Abstract
Pancreatogenic diabetes (PD) is a potentially fatal disease that occur secondary to pancreatic disorders. The current anti-diabetic therapy for PD is fraught with adverse effects that can increase morbidity. Here we investigated the efficacy of novel peptide nanomedicine: pancreatic polypeptide (PP) in sterically stabilized micelles (SSM) for management of PD. PP exhibits significant anti-diabetic efficacy but its short plasma half-life curtails its therapeutic application. To prolong and improve activity of PP in vivo, we evaluated the delivery of PP in SSM. PP-SSM administered to rats with PD, significantly improved glucose tolerance, insulin sensitivity and hepatic glycogen content compared to peptide in buffer. The studies established the importance of micellar nanocarriers in protecting enzyme-labile peptides in vivo and delivering them to target site, thereby enhancing their therapeutic efficacy. In summary, this study demonstrated that PP-SSM is a promising novel anti-diabetic nanomedicine and therefore should be further developed for management of PD.
Keywords: Pancreatic polypeptide, Sterically stabilized micelles, Pancreatogenic diabetes
INTRODUCTION
Pancreatogenic diabetes (PD), also known as type 3c diabetes mellitus (T3cDM), encompasses all diabetes mellitus cases that occur as a result of pancreatic diseases such as chronic pancreatitis (CP), hemochromatosis, pancreatic neoplasia, pancreatic resection and cystic fibrosis amongst others. CP accounts for approximately 76% of all pancreatogenic diabetes cases and causes progressive endocrine dysfunction leading to deficiency of insulin, glucagon, pancreatic polypeptide (PP) and incretin hormones such as gastric inhibitory peptide and glucagon like peptide-1 (GLP-1), resulting in the development of PD. CP induced PD is characterized primarily by impaired glucose tolerance and hepatic insulin resistance. Overall, PD accounts for approximately 8% of all diabetic patients worldwide (1–2% of diabetic patients in North America and 15–20% of diabetic patients in Southeast Asian countries).
Currently, there are no set guidelines for management of PD and medications such as insulin and its secretagogues, sulphonylureas, metformin, alpha glucosidase inhibitors and thiazolidinediones amongst others are commonly used. However, treatment of PD requires special consideration that limits the use of many of these drugs (discussed more elaborately in subsequent sections).
To this end, endocrine hormone pancreatic polypeptide has shown great promise as an anti-diabetic drug for treatment of PD secondary to CP. The peptide increases the expression of insulin receptors in the liver and thereby enables effective utilization of circulating insulin in the body. Therefore not surprisingly, hepatic insulin resistance in PD has mostly been attributed to the deficiency of pancreatic polypeptide in the body. Recently, this hormone was shown to improve insulin sensitivity and decrease insulin requirements in PD patients. However, the peptide suffers from the problem of short biological half-life that necessitates continuous administration of the peptide for obtaining therapeutic efficacy. Moreover, PP has the propensity to aggregate in aqueous media that further complicates effective delivery of the peptide. Researchers have endeavored to circumvent the issue of short half-life by synthesizing PP analogues that have better stability against in vivo degradation. However, immnogenicity/toxicity concerns of synthetic analogues must not be overlooked. In addition, chemical modification of peptide molecules escalates the already amplified manufacturing costs of these biomolecules. Furthermore, free peptide formulations have wide biodistribution due to their ability to easily extravasate out of circulation at normal vasculature and therefore these formulations cannot be passively targeted to diseased site. These peptide molecules can interact with their receptors expressed in various parts of the body and as a result lead to the development of undesirable effects and increase the dose required to obtain a therapeutic benefit.
We attempted to address the delivery problems of PP by associating the peptide with sterically stabilized micelles (SSM). SSM are polyethylene glycolated (PEGylated) phospholipid micelles that have been shown to increase the half-life of several peptides in vivo, prevent their aggregation in aqueous media and deliver them specifically to desired sites of action in their most active conformation for receptor interaction. Consequently, peptides delivered in SSM demonstrate enhanced therapeutic efficacy and reduced adverse effects. These peptide loaded micelles can be prepared with ease, scaled-up and lyophilized for long-term storage without using any lyo/cryoprotectant. We have recently prepared a formulation of PP in SSM (PP-SSM) that demonstrated decreased self-aggregation of peptide molecules and increased stability against proteolytic degradation in vitro. These promising results, prompted us to evaluate the efficacy of this novel nanomedicine in vivo for the treatment of pancreatogenic diabetes. In this study, we utilized a rat model of chronic pancreatitis induced pancreatogenic diabetes and determined the effectiveness of PP-SSM in improving glucose tolerance and insulin sensitivity compared to free peptide.
METHODS
The animal experiments were conducted in accordance to the University of Illinois at Chicago animal care committee guidelines and to the Guide for the Care and Use of Laboratory Animals, prepared by the Committee of Care and Use of Animals of the Institute of Laboratory Animal Resources, National Research Council. Care was taken to provide humane treatment to all animals used in the studies.
Materials
1,2-Distearoyl-sn-glycero-3-phosphatidylethanolamine-N-[methoxy(polyethyleneglycol)-2000] sodium salt (DSPE-PEG2000) was purchased from Lipoid GmbH (Ludwigshafen, Germany). Human pancreatic polypeptide was synthesized and purified to 95% purity (determined using reverse phase high performance liquid chromatography) by protein research laboratory, Research Resources Center at the University of Illinois at Chicago. All animals (6 weeks old male Wistar rats) were purchased from Charles River Laboratories. Phosphate buffered saline (PBS), pH 7.4 was purchased from Mediatech Inc, Manassas, VA. Normal saline (0.9% w/v sodium chloride injection USP) was purchased from Baxter Healthcare Corporation (Deerfield, IL). Freestyle lite glucose meter/strips, Humalog® (Insulin lispro injection), Humulin® (Regular human insulin injection), Ketamine hydrochloride® Injection USP and Anased® Injection (Xylazine hydrochloride) were obtained from pharmacy at University of Illinois at Chicago. L-arginine, D-glucose, amyloglucosidase and metformin hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO).
In vivo studies
The purpose of this study was to determine whether PP in the presence or absence of SSM exerts any significant anti-diabetic activity in a rat model of pancreatogenic diabetes. The formulation of PP in SSM was prepared as described earlier. Briefly, weighed quantity of DSPE-PEG2000 was dispersed in PBS and allowed to equilibrate for 1h in the dark at 25°C to form empty micelles. To these preformed micelles, a solution of PP in PBS was added and the resulting dispersion was equilibrated in the dark for 2h at 25°C to form PP-SSM. A lipid: peptide molar ratio of 30:1 was used to provide maximum peptide molecules per micelle.
Efficacy studies were conducted using PP-SSM/PP in buffer and controls such as buffer (PBS), empty micelles, insulin and metformin. The studies were conducted as per the following protocol: glucose tolerance and insulin sensitivity in normal rats was first determined (see below), followed by disease induction (described in subsequent section) and re-evaluation of glucose tolerance and insulin sensitivity. Animals were deemed to have impaired glucose tolerance when a significant difference in the blood glucose level was observed at the end of 1h of intraperitoneal glucose injection when compared to normal animals (i.e. animals before disease induction). Similarly, impaired insulin sensitivity was defined as significant difference in the percent decrease in blood glucose level at end of 30 minutes of intraperitoneal insulin injection when compared to normal animals. Treatment with PP/PP-SSM and controls was initiated when animals were found to demonstrate impaired glucose tolerance and insulin sensitivity. At the end of 5 consecutive days of treatment, glucose tolerance and insulin sensitivity was assessed again followed by quantification of hepatic glycogen content after glucose challenge. The schema for the efficacy studies has been presented in Figure 1.
Figure 1.
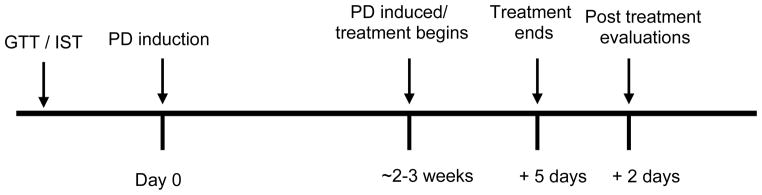
Schema for anti-diabetic efficacy studies in rats. Not according to scale. GTT: glucose tolerance test; IST: Insulin sensitivity test
Glucose tolerance and insulin sensitivity tests
At the start of the study, glucose tolerance and insulin sensitivity tests were performed to determine glucose homeostasis in normal animals. For glucose tolerance test (GTT), animals were fasted for a period of 8h but given free access to water. A 30% w/v D-glucose solution was prepared and injected intraperitoneally into the animals at a dose of 1 ml/100g body weight. Blood glucose was measured from the tail vein before glucose injection and at 30 and 60 minutes after injection. At the end of 60 minutes, all animals were provided food ad libitum. For insulin sensitivity test (IST), the animals were fasted for a period of 6h but given free access to water. Insulin solution was prepared from Humalog® using normal saline and injected into the animals intraperitoneally at a dose of 1 U/kg body weight. Blood glucose was measured from the tail vein before insulin injection and at 30 minutes after injection. At the end of 30 minutes, all animals were provided food ad libitum. Percent drop in blood glucose level upon insulin injection was calculated based on blood glucose level before insulin injection. All intraperitoneal injections as well as blood glucose testing were carried out by restraining the animals in DecapiCone™ for a brief period of time.
Induction of pancreatogenic diabetes in rats
After determining basal glucose tolerance and insulin sensitivity in animals, pancreatogenic diabetes was induced by daily intraperitoneal injection of L-arginine at a dose of 350 mg/kg body weight for a period of 2–3 weeks. Studies have shown that repeated injections of L-arginine to rats cause inflammation in the pancreas, fibrosis, progressive atrophy of acinar cells and development of lesions similar to that seen in human chronic pancreatitis. Concomitantly, insulin secretion from pancreas decreases and animals develop impaired glucose tolerance after 1 week of daily arginine injections. In this study, glucose tolerance and insulin sensitivity of the animals were determined at the end of each week. The animals were considered to have developed pancreatogenic diabetes when they demonstrated both impaired glucose tolerance and insulin sensitivity.
Anti-diabetic efficacy study
After induction of pancreatogenic diabetes, the animals were divided into six groups of five each such that the mean drop in percent blood glucose level, as determined from insulin sensitivity test, was similar across all groups. The animals were evaluated using PP/PP-SSM as treatment drugs, PBS/SSM as negative controls and metformin/insulin as positive controls.
PP dose of 200 μg/kg body weight was used in the study and PP-SSM samples were prepared as described above. The dose of PP used in the study was based on studies by Seymour et al. who found that a 5 day administration of 200 μg/kg body weight PP improved glucose tolerance in rats. Metformin solution was prepared in normal saline and injected to animals at a dose of 30 mg/kg body weight while insulin solution was prepared from Humulin® using normal saline and injected to animals at a dose of 0.5 U/kg body weight. Test drugs and controls were injected intravenously via the tail vein once daily for 5 consecutive days. In all cases, the injection volume was lower than 0.3 ml. Prior to the intravenous administration of control and treatment drugs, the animals were anesthetized by intraperitoneal injection of ketamine and xylazine (50 mg/kg and 5 mg/kg respectively). Glucose tolerance and insulin sensitivity were assessed at the end of 5 day treatment period.
Assessment of hepatic glycogen content
At the end of treatment period with PP/PP-SSM and controls and after evaluation of glucose tolerance and insulin sensitivity, the hepatic glycogen content in the rats was determined. For this, rats were injected with 30% D-glucose solution intraperitoneally at a dose of 1 ml/100g body weight. At 2h following glucose injection, the rats were sacrificed and their livers harvested. The hepatic glycogen content in the livers was measured using amyloglucosidase assay as described by Carr and Neff.
Data and Statistical analyses
All data are expressed as mean ± standard error of mean (S.E.M.). For the statistical analysis, student’s T-test was used and a value of p<0.05 was considered as statistical significance.
RESULTS
The purpose of this study was to evaluate the effectiveness of PP nanomedicine (PP-SSM) in treating pancreatogenic diabetes through assessment of glucose tolerance and insulin resistance. Glucose tolerance test and quantification of hepatic glycogen at the end of treatment period were conducted to determine whether treatment with PP/PP-SSM was able to overcome impaired glucose tolerance developed in the animals as a result of high dose of arginine injections. Insulin sensitivity test was performed to determine whether administration of PP-SSM overcomes insulin resistance. Insulin and metformin were used as positive controls to compare the efficacy of PP-SSM nanomedicine with the present standards of care.
Glucose tolerance test
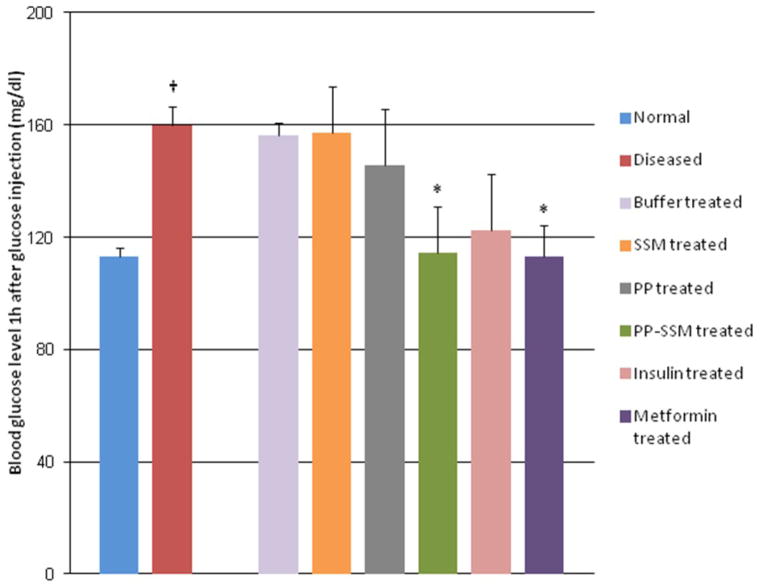
The mean blood glucose level of normal animals (before disease induction) was found to range between 102 – 118.6 mg/dl with an average of 113.1 ± 3.2 mg/dl across all groups after 1h of glucose injection. Upon daily intraperitoneal L-arginine injections for 2–3 weeks, the mean blood glucose level in the animals was significantly increased to an average of 160.1 ± 6.4 mg/dl after 1h of glucose injection (ranged between 146 – 176 mg/dl). This clearly demonstrated that these animals were not able to clear glucose from the blood stream as effectively as normal animals and therefore the animals were considered to have developed impaired glucose tolerance.
Treatment with free PP for five consecutive days led to a non-significant decrease in blood glucose levels after 1h of glucose challenge (145.8 ± 20 mg/dl) when compared to diseased animals while a significant decrease in blood glucose levels was observed for animals treated with PP nanomedicine (114.6 ± 16.5 mg/dl) (Figure 2). A similar significant improvement in glucose tolerance was also observed in the group of animals treated with metformin (113.2 ± 11.3 mg/dl). It must be noted that glucose tolerance in both PP-SSM and metformin treated groups were restored to normal levels, i.e. levels observed in animals before disease induction. All other control groups did not show any significant improvement in glucose tolerance at the end of treatment period. Blood glucose levels of animals after 1h of glucose challenge was noted to be 156.2 ± 4.6 mg/dl for buffer treated group; 157.4 ± 16.1 mg/dl for SSM treated group and 122.4 ± 20.2 mg/dl for insulin treated group.
Figure 2. Glucose tolerance in normal, diseased and drug treated animals.
Blood glucose levels at 60 minutes after glucose injection in normal, diseased and drug treated animals. † indicates statistically significant difference between normal and diseased animals (p < 0.05; n=5/group) while * represents statistically significant improvement in disposal of glucose from blood, in drug treated animals as compared to diseased animals. (p < 0.05; n=5/group).
Insulin sensitivity test
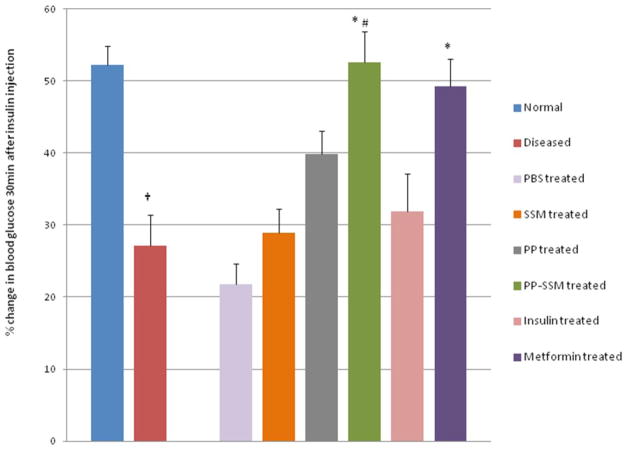
Insulin sensitivity of animals was evaluated as percent drop in blood glucose from basal level after 30 minutes of insulin injection. Normal animals (before disease induction) showed a drop of approximately 52.2 ± 2.7% upon insulin injection (ranged between 47.0 – 57.7% across the groups). However, a significant loss in insulin sensitivity was observed in the animals after 2–3 weeks of arginine injection. The mean percent drop in blood glucose was only 27.13 ± 4.3 (ranged between 24.1 – 32.1% across the groups) in animals injected with arginine. This significant loss in insulin sensitivity indicated the development of insulin resistance in the animals. At the point when animals displayed both impaired glucose tolerance as well as loss in insulin sensitivity; they were considered to have developed pancreatogenic diabetes and treatment with PP/PP-SSM and controls was thereafter started.
The animals that were treated with PP-SSM or metformin were found to significantly improve insulin sensitivity as compared to diseased animals. PP-SSM treated group demonstrated a 52.6 ± 4.3% drop in blood glucose level within 30 minutes of insulin injection while metformin treated group showed a drop of 49.2 ± 3.9% (Figure 3). As observed with glucose tolerance test, both PP-SSM and metformin treatment restored insulin sensitivity to normal levels, i.e. the response to insulin in animals before disease induction. All other treatment groups did not bring about any significant improvement in insulin sensitivity. The percent drop in blood glucose level was found to be 21.8 ± 2.8 for buffer treated group; 28.9 ± 3.4 for SSM treated group; 39.8 ± 3.2 for PP treated group and 31.9 ± 5.2 for insulin treated group. A significant difference in effectiveness between PP and PP-SSM treated animals was also observed. The study therefore demonstrated significantly higher efficacy of PP nanomedicine in improving insulin sensitivity in animals with PD as compared to PP in buffer.
Figure 3. Insulin sensitivity in normal, diseased and drug treated animals.
Insulin sensitivity represented as percent change in blood glucose level between 0 and 30 minutes after insulin injection for normal, diseased and treated animals. † represents statistically significant difference between normal and diseased animals; * indicates significant improvement in insulin sensitivity in treated animals compared to diseased animals (p<0.05, n=5/group) while # represents statistically significant enhancement of activity in PP-SSM treated group compared to PP treated group (p<0.05; n=5/group).
Assessment of hepatic glycogen content
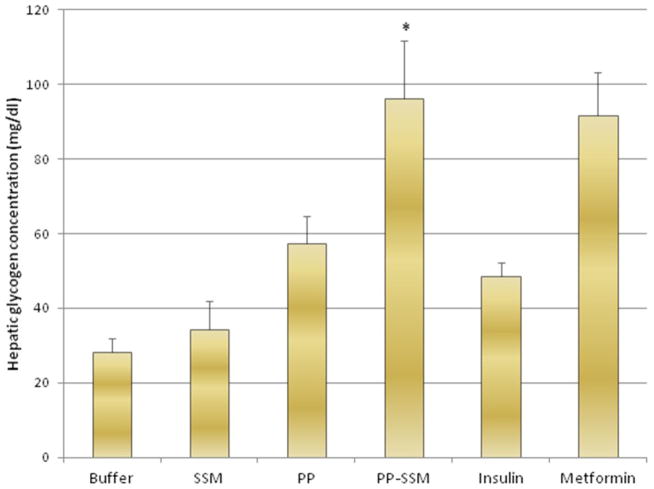
Hepatic glycogen content analysis at the end of treatment period revealed significantly higher glycogen content in livers of rats treated with PP-SSM (96.4 ± 15.2 mg/dl) compared to PP (57.4 ± 7.4 mg/dl). Animals treated with metformin also demonstrated higher hepatic glycogen content (91.6 ± 11.6 mg/dl) as compared to other treatment groups. Glycogen content in the livers was 28.2 ± 3.8 mg/dl for buffer treated group; 34.2 ± 7.6 mg/dl for SSM treated group and 48.6 ± 3.7 for insulin treated group (Figure 4). This study further corroborated the fact that PP-SSM nanomedicine is more effective in overcoming impaired glucose tolerance and insulin resistance in animals with pancreatogenic diabetes than peptide in aqueous media.
Figure 4. Glycogen content in livers of animals subjected to different treatments.
Hepatic glycogen content represented in mg/dl from animals treated with PP/PP-SSM and controls obtained at the end of efficacy study. * indicates statistically significant higher hepatic glycogen content in PP-SSM treated group compared to PP treated group (p<0.05 n=5/group).
DISCUSSION
PD is characterized by impaired glucose tolerance and loss of hepatic insulin sensitivity. Therefore to establish the anti-diabetic efficacy of PP-SSM for PD, assessment of glucose tolerance, insulin sensitivity as well as hepatic glycogen content was performed in diabetic animals.
Glucose tolerance test was conducted to determine the response of the animals to a high level of glucose in blood. This test measures both the capacity of the pancreas to secrete insulin and ability of the liver to respond to circulating insulin by clearing glucose from the blood stream. Therefore glucose tolerance test is used for the diagnosis of pancreatic endocrine function as well as insulin resistance in diabetes.
This study revealed that PP-SSM and metformin treated animals were able to significantly improve glucose tolerance as compared to diseased animals. The improvement in glucose tolerance observed in PP-SSM treated group can be attributed to the fact that PP in SSM due to its nanosize is passively targeted to its site of action i.e., to the liver via enhanced permeation and retention (EPR) effect observed through the liver fenestrations. In addition, it is postulated that PP in SSM is well-protected against proteolytic degradation in vivo as opposed to the formulation of peptide in buffer. It is believed that these two factors contribute toward greater accumulation of PP at the site of action and accounts for enhanced efficacy of PP when associated with SSM.
Insulin sensitivity test is used to measure the extent of insulin resistance in the body. Insulin resistance occurs as a result of reduced responsiveness of the body to given insulin. The etiologies for insulin resistance are mutation in insulin, presence of antibodies against insulin, decreased expression of insulin receptors in hepatic, muscle and adipose tissues, decreased binding of insulin to insulin receptors, mutations in the receptors, antibodies against insulin receptors, post receptor failure such as defective signal transduction and mutations in glucose transporter 4 (GLUT4) amongst others. Insulin resistance observed in pancreatogenic diabetes has been generally attributed to deficiency of PP which leads to decreased expression of insulin receptors in the liver. Therefore, exogenous administration of PP should be able to restore hepatic insulin sensitivity.
In this study, we observed that animals treated with PP-SSM or metformin had significantly higher sensitivity to insulin compared to other treatment groups. Furthermore, insulin sensitivity was restored to almost normal levels (as observed in animals before induction of the disease) in both PP-SSM and metformin treated groups. A significant increase in efficacy was also observed in PP-SSM treated group compared to peptide in buffer group which further corroborated the fact that SSM is able to effectively protect PP from enzymatic degradation in vivo and deliver it in its active conformation to the target site (liver), thereby eventually bringing about a better therapeutic effect than peptide alone.
Hepatic glycogen content assay was conducted to quantitate glycogen content in the liver after glucose challenge. This study was performed to assess the ability of liver to clear glucose from the blood and store it as glycogen in the liver. Our results indicated that livers of rats treated with PP-SSM had significantly higher glycogen content as compared to that of PP in buffer treated group. Animals treated with metformin also showed equivalent glycogen content in the liver as PP-SSM. This result is expected since metformin is known to increase the activity of glycogen synthase in the liver.
It must be noted that in all the studies, PP-SSM demonstrated equivalent therapeutic efficacy as metformin. However, the risk associated with metformin therapy such as lactic acidosis can be a grave concern in PD. In patients suffering from PD secondary to CP where alcoholism is the prime reason for development of CP, metformin is contraindicated. Additionally, such patients are prone to metabolic acidosis due to presence of acid-base imbalance observed in CP. Therefore metformin may not be very appropriate for management of PD in such patients. Insulin on the other hand, has been shown to increase the probability of development of pancreatic cancer, the risk of which is already elevated in CP patients. Therefore insulin or insulin secretagogues may not be suitable for PD therapy.
Anti-diabetic efficacy studies conducted using PP-SSM clearly demonstrated that efficacy of PP is significantly improved when it is delivered in SSM. PP nanomedicine due to its small size extravasates out of circulation only at the leaky vasculature of liver capillaries. This not only increases the dose of peptide reaching the target site but also reduces any adverse effect generated due to interaction of the peptide with receptors present in other parts of the body. The enhancement in therapeutic effect is also attributed to the fact that PEGylated micelles protect peptides from enzymatic degradation in vivo thus making them long-circulating and ultimately leading to greater accumulation of the peptide in target organs. In addition, SSM prevent self-aggregation of PP, therefore eliminates loss of peptide dose that is intended for therapy. Aggregated peptide formulations (PP in buffer), elicit much lower therapeutic effect compared to monomeric peptide formulations (PP-SSM), since peptide interacts with its receptors as monomers while aggregated peptides are rapidly cleared from the circulation. Association of PP with SSM not only provides PP in monomeric form but also presents it to the receptor in its most favorable conformation (improved alpha-helicity) for receptor interaction. We believe that all these factors together contribute toward improving the therapeutic efficacy of PP when it is delivered as a nanomedicine. With regards to the safety concern of PP-SSM, it must be pointed out that unlike small molecule drugs or free peptide in aqueous media, PP in SSM is not likely to show adverse effects as the peptide is passively targeted to its site of action. Moreover, both pancreatic polypeptide and PEGylated phospholipids used for preparation of PP nanomedicine have been approved by the Food and Drug Administration for use in humans. Therefore PP-SSM can be considered a safe nanomedicine for human use. Furthermore, PEG may prevent the interaction of peptide with immuno-stimulating factors, diminishing any possibility of immune reactions.
Taken together, these studies indicated that the novel formulation of pancreatic polypeptide in sterically stabilized phosholipid micelles demonstrated significant anti- diabetic activity in a rodent model of pancreatogenic diabetes. Based on these in vivo efficacy studies, we forsee a significant contribution of PP-SSM nanomedicine in the effective management of pancreatogenic diabetes without the risks associated with the current therapy.
Pancreatic polypeptide associated with sterically stabilized phospholipid micelles demonstrate better anti-diabetic efficacy compared to peptide in buffer for the treatment of pancreatogenic diabetes.
Acknowledgments
Funding: The study was supported in part by NIH grant CA121797 (HO), UIC university scholar (HO) and Van Doren (AB) awards.
This research project was conducted in the facility constructed with support from Research Facilities Improvement Program Grant number C06RR15482 from National Center for Research Resources NIH.
LIST OF ABBREVIATIONS
- CP
Chronic Pancreatitis
- DPSPE-PEG2000
1,2-Distearoyl-sn-glycero-3-phosphatidylethanolamine-N-[methoxy (polyethyleneglycol)-2000]
- EPR
Enhanced Permeation and Retention
- GLUT4
Glucose Transporter 4
- GLP-1
Glucagon-like Peptide -1
- GTT
Glucose Tolerance Test
- IST
Insulin Sensitivity Test
- PBS
Phosphate Buffered Saline
- PD
Pancreatogenic Diabetes
- PP
Pancreatic Polypeptide
- SEM
Standard Error of Mean
- SSM
Sterically Stabilized Micelles
- T3cDM
Type 3c Diabetes Mellitus
- VIP
Vasoactive Intestinal Peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes. Diabetes Care. 2011;34(suppl 1):S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui Y, Andersen DK. Pancreatogenic diabetes: Special considerations for management. Pancreatology. 2011 Jul 9;11(3):279–94. doi: 10.1159/000329188. [DOI] [PubMed] [Google Scholar]
- 3.Yki-Jarvinen H, Kiviluoto T, Taskinen MR. Insulin resistance is a prominent feature of patients with pancreatogenic diabetes. Metabolism. 1986 Aug;35(8):718–27. doi: 10.1016/0026-0495(86)90239-8. [DOI] [PubMed] [Google Scholar]
- 4.Nathan JD, Zdankiewicz PD, Wang J, Spector SA, Aspelund G, Jena BP, et al. Impaired hepatocyte glucose transport protein (GLUT2) internalization in chronic pancreatitis. Pancreas. 2001 Mar;22(2):172–8. doi: 10.1097/00006676-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Cui Y, Andersen DK. Diabetes and Pancreatic Cancer. Endocr Relat Cancer. 2012;19(5):F9–26. doi: 10.1530/ERC-12-0105. [DOI] [PubMed] [Google Scholar]
- 6.Brunicardi FC, Chaiken RL, Ryan AS, Seymour NE, Hoffmann JA, Lebovitz HE, et al. Pancreatic polypeptide administration improves abnormal glucose metabolism in patients with chronic pancreatitis. J Clin Endocrinol Metab. 1996 Oct;81(10):3566–72. doi: 10.1210/jcem.81.10.8855802. [DOI] [PubMed] [Google Scholar]
- 7.Sun YS, Brunicardi FC, Druck P, Walfisch S, Berlin SA, Chance RE, et al. Reversal of abnormal glucose metabolism in chronic pancreatitis by administration of pancreatic polypeptide. The American Journal of Surgery. 1986 Jan;151(1):130–40. doi: 10.1016/0002-9610(86)90023-1. [DOI] [PubMed] [Google Scholar]
- 8.Maeda H, Hanazaki K. Pancreatogenic diabetes after pancreatic resection. Pancreatology. 2011;11(2):268–76. doi: 10.1159/000328785. [DOI] [PubMed] [Google Scholar]
- 9.Rabiee A, Galiatsatos P, Salas-Carrillo R, Thompson MJ, Andersen DK, Elahi D. Pancreatic polypeptide administration enhances insulin sensitivity and reduces the insulin requirement of patients on insulin pump therapy. J Diabetes Sci Technol. 2011 Nov 1;5(6):1521–8. doi: 10.1177/193229681100500629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennig R, Kekis PB, Friess H, Adrian TE, Buchler MW. Pancreatic polypeptide in pancreatitis. Peptides. 2002 Feb;23(2):331–8. doi: 10.1016/s0196-9781(01)00605-2. [DOI] [PubMed] [Google Scholar]
- 11.Andersen DK. Mechanisms and emerging treatments of the metabolic complications of chronic pancreatitis. Pancreas. 2007 Jul;35(1):1–15. doi: 10.1097/mpa.0b013e31805d01b0. [DOI] [PubMed] [Google Scholar]
- 12.Seymour NE, Volpert AR, Andersen DK. Regulation of hepatic insulin receptors by pancreatic polypeptide in fasting and feeding. J Surg Res. 1996 Sep;65(1):1–4. doi: 10.1006/jsre.1996.9999. [DOI] [PubMed] [Google Scholar]
- 13.Kono T, Hanazaki K, Yazawa K, Ashizawa S, Fisher WE, Wang XP, et al. Pancreatic polypeptide administration reduces insulin requirements of artificial pancreas in pancreatectomized dogs. Artif Organs. 2005 Jan;29(1):83–7. doi: 10.1111/j.1525-1594.2004.29008.x. [DOI] [PubMed] [Google Scholar]
- 14.Adrian TE. Pancreatic polypeptide. J Clin Pathol Suppl (Assoc Clin Pathol) 1978;8:43–50. doi: 10.1136/jcp.s1-8.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adrian TE, Greenberg GR, Besterman HS, Bloom SR. Pharmacokinetics of pancreatic polypeptide in man. Gut. 1978 Oct;19(10):907–9. doi: 10.1136/gut.19.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee A, Onyuksel H. Human pancreatic polypeptide in a phospholipid based micellar formulation. Pharmaceutical Research. 2012;29(6):1698–711. doi: 10.1007/s11095-012-0718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellmann-Sickert K, Elling CE, Madsen AN, Little PB, Lundgren K, Gerlach LO, et al. Long-acting lipidated analogue of human pancreatic polypeptide is slowly released into circulation. J Med Chem. 2011 Apr 28;54(8):2658–67. doi: 10.1021/jm101357e. [DOI] [PubMed] [Google Scholar]
- 18.Tan TM, Field BC, Minnion JS, Cuenco-Shillito J, Chambers ES, Zac-Varghese S, et al. Pharmacokinetics, adverse effects and tolerability of a novel analogue of human pancreatic polypeptide, PP 1420. Br J Clin Pharmacol. 2012 Feb;73(2):232–9. doi: 10.1111/j.1365-2125.2011.04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee A, Onyuksel H. Peptide delivery using phospholipid micelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012 Jul 30;4(5):562–74. doi: 10.1002/wnan.1185. [DOI] [PubMed] [Google Scholar]
- 20.Sethi V, Rubinstein I, Kuzmis A, Kastrissios H, Artwohl J, Onyuksel H. Novel, biocompatible, disease modifying nanomedicine of VIP for rheumatoid arthritis. Mol Pharm. 2013;10(2):728–38. doi: 10.1021/mp300539f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnadas A, Rubinstein I, Sekosan M, Onyuksel H. Targeted delivery of paclitaxel to breast cancer by vasoactive intestinal peptide conjugated sterically stabilized phospholipid mixed micelles. AAPS J. 2004;6:M1191. [Google Scholar]
- 22.Dagar A, Kuzmis A, Rubinstein I, Sekosan M, Onyuksel H. VIP-targeted cytotoxic nanomedicine for breast cancer. Drug Delivery and Translational Research. 2012 Dec 01;2(6):454–62. doi: 10.1007/s13346-012-0107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim SB, Rubinstein I, Sadikot RT, Artwohl JE, Onyuksel H. A novel peptide nanomedicine against acute lung injury: GLP-1 in phospholipid micelles. Pharm Res. 2011 Mar;28(3):662–72. doi: 10.1007/s11095-010-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim SB, Banerjee A, Onyuksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J Control Release. 2012 Jun 12;163(1):34–45. doi: 10.1016/j.jconrel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Lim SB, Rubinstein I, Önyüksel H. Freeze drying of peptide drugs self-associated with long-circulating, biocompatible and biodegradable sterically stabilized phospholipid nanomicelles. Int J Pharm. 2008 May 22;356(1–2):345–50. doi: 10.1016/j.ijpharm.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durham HA, Truett GE. Development of insulin resistance and hyperphagia in zucker fatty rats. Am J Physiol Regul Integr Comp Physiol. 2006 Mar;290(3):R652–8. doi: 10.1152/ajpregu.00428.2004. [DOI] [PubMed] [Google Scholar]
- 27.Weaver C, Bishop AE, Polak JM. Pancreatic changes elicited by chronic administration of excess L-arginine. Exp Mol Pathol. 1994 Apr;60(2):71–87. doi: 10.1006/exmp.1994.1007. [DOI] [PubMed] [Google Scholar]
- 28.Fredstrom SB, Jessurun J, Gallaher DD. Pancreatitis induced in rats by repetitive administration of L-arginine. Pancreas. 2009 Apr;38(3):344–5. doi: 10.1097/MPA.0b013e318184ff83. [DOI] [PubMed] [Google Scholar]
- 29.Seymour NE, Volpert AR, Lee EL, Andersen DK, Hernandez C. Alterations in hepatocyte insulin binding in chronic pancreatitis: Effects of pancreatic polypeptide. Am J Surg. 1995 Jan;169(1):105,9. doi: 10.1016/s0002-9610(99)80117-2. discussion 110. [DOI] [PubMed] [Google Scholar]
- 30.Carr RS, Neff JM. Quantitative semi-automated enzymatic assay for tissue glycogen. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1984;77(3):447–9. doi: 10.1016/0305-0491(84)90258-x. [DOI] [PubMed] [Google Scholar]
- 31.Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25–37. doi: 10.1007/978-1-60761-609-2_3. [DOI] [PubMed] [Google Scholar]
- 32.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Controlled Release. 2000 Mar 1;65(1–2):271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 33.Insulin resistance [Internet] 2011 Available from: http://emedicine.medscape.com/article/122501-overview#aw2aab6b2b3aa.
- 34.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000 Dec;49(12):2063–9. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddi AS, Jyothirmayi GN. Effect of chronic metformin treatment of hepatic and muscle glycogen metabolism in KK mice. Biochem Med Metab Biol. 1992 Apr;47(2):124–32. doi: 10.1016/0885-4505(92)90016-r. [DOI] [PubMed] [Google Scholar]
- 36.Metformin and fatal lactic acidosis [Internet] 1998 Available from: http://www.medsafe.govt.nz/profs/PUarticles/5.htm.
- 37.Eovaldi B, Zanetti C. Non-anion gap metabolic acidosis in a patient with a pancreaticopleural fistula. J Am Osteopath Assoc. 2011 May;111(5):344–5. [PubMed] [Google Scholar]
- 38.Bonelli L, Aste H, Bovo P, Cavallini G, Felder M, Gusmaroli R, et al. Exocrine pancreatic cancer, cigarette smoking, and diabetes mellitus: A case-control study in northern italy. Pancreas. 2003 Aug;27(2):143–9. doi: 10.1097/00006676-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, et al. Pancreatitis and the risk of pancreatic cancer. international pancreatitis study group. N Engl J Med. 1993 May 20;328(20):1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]