Abstract
While a variety of natural and synthetic matrices have been used to influence embryonic stem cell (ESC) self-renewal or differentiation, and ESCs also deposit a rich matrix of their own, the mechanisms behind how extracellular matrix affects cell fate are largely unexplored. The ESC matrix is continuously remodeled by matrix metalloproteinases (MMPs), a process that we find is enhanced by the presence of mouse embryonic fibroblast feeders in a paracrine manner. Matrix remodeling by MMPs aids in the self-renewal of ESCs, as inhibition of MMPs inhibits the ability of ESCs to self-renew. We also find that addition of the interstitial collagenase MMP1 is sufficient to maintain long-term LIF-independent mESC self-renewal in a dose-dependent manner. This remarkable ability is due to the presence of endogenously produced self-renewal-inducing signals, including the LIF-family ligand CNTF, that are normally trapped within the ECM and become exposed upon MMP-induced matrix remodeling to signal through JAK and Stat3. These results uncover a new role for feeder cells in maintaining self-renewal and show that mESCs normally produce sufficient levels of autocrine-acting pro-self-renewal ligands.
Keywords: embryonic stem cell, extracellular matrix, matrix metalloproteinase, self-renewal, Stat3
Introduction
Interactions between stem cells and their extracellular microenvironment influence tissue generation, maintenance and repair [1], but comparatively little is known about the contribution of endogenous extracellular components to stem cells cultured in vitro. We previously found that downregulation of soluble secreted signaling caused mouse embryonic stem cells (mESCs) to exit their self-renewing state, and that extracellular matrix (ECM) remodeling was necessary to retain mESC self-renewal in the absence of soluble autocrine cues [2]. To further probe the effects of matrix remodeling on maintaining mESC self-renewal, here we assessed the mechanism and specificity involved in this phenomenon.
Once an adherent cell is attached to a substrate, it begins to lay down an extracellular matrix composed of several types of proteins and connective fibers. During cell culture, the matrix is also continuously remodeled, the most prominent class of endogenously secreted remodeling enzymes being matrix metalloproteinases (MMPs) [3]. One function of the matrix is to act as a reservoir of growth factors and cytokines [4], and remodeling can allow for the release of these trapped signaling proteins [5]. Matrix-bound proteins, including FGF4, have been shown to have a profound effect on self-renewal [6], but the role of remodeling proteins on mESC self-renewal is unknown.
While several methods have recently been described for maintaining the self-renewal of mouse embryonic stem cells (mESCs) [7–10], routine culture generally involves a feeder layer composed of mitotically inactivated mouse embryonic fibroblasts (MEFs) coupled with exogenous addition of the cytokine leukemia inhibitory factor (LIF) [11]. These conditions have also been used to reprogram somatic cells to pluripotency after ectopic expression of defined factors [12–14]. MEFs are known to contribute to mESC self-renewal by the secretion of LIF [15, 16], and here we investigated whether the feeder layer has an additional role in remodeling the ECM. We then expanded on this to study the effect of matrix remodeling in non-feeder-based ESC cultures, finding that addition of a single collagenase is sufficient to maintain ESC self-renewal, and went on to explore the mechanism behind this remarkable effect.
Materials and Methods
Cell culture
Mouse ESCs (CCE, V6.5 [17], ABJ1 (Oct4-GFP) [18], Sox2-GFP [19], 7xTCF-eGFP [20], H2A-GFP, and H2B-GFP [21] lines) were routinely cultured in medium consisting of DMEM supplemented with 15% defined fetal bovine serum (Hyclone), 4 mM L-glutamine, 1 mM non-essential amino acids, 1X penicillin-streptomycin, 100 μM β-mercaptoethanol (Sigma) and 10 ng/mL LIF (ESGRO, Chemicon). All cell culture reagents were from Invitrogen unless otherwise noted. Cells were grown at 37°C in a humidified incubator with 7.5% CO2. For serum-free culture, N2B27 medium with 10 ng/mL LIF and 10 ng/mL BMP-4 (R&D Systems) was used [22] and wells were pre-coated with gelatin. 129 EpiSCs derived from the epiblast of day E5.5 mouse embryos [23] were cultured in medium consisting of DMEM/F12 supplemented with 15% defined fetal bovine serum (Hyclone), 5% Knockout serum replacement (Invitrogen), 2 mM L-glutamine, 1 mM non-essential amino acids, 1X; penicillin-streptomycin, 100 μM β-mercaptoethanol (Sigma) and 3.5 ng/mL bFgf. For feeder-free experiments, EpiSCs were cultured in N2B27 medium supplemented with 20 ng/ml Activin A (R&D Systems) and 12 ng/ml bFgf (R&D Systems) [24] and wells were pre-coated with human plasma fibronectin (Millipore). Mouse embryonic fibroblasts were cultured in DMEM supplemented with 3% fetal bovine serum (Hyclone), 4 mM L-glutamine and 1X penicillin-streptomycin. Transwell assays were performed in 6-well plates using 3.0 μm polyester membrane inserts (Corning).
Culture media additives
The following concentrations of culture media additives were used for all experiments: MMP1 50 ng/ml (Peprotech), Ro 32-3555 50 μM (Tocris), collagenase 20 μg/ml (Sigma #C9722), MMP2 50 ng/ml (Peprotech), MMP3 50 ng/ml (Peprotech), Retinoic Acid 1 μM (Sigma), PD0325901 1 μM (Stemgent), Bay 11-7085 50 μM (Tocris), Dkk 100 ng/ml (Peprotech), IWP2 2 μM (Sigma), Wnt3a 100 ng/ml (Peprotech), JAK inhibitor I 1 μM (Calbiochem), SB431542 1 μM (Sigma), LIF blocking antibody 500 ng/ml (R and D Systems), CT-1 10 ng/ml (Peprotech), CNTF 10 ng/ml (Peprotech), OSM 10 ng/ml (Peprotech), CT-1 blocking antibody 7.5 μg/ml (R and D Systems), CNTF blocking antibody 7.5 μg/ml (20 μg/ml for 4 day experiments) (R and D Systems), OSM blocking antibody 7.5 μg/ml (R and D Systems). For shRNA knockdowns, candidate shRNA hairpins were cloned into a packaging vector for transfection into Phoenix cells and subsequent infection into mESCs. shRNA-containing cells were GFP sorted and immediately plated for knockdown verification or experimental use. Stat3 shRNA with a starting nucleotide of 1346 had the following sequence: 5′- TGCTGTTGACAGTGAGCGACTGAGTTGAATTATCAGCTTATAGTGAAGCCACAGATGTATAAGCTGATAATTCAACTCAGGTGCCTACTGCCTCGGA -3′. Stat3 shRNA with a starting nucleotide of 1413 had the following sequence: 5′- TGCTGTTGACAGTGAGCGCCAGAGGGTCTCGGAAATTTAATAGTGAAGCCACAGATGTATTAAATTTCCGAGACCCTCTGATGCCTACTGCCTCGGA -3′. gp130 shRNA with a starting nucleotide of 909 had the following sequence: 5′- TGCTGTTGACAGTGAGCGACACCATATAATTTATCAGTGATAGTGAAGCCACAGATGTATCACTGATAAATTATATGGTGGTGCCTACTGCCTCGGA -3′.
Quantitative RT-PCR
Cells were harvested using TrypLE Express trypsin replacement (Invitrogen) and total RNA was isolated using the RNeasy Mini Kit (Qiagen), according to manufacturer's instructions. RNA was converted to cDNA using the ProtoScript cDNA synthesis kit with Oligo(dT) primer (New England Biolabs), and quantitative PCR reactions were set up using the iQ SYBR green supermix (Bio-Rad), according to manufacturer's instructions. Reactions were run on a Bio-Rad C1000 thermal cycler using a CFX96 real-time system. Primers are listed in Supporting Information Table S1.
Flow cytometry
Reporter cell lines were harvested and incubated with propidium iodide to identify dead cells before performing flow cytometry. Direct intracellular immunostaining was performed with an Alexa Fluor 647-linked anti-mouse Nanog antibody (eBioscience). Internal fluorescent intensity for all samples was measured on a FACSCaliber flow cytometer (BD Biosciences). Cells sorted for further analysis were sorted on a MoFlo sorter.
RNA-sequencing
mRNA libraries were prepared from total RNA isolated with Trizol (Invitrogen) and purified with Dynabeads mRNA purification kit (Invitrogen). RNA was fragmented using Ambion RNA Fragmentation Reagents (Invitrogen), first strand cDNA was prepared using SuperScript III Reverse Transcriptase (Invitrogen), and second strand cDNA was prepared using Second Strand Buffer (Invitrogen) and DNA Polymerase I (New England Biolabs). Whole transcriptome mRNA sequencing of barcoded samples was performed on an Illumina GAIIx and data was processed according to the Illumina pipeline – Firecrest as the image analysis module, Bustard as the base calling module, and Bowtie for sequence alignment. Reads were mapped to the mouse reference genome and RPKM values were generated. Heat map was generated using the freely downloadable software Java Treeview and Cluster 3.0.
Pluripotency assays
For embryoid body formation, ESCs were harvested from culture and replated at 4 × 105 cells in a 60-mm ultra low attachment culture dish (Corning). Cells were grown in ESC medium with no LIF, and medium was replenished every two days. For blastocyst injections, V6.5 ESCs were grown off feeders in serum-containing media in the presence of LIF or MMP1 for five passages, then were injected into host blastocysts (C57/B6 × DBA F2). Cells grown under identical conditions in the absence of LIF or MMP1 were no longer viable by five passages.
Elisa
Enzyme-linked immunosorbent assay was performed on cells grown in the indicated conditions for 48 hours. Results were normalized by total protein content of the samples. Conditioned media was spun down using an Amicon 3 kD cutoff filter spin column and ECM samples were collected from cells grown for five days in the indicated conditions by adding Liberase DL (Roche) at 0.13 Wünsch units/ml for 30min at 37°C and collecting the digested supernatant. Phospho-ERK ELISA was purchased from R and D Systems, and assay was performed according to manufacturer's instructions. Phospho-Stat3 ELISA was purchased from RayBioTech, and assay was performed according to manufacturer's instructions. CNTF ELISA was purchased from Antibodies-online Inc., and assay was performed according to manufacturer's instructions. For MMP activity assay, the Amplite Universal Fluorimetric MMP Activity Assay Kit was purchased from AAT Bioquest, and assay was performed according to manufacturer's instructions.
Immunofluorescence
For activated Stat3, cells were incubated overnight with primary phospo-Stat3 antibody (Cell Signaling Technology) at 1:100 and secondary (anti-rabbit AF488, Invitrogen) was added for two hours at 2 μg/ml. Cells were counterstained with 1:100000 Hoechst (Sigma).
Statistical analysis
All results were analyzed by student's T-test and the resulting pairwise p-values are reported. Significance was established at p<0.05, and was evaluated up to the level of p<0.001.
Results
Paracrine-mediated matrix remodeling
Previous work from our group indicated the importance of endogenous extracellular matrix remodeling proteins on maintenance of ESC self-renewal [2]. Because MMPs are the predominant class of matrix remodeling proteins, and because proteomic analysis has shown that feeder cells secrete matrix remodeling proteins including MMPs [25, 26], we sought to determine whether MEF feeder cells secrete functional MMPs that may contribute to mESC self-renewal in co-culture systems. We found that MEFs express and secrete high levels of active MMPs as compared to mESCs (Fig. 1A-B, Supporting Information Fig. S1A), and that this production is enhanced further when secreted signals from mESCs are present in the MEF culture media, using mESCs grown on a transwell insert (Fig. 1A). This indicates a paracrine-mediated effect in which mESCs induce MEFs to produce high levels of MMPs. To determine the specificity of this mESC-induced effect, we performed an identical experiment with mouse epiblast stem cell (EpiSC)-secreted factors instead of mESC-secreted factors and found that this transwell co-culture system did not upregulate MMP expression by MEFs (Fig. 1C), indicating that the induction is not a generic phenomenon but that mESC-secreted factors specifically induce MEFs to upregulate MMPs.
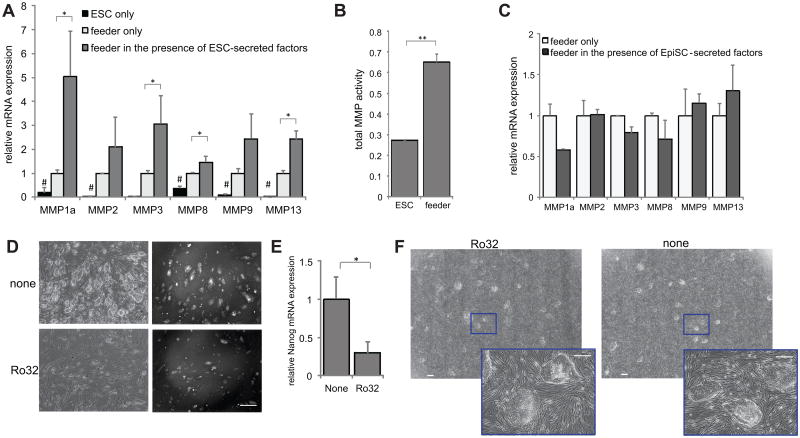
Figure 1.
MMP paracrine effect. (A) mRNA expression levels in ESCs, feeders, or feeders above which ESCs were growing on a transwell insert. (B) Total MMP activity for day 0-2 cultures, analyzed fluorimetrically. (C) mRNA expression levels in feeders or feeders above which EpiSCs were growing on a transwell insert. (D) Images of Oct4-GFP ESCs growing on feeders for two passages in the presence or absence of the MMP inhibitor Ro32-3555. Left panels show phase and right panels show GFP. Scale bar = 400μm. (E) mRNA expression levels of Nanog from sorted H2B-GFP mESCs grown for 4 days on feeders in the absence or presence of Ro32-3555. (F) Images of EpiSCs growing on feeders after two passages in the presence or absence of Ro32-3555. Scale bars = 400 μm. **=p<0.001, * =p<0.05; #=p<0.05 for all pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
We next sought to determine whether MEF-produced MMPs affect mESCs. We found that adding the collagenase-specific small molecule MMP inhibitor Ro32-3555 to a culture of mESCs and MEFs had no effect on MEF survival, but did lead to a decrease in stem cell number over serial passages, consistent with a gradual loss of self-renewal (Fig. 1D, Supporting Information Fig. S1B). Expression levels of the important self-renewal marker Nanog also decreased in mESCs grown on feeders in the presence of Ro32-3555 (Fig. 1E). However, EpiSCs grown on MEFs were not obviously affected in terms of cell number or morphology after serial passage in the presence of Ro32-3555 (Fig. 1F). This indicates that inhibition of collagen-specific MMPs in the presence of other soluble cues affects mESCs but not mEpiSCs, suggesting a potentially novel mechanism by which MEFs enhance ESC self-renewal. Because the effects of MMP inhibition may include growth or replating inefficiency and not merely a decrease in self-renewal capability, we next sought to examine the role of MMPs in mESC self-renewal specifically by adding MMP to pure mESC cultures at levels consistent with the levels secreted by MEFs.
MMP1 addition enhances mESC self-renewal
Because MMP1a is the most highly expressed metalloproteinase in the mESC-MEF paracrine system (Fig. 1A), and it is a collagenase, the inhibition of which was found to affect mESCs specifically, we added exogenous MMP1 to ESC cultures to assess the effect of matrix remodeling on self-renewal in the absence of feeders, using concentrations on the same order as those present in feeder-conditioned media (Supporting Information Fig. S1A). We found that addition of MMP1 was able to maintain mESC colony morphology in serum-based media in the absence of LIF after four days of growth (Fig. 2A), with relatively high expression of self-renewal markers Nanog and Rex1 and low expression of the early differentiation marker Fgf5 (Fig. 2B). MMP1, also known as interstitial collagenase, acts by cleaving collagen type I, II, and III. To ensure that the maintenance of mESC self-renewal was a functional effect of MMP1 activity, we also added type I crude collagenase to mESC cultures in the absence of LIF and found a similar upregulation of self-renewal markers and downregulation of Fgf5 (Fig. 2B, Supporting Information Fig. S2A).
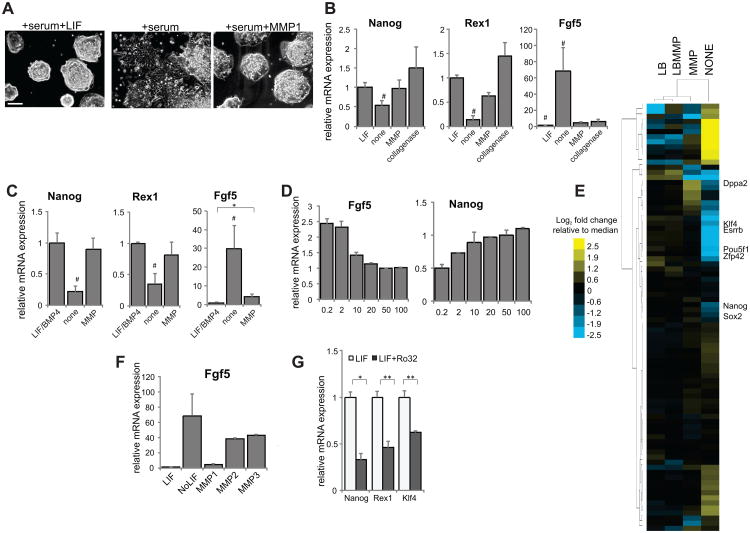
Figure 2.
Acute effects of collagenase addition. (A) Representative images of mESCs after four days of growth in the indicated conditions. Scale bar = 200μm. (B) mRNA expression levels of self-renewal and differentiation markers after four days in the indicated conditions in the presence of serum. (C) mRNA expression levels of self-renewal and differentiation markers after four days in the indicated conditions in serum-free media. (D) mRNA expression levels of Fgf5 and Nanog, x-axis numbers indicate the MMP1 concentration added in ng/ml. (E) Heatmap comparing RNA-seq data for genes in the gene ontology list of stem cell maintenance-related genes from cultures of cells grown with LIF/BMP4 (LB), MMP1, neither, or both, clustered hierarchically using a centroid linkage after median normalization and log2 transformation. (F) Fgf5 mRNA expression from cells grown in the presence of other recombinant MMPs. (G) mRNA expression levels of self-renewal markers for cells grown for two passages in the presence or absence of Ro32-3555. **=p<0.001, * =p<0.05; #=p<0.05 for all pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
A similar result was observed in serum-free N2B27 media, where cells with MMP1 added for five days had a similar expression pattern to cells with added LIF and BMP4 (self-renewal media), which was distinct from the pattern seen in N2B27 (differentiation media) alone (Fig. 2C, Supporting Information Fig. S2B), an effect that was found to be dose-dependent (Fig. 2D). These similarities in expression pattern extended more broadly, as high-throughput RNA sequencing data allowed us to cluster the GO terms associated with stem cell maintenance, and the MMP condition was found to cluster more tightly with the LIF/BMP4 condition than with the no addition condition (Fig. 2E), while the correlation between all gene expression data displayed a similar trend (Supporting Information Fig. S3).
To determine whether the addition of MMP was able to overcome active induction of differentiation, we added retinoic acid (RA) in the presence or absence of MMP, and found that MMP inhibits the ability of RA to induce differentiation, in terms of both protein and mRNA expression (Supporting Information Fig. S4A-B). To determine whether other MMPs have the same effect, we added a gelatinase (MMP2) and a stromelysin (MMP3), and found that they were not able to halt differentiation (Fig. 2F), while addition of collagenase was able to maintain expression of self-renewal markers (Fig. 2B), indicating that cleavage of collagen is an integral event in MMP1-mediated self-renewal. These results indicate that MMP1 is sufficient to maintain short-term self-renewal, and we also find that MMP inhibition causes cells to lose expression of self-renewal markers even in the presence of LIF (Fig. 2G), which, along with the fact that MMP inhibition affects mESC self-renewal in feeder-based cultures (Fig. 1D), indicates that the presence of MMPs is required. We went on to determine the ability of MMP to maintain long-term self-renewal of pluripotent stem cells.
Long-term LIF-independent ESC self-renewal
We were able to maintain mESCs for several passages in the complete absence of LIF but presence of MMP1 in serum-containing media, with indistinguishable morphology (Fig. 3A) and similar growth kinetics (Fig. 3B) and protein levels (Fig. 3C) to mESCs grown in the presence of LIF. In serum-free media, we were also able to maintain mESCs for up to 3 passages in the presence of MMP1, whereas cells could not be maintained in serum-free media alone for more than one passage (Supporting Information Fig. S5). At twenty passages with no LIF but with MMP, we found very similar gene expression patterns to cultures grown in LIF (Fig. 3D), with the exception of a modest increase in Fgf5. However, Fgf5 is a very sensitive early differentiation marker that increases up to 60-fold after just 5 days in media with no LIF or MMP1 (Fig. 2B), so a slight upregulation after 20 passages does not indicate significant differentiation.
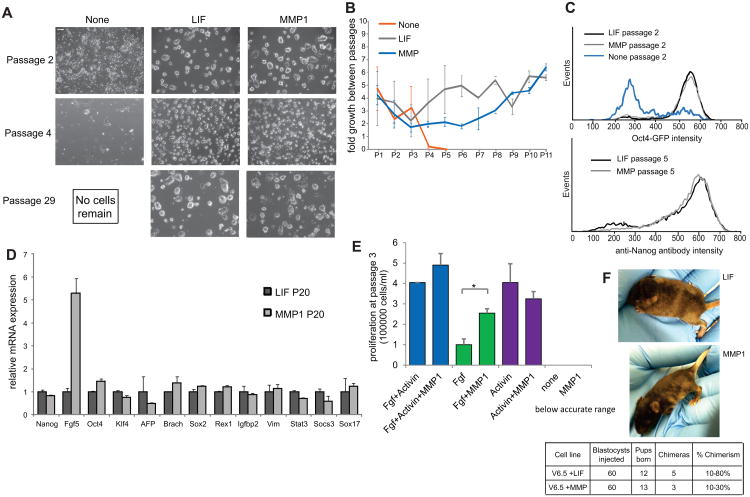
Figure 3.
Long-term maintenance of self-renewal by MMP1 addition. (A) Representative images of cells grown under the indicated conditions for the indicated number of passages. Scale bar = 200μm (B) Fold growth of cells during each passage over the course of 11 passages in the indicated conditions. (C) Flow cytometry histograms of Oct4-GFP reporter mESCs after two passages with indicated additions (top) and of Nanog immunofluorescent staining intensity after five passages with indicated additions (bottom). (D) mRNA expression levels of a broad marker panel after 20 passages in serum-containing media with added LIF or MMP1. (E) Counts of EpiSCs seeded at equal density and cultured for three passages in N2B27 medium supplemented with various combinations of Fgf, Activin and MMP1, n=2. (F) Images of mice 12 days after birth from blastocysts injected with mESCs grown in the presence of LIF or MMP1, with quantification of chimera generation and percent chimerism. Mice normally have black coat colour, and injected mESCs are from an agouti background. * =p<0.05; all data represents averages of at least three independent experiments and error bars represent SD.
To assess whether the ability of MMP1 to maintain self-renewal is universal among pluripotent stem cells, we added MMP1 to EpiSCs cultured in feeder-free conditions. While MMP1 alone was not able to maintain EpiSC self-renewal over multiple passages (Fig. 3E), its addition did enhance the ability of Fgf2 to maintain EpiSC proliferation for up to three passages (Fig. 3E, Supporting Information Fig. S6A), indicating that signaling pathways pertaining to EpiSC growth may also be activated downstream of MMP1. Consistent with this, we find that collagenase inhibition does cause a growth defect in EpiSCs in the absence of a feeder layer (Supporting Information Fig. S6B). To determine whether the same soluble signal that contributes to ESC self-renewal also acts to enhance EpiSC growth, we cultured EpiSCs in media conditioned by ESCs grown in the presence or absence of MMP1, and found no growth enhancement in ESC+MMP1-conditioned media (Supporting Information Fig. S6C), which is not surprising given the different pathways involved in mediating the self-renewal of ESCs versus EpiSCs. Because the effect of MMP1 on the maintenance of pluripotency is more pronounced and works independently in ESCs, we chose to focus on these cells to determine the extent to which pluripotency was maintained and the mechanisms involved.
To assess developmental potential in addition to self-renewal, we made embryoid bodies from mESCs cultured for 5 passages in media containing LIF or MMP and found normal embryoid body formation as well as normal expression patterns of differentiation markers under both conditions (Supporting Information Fig. S7), and blastocyst injections from cells grown in media containing either LIF or MMP1 yielded chimeric offspring (Fig. 3F). The ability of MMP to maintain mESC self-renewal and pluripotent potential could be acting on the endogenous ECM by activating or repressing various signaling pathways that enhance or reduce the ability of ESCs to self-renew, so we next went on to identify the relevant pathways involved.
Mechanism of MMP-mediated self-renewal
Several novel mechanisms have recently been uncovered that allow for the maintenance of mESC self-renewal in the absence of LIF, including simultaneous inhibition of ERK and GSK3 [7], inhibition of NF-κB signaling [9], inhibition of Src signaling [10], and induction of the endogenous Wnt signaling pathway [27], which enhances self-renewal but still requires LIF. We thus examined whether any of these mechanisms were contributing to the phenotype seen in the presence of MMP1. We found that MMP1 addition does not inhibit ERK signaling, as assessed by the presence of active ERK (Fig. 4A), nor does it inhibit expression of downstream NF-κB targets (Fig. 4B), indicating that MMP1 does not act through either of these signaling pathways. Smad2/3 is also not involved in MMP-mediated self-renewal, as Smad inhibition in the presence of MMP does not affect expression levels of self-renewal markers, and actually decreases Fgf5 expression (Supporting Information Fig. S8A). This result also indicates that the cells have not transitioned to a more EpiSC-like state in which self-renewal is mediated through Alk4/5/7 instead of Stat3 [23, 28].
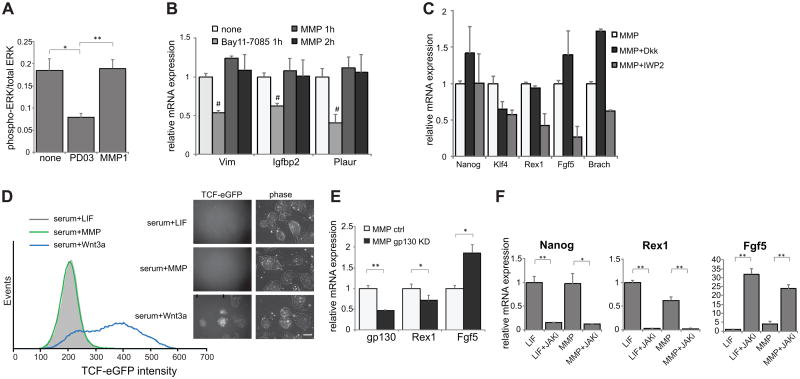
Figure 4.
Signaling pathways examined in terms of MMP-mediated self-renewal. (A) Phosphorylated ERK levels with the indicated additions, analyzed by ELISA (PD03 = PD0325901, ERK inhibitor). (B) mRNA expression levels of NF-κB downstream target genes after addition of the NF-κB inhibitor Bay11-7085, or of MMP1 for the indicated number of hours. (C) mRNA expression levels of self-renewal and differentiation markers in the presence of Wnt pathway inhibitors. (D) Flow cytometry histogram of Wnt reporter activation after two days of the indicated additions to mESCs grown on feeders. Images are representative fluorescent and phase images. Scale bar = 200μm (E) mRNA expression levels after shRNA-mediated gp130 knockdown in the presence of MMP compared to levels using a control shRNA vector. (F) mRNA expression levels of self-renewal and differentiation markers after addition of a JAK inhibitor. **=p<0.001, * =p<0.05; #=p<0.05 for all pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
Endogenous Wnt signaling has recently been shown to be important for mESC self-renewal [27], and Wnt has also been shown to bind to the extracellular matrix and heparan sulfate proteoglycans [29, 30]. It thus represented an attractive candidate for contributing to MMP1-mediated self-renewal. However, we found that inhibition of either extracellular Wnt or of the export of Wnt to the extracellular domain, by the protein Dkk1 [31] or by the small molecule inhibitor IWP2, respectively, caused neither a drastic reduction in levels of self-renewal markers nor a significant increase in levels of differentiation markers (Fig. 4C). We also found that TCF-eGFP Wnt mESCs [20] that report on Wnt pathway activation were not activated in the presence of MMP1 (Fig. 4D), nor were canonical downstream Wnt targets upregulated after short-term stimulation with MMP1 (Supporting Information Fig. S8B). Thus, it is unlikely that the primary stimulus causing this phenotype is due to endogenous Wnt signaling.
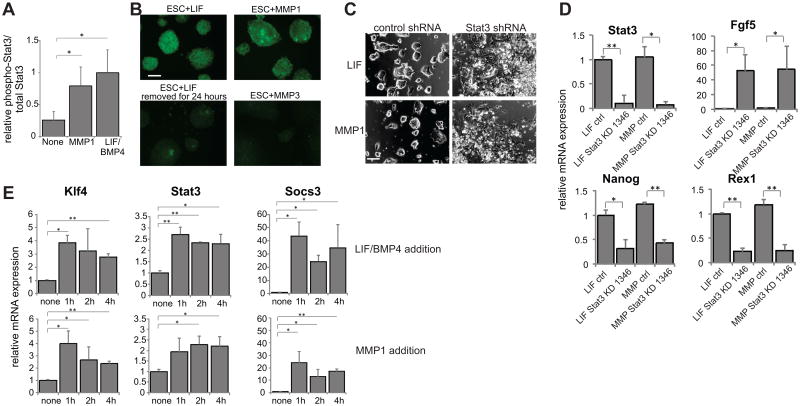
The most well-studied and first-described mechanism of maintaining mESC self-renewal involves signaling of LIF [15] through the homodimeric gp130-LIFR receptor complex to activate JAK, which then activates the transcription factor Stat3 [32] to transcribe important self-renewal genes, including Oct4 and Nanog [33]. We thus assessed whether MMP1 was causing a similar signaling pathway to be activated, perhaps by allowing an endogenous ligand to be released from the extracellular matrix to initiate the pathway. We found that gp130, a common receptor subunit for many extracellular LIF-family signaling proteins [34], is required for MMP1-mediated self-renewal, as its knockdown shifts transcription toward a more differentiated state (Fig. 4E). Likewise, signaling through JAK is essential for this phenotype, as its inhibition causes rapid differentiation in the presence of either MMP1 or LIF (Fig. 4F). Stat3 is also important in MMP1-mediated self-renewal, as it is shown to be active in the presence of MMP1 (Fig. 5A-B) and its knockdown causes differentiation of mESCs in the presence of MMP1, as assessed via morphology (Fig. 5C) and gene expression (Fig. 5D, Supporting Information Fig. S8C). The activation of Stat3 is known to rapidly cause transcriptional upregulation of several downstream targets, including Socs3, Klf4, and Stat3 itself [35]. We also find that, as soon as 1 hour after stimulation with either LIF or MMP1, transcript levels of all three genes increase significantly (Fig. 5E), indicating that Stat3 is being activated in both cases. Thus, addition of MMP triggers a signaling cascade that acts through the gp130-JAK-Stat3 pathway, with similar sensitivity to inhibition and activation kinetics as the pathway stimulated by exogenous LIF addition.
Figure 5.
Stat3 is required for MMP-mediated self-renewal. (A) Phosphorylated (T705) Stat3 levels with the indicated additions, analyzed by ELISA. (B) Immunofluorescence staining for phosphorylated (T705) Stat3 in cells grown in the indicated conditions. Scale bar = 200μm. (C) Representative images of cells after two passages with added LIF or MMP1 with a control shRNA construct or Stat3 shRNA, construct 1346. Scale bar = 200μm. (D) mRNA expression levels of self-renewal and differentiation markers in cells grown in indicated conditions with a control shRNA or Stat3 shRNA, construct 1346. (E) mRNA expression levels of Stat3 downstream targets after short-term addition of LIF/BMP4 or MMP1. **=p<0.001, *=p<0.05 for pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
Because inhibition of essential components of the LIF-mediated self-renewal pathway is able to inhibit MMP1-mediated self-renewal, and the fact that LIF is known to be secreted and detected by mESCs in an autocrine manner [36, 37], it is possible that endogenous LIF itself is responsible for the MMP1-mediated phenotype. To assess this, we added a blocking antibody to LIF and found that the antibody was able to successfully block LIF-mediated self-renewal (Fig. 6A, Supporting Information Fig. S9A), whereas it had no effect on MMP1-mediated self-renewal in terms of morphology or marker expression (Fig. 6A-B). We therefore explored other candidate ligands that could be released from the matrix by MMP.
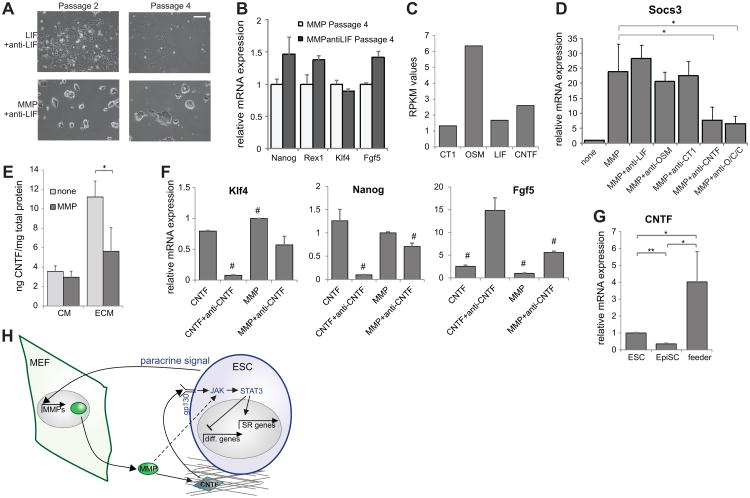
Figure 6.
Endogenous gp130-JAK-Stat3 signal. (A) Representative images of ESCs growing for the indicated number of passages in the presence of a LIF blocking antibody with added LIF or added MMP1. Scale bar = 200μm. (B) mRNA expression levels of indicated markers after four passages in the presence of MMP1 with or without LIF-blocking antibody. (C) RPKM values from normalized RNA-sequencing data for gp130 ligands in self-renewing mESCs. (D) mRNA expression levels of a Stat3 target gene after 1 hour of indicated additions. O/C/C indicates blocking the three ligands OSM, CT1, and CNTF. (E) CNTF levels recovered from conditioned media and solubilized ECM samples from cultures grown in the indicated conditions. (F) mRNA expression levels of self-renewal and differentiation markers in cells grown for four days in serum-free media with the indicated additions. (G) CNTF mRNA expression levels in the indicated cell types. (H) Model depicting the effect of matrix remodeling on mESCs. Dotted line indicates action of an unknown pathway. **=p<0.001, * =p<0.05; #=p<0.05 for all pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
Other ligands in the LIF family that use the gp130 receptor and are both secreted by mESCs and have the appropriate receptors expressed include IL-11, oncostatin M (OSM), ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), and cardiotrophin-like cytokine factor 1 (CLCF1) [34]. Of these, OSM [38], CNTF [39], and CT-1 [40] have been shown to be able to maintain ESC self-renewal, and they are detectably expressed in our system at similar levels relative to one another (Fig. 6C). These three proteins are all also able to activate Stat3, as the Stat3 downstream target Socs3 shows significant upregulation after 1 hour in the presence of any of these ligands, and blocking the ligands using blocking antibodies inhibits this activity (Supporting Information Fig. S9B). While blocking any of the ligands in the presence of MMP did not reduce the Socs3 expression level to its baseline value (Fig. 6D), blocking CNTF did have a significant impact on Socs3 expression levels, and this block also affected the more modest MMP-induced upregulation of other Stat3 transcriptional targets (Supporting Information Fig. S9C).
To further explore the role of CNTF specifically, we measured levels of CNTF in ESC-conditioned media and solubilized ECM with or without the addition of MMP for 24h. We found that CNTF levels in the ECM decreased in the presence of MMP (Fig. 6E), as expected if CNTF is being released. While levels of CNTF in the conditioned media did not concomitantly increase with MMP addition (Fig. 6E), this could be due to binding of the protein at the cell surface or uptake by the cell. To demonstrate the functionality of CNTF in MMP-mediated self-renewal, we added a CNTF blocking antibody and showed that it was able to fully block self-renewal mediated by recombinant CNTF and partially block MMP-mediated self-renewal (Fig. 6F), indicating that matrix-bound endogenous CNTF is partly responsible for MMP1-mediated self-renewal.
To determine whether CNTF may be playing a role in feeder-mediated self-renewal, we compared expression levels in ESCs, EpiSCs, and feeders. We found that, relative to CNTF expression levels in ESCs, EpiSCs show lower expression (Fig. 6G), as expected as EpiSCs do not respond to signals that act through gp130. However, feeder cells show increased CNTF expression relative to that in ESCs (Fig. 6G), which may indicate another mechanism by which feeder-secreted MMPs enhance ESC self-renewal—by releasing the feeders' endogenously secreted ligands as well as those secreted by ESCs. Together, these data indicate that cell-secreted CNTF is partially but not entirely responsible for the activation of Stat3 downstream of MMP addition, showing a novel role for ESC-produced CNTF while also implicating an additional cell-produced signal in MMP-mediated self-renewal (Fig. 6H).
Discussion
Regulation of cell signaling at the level of the ECM has been shown to occur in many contexts. Embryonic stem cells have been shown to maintain self-renewal on softer substrates approaching the stiffness of the cells themselves [8], and substrate stiffness has been shown to affect neuronal stem cell differentiation [41] and the availability of autocrine signals [42]. In another example, reducing the sulfation of heparan sulfate proteoglycans in the ECM was shown to help maintain self-renewal of mESCs by reducing signaling from autocrine Fgf4 ligands [6]. Here, we find that the ECM also harbors mESC-secreted CNTF that can be released to act through the gp130-JAK-Stat3 pathway (Fig. 6H), in addition to other signal(s) that also act through JAK and Stat3, thus providing the first evidence for endogenously secreted factors that are produced at sufficient levels to maintain ESC self-renewal. The role of MMP1 in aiding self-renewal may extend to EpiSCs even though JAK-Stat3 signaling does not play a role in EpiSC self-renewal. It is likely that multiple types of signals may be released or activated upon MMP1 addition, and that the identity of the signals is likely to be determined by the cell type and the specific endogenous matrix it creates. Though MMP1 was not able to maintain EpiSC self-renewal by itself, other MMPs with distinct substrates may be more effective for these cells.
The actions of ECM-based signals require dynamic regulation of the ECM, which is performed endogenously by matrix metalloproteinases, both in vitro and in vivo during development [43]. For example, MMPs play a role in embryo implantation [44] and are essential for activation of hematopoietic stem cells [45]. For mESCs, though endogenous secretion levels of MMPs are low, co-culture with MEFs, which we show secrete high levels of MMPs, enhances mESC self-renewal. The ability of MEFs to maintain self-renewal was partly clarified by isolation of LIF as a diffusible factor produced by stromal cells [15], but the observation that many mESC lines self-renew better with feeders than with serum+LIF suggests that MEFs have other roles besides secreting LIF. Here we find a novel role for MEFs in their secretion of MMPs, a mechanism that could be important in other co-culture systems to release cell-type-specific secreted factors from the extracellular matrix. We also find that secretion of MMPs by MEFs is enhanced in the presence of ESCs, due to the presence of an ESC-secreted factor. While the identity of this factor or factors remains unknown, ESCs are known to secrete high levels of many growth factors [46] that have been shown to upregulate MMP expression and/or secretion levels in other contexts, such as VEGF [47] and TGFβ family ligands [48].
The identification of stem-cell-secreted factors that are able to maintain ESC self-renewal upon release or activation by exogenous MMP1 indicates the importance of endogenously produced extracellular matrix-associated ligands. Autocrine ligands have been identified that contribute to mESC self-renewal, including LIF [37] and Wnt3a [27]. However, autocrine levels of LIF are not high enough to maintain self-renewal in normal cultures, and autocrine Wnt, while necessary for maintenance of self-renewal, is not sufficient to maintain self-renewal in the absence of LIF. Though we find that cell-secreted CNTF normally bound within the matrix can be released to aid in mESC self-renewal, other factors or signals are likely to also be released or activated during MMP-mediated matrix remodeling. Other possible mechanisms involve the activation of integrins or E-cadherin by ECM components, as these signals have been shown to activate Stat3 in other systems [49, 50]. The secretion and sequestering of functional autocrine-acting ligands likely extends beyond those that act on ESCs, and the specific mechanisms by which they can be removed and activated may vary in different systems. This study shows that the extracellular matrix is an often-overlooked but major source of signaling molecules, and opens up the possibility for further studies on the diverse effects of extracellular matrix remodeling on maintaining and directing stem cell fate.
Summary
In this study, we show that extracellular matrix remodeling by matrix metalloproteinase 1 is sufficient for maintenance of LIF-independent mESC self-renewal through the JAK-Stat3 signaling pathway. In ESC co-culture systems with an embryonic fibroblast feeder layer, the MMPs secreted by feeders act in a paracrine manner to enhance ESC self-renewal, further indicating the importance of extracellular matrix remodeling in embryonic stem cell maintenance. Mechanistically, MMPs act to promote ESC self-renewal by indirectly inducing signaling through JAK and Stat3, thus promoting expression of Nanog and other key self-renewal markers. Upstream, this signaling is mediated at least in part by ESC-secreted ligands including CNTF that are normally trapped within the endogenous ECM and are released upon matrix remodeling to bind to receptors on the cell surface.
Supplementary Material
Supplementary Figure 1. Secretion of MMP from feeder cells. (A) Secreted protein levels of MMP2 in conditioned media of indicated cultures, analyzed by ELISA. Concentrations are normalized by final cell density. (B) Fraction of ESCs in the total cell population after two days of growth on a feeder layer in the presence or absence of Ro32. Data was obtained using two separate ESC cell lines with GFP conjugated to histone H2A or H2B (to ease cell counting). **=p<0.001,*=p<0.05.
Supplementary Figure 2. Additional marker expression with MMP addition. (A) mRNA expression levels in the indicated conditions in serum-containing media. (B) mRNA expression levels in the indicated conditions in serum-free media. * =p<0.05 and #=p<0.05 for all pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
Supplementary Figure 3. Correlation between all genes expressed in the RNA-sequencing dataset in the indicated conditions.
Supplementary Figure 4. MMP1 overcomes RA differentiation stimulus. (A) Flow cytometry histogram of Sox2-GFP reporter mESCs fluorescent intensity after growth in the indicated conditions (RA = retinoic acid). (B) mRNA expression in the presence of RA with or without MMP1. #=p<0.05 for all pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
Supplementary Figure 5. Images of representative morphology of cells grown in serum-free N2B27 media with the indicated additions. Labels at left indicate passage number. Scale bar = 200μm.
Supplementary Figure 6. EpiSCs cultured in the presence or absence of MMP1. (A) Representative images of EpiSCs seeded at equal density and cultured for three passages in N2B27 medium supplemented with various combinations of Fgf, Activin and MMP1. (B) Counts and representative images of EpiSCs seeded at equal density and cultured for two passages in EpiSC medium supplemented with the MMP inhibitor Ro32-555 or an equal volume of DMSO, n=2, **=p<0.001. (C) Counts and representative images of EpiSCs seeded at equal density and cultured for two passages in a 1:1 ratio of EpiSC medium and conditioned medium from ESCs treated with recombinant MMP1, n=2.
Supplementary Figure 7. mRNA expression level timecourse from embryoid bodies grown for the indicated number of days with no LIF or MMP1, after five passages in media supplemented with either LIF or MMP1. Image shows representative embryoid bodies from MMP1 condition on day 5. Scale bar = 400μm.
Supplementary Figure 8. Additional evidence regarding signaling pathways involved in MMP-mediated self-renewal. (A) mRNA expression of differentiation and self-renewal markers in the presence or absence of SB431542, an Alk4/5/7 inhibitor of Activin/Nodal signaling. (B) mRNA expression levels of Wnt downstream target genes after short-term addition of MMP for the indicated number of hours. (C) mRNA expression levels of self-renewal and differentiation markers in cells grown in indicated conditions with a control shRNA or Stat3 shRNA, construct 1413. **=p<0.001,*=p<0.05.
Supplementary Figure 9. LIF-family ligands in MMP1-mediated self-renewal. (A) mRNA expression levels of self-renewal and differentiation markers after addition of a LIF-blocking antibody to cells with added LIF or added MMP1. (B) mRNA expression levels of a Stat3 target gene after 1 hour of indicated additions. (C) mRNA expression levels of Stat3 downstream target genes after 1 h in the indicated conditions. * =p<0.05; ##=p<0.001, #=p<0.05 for pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
Supplementary Table 1. Forward and reverse primers used for quantitative RT-PCR.
Acknowledgments
We thank the Massachusetts Institute of Technology Flow Cytometry Core; the BioMicro Center and Fugen Li for RNA sequencing data and interpretation; Daley lab (ABJ1), Boyer lab (H2A) and Derk ten Berge (7xTCF-eGFP) for providing reporter mESCs; Kibibi Ganz for blastocyst injections; and Laurie Boyer for helpful discussions. This work was supported by the National Institutes of Health Grant EB007278 and the Singapore-MIT Alliance to JV, a Sir Henry Wellcome Postdoctoral Fellowship to TWT, and by NIH grant HD 045022 to RJ.
Funding: This work was supported by the National Institutes of Health Grant EB007278 and the Singapore-MIT Alliance to JV and by NIH grant HD 045022 to RJ.
Footnotes
Author contributions: Laralynne Przybyla: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing
Thorold Theunissen: Collection of data, data analysis and interpretation, manuscript editing
Rudolf Jaenisch: Experimental design, manuscript editing
Joel Voldman: Conception and design, assembly of data, data interpretation, manuscript writing
Disclosure of potential conflicts of interest: The authors declare no potential conflicts of interest.
References
- 1.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 2.Przybyla LM, Voldman J. Attenuation of extrinsic signaling reveals the importance of matrix remodeling on maintenance of embryonic stem cell self-renewal. Proc Natl Acad Sci USA. 2012;109(3):835–840. doi: 10.1073/pnas.1103100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends in Genetics. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO. The Extracellular Matrix: Not Just Pretty Fibrils. Science. 2009;326(5957):1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. The Faseb Journal. 1997;11(1):51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 6.Lanner F, Lee KL, Sohl M, et al. Heparan Sulfation–Dependent Fibroblast Growth Factor Signaling Maintains Embryonic Stem Cells Primed for Differentiation in a Heterogeneous State. Stem Cells. 2010;28(2):191–200. doi: 10.1002/stem.265. [DOI] [PubMed] [Google Scholar]
- 7.Ying QL, Wray J, Nichols J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453(7194):519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhury F, Li Y, Poh YC, et al. Soft Substrates Promote Homogeneous Self-Renewal of Embryonic Stem Cells via Downregulating Cell-Matrix Tractions. PLOS One. 2010;5(12):e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutta D, Ray S, Home P, et al. Self-Renewal Versus Lineage Commitment of Embryonic Stem Cells: Protein Kinase C Signaling Shifts the Balance. Stem Cells. 2011;29(4):618–628. doi: 10.1002/stem.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Zhu L, Yang A, et al. Calcineurin-NFAT Signaling Critically Regulates Early Lineage Specification in Mouse Embryonic Stem Cells and Embryos. Cell Stem Cell. 2011;8(1):46–58. doi: 10.1016/j.stem.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Tremml G, Singer M, Malavarca R. Culture of mouse embryonic stem cells. Curr Protoc Stem Cell Biol. 2008;Chapter 1 doi: 10.1002/9780470151808.sc01c04s5. Unit 1C.4. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 14.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 15.Smith AG, Heath JK, Donaldson DD, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 16.Williams RL, Hilton DJ, Pease S, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336(6200):684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 17.Eggan K, Akutsu H, Loring J, et al. Hybrid Vigor, Fetal Overgrowth, and Viability of Mice Derived by Nuclear Cloning and Tetraploid Embryo Complementation. PNAS. 2001;98(11):6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bortvin A, Goodheart M, Liao M, et al. Dppa3/Pgc7/stella is a maternal factor and is not required for germ cell specification in mice. BMC Dev Biol. 2004;4:2. doi: 10.1186/1471-213X-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stadtfeld M, Maherali N, Breault DT, et al. Defining Molecular Cornerstones during Fibroblast to iPS Cell Reprogramming in Mouse. Cell Stem Cell. 2008;2(3):230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ten Berge D, Koole W, Fuerer C, et al. Wnt Signaling Mediates Self-Organization and Axis Formation in Embryoid Bodies. Cell Stem Cell. 2008;3(5):508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foudi A, Hochedlinger K, Van Buren D, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. NAT Biotechnol. 2009;27(1):84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying QL, Nichols J, Chambers I, et al. BMP Induction of Id Proteins Suppresses Differentiation and Sustains Embryonic Stem Cell Self-Renewal in Collaboration with STAT3. Cell. 2003;115(3):281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 23.Tesar PJ, Chenoweth JG, Brook FA, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448(7150):196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 24.Guo G, Yang J, Nichols J, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136(7):1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talbot NC, Sparks WO, Powell AM, et al. Quantitative and semiquantitative immunoassay of growth factors and cytokines in the conditioned medium of STO and CF-1 mouse feeder cells. In Vitro Cell Dev Biolanim. 2012;48(1):1–11. doi: 10.1007/s11626-011-9467-7. [DOI] [PubMed] [Google Scholar]
- 26.Prowse ABJ, McQuade LR, Bryant KJ, et al. Identification of Potential Pluripotency Determinants for Human Embryonic Stem Cells Following Proteomic Analysis of Human and Mouse Fibroblast Conditioned Media. J Proteome Res. 2007;6(9):3796–3807. doi: 10.1021/pr0702262. [DOI] [PubMed] [Google Scholar]
- 27.ten Berge D, Kurek D, Blauwkamp T, et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13(9):1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brons IGM, Smithers LE, Trotter MWB, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448(7150):191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 29.Schryver B, Hinck L, Papkoff J. Properties of Wnt-1 protein that enable cell surface association. Oncogene. 1996;13(2):333–342. [PubMed] [Google Scholar]
- 30.Fuerer C, Habib SJ, Nusse R. A study on the interactions between heparan sulfate proteoglycans and Wnt proteins. Developmental Dynamics. 2010;239(1):184–190. doi: 10.1002/dvdy.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berendsen AD, Fisher LW, Kilts TM, et al. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. Proc Natl Acad Sci USA. 2011;108(41):17022–17027. doi: 10.1073/pnas.1110629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirai H, Karian P, Kikyo N. Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochemical Journal. 2011;438(1):11–23. doi: 10.1042/BJ20102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Xu H, Yuan P, et al. Integration of External Signaling Pathways with the Core Transcriptional Network in Embryonic Stem Cells. Cell. 2008;133(6):1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochemical Journal. 2003;374(1):1. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourillot P, Aksoy I, Schreiber V, et al. Novel STAT3 Target Genes Exert Distinct Roles in the Inhibition of Mesoderm and Endoderm Differentiation in Cooperation with Nanog. Stem Cells. 2009;27(8):1760–1771. doi: 10.1002/stem.110. [DOI] [PubMed] [Google Scholar]
- 36.Davey RE, Onishi K, Mahdavi A, et al. LIF-mediated control of embryonic stem cell self-renewal emerges due to an autoregulatory loop. FASEB J. 2007;21(9):2020–2032. doi: 10.1096/fj.06-7852com. [DOI] [PubMed] [Google Scholar]
- 37.Zandstra PW, Le HV, Daley GQ, et al. Leukemia inhibitory factor (LIF) concentration modulates embryonic stem cell self-renewal and differentiation independently of proliferation. Biotechnol Bioeng. 2000;69(6):607–617. [PubMed] [Google Scholar]
- 38.Yoshida K, Chambers I, Nichols J, et al. Maintenance of the pluripotential phenotype of embryonic stem cells through direct activation of gp130 signalling pathways. Mechanisms of Development. 1994;45(2):163–171. doi: 10.1016/0925-4773(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 39.Conover JC, Ip NY, Poueymirou WT, et al. Ciliary neurotrophic factor maintains the pluripotentiality of embryonic stem cells. Development. 1993;119(3):559–565. doi: 10.1242/dev.119.3.559. [DOI] [PubMed] [Google Scholar]
- 40.Pennica D, Shaw KJ, Swanson TA, et al. Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J Biol Chem. 1995;270(18):10915–10922. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- 41.Keung AJ, Juan-Pardo EM de, Schaffer DV, et al. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells. 2011;29(11):1886–1897. doi: 10.1002/stem.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells RG, Discher DE. Matrix Elasticity, Cytoskeletal Tension, and TGF-{beta}: The Insoluble and Soluble Meet. Sci Signal. 2008;1(10):pe13. doi: 10.1126/stke.110pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14(17):2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 44.Alexander CM, Hansell EJ, Behrendtsen O, et al. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122(6):1723–1736. doi: 10.1242/dev.122.6.1723. [DOI] [PubMed] [Google Scholar]
- 45.Heissig B, Hattori K, Dias S, et al. Recruitment of Stem and Progenitor Cells from the Bone Marrow Niche Requires MMP-9 Mediated Release of Kit-Ligand. Cell. 2002;109(5):625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo Y, Barbara GE, Hal EB. Murine Embryonic Stem Cells Secrete Cytokines/Growth Modulators That Enhance Cell Survival/Anti-Apoptosis and Stimulate Colony Formation of Murine Hematopoietic Progenitor Cells. Stem Cells. 2006;24(4):850–856. doi: 10.1634/stemcells.2005-0457. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh S, Basu M, Roy SS. ETS-1 protein regulates vascular endothelial growth factor-induced matrix metalloproteinase-9 and matrix metalloproteinase-13 expression in human ovarian carcinoma cell line SKOV-3. J Biol Chem. 2012;287(18):15001–15015. doi: 10.1074/jbc.M111.284034. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol. 2004;25(5):1375–1382. [PubMed] [Google Scholar]
- 49.Guo W, Pylayeva Y, Pepe A, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126(3):489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 50.Hawkins K, Mohamet L, Ritson S, et al. E-cadherin and, in Its Absence, N-cadherin Promotes Nanog Expression in Mouse Embryonic Stem Cells via STAT3 Phosphorylation. Stem Cells. 2012;30(9):1842–1851. doi: 10.1002/stem.1148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Secretion of MMP from feeder cells. (A) Secreted protein levels of MMP2 in conditioned media of indicated cultures, analyzed by ELISA. Concentrations are normalized by final cell density. (B) Fraction of ESCs in the total cell population after two days of growth on a feeder layer in the presence or absence of Ro32. Data was obtained using two separate ESC cell lines with GFP conjugated to histone H2A or H2B (to ease cell counting). **=p<0.001,*=p<0.05.
Supplementary Figure 2. Additional marker expression with MMP addition. (A) mRNA expression levels in the indicated conditions in serum-containing media. (B) mRNA expression levels in the indicated conditions in serum-free media. * =p<0.05 and #=p<0.05 for all pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
Supplementary Figure 3. Correlation between all genes expressed in the RNA-sequencing dataset in the indicated conditions.
Supplementary Figure 4. MMP1 overcomes RA differentiation stimulus. (A) Flow cytometry histogram of Sox2-GFP reporter mESCs fluorescent intensity after growth in the indicated conditions (RA = retinoic acid). (B) mRNA expression in the presence of RA with or without MMP1. #=p<0.05 for all pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
Supplementary Figure 5. Images of representative morphology of cells grown in serum-free N2B27 media with the indicated additions. Labels at left indicate passage number. Scale bar = 200μm.
Supplementary Figure 6. EpiSCs cultured in the presence or absence of MMP1. (A) Representative images of EpiSCs seeded at equal density and cultured for three passages in N2B27 medium supplemented with various combinations of Fgf, Activin and MMP1. (B) Counts and representative images of EpiSCs seeded at equal density and cultured for two passages in EpiSC medium supplemented with the MMP inhibitor Ro32-555 or an equal volume of DMSO, n=2, **=p<0.001. (C) Counts and representative images of EpiSCs seeded at equal density and cultured for two passages in a 1:1 ratio of EpiSC medium and conditioned medium from ESCs treated with recombinant MMP1, n=2.
Supplementary Figure 7. mRNA expression level timecourse from embryoid bodies grown for the indicated number of days with no LIF or MMP1, after five passages in media supplemented with either LIF or MMP1. Image shows representative embryoid bodies from MMP1 condition on day 5. Scale bar = 400μm.
Supplementary Figure 8. Additional evidence regarding signaling pathways involved in MMP-mediated self-renewal. (A) mRNA expression of differentiation and self-renewal markers in the presence or absence of SB431542, an Alk4/5/7 inhibitor of Activin/Nodal signaling. (B) mRNA expression levels of Wnt downstream target genes after short-term addition of MMP for the indicated number of hours. (C) mRNA expression levels of self-renewal and differentiation markers in cells grown in indicated conditions with a control shRNA or Stat3 shRNA, construct 1413. **=p<0.001,*=p<0.05.
Supplementary Figure 9. LIF-family ligands in MMP1-mediated self-renewal. (A) mRNA expression levels of self-renewal and differentiation markers after addition of a LIF-blocking antibody to cells with added LIF or added MMP1. (B) mRNA expression levels of a Stat3 target gene after 1 hour of indicated additions. (C) mRNA expression levels of Stat3 downstream target genes after 1 h in the indicated conditions. * =p<0.05; ##=p<0.001, #=p<0.05 for pairwise comparisons, all data represents averages of at least three independent experiments and error bars represent SD.
Supplementary Table 1. Forward and reverse primers used for quantitative RT-PCR.