Abstract
Background
Although many retrospective studies suggest resection of the primary tumor improves survival in metastatic breast cancer, animal studies suggest resection induces metastasis. Moreover, there has been no critical evaluation of how well animal studies actually model metastatic breast cancer. We utilized our newly established orthotopic cancer implantation under direct vision model to evaluate the hypothesis that primary tumor resection improves survival in metastatic breast cancer by reduction of overall tumor burden and improved immune responsiveness.
Methods
Murine mammary adenocarcinoma 4T1-luc2 cells that can be visualized by bioluminescence were implanted orthotopically into Balb/c mice under direct vision. Resection of the primary tumors at Days 6, 10, and 28 were compared to sham resection of the contralateral normal mammary gland and observation alone. Tumor burden was quantified by bioluminescence. Tumor draining lymph nodes were identified by intradermal injection of lymphazurin, and primary tumors, lymph nodes and lungs were examined pathologically. Kaplan-Meier survival analyses were performed. Splenocyte myeloid derived suppressor cells (MDSCs) and CD4 or CD8 single positive T lymphocytes were quantified by flow cytometry.
Results
Tumors invaded locally, metastasized to regional lymph nodes, and then to distant organs, with subsequent mortality. Surgical stress increased tumor burden only transiently without affecting survival. When primary tumor resection decreased overall tumor burden substantially, further growth of metastatic lesions did not increase overall tumor burden compared to observation and survival was improved, which was not the case when resection did not significantly reduce overall tumor burden. Decreasing overall tumor burden through resection of the primary tumor resulted in decreased splenic MDSC numbers and increased CD4 and CD8 cells, suggesting the potential for an improved immunological response against cancer.
Conclusions
Decreasing overall tumor burden through resection of the primary breast tumor decreased MDSCs, increased CD4 and CD8 cells, and improved survival.
Introduction
Because breast cancer is the second leading cause of cancer death among women in the United States and five year survival for metastatic disease is only 26%,1 advances in the management of metastatic breast cancer is expected to have a great impact. Without data from prospective, randomized controlled clinical trials, the role of surgical resection of the primary tumors in patients with metastatic breast cancer can only be based on retrospective studies.2 Although they have limitations, such as potential selection bias,3 many such clinical studies suggest that resection of the primary tumor improves the overall survival of these patients.4–11 Studies have been conducted in animal models of metastatic breast cancer as well to further investigate in vivo the potential biological mechanisms underlying the clinical results. However, conflicting conclusions have been reached with different animal experiments.12–15
Folkman demonstrated that the primary tumor actively secretes angiostatin, which suppresses the angiogenic activity of metastatic cancer, and that resection of the primary tumor removes that suppression and thus increases angiogenesis and growth of metastatic lesions.16 Fisher demonstrated that animals with metastatic disease were immunologically compromised, and that surgical stress releases growth factors, which in turn stimulate proliferation of metastasized cancer cells.17 Moreover, it was shown that surgical injury enhances the expression of genes that promote breast cancer metastasis to the lung,12 and that surgical resection of breast cancer increases cancer cell presence in sentinel lymph nodes.13 However, these studies using rodent models did not evaluate translational clinical endpoints such as disease free or even overall survival, nor did they discuss the clinical reports that contradicted the results of their animal experiments.
In contrast, the primary tumor has been shown to play a role in promoting metastatic proliferation through metastatic lesions self-seeding back into the primary tumor, 14 thus its removal may suppress metastatic growth by dividing the metastatic progression sequence. Primary tumor resection has also been shown to improve immune responsiveness against cancer, even in the presence of metastatic disease.15 In addition to the discrepancies in the results of animal studies and retrospective clinical studies, most of these studies also did not evaluate translational clinical endpoints nor did they address the contradictory findings of other animal studies. There has been no critical evaluation of how adequately these animal experiments model human breast cancer in the first place.18–27
To address these contradictions and deficiencies, we have now utilized a newly developed orthotopic implantation under direct vision method in immune intact syngeneic mice as a metastatic breast cancer model. We evaluated the effects of surgical stress and primary tumor resection on overall tumor burden, cancer progression, host immune response parameters, and overall survival.
Methods
Animal Models
Virginia Commonwealth University Institutional Animal Care and Use Committee (IACUC) approval was obtained for all experiments. Female Balb/c mice, 12 weeks of age and weighing approximately 20g, were obtained from Harlan Laboratories (Frederick, MD). The 4T1-luc2, estrogen and progesterone receptor negative, HER2 positive mammary adenocarcinoma cell line derived from Balb/c mice and genetically manipulated to overexpress the firefly luciferase gene was obtained from Caliper Life Sciences (Hopkinton, MA). The cells were cultured in RPMI media, suspended at a concentration of 1×107 cells/ml and 10 µL of this solution were then implanted as described below.
All cell implantations were performed under isoflurane anesthesia using sterile technique. A 5 mm incision was made medial to the nipple, and a cotton swab was used to expose the mammary gland. The cells were implanted directly into the mammary gland under direct vision, using ×10 microscopic magnification, and the wound was closed with a nylon suture. The Balb/c mice have 5 mammary glands on each side, and the right chest mammary gland was used for implantations. Xenogen IVIS® 200 and Living Image® software (Caliper Life Sciences, Hopkinton, MA) were used to quantify the photon/sec emitted by 4T1-luc2 cells after intraperitoneal injection of 200 µL (150 mg/kg) of luciferin (Fisher Scientific, Inc.), which enables quantification of tumor burden and cancer progression live in vivo. Pathological analyses of tumors, lymph nodes and metastatic sites were performed after formalin fixation. The slide sections and hematoxylin and eosin (H&E) staining were performed by Virginia Commonwealth University Health System Anatomic Pathology Research Services (APRS).
To evaluate the effect of surgical stress and primary tumor resection on tumor burden, cancer progression, and survival, the mice were randomized to the following groups (8 mice per group) six days after implantation, when distant metastases were confirmed by bioluminescence: Observation (Obs): No operative manipulation was performed; Resection of the Primary Tumor (Resect): Ten days after implantation the entire tumor was resected and the incision was closed (This was performed at Days 6, 10 or 28 after implantation in another experiment); and Surgical stress (Sham): Ten days after implantation to the right chest, the entire left chest normal mammary gland (contralateral side to the tumor) was resected and the incision was closed. To evaluate the effect of cancer progression on the host immune parameters, spleens were harvested 7, 18 and 21 days after tumor implantation, and were compared to control mice (5 Balb/c tumor-free mice per group). To evaluate the effect of primary tumor resection on host immune parameters, spleens were harvested 8 or 15 days after Sham operation or Resection performed on Day 10 (5 mice per group).
Analysis of splenocytes for MDSC and T cell subsets
Spleens were harvested in RPMI, weighed, and crushed through a cell strainer. Splenocytes were resuspended in 1× ammonium chloride solution to lyse red blood cells and stained with 0.04% trypan blue to exclude dead cells. Viable cell numbers were counted under a light microscope using a hemacytometer. Cells were brought to 1×106 in 100 µl and stained for 30 min with anti-mouse CD11b (FITC-conjugated) and anti-mouse Ly-6G/Ly-6C(Gr-1) (PE-conjugated) (Biolegend, San Diego, CA). Unstained cells were used as a negative control, and rat IgG2b,κ was used as the isotype control. Staining with CD11b alone or Gr-1 alone was used as single color positive controls. Stained cells were fixed with 2% paraformaldehyde and analyzed (25,000 cells per sample) within 7 days of staining on an ELITE Beckman Coulter flow cytometer. For assessment of CD4+ and CD8+ T cells, fluorescently labeled Abs directed against CD4 (GK1.5) and CD8 (53–6.7) from Pharmingen (San Diego, CA), were used and flow cytometry performed as above. Appropriate isotype controls were used in all cases.
Statistical analysis
For photon emission, percentage of MDSC, CD4 and CD8 cells, Student's t-test was used, and Kaplan-Meier statistical analyses were utilized to compare survival.
Results
Our orthotopic implantation method produced quantifiable cancer progression, which started locally, metastasized to regional lymph nodes, then to distant organs
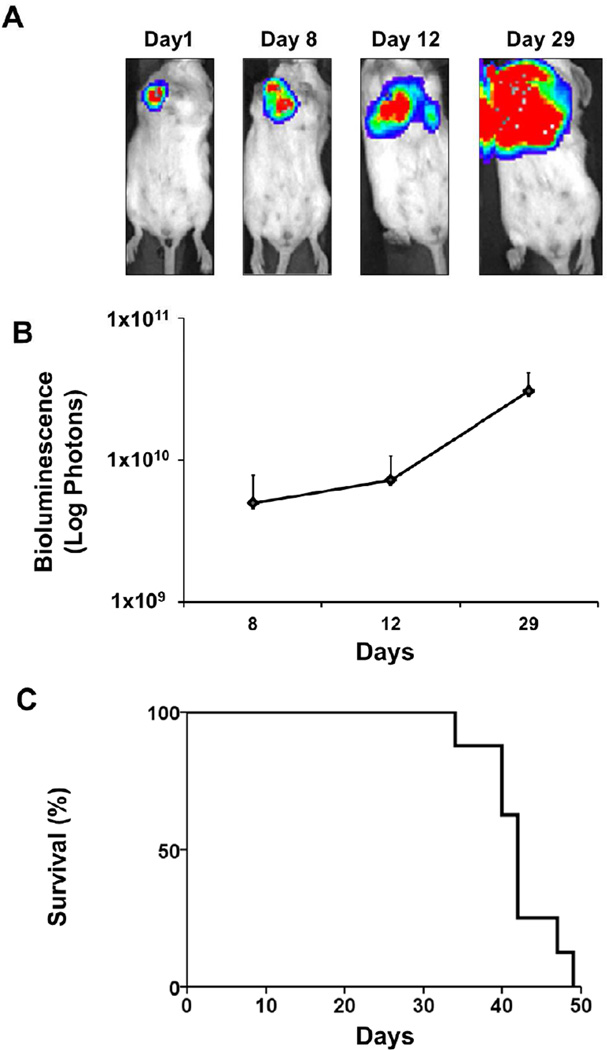
First, we evaluated whether orthotopic implantation under direct vision into the chest mammary glands of immune-competent mice result in a pattern of cancer progression similar to human breast cancer. After implantation of the bioluminescent 4T1-luc2 cells, tumor growth surveillance was performed noninvasively both visually (Fig 1A) and quantitatively by bioluminescence (Fig 1B). The bioluminescence images from the body surface demonstrate that the implanted 4T1-luc2 cells metastasized to regional lymph nodes by Day 8 after implantation and then to the lungs by Day 12. However, by that time the primary chest tumor was so enlarged that it obscured the evaluation of the metastatic lesions (Fig 1A). We observed an increase of photons emitted from viable cancer cells that reflects an increase in overall tumor burden (Fig 1B). Fig 1C shows the survival curve of this model.
FIGURE 1. Our orthotopic implantation model allows for quantification of overall tumor burden and survival analysis.
(A) Representative bioluminescent images after orthotopic implantation of 1×105 4T1-luc2 cells. Bioluminescence images at 1, 8, 12 and 29 days after implantation demonstrate progressive tumor growth and metastatic spread. (B) The total tumor burden is quantified as total photons measured by bioluminescent technology as cancer progressed after implantation. (C) Kaplan-Meier survival curve of this model.
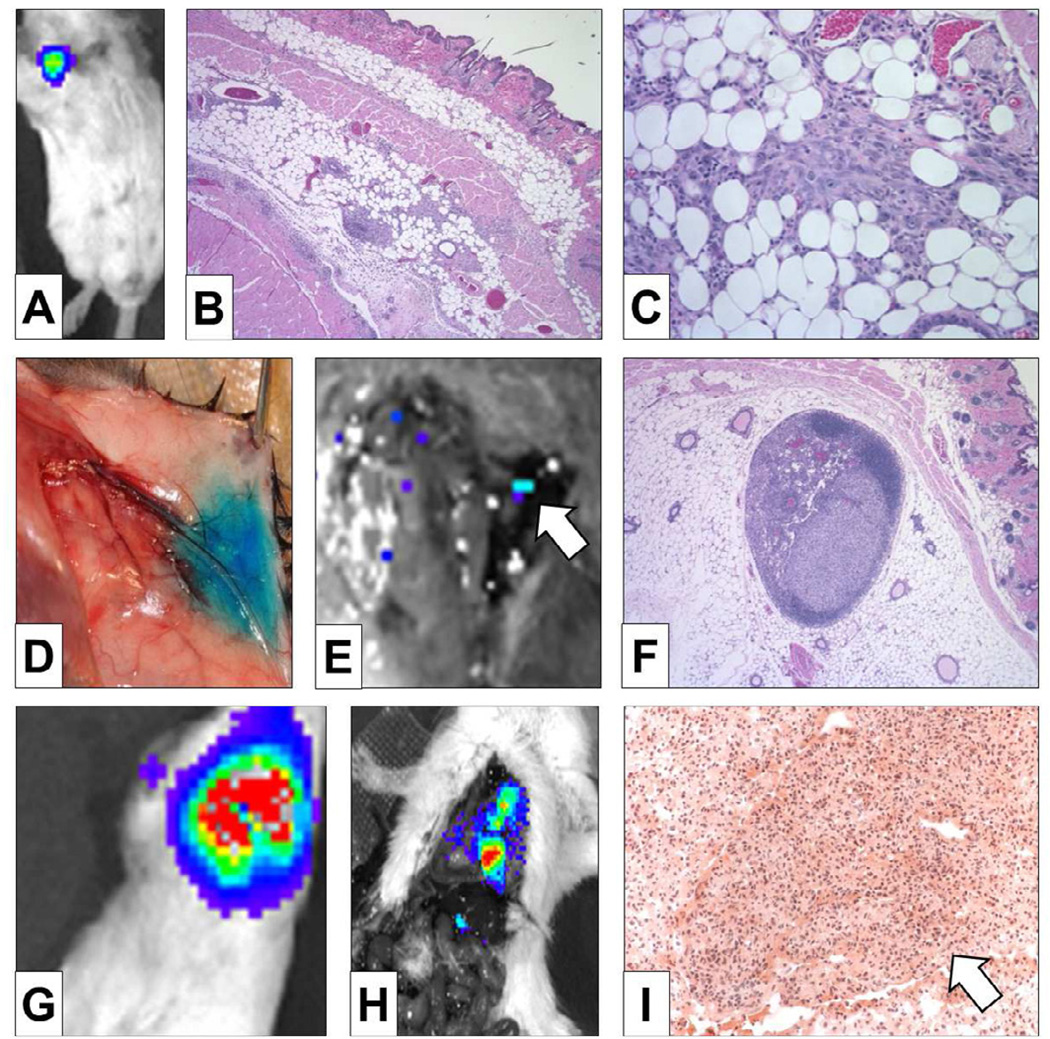
As is the case for human breast cancer, implanted 4T1-luc2 cells grow and invade locally within the mammary gland (Fig 2A-C). The mammary gland lymphatic drainage as mapped by Lymphazurin injection was to the axillary lymph node basin (Fig 2D), to which the tumor metastasized before the distant organs, as confirmed by bioluminescence (Fig 2E) and pathologic examination of the axillary lymph nodes (Fig 2F). Note that the bioluminescence signal was only detected in lymph nodes and not in the lung when the primary tumor was removed 4 days after implantation (Fig 2E). Similar to human breast cancer progression, distant lung metastasis was observed after lymph node metastasis, which was confirmed by bioluminescence and pathologic examination of the lungs (Fig 2G-I). These metastatic lesions in the lung formed tumors, unlike lesions produced by tail vein injection of cancer cells, which is the most commonly used lung metastasis model (Fig 2I).
FIGURE 2. Our orthotopic implantation model mimics the human pattern of breast cancer progression.
Our model produced mammary gland tumors as demonstrated by bioluminescence (A) and confirmed by H&E staining (x40 (B), ×100 (C)). Subdermal injection of Lymphazurin demonstrates lymphatic drainage to the axillary lymph node basin (D), to which 4T1-luc2 tumors regionally metastasized as confirmed by bioluminescence (white arrow in E) and by H&E staining of an axillary lymph node (F). The lack of bioluminescent uptake in the lung fields demonstrates that lymph node metastasis occurred before distant lung metastasis (E). Non-invasive bioluminescence demonstrates lung metastasis after removal of the primary tumor (G), which was confirmed by bioluminescent lesions in the lung on thoracotomy after primary tumor resection (H). H&E staining demonstrates a metastatic lung tumor (white arrow in I).
Both surgical stress and removal of the primary tumor aggravate growth of metastatic lesions
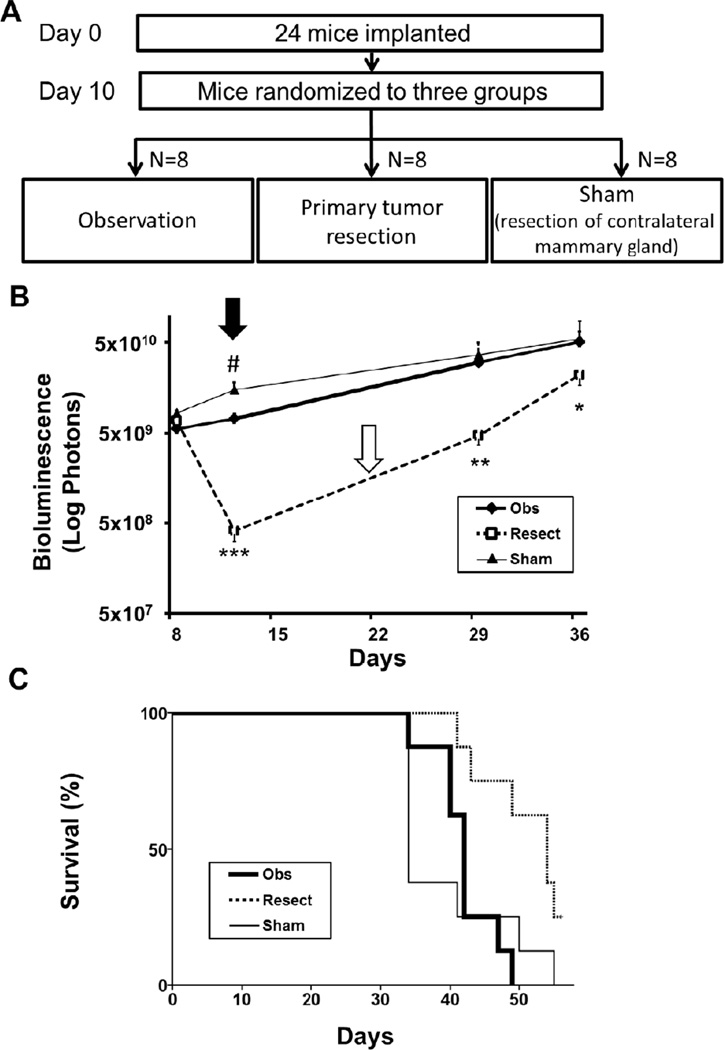
To investigate the effect of surgical stress on growth of metastatic lesions, we compared the quantified overall tumor burden after contralateral normal mammary gland resection (Sham group) to observation alone (Obs group) (Fig 3). Mice were randomized after implantation to ensure there were no differences in tumor burden prior to interventions (Fig 3A). Tumor burden was measured 2 days after the procedure, which was significantly greater in the Sham group compared to the Obs group (Fig 3B). This is in agreement with Fisher’s notion that surgical stress significantly increases tumor burden.17 However, this difference became negligible by 19 days after the Sham operation.
FIGURE 3. Primary tumor resection in metastatic breast cancer significantly prolongs survival despite the observed Fisher and Folkman effects.
(A) Study design: Twenty-four mice ten days after implantation with 1×105 4T1-luc2 cells were randomized to Observation (Obs), Resection of primary tumor (Resect), and Resection of the contralateral mammary gland (Sham) groups (N = 8 per group). (B) Overall tumor burden was quantified by bioluminescence. There was no statistical difference in overall tumor burden between the groups at the time of randomization. Tumor burden was significantly higher in the Sham group (thin line) compared to the Obs group (bold line) on day 12 (Fisher effect (black arrow), #P < .001), but there was no significant difference by day 29. The rate of increase in tumor burden was significantly faster after removal of the primary tumor (Resect group (dotted line); *P < .05, **P < .001, ***P <. 001), with a slope of 11.4 and 4.7 in the Resect group compared to 4.2 and 1.7 in the Obs group (Folkman effect (white arrow)). (C) Kaplan-Meier analysis demonstrates a significant improvement in survival after resection (dotted line) vs. Sham (thin line) (P < .01) and vs. Obs (bold line) (P = .01). No difference in survival was observed between the Sham and Obs groups.
To investigate the effect of removal of the primary tumor on metastatic growth, we compared the increase in overall tumor burden after resection of the implanted primary tumor (Resect group) to the Obs group (Fig 3A, B). Although resection of the primary tumor at day 10 significantly reduced overall tumor burden, there was a rapid increase in the growth rate of the remaining metastatic lesions when compared to the Obs group (Fig 3B). Specifically, comparing the Resect versus the Obs group, the slope of increase in tumor burden between day 12 and 29 was 11.4 versus 4.2, and between day 29 and 36 was 4.7 versus 1.7, respectively (Fig 3B). This is in agreement with Folkman’s theory that removal of the primary tumor accelerates growth of metastatic lesions.16
To address the question whether primary tumor resection in animals with metastatic breast cancer has an effect on survival, overall survival was compared between the three groups (Resect, Sham and Obs) while controlling for overall tumor burden (Fig 3A, C). Kaplan-Meier analysis demonstrates that there was no significant difference in survival between the Sham and Obs groups (P = .83). However, there was a significant improvement in survival in the Resect compared to Obs (P < .01) and Sham groups (P < .01).
Only primary tumor resection that significantly reduces overall tumor burden improves survival
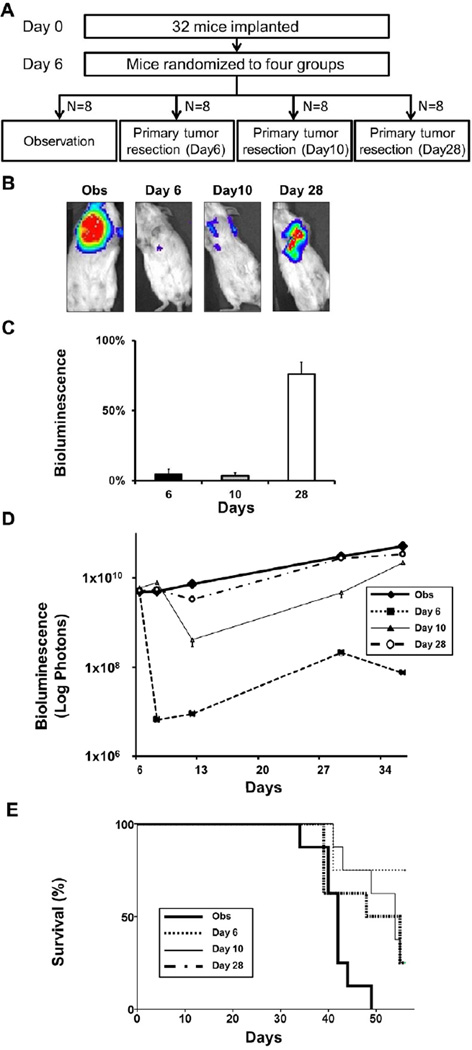
Next, we investigated the effect of overall tumor burden reduction on survival by comparing resection of the primary tumor at different time points after implantation. Because metastatic lesions grow over time, the primary tumor represents a progressively smaller percentage of the overall tumor burden. Primary tumor resection was performed 6, 10, or 28 days after implantation to achieve different percentages of overall tumor burden reduction (Fig 4A). After primary tumor resection at Day 28, 76% of the overall tumor burden remained as metastatic lesions, whereas only 4% remained after Day 6 or Day 10 primary tumor resection (Fig 4B, C). When compared to the Obs group, only the Day 28 resection group failed to significantly reduce overall tumor burden (Fig 4D). Kaplan-Meier analysis demonstrated that only the groups in which overall tumor burden was substantially reduced by primary tumor resection (Day 6 and Day 10 groups) had significantly improved survival compared to Obs (Fig 4E). Conversely, primary tumor resection that did not significantly reduce overall tumor burden, as in the Day 28 group, did not improve survival.
FIGURE 4. Only primary tumor resection that significantly reduces overall tumor burden improves survival.
(A) Study design: Thirty two mice six days after implantation with 1×105 4T1-luc2 cells were randomized into 4 groups (N = 8 per group): observation (Obs), primary tumor resection on day 6 (Day 6), tumor resection on day 10 (Day 10), and tumor resection on day 28 (Day 28), again controlling for overall tumor burden. (B) Representative bioluminescence imaging of a mouse from the Obs group, and immediately after resection of primary tumor of Day 6, Day 10, and Day 28 groups. (C) The percentage of remaining overall tumor burden after primary tumor resection for Day 6, Day 10 and Day 28 groups was quantified by bioluminescence. The percentage of overall tumor burden was calculated by dividing the quantification of remaining metastatic lesions by the overall tumor burden before resection (N = 8). (D) Time course of overall tumor burden quantified by bioluminescence for each group. There was no significant difference in overall tumor burden at randomization and only the Day 28 group (bold dotted line) did not reduce overall tumor burden significantly at the time of resection. (E) Kaplan-Meier analysis showed that only Day 28 (bold dotted line) failed to significantly improve survival compared to observation (Obs (bold line) vs. Day 6 (thin dotted line) P < .01, Obs vs. Day 10 (thin line) P < .01).
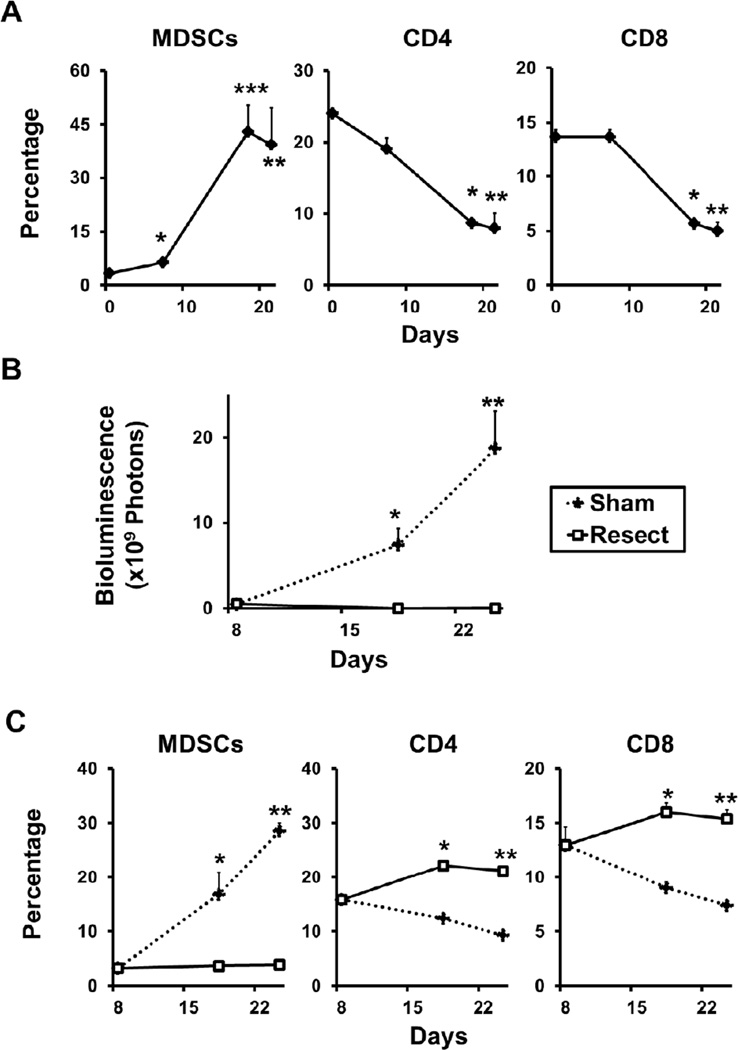
Primary tumor resection reverses the immunosuppressive effects of cancer progression
Because MDSCs have been previously shown to increase in metastatic breast cancer and can suppress the antitumor function of T-cells,15, 28 we investigated the effect of decreasing tumor burden by resection on the number of MDSCs, CD4, and CD8 cells in the spleen. After implantation, the percentage of MDSCs significantly increased with cancer progression, while CD4 and CD8 cells decreased (Fig 5A), which is in agreement with previous reports.15, 28 Eighteen days after implantation, the Resect group demonstrated a significant reduction in the overall tumor burden compared to the Sham group (Fig 5B). The increase in MDSCs with cancer progression was completely prevented by primary tumor resection (Fig 5C left panel). In contrast, CD4 and CD8 cells were increased by resection of the primary tumor (Fig 5C middle and right panel). These findings suggest an association between the reduction of overall tumor burden by primary tumor resection and restoration of anti-cancer immune responsiveness.
FIGURE 5. Reduction of overall tumor burden by primary tumor resection reverses the immunosuppressive effects of cancer progression.
(A) The spleens were harvested untreated mice and from mice 7, 18, and 21 days after implantation (N = 5 per group). Flow cytometry analysis of splenocytes was performed to quantify MDSCs and CD4 and CD8 positive T cells. MDSCs increased (*P < .05, **P<.01, ***P<.05, N = 3), while CD4 (*P < .001, **P < .01) and CD8 positive cells (*P < .01, **P < .01) decreased significantly in the spleen, 18 and 21 days after implantation. (B) After controlling for overall tumor burden 8 days implantation, mice were randomized to 2 groups (N = 5): Resection of primary tumor (Resect), and contralateral mammary gland resection (Sham), both performed on day 10. The Resect group showed significantly reduced overall tumor burden compared to Sham as demonstrated by bioluminescence. (*P < .01, P < 0.01, respectively) (C) Spleens of the Resect and Sham groups were harvested 8, 18 and 25 days after implantation (N = 5 per group). The Resect group suppressed the increase in MDSCs (left panel; *P < .01, **P < .001) and increased CD4 (middle panel; *P < .001, **P < .001) and CD8 (right panel; *P < .001, **P < .001) positive splenocytes compared to Sham.
Discussion
The dogma against resection of the primary tumor in metastatic breast cancer is that it is unlikely to affect metastatic lesions in a beneficial way, and might worsen survival because surgical stress could induce metastatic proliferation as reported by Fisher.17 Moreover, resection of the primary tumor could remove inhibition of tumor-induced angiogenesis, leading to increased growth of metastatic foci, as reported by Folkman.16 The assumptions of these two classic effects, together with other recent reports of increased metastatic proliferation after removal of the primary tumor,12, 13 are contradicted by retrospective reports indicating that resection of the primary breast tumor improves survival.3 However, our results may explain these contradictions and suggest that although the Fisher and Folkman effects can be observed in animal models, they may not be the key determinants of survival.
We observed that surgical stress induced a brief period of increased tumor growth, and resection of the primary tumor accelerated metastatic progression. However, previous reports evaluated the impact on metastatic proliferation by counting metastatic lesions in the lung at necropsy,12, 16, 17, 29 and not by monitoring tumor progression in the whole animal in vivo over time, as was done in this study utilizing bioluminescence technology. It was previously not appreciated that the effect of surgical stress is transient and the effect on overall tumor burden dissipates outside of the period of surgical stress. Likewise, because tumor burden is greatly reduced by primary tumor resection, even with the effect of a rapid increase of metastatic proliferation, overall tumor burden after resection remains significantly less. Therefore, although the Fisher and Folkman effects can impact metastatic proliferation, our data demonstrate that they did not have a significant impact on overall tumor burden. We also found that only primary tumor resection that significantly reduces overall tumor burden improves survival. Our results are consistent with some retrospective studies suggesting that certain patient subpopulations with metastatic breast cancer may benefit from resection of the primary tumor.4–11
Decreasing tumor burden in our syngeneic tumor model through resection of the primary tumor improved survival, which may at least in part, result from reversing the detrimental effects of cancer progression on the immune response, such as the increase of MDSCs, which inhibit T cell antitumor function.15, 28 As cancer progressed and tumor burden increased, MDSCs increased and CD4 and CD8 cells decreased in the spleen. Decreasing tumor burden by resection of the primary site suppressed the increase in MDSCs and the decrease in CD4 and CD8 cells. Our results support similar findings of improved immune function after primary tumor resection in mice.15
Our results are based on a mouse model and to suggest its direct application to clinical decisions or practice would be premature. Also, it has been suggested that human breast cancers found to be metastatic at the time of first diagnosis are biologically aggressive cancers and their clinical outcomes are typically driven by the biology of the tumor. In this study, we were able to evaluate the effect of resection of the primary tumor in the setting of metastasis, eliminating the bias of heterogeneous biology by utilizing an animal model in which identical cancer cells are implanted into each subject. Our results may help to explain decades of animal and retrospective clinical data and to guide future animal and clinical research.
In order to know whether resection of the primary tumor improves survival in metastatic breast cancer patients, a prospective controlled randomized trial must be conducted. In March, 2011, the Eastern Cooperative Oncology Group opened a clinical trial to address this question. ECOG E2108 (NCT01242800) is a phase III, multicenter, prospective, randomized trial, which will compare surgical resection of the primary tumor to medical therapy alone in patients who have responded to initial systemic therapy, and will evaluate overall survival as the primary endpoint. This trial is expected to provide further guidance on the role of primary tumor resection in metastatic breast cancer in humans. For now, selecting patients who may benefit from resection remains a clinical and research challenge.
In summary, we evaluated orthotopic surgical implantation in immune intact mice as an appropriate translational model to evaluate the role of primary tumor resection in metastatic breast cancer. Second, our data confirmed the well reported findings of Drs. Fisher and Folkman, but did so in a manner that also explained the results, which had been previously thought to be conflicting. The Fisher and Folkman effects in themselves did not determine survival in our model. Instead, overall tumor burden was the primary determinant. Decreasing tumor burden through primary tumor resection may improve survival by decreasing MDSCs and increasing CD4 and CD8 cells.
Acknowledgements
The authors thank Dr. Ronald C. Merrell, Dr. James P. Neifeld, and Dr. Michael G. Sarr for providing a critical review and valuable input to improve this manuscript. The authors also thank the Virginia Commonwealth University Health System Anatomic Pathology Research Services (APRS) Director Dr. Jorge A. Almenara and histotechnologists for technical assistance in the tissue processing, sectioning and staining.
This work was supported by NIH grants (R01CA160688, and K12HD055881) and Susan G. Komen for the Cure (Investigator Initiated Research Grant (12222224) and Career Catalyst Research Grant KG090510) to KT, and NCI grant R01CA61774 to SS. MN is a Japan Society for the Promotion of Science Postdoctoral Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeSantis C, Siegel R, Bandi P, et al. Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Leung AM, Vu HN, Nguyen KA, et al. Effects of surgical excision on survival of patients with stage IV breast cancer. J Surg Res. 161:83–88. doi: 10.1016/j.jss.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 3.Cady B, Nathan NR, Michaelson JS, et al. Matched pair analyses of stage IV breast cancer with or without resection of primary breast site. Ann Surg Oncol. 2008;15:3384–3395. doi: 10.1245/s10434-008-0085-x. [DOI] [PubMed] [Google Scholar]
- 4.Rao R, Feng L, Kuerer HM, et al. Timing of surgical intervention for the intact primary in stage IV breast cancer patients. Ann Surg Oncol. 2008;15:1696–1702. doi: 10.1245/s10434-008-9830-4. [DOI] [PubMed] [Google Scholar]
- 5.Rapiti E. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol. 2006;24:2743–2749. doi: 10.1200/JCO.2005.04.2226. [DOI] [PubMed] [Google Scholar]
- 6.Khan Does Aggressive Local Therapy Improve Survival In Metastatic Breast Cancer? Curr Surg. 2004;61:251–255. doi: 10.1067/msy.2002.127544. [DOI] [PubMed] [Google Scholar]
- 7.Gnerlich J, Jeffe DB, Deshpande AD, et al. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988–2003 SEER data. Ann Surg Oncol. 2007;14:2187–2194. doi: 10.1245/s10434-007-9438-0. [DOI] [PubMed] [Google Scholar]
- 8.Fields RC, Jeffe DB, Trinkaus K, et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol. 2007;14:3345–3351. doi: 10.1245/s10434-007-9527-0. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael AR, Anderson ED, Chetty U, et al. Does local surgery have a role in the management of stage IV breast cancer? Eur J Surg Oncol. 2003;29:17–19. doi: 10.1053/ejso.2002.1339. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard DK, Shetty PB, Hilsenbeck SG, et al. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg. 2008;247:732–738. doi: 10.1097/SLA.0b013e3181656d32. [DOI] [PubMed] [Google Scholar]
- 11.Babiera GV, Rao R, Feng L, et al. Effect of primary tumor extirpation in breast cancer patients who present with stage IV disease and an intact primary tumor. Ann Surg Oncol. 2006;13:776–782. doi: 10.1245/ASO.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Al-Sahaf O, Wang JH, Browne TJ, et al. Surgical injury enhances the expression of genes that mediate breast cancer metastasis to the lung. Ann Surg. 252:1037–1043. doi: 10.1097/SLA.0b013e3181efc635. [DOI] [PubMed] [Google Scholar]
- 13.Tvedskov TF, Jensen MB, Balslev E, et al. Stage migration after introduction of sentinel lymph node dissection in breast cancer treatment in Denmark: a nationwide study. Eur J Cancer. 47:872–878. doi: 10.1016/j.ejca.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danna EA, Sinha P, Gilbert M, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–2211. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J. New perspectives in clinical oncology from angiogenesis research. Eur J Cancer. 1996;32A:2534–2539. doi: 10.1016/s0959-8049(96)00423-6. [DOI] [PubMed] [Google Scholar]
- 17.Fisher ER, Fisher B. Experimental studies of factors influencing the development of hepatic metastases. XIII. Effect of hepatic trauma in parabiotic pairs. Cancer Res. 1963;23:896–900. [PubMed] [Google Scholar]
- 18.Schuh J. Trials, Tribulations, and Trends in Tumor Modeling in Mice. Toxicologic Pathology. 2004;32:53–66. doi: 10.1080/01926230490424770. [DOI] [PubMed] [Google Scholar]
- 19.Burger MM. UICC study group on basic and clinical cancer research: Animal models for the natural history of cancer. Meeting held at Woods Hole, MA (USA), June 21–23, 1999. International Union Against Cancer. Int J Cancer. 2000;85:303–305. doi: 10.1002/(sici)1097-0215(20000201)85:3<303::aid-ijc1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JI, Decker S, Zaharevitz D, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bibby MC, Sleigh NR, Loadman PM, et al. Potentiation of EO9 anti-tumour activity by hydralazine. Eur J Cancer. 1993;29A:1033–1035. doi: 10.1016/s0959-8049(05)80218-7. [DOI] [PubMed] [Google Scholar]
- 22.Ottewell PD, Coleman RE, Holen I. From genetic abnormality to metastases: murine models of breast cancer and their use in the development of anticancer therapies. Breast Cancer Res Treat. 2006;96:101–113. doi: 10.1007/s10549-005-9067-x. [DOI] [PubMed] [Google Scholar]
- 23.Gravekamp C, Sypniewska R, Gauntt S, et al. Behavior of metastatic and nonmetastatic breast tumors in old mice. Exp Biol Med (Maywood) 2004;229:665–675. doi: 10.1177/153537020422900711. [DOI] [PubMed] [Google Scholar]
- 24.T Corbett FV, LoRusso P, et al. In: In vivo methods for screening and preclinical testing. Teicher B, editor. Totowa, NJ: Humana Press, Inc; 1998. [Google Scholar]
- 25.Tao K, Fang M, Alroy J, et al. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller FR. Tumor subpopulation interactions in metastasis. Invasion Metastasis. 1983;3:234–242. [PubMed] [Google Scholar]
- 27.Lelekakis M, Moseley JM, Martin TJ, et al. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17:163–170. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- 28.Le HK, Graham L, Cha E, et al. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9:900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 29.O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]