in human endotoxemia. Physiol Genomics. 2010;42(1):5–19. doi: 10.1152/physiolgenomics.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. A physiological model for autonomic heart rate regulation in human endotoxemia. Shock. 2011;35(3):229–239. doi: 10.1097/SHK.0b013e318200032b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong X, Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Agent-based modeling of endotoxin-induced acute inflammatory response in human blood leukocytes. PLoS One. 2010;5(2):e9249. doi: 10.1371/journal.pone.0009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Modeling the influence of circadian rhythms on the acute inflammatory response. J Theor Biol. 2010;264(3):1068–1076. doi: 10.1016/j.jtbi.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Scheff JD, Mavroudis PD, Calvano SE, Lowry SF, Androulakis IP. Modeling autonomic regulation of cardiac function and heart rate variability in human endotoxemia. Physiol Genomics. 2011;43(16):951–964. doi: 10.1152/physiolgenomics.00040.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rassias AJ, Holzberger PT, Givan AL, Fahrner SL, Yeager MP. Decreased physiologic variability as a generalized response to human endotoxemia. Crit Care Med. 2005;33(3):512–519. doi: 10.1097/01.ccm.0000155908.46346.ed. [DOI] [PubMed] [Google Scholar]
- 23.Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Moller K. Human endotoxemia as a model of systemic inflammation. Curr Med Chem. 2008;15(17):1697–1705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- 24.Buttenschoen K, Kornmann M, Berger D, Leder G, Beger HG, Vasilescu C. Endotoxemia and endotoxin tolerance in patients with ARDS. Langenbecks Arch Surg. 2008;393(4):473–478. doi: 10.1007/s00423-008-0317-3. [DOI] [PubMed] [Google Scholar]
- 25.Shanker B-A, Coyle SM, Reddell MT, Choi CW, Calvano J, Macor MA, Calvano SE, Lowry SF. Modeling the human injury response. Journal of the American College of Surgeons. 211(Supplement 1)(3):S53–S54. [Google Scholar]
- 26.Lowry SF. The stressed host response to infection: the disruptive signals and rhythms of systemic inflammation. Surg Clin North Am. 2009;89(2):311–326. doi: 10.1016/j.suc.2008.09.004. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deans KJ, Haley M, Natanson C, Eichacker PQ, Minneci PC. Novel therapies for sepsis: a review. J Trauma. 2005;58(4):867–874. doi: 10.1097/01.ta.0000158244.69179.94. [DOI] [PubMed] [Google Scholar]
- 28.Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2(5):391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 29.Freeman BD, Natanson C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin Investig Drugs. 2000;9(7):1651–1663. doi: 10.1517/13543784.9.7.1651. [DOI] [PubMed] [Google Scholar]
- 30.Vodovotz Y, Constantine G, Rubin J, Csete M, Voit EO, An G. Mechanistic simulations of inflammation: current state and future prospects. Math Biosci. 2009;217(1):1–10. doi: 10.1016/j.mbs.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day J, Rubin J, Vodovotz Y, Chow CC, Reynolds A, Clermont G. A reduced mathematical model of the acute inflammatory response II. Capturing scenarios of repeated endotoxin administration. J Theor Biol. 2006;242(1):237–256. doi: 10.1016/j.jtbi.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Kumar R, Clermont G, Vodovotz Y, Chow CC. The dynamics of acute inflammation. J Theor Biol. 2004;230(2):145–155. doi: 10.1016/j.jtbi.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds A, Rubin J, Clermont G, Day J, Vodovotz Y, Bard Ermentrout G. A reduced mathematical model of the acute inflammatory response: I. Derivation of model and analysis of anti-inflammation. J Theor Biol. 2006;242(1):220–236. doi: 10.1016/j.jtbi.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Arciero JC, Ermentrout GB, Upperman JS, Vodovotz Y, Rubin JE. Using a mathematical model to analyze the role of probiotics and inflammation in necrotizing enterocolitis. PLoS One. 2010;5(4):e10066. doi: 10.1371/journal.pone.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riviere B, Epshteyn Y, Swigon D, Vodovotz Y. A simple mathematical model of signaling resulting from the binding of lipopolysaccharide with Toll-like receptor 4 demonstrates inherent preconditioning behavior. Math Biosci. 2009;217(1):19–26. doi: 10.1016/j.mbs.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieman G, Brown D, Sarkar J, Kubiak B, Ziraldo C, Dutta-Moscato J, Vieau C, Barclay D, Gatto L, Maier K, Constantine G, Billiar TR, Zamora R, Mi Q, Chang S, Vodovotz Y. A two-compartment mathematical model of endotoxin-induced inflammatory and physiologic alterations in swine. Crit Care Med. 2012;40(4):1052–1063. doi: 10.1097/CCM.0b013e31823e986a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres A, Bentley T, Bartels J, Sarkar J, Barclay D, Namas R, Constantine G, Zamora R, Puyana JC, Vodovotz Y. Mathematical modeling of posthemorrhage inflammation in mice: studies using a novel, computer-controlled, closed-loop hemorrhage apparatus. Shock. 2009;32(2):172–178. doi: 10.1097/SHK.0b013e318193cc2b. [DOI] [PubMed] [Google Scholar]
- 38.Yang Q, Calvano SE, Lowry SF, Androulakis IP. A dual negative regulation model of Toll-like receptor 4 signaling for endotoxin preconditioning in human endotoxemia. Math Biosci. 2011;232(2):151–163. doi: 10.1016/j.mbs.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Daun S, Rubin J, Vodovotz Y, Roy A, Parker R, Clermont G. An ensemble of models of the acute inflammatory response to bacterial lipopolysaccharide in rats: results from parameter space reduction. J Theor Biol. 2008;253(4):843–853. doi: 10.1016/j.jtbi.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Li NY, Vodovotz Y, Kim KH, Mi Q, Hebda PA, Abbott KV. Biosimulation of acute phonotrauma: an extended model. Laryngoscope. 2011;121(11):2418–2428. doi: 10.1002/lary.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown BN, Price IM, Toapanta FR, DeAlmeida DR, Wiley CA, Ross TM, Oury TD, Vodovotz Y. An agent-based model of inflammation and fibrosis following particulate exposure in the lung. Math Biosci. 2011;231(2):186–196. doi: 10.1016/j.mbs.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li NY, Vodovotz Y, Hebda PA, Abbott KV. Biosimulation of inflammation and healing in surgically injured vocal folds. Ann Otol Rhinol Laryngol. 2010;119(6):412–423. doi: 10.1177/000348941011900609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li NY, Verdolini K, Clermont G, Mi Q, Rubinstein EN, Hebda PA, Vodovotz Y. A patient-specific in silico model of inflammation and healing tested in acute vocal fold injury. PLoS One. 2008;3(7):e2789. doi: 10.1371/journal.pone.0002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mi Q, Riviere B, Clermont G, Steed DL, Vodovotz Y. Agent-based model of inflammation and wound healing: insights into diabetic foot ulcer pathology and the role of transforming growth factor-beta1. Wound Repair Regen. 2007;15(5):671–682. doi: 10.1111/j.1524-475X.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 45.An G. In silico experiments of existing and hypothetical cytokine-directed clinical trials using agent-based modeling. Crit Care Med. 2004;32(10):2050–2060. doi: 10.1097/01.ccm.0000139707.13729.7d. [DOI] [PubMed] [Google Scholar]
- 46.An G. Introduction of an agent-based multi-scale modular architecture for dynamic knowledge representation of acute inflammation. Theor Biol Med Model. 2008;5:11. doi: 10.1186/1742-4682-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An G, Mi Q, Dutta-Moscato J, Vodovotz Y. Agent-based models in translational systems biology. Wiley Interdiscip Rev Syst Biol Med. 2009;1(2):159–171. doi: 10.1002/wsbm.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim M, Christley S, Alverdy JC, Liu D, An G. Immature oxidative stress management as a unifying principle in the pathogenesis of necrotizing enterocolitis: insights from an agent-based model. Surg Infect (Larchmt) 2012;13(1):18–32. doi: 10.1089/sur.2011.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seal JB, Alverdy JC, Zaborina O, An G. Agent-based dynamic knowledge representation of Pseudomonas aeruginosa virulence activation in the stressed gut: Towards characterizing host-pathogen interactions in gut-derived sepsis. Theor Biol Med Model. 2011;8:33. doi: 10.1186/1742-4682-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mi Q, Constantine G, Ziraldo C, Solovyev A, Torres A, Namas R, Bentley T, Billiar TR, Zamora R, Puyana JC, Vodovotz Y. A dynamic view of trauma/hemorrhage-induced inflammation in mice: principal drivers and networks. PLoS One. 2011;6(5):e19424. doi: 10.1371/journal.pone.0019424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature. 2005;437(7061):1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 52.Yang EH, Almon RR, Dubois DC, Jusko WJ, Androulakis IP. Identification of global transcriptional dynamics. PLoS One. 2009;4(7):e5992. doi: 10.1371/journal.pone.0005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aldridge BB, Burke JM, Lauffenburger DA, Sorger PK. Physicochemical modelling of cell signalling pathways. Nat Cell Biol. 2006;8(11):1195–1203. doi: 10.1038/ncb1497. [DOI] [PubMed] [Google Scholar]
- 54.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 55.Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24(8):444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 56.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 57.van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97(3):713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Poll T. Effects of Catecholamines on the Inflammatory Response. Sepsis. 2000;4:159–167. [Google Scholar]
- 59.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9(5–8):125–134. [PMC free article] [PubMed] [Google Scholar]
- 60.Alvarez SM, Katsamanis Karavidas M, Coyle SM, Lu SE, Macor M, Oikawa LO, Lehrer PM, Calvano SE, Lowry SF. Low-dose steroid alters in vivo endotoxin-induced systemic inflammation but does not influence autonomic dysfunction. J Endotoxin Res. 2007;13(6):358–368. doi: 10.1177/0968051907086465. [DOI] [PubMed] [Google Scholar]
- 61.van der Poll T, Barber AE, Coyle SM, Lowry SF. Hypercortisolemia increases plasma interleukin-10 concentrations during human endotoxemia--a clinical research center study. J Clin Endocrinol Metab. 1996;81(10):3604–3606. doi: 10.1210/jcem.81.10.8855809. [DOI] [PubMed] [Google Scholar]
- 62.Jan BU, Coyle SM, Oikawa LO, Lu SE, Calvano SE, Lehrer PM, Lowry SF. Influence of acute epinephrine infusion on endotoxin-induced parameters of heart rate variability: a randomized controlled trial. Ann Surg. 2009;249(5):750–756. doi: 10.1097/SLA.0b013e3181a40193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramakrishnan R, DuBois DC, Almon RR, Pyszczynski NA, Jusko WJ. Fifth-generation model for corticosteroid pharmacodynamics: application to steady-state receptor down-regulation and enzyme induction patterns during seven-day continuous infusion of methylprednisolone in rats. J Pharmacokinet Pharmacodyn. 2002;29(1):1–24. doi: 10.1023/a:1015765201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coogan AN, Wyse CA. Neuroimmunology of the circadian clock. Brain Res. 2008;1232:104–112. doi: 10.1016/j.brainres.2008.07.087. [DOI] [PubMed] [Google Scholar]
- 65.Haimovich B, Calvano J, Haimovich AD, Calvano SE, Coyle SM, Lowry SF. In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Crit Care Med. 2010;38(3):751–758. doi: 10.1097/CCM.0b013e3181cd131c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pollmacher T, Mullington J, Korth C, Schreiber W, Hermann D, Orth A, Galanos C, Holsboer F. Diurnal variations in the human host response to endotoxin. J Infect Dis. 1996;174(5):1040–1045. doi: 10.1093/infdis/174.5.1040. [DOI] [PubMed] [Google Scholar]
- 67.Chakraborty A, Krzyzanski W, Jusko WJ. Mathematical modeling of circadian cortisol concentrations using indirect response models: Comparison of several methods. Journal of Pharmacokinetics and Biopharmaceutics. 1999;27(1):23–43. doi: 10.1023/a:1020678628317. [DOI] [PubMed] [Google Scholar]
- 68.Lightman SL, Conway-Campbell BL. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci. 2010;11(10):710–718. doi: 10.1038/nrn2914. [DOI] [PubMed] [Google Scholar]
- 69.McNally JG, Muller WG, Walker D, Wolford R, Hager GL. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science. 2000;287(5456):1262–1265. doi: 10.1126/science.287.5456.1262. [DOI] [PubMed] [Google Scholar]
- 70.Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11(9):1093–1102. doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McMaster A, Jangani M, Sommer P, Han N, Brass A, Beesley S, Lu W, Berry A, Loudon A, Donn R, Ray DW. Ultradian cortisol pulsatility encodes a distinct, biologically important signal. PLoS One. 2011;6(1):e15766. doi: 10.1371/journal.pone.0015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheff JD, Kosmides AK, Calvano SE, Lowry SF, Androulakis IP. Pulsatile glucocorticoid secretion: origins and downstream effects. IEEE Trans Biomed Eng. 2011;58(12):3504–3507. doi: 10.1109/TBME.2011.2162236. [DOI] [PubMed] [Google Scholar]
- 73.Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Transcriptional implications of ultradian glucocorticoid secretion in homeostasis and in the acute stress response. Physiol Genomics. 2012;44(2):121–129. doi: 10.1152/physiolgenomics.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmidt HB, Werdan K, Muller-Werdan U. Autonomic dysfunction in the ICU patient. Curr Opin Crit Care. 2001;7(5):314–322. doi: 10.1097/00075198-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Gholami M, Mazaheri P, Mohamadi A, Dehpour T, Safari F, Hajizadeh S, Moore KP, Mani AR. Endotoxemia is Associated With Partial Uncoupling of Cardiac Pacemaker From Cholinergic Neural Control in Rats. Shock. 2012;37(2):219–227. doi: 10.1097/SHK.0b013e318240b4be. [DOI] [PubMed] [Google Scholar]
- 76.Godin PJ, Fleisher LA, Eidsath A, Vandivier RW, Preas HL, Banks SM, Buchman TG, Suffredini AF. Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Crit Care Med. 1996;24(7):1117–1124. doi: 10.1097/00003246-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 77.Rassias AJ, Guyre PM, Yeager MP. Hydrocortisone at stress-associated concentrations helps maintain human heart rate variability during subsequent endotoxin challenge. J Crit Care. 2011 doi: 10.1016/j.jcrc.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sayk F, Vietheer A, Schaaf B, Wellhoener P, Weitz G, Lehnert H, Dodt C. Endotoxemia causes central downregulation of sympathetic vasomotor tone in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R891–898. doi: 10.1152/ajpregu.90444.2008. [DOI] [PubMed] [Google Scholar]
- 79.Jan BU, Coyle SM, Macor MA, Reddell M, Calvano SE, Lowry SF. Relationship of basal heart rate variability to in vivo cytokine responses after endotoxin exposure. Shock. 2010;33(4):363–368. doi: 10.1097/SHK.0b013e3181b66bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kox M, Ramakers BP, Pompe JC, van der Hoeven JG, Hoedemaekers CW, Pickkers P. Interplay between the acute inflammatory response and heart rate variability in healthy human volunteers. Shock. 2011;36(2):115–120. doi: 10.1097/SHK.0b013e31821c2330. [DOI] [PubMed] [Google Scholar]
- 81.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 82.Task Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 83.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 84.deBoer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am J Physiol. 1987;253(3 Pt 2):H680–H689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- 85.Karemaker JM. Autonomic integration: the physiological basis of cardiovascular variability. J Physiol. 1999;517(Pt 2):316. doi: 10.1111/j.1469-7793.1999.0316t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dick TE, Molkov YI, Nieman G, Hsieh YH, Jacono FJ, Doyle J, Scheff JD, Calvano SE, Androulakis IP, An G, Vodovotz Y. Linking Inflammation, Cardiorespiratory Variability, and Neural Control in Acute Inflammation via Computational Modeling. Front Physiol. 2012;3:222. doi: 10.3389/fphys.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47(4):658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 88.Stys A, Stys T. Current clinical applications of heart rate variability. Clin Cardiol. 1998;21(10):719–724. doi: 10.1002/clc.4960211005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Annane D, Trabold F, Sharshar T, Jarrin I, Blanc AS, Raphael JC, Gajdos P. Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. Am J Respir Crit Care Med. 1999;160(2):458–465. doi: 10.1164/ajrccm.160.2.9810073. [DOI] [PubMed] [Google Scholar]
- 90.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96(9):3224–3232. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 91.Batchinsky AI, Skinner JE, Necsoiu C, Jordan BS, Weiss D, Cancio LC. New measures of heart-rate complexity: effect of chest trauma and hemorrhage. J Trauma. 2010;68(5):1178–1185. doi: 10.1097/TA.0b013e3181bb98a6. [DOI] [PubMed] [Google Scholar]
- 92.Cancio LC, Batchinsky AI, Salinas J, Kuusela T, Convertino VA, Wade CE, Holcomb JB. Heart-rate complexity for prediction of prehospital lifesaving interventions in trauma patients. J Trauma. 2008;65(4):813–819. doi: 10.1097/TA.0b013e3181848241. [DOI] [PubMed] [Google Scholar]
- 93.Morris JA, Jr, Norris PR, Waitman LR, Ozdas A, Guillamondegui OD, Jenkins JM. Adrenal insufficiency, heart rate variability, and complex biologic systems: a study of 1,871 critically ill trauma patients. J Am Coll Surg. 2007;204(5):885–892. doi: 10.1016/j.jamcollsurg.2007.01.019. discussion 892-883. [DOI] [PubMed] [Google Scholar]
- 94.Riordan WP, Jr, Norris PR, Jenkins JM, Morris JA., Jr Early loss of heart rate complexity predicts mortality regardless of mechanism, anatomic location, or severity of injury in 2178 trauma patients. J Surg Res. 2009;156(2):283–289. doi: 10.1016/j.jss.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 95.Ahmad S, Ramsay T, Huebsch L, Flanagan S, McDiarmid S, Batkin I, McIntyre L, Sundaresan SR, Maziak DE, Shamji FM, Hebert P, Fergusson D, Tinmouth A, Seely AJ. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One. 2009;4(8):e6642. doi: 10.1371/journal.pone.0006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fairchild KD, Srinivasan V, Moorman JR, Gaykema RP, Goehler LE. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R330–R339. doi: 10.1152/ajpregu.00487.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang J, Wang Y, Jiang D, Zhou J, Huang X. The sympathetic-vagal balance against endotoxemia. J Neural Transm. 2010;117(6):729–735. doi: 10.1007/s00702-010-0407-6. [DOI] [PubMed] [Google Scholar]
- 98.Schmidt H, Saworski J, Werdan K, Muller-Werdan U. Decreased beating rate variability of spontaneously contracting cardiomyocytes after co-incubation with endotoxin. J Endotoxin Res. 2007;13(6):339–342. doi: 10.1177/0968051907086233. [DOI] [PubMed] [Google Scholar]
- 99.Takayama K, Yuhki K, Ono K, Fujino T, Hara A, Yamada T, Kuriyama S, Karibe H, Okada Y, Takahata O, Taniguchi T, Iijima T, Iwasaki H, Narumiya S, Ushikubi F. Thromboxane A2 and prostaglandin F2alpha mediate inflammatory tachycardia. Nat Med. 2005;11(5):562–566. doi: 10.1038/nm1231. [DOI] [PubMed] [Google Scholar]
- 100.Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 1996;24(7):1107–1116. doi: 10.1097/00003246-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 101.Pincus SM. Greater signal regularity may indicate increased system isolation. Math Biosci. 1994;122(2):161–181. doi: 10.1016/0025-5564(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 102.Lowry SF, Calvano SE. Challenges for modeling and interpreting the complex biology of severe injury and inflammation. J Leukoc Biol. 2008;83(3):553–557. doi: 10.1189/jlb.0607377. [DOI] [PubMed] [Google Scholar]
- 103.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 104.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, Ashok M, Goldstein RS, Chavan S, Pavlov VA, Metz CN, Yang H, Czura CJ, Wang H, Tracey KJ. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35(12):2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 105.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 106.Hashiramoto A, Yamane T, Tsumiyama K, Yoshida K, Komai K, Yamada H, Yamazaki F, Doi M, Okamura H, Shiozawa S. Mammalian clock gene Cryptochrome regulates arthritis via proinflammatory cytokine TNF-alpha. J Immunol. 2010;184(3):1560–1565. doi: 10.4049/jimmunol.0903284. [DOI] [PubMed] [Google Scholar]
- 107.Motzkus D, Albrecht U, Maronde E. The human PER1 gene is inducible by interleukin-6. J Mol Neurosci. 2002;18(1–2):105–109. doi: 10.1385/JMN:18:1-2:105. [DOI] [PubMed] [Google Scholar]
- 108.Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. 2007;47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- 109.Mavroudis PD, Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Entrainment of peripheral clock genes by cortisol. Physiol Genomics. 2012;44(11):607–621. doi: 10.1152/physiolgenomics.00001.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petrovsky N, Harrison LC. The chronobiology of human cytokine production. Int Rev Immunol. 1998;16(5–6):635–649. doi: 10.3109/08830189809043012. [DOI] [PubMed] [Google Scholar]
- 111.Donadio V, Cortelli P, Falzone F, Bugiardini E, Giuliani A, Misciali C, Montagna P, Calza L, Liguori R. Isolated generalised anhidrosis induced by postganglionic sympathetic skin nerve fibre degeneration: an incomplete Ross syndrome? J Neurol Neurosurg Psychiatry. 2008;79(8):959–961. doi: 10.1136/jnnp.2007.142802. [DOI] [PubMed] [Google Scholar]
- 112.Brown EN, Meehan PM, Dempster AP. A stochastic differential equation model of diurnal cortisol patterns. Am J Physiol Endocrinol Metab. 2001;280(3):E450–E461. doi: 10.1152/ajpendo.2001.280.3.E450. [DOI] [PubMed] [Google Scholar]
- 113.Charloux A, Gronfier C, Lonsdorfer-Wolf E, Piquard F, Brandenberger G. Aldosterone release during the sleep-wake cycle in humans. Am J Physiol. 1999;276(1 Pt 1):E43–E49. doi: 10.1152/ajpendo.1999.276.1.E43. [DOI] [PubMed] [Google Scholar]
- 114.Seydnejad SR, Kitney RI. Modeling of Mayer waves generation mechanisms. IEEE Eng Med Biol Mag. 2001;20(2):92–100. doi: 10.1109/51.917729. [DOI] [PubMed] [Google Scholar]
- 115.Octavio JA, Rodriguez AE, Misticchio F, Marcano A, Jimenez J, Moleiro F. Circadian profiles of heart rate and its instantaneous variability in patients with chronic Chagas' disease. Rev Esp Cardiol. 2004;57(2):130–137. [PubMed] [Google Scholar]