Abstract
Retinopathy of prematurity (ROP) and infantile hemangiomas are vascular disorders that may share common mechanisms. This study examined a potential clinical association between these disorders in populations of preterm infants at two hospitals in the U.S. and Hungary. Clinically collected data from infants with gestational ages less than 32 weeks born between May 1, 2007 and December 31, 2010 seen in the University of Iowa Children’s Hospital or the Department of Obstetrics and Gynecology, University of Pécs, were abstracted from electronic medical records and entered into a study database. Demographic and clinical variables were examined as potential covariates to the disorders of interest. Data were initially analyzed by center and then combined through meta-analysis. Six hundred eighty-four subjects were studied, 236 from Pécs and 448 from Iowa. There were no significant demographic differences between populations. Univariate analysis on each study population yielded covariates to ROP in each population, including infantile hemangioma, which were entered into a logistic regression model. These models were combined through random effects meta-analysis and demonstrated a significant relationship between infantile hemangioma and ROP (odds ratio=1.84, 95% confidence interval 1.08–3.12).
Conclusion
Infantile hemangioma and ROP co-occur in premature infant populations. Further studies are needed to investigate the pathogenesis of both disorders.
Keywords: Angiogenesis, Hemangioma, Preterm infants, Retinopathy of prematurity, Vascular endothelial growth factor, Vasculogenesis
Introduction
Retinopathy of prematurity (ROP) is an eye disease that affects the retina of the preterm infant and can cause impairment or loss of vision. It occurs in the setting of incomplete retinal vascularization at birth, a normal developmental stage in preterm infants [25, 32]. Normal visual development requires the relatively hypoxic environment of the fetus in utero [7]. When a premature infant is exposed to higher ambient oxygen levels postnatally, oxygen-sensitive growth inhibitors may halt the development of retinal vasculature before it is complete [20, 34, 36]. Compensatory cellular mechanisms stimulate continued development but are not well controlled. The result can be overgrowth of vasculature, which invades inappropriate parts of the eye and may result in fibrous scar tissue, which can damage vision [33, 39]. ROP occurs in 35–60% of very low birth weight infants and is one of the top three causes of blindness in children [13, 19, 23].
Infantile hemangiomas (IHs) are benign vascular tumors that develop in infancy or early childhood. Their growth phase is marked by rapid angiogenesis through exuberant proliferation of the vascular endothelium [6, 10, 14, 40]. IHs vary in size, number, and location, and they often regress without treatment [14]. A common tumor, IH occurs in approximately 5% of infants and affects preterm populations disproportionately [1, 6, 10, 21, 29]. Although most IHs resolve spontaneously, they can become problematic in an estimated 10–20% of cases; this occurs if their growth becomes excessive or if they obstruct vision, breathing, or eating [4, 10, 17].
Both IH and ROP are disorders of vascular proliferation. Although IH pathogenesis is less well understood, researchers speculate it, too, may be caused by interrupted vascular development resulting in inappropriate cell proliferation [3, 15, 22, 26, 28, 40]. It is now known that hypoxia-induced mediators, such as vascular endothelial growth factor, can stimulate vasculogenesis in both ROP and IH [31]. Blockade of β-adrenergic receptors with propranolol promotes regression of IHs [6, 11]; propranolol has also shown promise for treatment of an animal model of ROP [37], but this finding has not yet been replicated by others [5]. Studying IH and ROP may further increase our understanding of the mechanisms of normal and abnormal vasculogenesis. A recent study of very-low-birth-weight infants found a significant association between IH and ROP [35].
We undertook the present study to examine the concordance between IH and ROP in populations of preterm infants in the U.S. and Hungary. We hypothesized that preterm infants with IH are more likely to also have ROP than are those without IH. Such an association would lend credence to the idea that these two disorders may have shared pathogenic mechanisms. If this is the case, studies of strategies for preventing or ameliorating ROP should consider stratification of subjects at enrollment based on the presence or absence of IH. In addition, examination of the potential association between IH and ROP may provide new clues to the regulation of vasculogenesis and the pathobiology of these disorders.
Methods
This study was conducted on infants admitted to the neonatal intensive care units of the University of Iowa Children’s Hospital and the Department of Obstetrics and Gynecology, University of Pécs. The study was done in these two centers to increase the generalizability of the results. The subjects were all infants with gestational age less than 32 weeks born between May 1, 2007 and December 31, 2010 for whom complete records were available. The study was based on analysis of data collected prospectively for clinical purposes. The study protocol was approved by the institutional review board at each center. Data were collected from the patients’ electronic medical records and the local NICU databases. At the University of Iowa, additional data were collected from the records of follow-up clinic visits.
Demographic information, including gestational age, gender, birth weight, date of death if applicable, and hospital of birth were collected and maintained in the password-secured REDCap™ database of the University of Iowa Institute for Clinical and Translational Science [18].
Clinical data collected and added to this database included number of hyperglycemia episodes (glucose >150 mg/dl twice in 24 hours), insulin exposure, highest serum bilirubin level, transfusion of blood products, type and duration of respiratory support, bacterial or fungal infection (sepsis, meningitis, or urinary tract infection proven by culture and treated for at least 7 days with antimicrobial agents), enteral nutrition source (breast milk feeding – any in Iowa, exclusive in Pécs), exposure to antenatal corticosteroid, and postnatal treatment with systemic corticosteroid, erythropoietin, or methylxanthine.
Data on retinopathy of prematurity (ROP) were collected by recording the highest stage of the disease diagnosed in either eye throughout the infant’s hospitalization. The ophthalmological examinations were performed by trained pediatric ophthalmologists, and the 2005 International Classification of ROP was used in both centers [25].
At the University of Iowa, data on the number and size of hemangiomas were collected by reviewing physical examination records from the period of hospitalization and follow-up clinic visits. At the time the examinations were performed, the present study had not yet been conceived. The date of the examination with the greatest number of hemangiomas was recorded, as well as their number, size, and location. The IH data were extracted from the examination records by an individual (RMH) who had no knowledge of the patients’ ROP diagnoses. The data on hemangioma number and size were collected in Pécs only from the discharge examination.
The primary goal of this study was to look for association between IH and ROP, so infants with missing data on either disorder were excluded. At the University of Iowa, patients without follow-up clinic visits were also excluded, as the records of physical examination at follow-up were the most reliable source of data on hemangiomas.
Data from Pécs and Iowa infants were initially analyzed separately. Chi-square tests were used to assess independence between IH and ROP. Clinical covariates were screened for relation to IH or ROP using t-tests or Mann-Whitney U tests for continuous variables and chi-square tests for categorical variables. Covariates related to either at the p≤0.10 level were included in logistic regression models to adjust for possible confounders. In these models, ROP was treated as the outcome variable and IH and other covariates as explanatory variables. The adjusted results of the separate analyses were then combined using random-effects meta-analysis [9]. Data were combined at the patient level between the Iowa and Pécs sites, and the relationship between ROP and IH was examined in this dataset as well. The relationship between ROP stage and IH risk was examined in the populations from both sites separately using the Wilcoxon rank-sum test.
Results
Between May 1, 2007 and December 31, 2010, a total of 897 infants with gestational ages less than 32 weeks were admitted to the NICUs at the University of Iowa Children’s Hospital and the University of Pécs Obstetrics and Gynecology Hospital. Of these, 374 inborn and 75 outborn infants were admitted to UI Children’s Hospital NICU, and 285 inborn infants were admitted to the University of Pécs NICU. Infants who died before discharge (n=88), did not attend follow-up visits (n=122), or were outborn and missing ophthalmology records from the transferring hospital (n=2) were excluded. 684 subjects were included in the analysis, 448 from Iowa and 236 from Pécs.
There were no significant demographic differences between the study populations from Iowa and Pécs (Table 1). Hemangiomas were more common in the Iowa population than among the Pécs infants (21.9% vs 7.6%, p<0.001).
Table 1.
Patient characteristics and data on infantile hemangioma (IH) and retinopathy of prematurity (ROP) from the University of Iowa Children’s Hospital and the University of Pécs Obstetrics and Gynaecology Hospital
| All(n=684) | Iowa(n=448) | Pécs (n=236) | p-value | |
|---|---|---|---|---|
| Birth weight, g (SD) | 1124 (349) | 1138 (384) | 1098 (269) | 0.11 |
| Gestational age, wk (SD) | 27.9 (2.2) | 27.9 (2.3) | 28.0 (2.1) | 0.60 |
| Female, % | 51.5 | 52.5 | 49.6 | 0.52 |
| ROP, % | 39.0 | 39.1 | 39.0 | 1.00 |
| Stage 1 | 77 | 66 | 11 | |
| Stage 2 | 138 | 83 | 55 | |
| Stage >=3 | 52 | 26 | 26 | |
| IH, % | 17.0 | 21.9 | 7.6 | <0.001 |
| 1 | 67 | 54 | 13 | |
| 2–5 | 39 | 35 | 13 | |
| >5 | 10 | 9 | 1 | |
| IH size | ||||
| <0.2 cm | 75 | 75 | 0 | |
| 0.2–0.9 cm | 55 | 54 | 1 | |
| >= 1.0 cm | 51 | 35 | 16 | |
| ROP only, % | 30.1 | 28.1 | 33.9 | |
| IH only, % | 8.0 | 10.9 | 2.5 | |
| ROP + IH, % | 8.9 | 10.9 | 5.1 | |
| No ROP and no IH, % | 52.9 | 50.0 | 58.5 | |
After univariate analysis of potential covariates in the Iowa population, hyperglycemia, transfusion, infection, breast feeding, postnatal corticosteroids, gestational age, birth weight, highest bilirubin, and days on oxygen were all related significantly (p≤0.10) to IH or ROP and so were eligible for entry into a logistic regression model (Table 2). In the Pécs population, all these variables as well as sex, antenatal steroids, and erythropoietin exposure were eligible for inclusion. Stepwise logistic regression modeling was used in each population for all covariates to build a final model.
Table 2.
Factors associated with ROP
|
|
||||||
|---|---|---|---|---|---|---|
| Iowa | Pécs | |||||
|
| ||||||
| No ROP (n=273) | ROP (n=175) | No ROP (n=144) | ROP (n=92) | |||
|
| ||||||
| Dichotomous Variables
| ||||||
| N(%) | P-value | N(%) | P-value | |||
| Female sex | 122 (44.7) | 91 (52.0) | 0.157 | 64 (44.4) | 55 (59.8) | 0.030 |
| Any hyperglycemia | 65 (23.8) | 112 (64.0) | 0.000 | 23 (16.0) | 39 (42.4) | <0.001 |
| Insulin treatment | 0 (0) | 6 (3.4) | 0.008 | 7 (4.9) | 25 (27.2) | <0.001 |
| Transfusion | 165 (60.4) | 164 (93.7) | <0.001 | 105 (72.9) | 80 (87.0) | 0.017 |
| Any infection | 50 (18.3) | 79 (45.1) | <0.001 | 95 (66.0) | 75 (81.5) | 0.014 |
| Exclusive breast feeding | 124 (45.4) | 34 (19.4) | <0.001 | 140 (97.2) | 83 (90.2) | 0.045 |
| Antenatal corticosteroids | 246 (90.1) | 147 (84.5) | 0.103 | 79 (54.9) | 38 (41.3) | 0.058 |
| Postnatal corticosteroids | 78 (28.6) | 114 (65.1) | <0.001 | 13 (9.0) | 49 (53.3) | <0.001 |
| Erythropoietin exposure | 0 (0) | 3 (1.7) | 0.115 | 68 (47.2) | 71 (77.2) | <0.001 |
| Methylxanthine exposure | 260 (95.2) | 172 (98.3) | 0.151 | 59 (41.0) | 48 (52.2) | 0.121 |
| At least 1 hemangioma | 49 (17.9) | 49 (28.0) | 0.017 | 6 (4.2) | 12 (13.0) | 0.024 |
|
| ||||||
| Continuous Variables
|
||||||
| Median (SD) | P-value | Median (SD) | P-value | |||
|
| ||||||
| Gestational age, wk | 29 (1.6) | 26 (2.1) | <0.001 | 29 (1.6) | 26 (2.0) | <0.001 |
| Birth weight, g | 1328 (340.4) | 835 (287.9) | <0.001 | 1260 (220.5) | 890 (240.9) | <0.001 |
| Number of hyperglycemic episodes | 0 (0.9) | 1 (3.5) | <0.001 | 0 (0.7) | 0 (3.2) | <0.001 |
| Highest bilirubin, mg/dl | 8.2 (2.3) | 7.3 (2.5) | <0.001 | 10.2 (3.1) | 8.1 (3.1) | <0.001 |
| Duration of oxygen therapy, d | 60 (109.7) | 219 (163.9) | <0.001 | 7 (23.1) | 54.5 (28.5) | <0.001 |
| Number of infections | 1 (0.6) | 1 (1) | 0.217 | |||
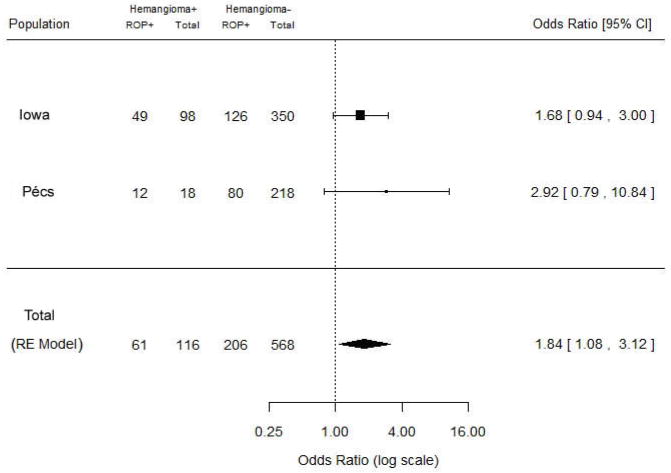
After stepwise regression, hemangioma remained in the logistic regression model in each population but showed only a trend toward significant relation to ROP (adjusted OR for Iowa 1.68 [95% CI 0.94–3.00] and for Pécs 2.92 [0.79–10.8]) (Table 3). When the corrected associations from the two populations were combined through random effects meta-analysis, a significant relationship between IH and ROP was detected (OR=1.84, 95% CI 1.08–3.12) (Fig. 1).
Table 3.
Relationship between ROP and IH
| Iowa | Hemangioma
|
Pécs | Hemangioma
|
||||
|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | ||||
| ROP | No | 224 | 49 | ROP | No | 138 | 6 |
| Yes | 126 | 49 | Yes | 80 | 12 | ||
|
| |||||||
| 95% | CI | 95% | CI | ||||
|
| |||||||
| OR | 1.78 | 1.13 | 2.80 | OR | 3.39 | 1.25 | 10.2 |
| aOR | 1.68 | 0.94 | 3.00 | aOR | 2.92 | 0.79 | 10.8 |
aOR is adjusted odds ratio. The odds ratio was adjusted for gestational age, days on oxygen, and postnatal corticosteroids. In Iowa, it was also adjusted for birth weight and exclusive human milk feeding. In Pécs, adjustments were made for sex, highest bilirubin, insulin treatment, and antenatal corticosteroids.
Fig. 1.
Forest plot for meta-analysis of data from current study by study site
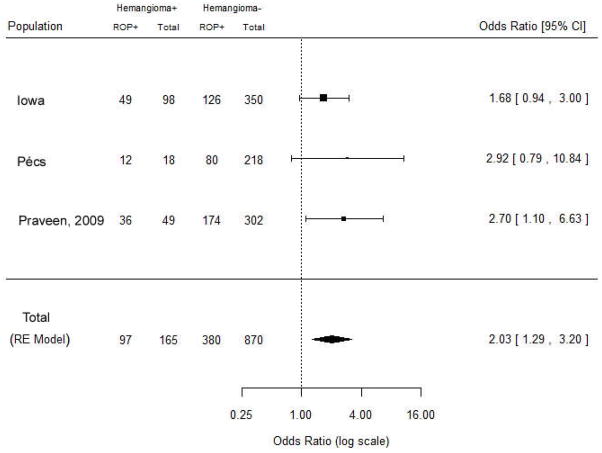
When the results from Praveen et al [35] were combined with our study population, the relationship between IH and ROP was even more strongly expressed (OR=2.03, 95% CI 1.29–3.20) (Fig. 2). These combined results suggest that IH and ROP do not occur independently in preterm infants. The risk of IH increased significantly with increasing ROP stage in both the Iowa and Pécs populations (results not shown).
Fig. 2.
Forest plot for meta-analysis of data from current study and study of Praveen et al [35]
Discussion
Our results support the hypothesized relationship between ROP and IH. IH was one of several variables in our model associated with ROP. Some of the covariates related to ROP we found were also identified in the analysis of ROP and IH association by Praveen et al [35], including gestational age and postnatal corticosteroid use. We found several other covariates not examined by Praveen et al to be significant, and we included these in our model; these were exclusive breastfeeding, duration of oxygen exposure, hyperglycemia, and insulin treatment.
Many of the variables that were significantly correlated with ROP were expected. Birth weight, gestational age, and oxygen exposure were covariates in our model that were previously known to be linked to ROP risk [2, 8, 12, 16, 27, 30]. Other variables, such as erythropoietin, breastfeeding, and hyperglycemia, are less clinically established covariates to ROP but were found to be linked to ROP in one or both of our populations [24, 38].
While most variables held similar significance in both the Iowa and Pécs populations, a few did not. For example, erythropoietin was significantly correlated to ROP in Pécs, where it was given to 139 infants, but not in Iowa, where only 3 received the drug.
There are several limitations to our study. Infants were screened for ROP using standardized ophthalmologic protocols in both hospitals. Whereas the case finding for ROP should be quite complete, we suspect this is not the case of IH in our centers. Most IHs do not cause clinically significant problems and may be overlooked in care provider notes. Further, diagnosis of IH can be easily confused with other vascular anomalies, and misdiagnosis is relatively common [17]. Because hemangioma data collection is not standardized, it is possible that physicians in Iowa and Pécs differed in their recording practices. For example, 46% of all Iowa hemangiomas were <0.2 cm, but none of this size were noted in Pécs. This likely reflects a difference between the centers in the size threshold for recording the presence of hemangiomas. Another potential source of ascertainment bias in the diagnosis of IH is the difference between centers in the timing of skin examinations. In Pécs, IHs were noted only on the discharge examination, whereas at Iowa, IHs were sought and recorded both at discharge and on multiple follow-up examinations, all of which were reviewed for this study. The impact of ascertainment bias, if it existed, should have been minimized by analyzing the Iowa and Pécs data separately, and the association between ROP and IH was found in both populations.
Our results show that ROP and IH are likely to co-occur, but prospective studies are needed to confirm and more clearly define this association. To improve our understanding of the association of ROP and IH, further investigation into the pathogenesis of both disorders is needed. Greater knowledge of the biology of vasculogenesis in ROP and IH will increase our understanding of any overlapping mechanisms between ROP and IH.
Conclusions
Preterm infants with IH are more likely to also have ROP than are infants without IH. Further study of these conditions and their association may shed new light on the role of abnormal vasculogenesis in these disorders and on common mechanisms of pathogenesis in IH and ROP.
Acknowledgments
This research was supported by grant UL1 RR024979 from the National Institutes of Health and grant OTKA 78480 from the Országos Tudományos Kutatási Alapprogramok (Hungarian Scientific Research Fund). We thank Gretchen A. Cress, RN, BSN, Erin M. Reynolds, PhD, MPH, and Heather A. Davis, MLIS for advice on setting up the database used for this study.
Abbreviations
- CI
confidence interval
- IH
infantile hemangioma
- OR
odds ratio
- ROP
retinopathy of prematurity
Footnotes
The authors declare that they have no financial relationships and no conflict of interest relevant to this article to disclose.
References
- 1.Amir J, Metzker A, Krikler R, Reisner SH. Strawberry hemangioma in preterm infants. Pediatr Dermatol. 1986;3(4):331–332. doi: 10.1111/j.1525-1470.1986.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 2.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38(7):397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boscolo E, Mulliken JB, Bischoff J. VEGFR-1 mediates endothelial differentiation and formation of blood vessels in a murine model of infantile hemangioma. Am J Pathol. 2011;179(5):2266–2277. doi: 10.1016/j.ajpath.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boye E, Jinnin M, Olsen BR. Infantile hemangioma: challenges, new insights, and therapeutic promise. J Craniofac Surg. 2009;20(Suppl 1):678–684. doi: 10.1097/SCS.0b013e318193d6c1. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Joyal JS, Hatton CJ, Juan AM, Pei DT, Hurst CG, Xu D, Stable A, Hellstrom A, Smith LEH. Propranolol inhibition of β-adrenergic receptor does not suppress pathologic neovascularization in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2012;53(6):2968–2977. doi: 10.1167/iovs.12-9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131(1):99–108. doi: 10.1542/peds.2012-1128. [DOI] [PubMed] [Google Scholar]
- 7.Cringle SJ, Yu DY. Oxygen supply and consumption in the retina: implications for studies of retinopathy of prematurity. Doc Ophthalmol. 2010;120(1):99–109. doi: 10.1007/s10633-009-9197-2. [DOI] [PubMed] [Google Scholar]
- 8.Crosse VM, Evans PJ. Prevention of retrolental fibroplasia. AMA Arch Ophthalmol. 1952;48(1):83–87. doi: 10.1001/archopht.1952.00920010086012. [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341(3):173–181. doi: 10.1056/NEJM199907153410307. [DOI] [PubMed] [Google Scholar]
- 11.Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, Chun RH, Garzon MC, Holland KE, Liberman L, MacLellan-Tobert S, Mancini AJ, Metry D, Puttgen KB, Seefeldt M, Sidbury R, Ward KM, Blei F, Baselga E, Cassidy L, Darrow DH, Joachim S, Known EKM, Martin K, Perkins J, Siegel DH, Boucek RJ, Frieden IJ. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131(1):128–140. doi: 10.1542/peds.2012-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ertl T, Gyarmati J, Gaál V, Szabo I. Relationship between hyperglycemia and retinopathy of prematurity in very low birth weight infants. Biol Neonate. 2006;89(1):56–59. doi: 10.1159/000088199. [DOI] [PubMed] [Google Scholar]
- 13.Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M, Tung B Early Treatment for Retinopathy of Prematurity Cooperative Group. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116(1):15–23. doi: 10.1542/peds.2004-1413. [DOI] [PubMed] [Google Scholar]
- 14.Greenberger S, Bischoff J. Infantile hemangioma – mechanism(s) of drug action on a vascular tumor. Cold Spring Harb Perspect Med. 2011;1(1):a006460. doi: 10.1101/cshperspect.a006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberger S, Yuan S, Walsh LA, Boscolo E, Kang KT, Matthews B, Mulliken JB, Bischoff J. Rapamycin suppresses self-renewal and vasculogenic potential of stem cells isolated from infantile hemangioma. J Invest Dermatol. 2011;131(12):2467–2276. doi: 10.1038/jid.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunn TR, Easdown J, Outerbridge EW, Aranda JV. Risk factors in retrolental fibroplasia. Pediatrics. 1980;65(6):1096–1100. [PubMed] [Google Scholar]
- 17.Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B, Hopper AJ, Frieden IJ. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118(3):882–887. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thieklke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatton DD, Schwietz E, Boyer B, Rychwalski P. Babies count: the national registry for children with visual impairments, birth to 3 years. J AAPOS. 2007;11(4):351–355. doi: 10.1016/j.jaapos.2007.01.107. [DOI] [PubMed] [Google Scholar]
- 20.Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, Albertsson-Wikland K, Carlsson B, Niklasson A, Sjodell L, LeRoith D, Senger DR, Smith LE. Low IGF-1 supresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA. 2001;98(10):5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B, Nopper AJ, Frieden IJ Hemangioma Investigator Group. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr. 2007;150(3):291–294. doi: 10.1016/j.jpeds.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Holland KE, Drolet BA. Infantile hemangioma. Pediatr Clin North Am. 2010;57(5):1069–1083. doi: 10.1016/j.pcl.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Hussain N, Clive J, Bhandari V. Current incidence of retinopathy of prematurity, 1989–1997. Pediatrics. 1999;104(3):e26. doi: 10.1542/peds.104.3.e26. [DOI] [PubMed] [Google Scholar]
- 24.Hylander MA, Strobino DM, Pezzullo JC, Dhanireddy R. Association of human milk feedings with a reduction in retinopathy of prematurity among very low birthweight infants. J Perinatol. 2001;21(6):356–362. doi: 10.1038/sj.jp.7210548. [DOI] [PubMed] [Google Scholar]
- 25.International Committee for the Classification of Retinopathy of Prematurity . The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 26.Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14(11):1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaempf JW, Kaempf AJ, Wu Y, Stawarz M, Niemeyer J, Grunkemeier G. Hyperglycemia, insulin and slower growth velocity may increase the risk of retinopathy of prematurity. J Perinatol. 2011;31(4):251–257. doi: 10.1038/jp.2010.152. [DOI] [PubMed] [Google Scholar]
- 28.Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118(7):2592–2599. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol. 2008;25(2):168–173. doi: 10.1111/j.1525-1470.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 30.Kinsey VE. Retrolental fibroplasia: cooperative study of retrolental fibroplasia and the use of oxygen. AMA Arch Ophthalmol. 1956;56(4):481–543. [PubMed] [Google Scholar]
- 31.Kleinman ME, Greives MR, Churgin SS, Blechman KM, Chang EI, Ceradini DJ, Tepper OM, Gurtner GC. Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma. Arterioscler Thromb Vasc Biol. 2007;27(12):2664–2670. doi: 10.1161/ATVBAHA.107.150284. [DOI] [PubMed] [Google Scholar]
- 32.Madan A, Jan JE, Good WV. Visual development in preterm infants. Dev Med Child Neurol. 2005;47(4):276–280. doi: 10.1017/s0012162205000514. [DOI] [PubMed] [Google Scholar]
- 33.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36(6):724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114(10):1210–1228. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 35.Praveen V, Vidavalur R, Rosenkrantz TS, Hussain N. Infantile hemangiomas and retinopathy of prematurity: possible association. Pediatrics. 2009;123(3):e484–489. doi: 10.1542/peds.2007-0803. [DOI] [PubMed] [Google Scholar]
- 36.Raghuveer TS, Bloom BT. A paradigm shift in the prevention of retinopathy of prematurity. Neonatology. 2011;100(2):116–129. doi: 10.1159/000322848. [DOI] [PubMed] [Google Scholar]
- 37.Ristori C, Filippi L, Dal Monte M, Martini D, Cammalleri M, Fortunato P, la Marca G, Fiorini P, Bagnoli P. Role of the adrenergic system in a mouse model of oxygen-induced retinopathy: antiangiogenic effects of β-adrenoreceptor blockade. Invest Ophthalmol Vis Sci. 2011;52(1):155–170. doi: 10.1167/iovs.10-5536. [DOI] [PubMed] [Google Scholar]
- 38.Romagnoli C, Tesfagabir MG, Giannantonio C, Papacci P. Erythropoietin and retinopathy of prematurity. Early Hum Dev. 2011;87(Suppl 1):S39–42. doi: 10.1016/j.earlhumdev.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 39.Sapieha P, Joyal JS, Rivera JC, Kermorvant-Duchemin E, Sennlaub F, Hardy P, Lachapelle P, Chemtob S. Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life. J Clin Invest. 2010;120(9):3022–3032. doi: 10.1172/JCI42142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y, Flint AF, Mulliken JB, Wu JK, Bischoff J. Endothelial progenitor cells in infantile hemangioma. Blood. 2004;103(4):1373–1375. doi: 10.1182/blood-2003-08-2859. [DOI] [PubMed] [Google Scholar]