Abstract
Objective
To evaluate associations between traditional cardiovascular disease (CVD) risk factors, inflammatory markers, and markers of HIV disease activity with ultrasonographic measures of CVD risk in patients with HIV who are not receiving antiretroviral therapy (ART).
Design
Cross-sectional, baseline evaluation of ART-naïve HIV-infected individuals without known CVD or diabetes mellitus enrolled in a randomized ART treatment trial.
Methods
Prior to ART initiation, carotid artery intima-media thickness (CIMT) and brachial artery flow-mediated dilation (FMD) were measured. Additional parameters included CD4 cell count, HIV viral load, body composition, lipoproteins, and inflammatory markers. Associations with common CIMT, bifurcation CIMT, presence of carotid artery lesions, and brachial artery FMD were evaluated.
Results
The 331 enrolled subjects were a median (1st–3rd quartile) of 36 (28–45) years old. Common and bifurcation CIMT values were higher and lesions more prevalent with older age (p <0.001). FMD was lower with older age (p =0.009). Those with a Framingham Risk Score >6%/10 years (N =44) had higher common and bifurcation CIMT (p <0.001), carotid lesion prevalence (p <0.001), and lower FMD (p =0.035). Independent associations with common CIMT were identified for increasing age, height, weight, small LDL particles, and black race; these were similar for bifurcation CIMT. Presence of carotid artery lesions was associated with increasing age, presence of metabolic syndrome, interleukin-6, and lower HIV-1 RNA.
Conclusions
In a contemporary cohort of ART-naive HIV-infected individuals, ultrasonographic measures of CVD risk were more strongly associated with traditional risk factors than CD4 cell counts, HIV replication, or inflammatory markers.
Keywords: atherosclerosis, carotid arteries, endothelial function, human immunodeficiency virus, inflammation
Cardiovascular disease (CVD) is a leading cause of mortality among individuals with human immunodeficiency virus (HIV) infection [1,2]. Although there is evidence that HIV-infected individuals are at increased CVD risk, most of the studies evaluating the associations between HIV-infection and CVD have been conducted in individuals receiving antiretroviral therapy (ART). Several commonly used ART agents have been associated with increased risk for myocardial infarction and metabolic abnormalities that increase CVD risk, such as dyslipoproteinemia and insulin resistance [3–5]. Given the relatively low CVD risk in most HIV-infected individuals in the United States and the confounding effects of ART on CVD risk, it is unclear if HIV infection or its treatment notably increases CVD risk. Prior to the era of active ART, HIV infection also was associated with premature coronary heart disease and atherogenic dyslipoproteinemia, characterized by hypertriglyceridemia and small low-density lipoproteins, as well as increased serological markers of inflammation [6]. However, studies characterizing CVD risk factors in ART-naïve patients tended to be small, included highly selected patients who mostly were of white ethnicity, and no longer reflect the general health and diversity of modern patients considering initiation of ART. Furthermore, subclinical arterial disease has not been well-characterized in HIV-infected patients not on ART. The purpose of this report is to evaluate the associations between traditional CVD risk factors, inflammatory markers, and markers of HIV disease activity with ultrasonographic measures of CVD risk (carotid artery intima-media thickness [CIMT] and brachial artery flow-mediated vasodilation [FMD]), in patients with HIV who are not receiving ART. CIMT and FMD respectively are measures of arterial structure and function that independently predict future CVD events in individuals without known CVD [7–13].
Methods
Study participants and design
This was a cross-sectional, baseline evaluation of ART-naïve HIV-infected individuals enrolled in a randomized ART treatment trial (AIDS Clinical Trials Group Study A5257) who agreed to undergo additional CVD testing. This CVD sub-study (AIDS Clinical Trials Group Study A5260 s) was approved by the Institutional Review Boards at all 26 participating sites. All subjects provided written informed consent. Subjects were required to be ≥18 years of age and have HIV-1 infection, documented by any licensed enzyme-linked immunosorbent assay test kit and confirmed by Western blot prior to study entry. They were required to be ART-naïve (defined as ≤10 days of ART at any time prior to entry) and have screening HIV-1 ribonucleic acid (RNA) >1000 copies/ mL within 90 days prior to study. The major exclusion criteria for A5260 s were known CVD (history of myocardial infarction, coronary artery bypass graft surgery, percutaneous coronary intervention, stroke, transient ischemic attack, and peripheral arterial disease), diabetes mellitus, uncontrolled thyroid disease, and use of lipid-lowering medications.
Multiple demographic, anthropomorphic, and laboratory parameters were measured, including body composition (by whole body dual-energy x-ray absorptiometry and single slice non-contrast abdominal computed tomography at the L4–L5 level), lipoproteins, inflammatory biomarkers, adipocytokines, and immune activation. Predicted 10-year risk of coronary death/myocardial infarction was estimated using the Framingham risk score (FRS) and considered to be moderate/high if ≥6%. Prior to ART initiation, CIMT and FMD images were obtained by ultrasonography. All sonographers underwent centralized training and certification by core imaging laboratories at the University of Southern California (CIMT) and the University of Wisconsin (FMD).
Carotid artery ultrasonography
As previously described, B-mode images of the distal common carotid artery (CCA) and the carotid artery bifurcation were acquired with a high-resolution linear array ultrasound transducer with superimposed simultaneous electrocardiographic tracing [14–16]. Lying supine, subjects were positioned so the neck was extended to present the optimal angle for ultrasound examination. The right CCA was imaged in cross-section and the transducer moved laterally until the jugular vein and the CCA were stacked with the former above the latter; the transducer was then rotated around the central image line 90 degrees maintaining the jugular vein stacked above the CCA and bifurcation while obtaining a longitudinal view of both vessels emphasizing the far wall. Carotid artery images were sent electronically to the University of Southern California core imaging laboratory for quality control and interpretation by a single, experienced technician. Carotid artery lesions were defined as focal regions of CIMT ≥1.5 mm and along with CIMT of the far walls of the right CCA and bifurcation were measured using an in-house developed automated edge detection program (Patents 2005, 2006, 2011) [14–18]. Paired ultrasound and phantom scans were obtained at baseline. The within-group coefficient of variance of paired baseline CCA CIMT scans and paired baseline bifurcation CIMT scans were 1.09% and 1.27%, respectively. Using this technique, the mean (standard deviation) far wall right CCA IMT among 128 non-diabetic men aged 35.6 (9.5) years was 0.68 (0.13) mm; it was 0.65 (0.11) among 199 non-diabetic women aged 35.7 (8.5) years [19].
Brachial artery reactivity testing
As previously described, subjects were required to fast, not smoke, or drink caffeinated products for at least 8 hours prior to testing [20,21]. After resting supine for 10-minutes in a temperature-controlled room, a blood pressure cuff was placed on the widest part of proximal right forearm approximately 1 cm distal to the antecubital fossa. The arm was extended 90° from the thorax and placed on an arm board with the elbow positioned downwards and the hand rotated so thumb pointed towards the ceiling. Using a high resolution linear array vascular ultrasound transducer, the brachial artery was located above the elbow and scanned in longitudinal sections with the focus zone set to the depth of the far wall. Time-gain compensation and overall gain settings were adjusted to optimize images of the lumen/arterial wall interface. Extra-vascular landmarks were identified and labeled to assure that the imaged segment of the brachial artery is reproduced within and between studies. After recording baseline B-mode images of the brachial artery and spectral Doppler images of flow, the forearm cuff was inflated to 250 mmHg for 5 minutes to induce reactive hyperemia. Immediately after deflation, spectral Doppler images are obtained to verify hyperemia. FMD of the brachial artery was measured 60 and 90 seconds after cuff deflation. The relative FMD (%) was calculated as the ratio between the largest post-cuff release and the baseline diameter. Images were sent electronically to the University of Wisconsin core imaging laboratory for quality control and interpretation by a single, experienced technician using Access Point Web software (Freeland Systems, Westminster, CO). Phantom scans were obtained at baseline; paired brachial artery FMD scans were performed at week 24. Blinded, paired readings of 25 FMD studies showed a median difference of 0.20% (−0.47–0.49%). Using this technique, the mean (standard deviation) FMD among 24 non-diabetic men aged 32.0 (6.0) years was 4.78 (2.75) %; it was 6.09 (3.23) % among 42 non-diabetic women aged 34.6 (6.6) years.
Laboratory testing
Fasting (at least 8 hours) blood samples were obtained and sent to core laboratories for analysis. Standard chemistry testing was performed at the individual sites. Lipoproteins were quantified and characterized by Nuclear Magnetic Resonance Spectroscopy at LipoScience. Inflammatory biomarkers and adipocytokines were measured at the University of Vermont Laboratory for Clinical Biochemistry on plasma stored at −70°C; they included high-sensitivity C-reactive protein by nephelometry (Siemens BNII Nephelometer, Siemens Healthcare, Indianapolis, IN) and interleukin-6, adiponectin, leptin by enzyme-linked immunosorbent assay (R&D Elisa, R&D Systems, Minneapolis, MN). Pro-inflammatory high-density lipoprotein (HDL) was measured by using a cell-free oxidation assay at the University of California-Los Angeles.
Data analysis
Continuous variables are described as medians (1st–3rd quartile, Q1-Q3); categorical variables are presented as percentages. Associations between traditional risk factors, HIV-related measures, and other markers were examined for CCA CIMT, bifurcation CIMT, presence of carotid artery lesions, FMD and brachial artery diameter. Univariate associations were evaluated by non-parametric k-sample tests and tests of non-zero Spearman correlation. Associations that were nominally significant (p <0.05) were included in adjusted analyses. Adjusted analyses used multivariable linear and logistic regression modeling with candidate variable selection based on the Akaiki Information Criterion. Final model selection was done manually with clinical input and consideration of collinearity and final model R2 values. Parameter estimates (95% confidence intervals) for the presence of carotid lesions are presented as odds ratios; otherwise estimates represent average shifts in the respective endpoints per unit changes or population subgroups as described. Body size is represented by height and weight; dual-energy x-ray absorptiometry, abdominal computed tomography, and other anthropomorphic measures did not substantively alter the models.
Results
Subject characteristics**
(Table 1, Fig. 1)Of the 331 participants, 89% were male, 44% were white, 32% were black, and 20% were of Hispanic race/ethnicity; 23% had a prior AIDS diagnosis. HIV-1 RNA was 4.5 (4.0–5.1) log10copies/mL and CD4 cell counts were 349 (207–455)/mm3. There were 38% current smokers and 22% former smokers. Except for a mildly low HDL cholesterol of 38 (31–45) mg/dL, distributions of traditional CVD risk factors were typical of a healthy population. The FRS was 1.0 (1.0–3.0) %; 87% had FRS <6%. Higher viral loads (r-0.22, p <0.001) and lower CD4 cell counts (r =−0.16, p =0.004) were associated with higher resting heart rates.
Table 1.
Subject Characteristics.
| Parameter | Median | Q1 – Q3 |
|---|---|---|
| Age (years) | 36 | 28 – 45 |
| HIV-1 RNA (log10 copies/mL) | 4.5 | 4.0 – 5.1 |
| CD4 cell count (/mm3) | 349 | 207 – 455 |
| Time since HIV diagnosis (months) | 5.7 | 2.4 – 31.5 |
| Framingham risk score (% 10-year risk) | 1 | 1 – 3 |
| Systolic blood pressure (mmHg) | 117 | 108 –125 |
| Diastolic blood pressure (mmHg) | 75 | 68 – 80 |
| Creatinine (mg/dL) | 0.9 | 0.8 – 1.0 |
| Fasting glucose (mg/dL) | 84 | 77 – 91 |
| Body Size and Composition | ||
| Height (cm) | 175 | 169 – 180 |
| Weight (kg) | 77 | 67 – 87 |
| Body-mass index (kg/m2) | 25 | 22 – 28 |
| Waist circumference (cm) | 88 | 81 – 98 |
| Total body fat mass (kg) | 17 | 11 – 24 |
| Total body lean mass (kg) | 56 | 50 – 63 |
| Lipids and Lipoproteins | ||
| Total cholesterol (mg/dL) | 155 | 134 – 180 |
| Triglycerides (mg/dL) | 102 | 70 – 148 |
| HDL cholesterol (mg/dL) | 38 | 31 – 45 |
| Calculated low-density lipoprotein cholesterol (mg/dL) | 93 | 74 –115 |
| Total LDL particles (nmol/L) | 1076 | 884 –1286 |
| Small LDL particles (nmol/L) | 669 | 449 – 862 |
| Mean LDL particle size (nm) | 20.5 | 20.1 – 20.9 |
| Total HDL particles (umol/L) | 29.3 | 25.7 – 32.9 |
| Small HDL particles (umol/L) | 16.6 | 13.1 – 19.6 |
| Inflammatory Markers and Adipocytokines | ||
| Pro-inflammatory HDL (log10 FU/min) | 4.3 | 4.3 – 4.3 |
| C-reactive protein(mg/L) | 1.4 | 0.7 – 3.0 |
| Interleukin-6 (pg/mL) | 1.8 | 1.2 – 3.0 |
| Adiponectin (ng/mL) | 7780 | 5355 – 11540 |
| Leptin (pg/mL) | 5288 | 3017 – 9558 |
| Ultrasonographic Markers | ||
| Common CIMT (mm) | 0.64 | 0.59 – 0.70 |
| Bifurcation CIMT (mm) | 0.74 | 0.65 – 0.82 |
| Brachial artery FMD (%) | 4.5 | 3.0 – 6.3 |
| Brachial artery diameter (mm) | 4.25 | 3.84 – 4.55 |
Fig. 1.
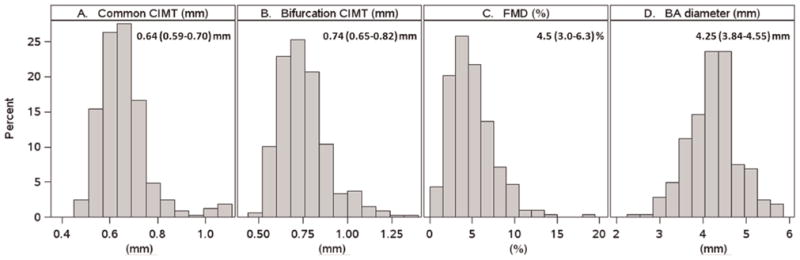
Distributions of CVD measures.
Carotid artery intima-media thickness**
(Figures 1 and 2)Common and bifurcation CIMT were 0.64 (0.59–0.70) and 0.74 (0.65–0.82) mm, respectively (Fig. 1). In univariate analyses, CCA and bifurcation CIMT were associated with classic CVD risk factors (nominal p <0.05). Higher CIMT was associated with older age, non-Hispanic ethnicity, medium/high FRS, higher systolic and diastolic blood pressure, higher total cholesterol, LDL cholesterol and non-HDL cholesterol, longer smoking history, higher fasting glucose (CCA only), higher measures of body size and composition (body-mass index, weight, height, waist circumference, limb fat, trunk fat, visceral adipose tissue, subcutaneous adipose tissue, total abdominal adipose tissue, total body fat, total body lean mass and upper extremity fat), lower estimated glomerular filtration rate (eGFR), and presence of metabolic syndrome. Higher CIMT also was associated with higher levels of total and small LDL particles, small HDL particles (bifurcation only), lower pro-inflammatory HDL, and higher leptin. There was some evidence of associations with HIV-1 disease; lower baseline HIV-1 RNA was associated with higher CIMT, prior AIDS diagnosis (CCA only), and longer time since HIV diagnosis (bifurcation only). Of note, significant associations between CIMT and CD4 cell count were not observed. A longer smoking history was associated with increasing age (p =0.021).
Fig. 2.
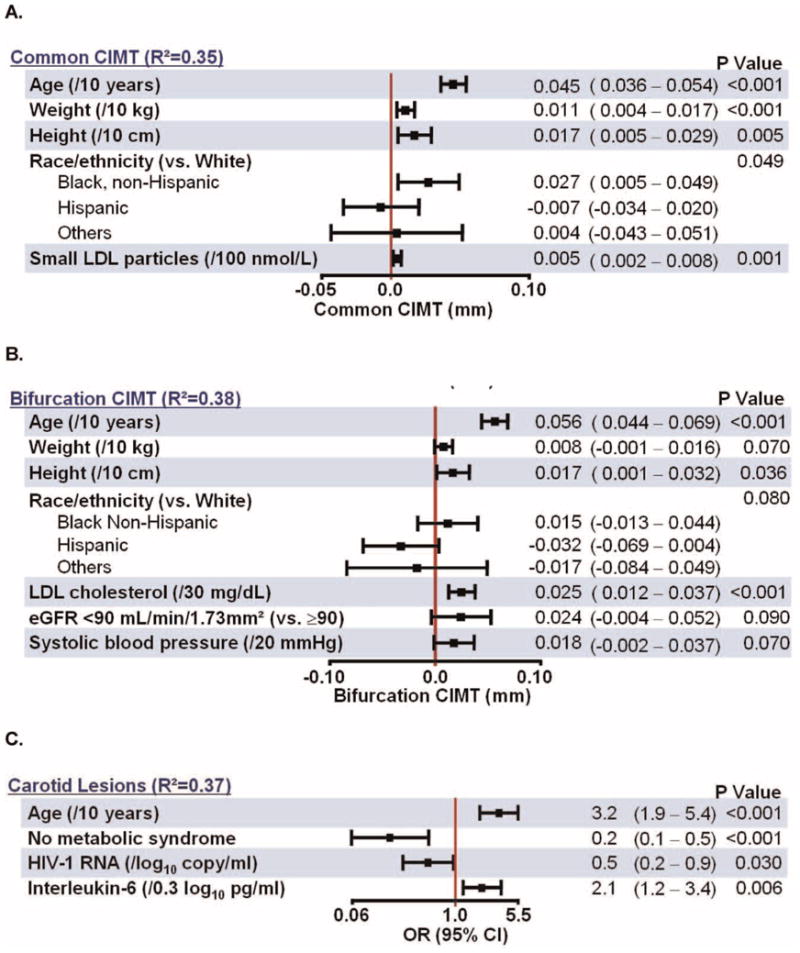
Multivariable Models for Carotid Ultrasound Outcomes.
In multivariable analyses, independent associations with increasing CCA CIMT (Fig. 2A) were observed for older age, larger body size, and higher levels of small LDL particles as well as non-Hispanic black race/ethnicity (compared to White). Independent associations with increasing bifurcation CIMT were similar (Fig. 2B) and were observed for older age, larger body size and LDL cholesterol, with positive trends as indicated. In a separate model, moderate/high FRS was independently associated with CCA (p =0.004) but not bifurcation (p =0.100) CIMT (data not shown).
Carotid artery lesions**
(Fig. 2C)There were 27 (8%) subjects who had carotid artery lesions. Subjects with lesions tended to be older (48 versus 34 years, p <0.001) and were more likely to have moderate/high FRS (41% compared to 11%, p <0.001); 44% of those with lesions were ≥50 years old (p <0.001, data not shown) and 41% with lesions had moderate/high FRS ≥6% (p <0.001, data not shown). In univariate analyses, presence of carotid artery lesions was associated (nominal p <0.05) with older age, higher systolic and diastolic blood pressure, presence of metabolic syndrome, higher small LDL particles, higher levels of visceral adipose tissue, higher levels of interleukin-6, and lower HIV-RNA. In adjusted analyses, age, level of interleukin-6, presence of metabolic syndrome, and lower HIV-1 RNA level remained independently associated with the presence of lesions (Fig. 2C).
Brachial artery FMD and diameter**
(Figs. 1 and 3)Median FMD was 4.5 (3.0–6.3) % (Fig. 1). In univariate analyses, higher maximum FMD (indicative of lower CVD risk) was associated (nominal p <0.05) with younger age, lower FRS, higher measures of body composition (body-mass index, trunk fat, upper extremity fat, total body fat), shorter height, smaller brachial artery diameter, higher levels of interleukin-6, and higher HIV-1 RNA. In multivariable analyses (Fig. 3A), independent associations with higher FMD were observed for smaller brachial artery diameter, increasing weight, and decreasing height. In a separate model, moderate/high FRS was independently associated with lower FMD (p =0.035, data not shown).
Fig. 3.
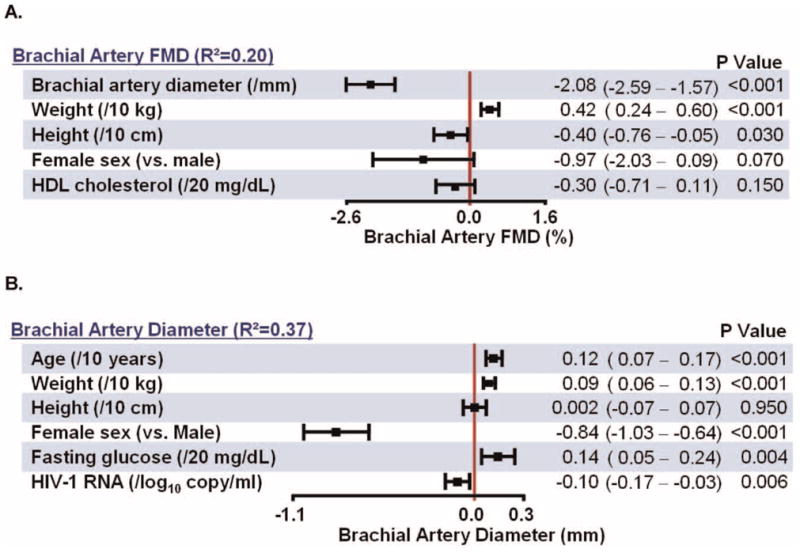
Multivariable Models for Brachial Artery Ultrasound Outcomes.
Given the strong dependence of FMD on brachial artery diameter, the latter associations were explored in detail. In univariate analyses, larger diameter (indicative of higher CVD risk) was associated (nominal p <0.05) with older age, male sex, lower baseline HIV-1 RNA, higher baseline CD4 cell count, medium/high FRS, higher systolic and diastolic blood pressures, metabolic syndrome, higher measures of body size and composition (BMI, weight, height, mid-waist circumference, trunk fat, total body fat, total body lean mass, visceral adipose tissue, subcutaneous adipose tissue, and total abdominal adipose tissue), higher glucose, higher creatinine, higher levels of small LDL and HDL particles, and lower levels of pro-inflammatory HDL and interleukin-6. Of note, the association of brachial artery diameter and total body lean mass was much stronger (ρ =0.47, p <0.001) than its associations with height, weight, body-mass index, and waist circumference (ρ =0.23–0.35) and any fat depot (ρ =0.04–0.14). Lean body mass was strongly associated with height (ρ =0.63) and weight (ρ =0.80) (both p <0.001). In multivariable analyses (Fig. 3B), independent associations with larger brachial artery diameter were observed for increasing age, weight, male sex, fasting glucose and lower HIV-1 RNA level.
Discussion
In a contemporary cohort of HIV-infected ART-naive individuals without advanced HIV disease, ultrasonographic measures of CVD risk were more strongly associated with traditional risk factors such as aging, body size, and lipoprotein measurements, rather than CD4 cell count, viral replication, inflammatory markers, and cytokines. Several aspects of this study and findings are notable and important for understanding CVD risk in contemporary patients with HIV infection. This is the first large study to evaluate CVD risk among HIV-infected, but treatment-naïve individuals ready to initiate ART. Given the complex interplay between HIV infection and treatment on CVD risk factors and CVD risk, understanding the associations with arterial disease prior to ART initiation is important for understanding why patients with HIV infection appear to be at increased CVD risk compared to HIV-negative individuals. This is the only study of its kind to simultaneously evaluate CIMT and FMD along with multiple, putative markers of CVD risk including advanced lipoprotein testing, inflammatory markers, immune activation, HIV disease activity. Its multicenter nature with strict quality control enhanced the reliability of the data and our ability to identify associations. A medium or high 10-year predicted CVD risk was associated with increased CIMT in the common and bifurcation carotid artery segments, more carotid artery lesions, lower brachial artery FMD and larger brachial artery diameter – each of the ultrasound measures of increased CVD risk, demonstrating the internal validity of our findings.
By demonstrating that modifiable risk factors such as increased body size and lipoprotein measures are the major associates of increased CIMT, carotid artery lesions, and impaired FMD, these parameters can be targeted for early preventive lifestyle and if necessary, pharmacological interventions to reduce future CVD risk in patients initiating ART. Indeed, a recent randomized clinical trial demonstrated that a focused dietary intervention significantly reduced LDL cholesterol in HIV-infected patients beginning their first ART regimen [22], the magnitude of which would be expected to significantly reduce long-term CVD risk. AIDS Clinical Trials Group Study A5078, a longitudinal, observational investigation showed that in cross-section, traditional risk factors overshadowed the impact of HIV protease inhibitor exposure, and that progression of CCA CIMT was similar among age- and risk-factor matched individuals with and without HIV infection [23,24]. Our results confirm and extend these findings, showing that traditional CVD risk factors, but not markers of inflammation and HIV disease activity are strongly associated with bifurcation CIMT, carotid artery lesions, and brachial artery FMD in a low CVD risk population.
Despite a wide range of CD4 cell counts among our participants, CD4 cell counts were not independently associated with any ultrasonographic marker of CVD risk. This may be due to the relatively small sample size since within the larger sample sizes of the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study, a CD4 cell count <200 cells/mm3 was independently associated with increased carotid artery lesion prevalence and with increased carotid arterial stiffness in HIV-infected vs. HIV-uninfected individuals [17,25]. However, these individuals were older than those in our study and most were ART-experienced. It has been hypothesized that starting ART in subjects with higher cell counts would reduce CVD risk [26].
The effects of HIV viral load on arterial function and disease are less clear. Lower HIV loads were weakly associated with carotid artery lesions but neither CIMT measure; and higher viral loads were associated with smaller brachial artery diameters, but not worse FMD. Active infection may invalidate the observational association between smaller arteries and lower CVD risk, perhaps because of sympathetic activation and attendant vasoconstriction [8]. Indeed, higher viral loads were associated with higher heart rates. In ACTG Study A5152 s, a randomized clinical trial of 3 ART regimens in treatment-naïve patients, effective ART improved FMD, reduced heart rates, and increased brachial artery diameters [20]. Improvement in FMD was related to the reduction in HIV load, indicating that treating HIV infection improves endothelial function and relieves vasoconstriction that accompanies untreated HIV infection [20]. In the Study to Understand the Natural History of HIV/AIDS in the ERA of Effective Therapy, suppression of plasma HIV-1 RNA viral load to <400 copies/ml was associated with decreased progression of CCA CIMT over a 2-year period [27]. The possibility of increased CVD risk with treatment interruption was observed in the SMART study [28]. Therefore, treatment of HIV infection may reduce CVD risk, as does treatment of CVD risk factors.
Limitations of this study include the absence of an HIV-negative or HIV treatment-experienced control group and the young age of the participants, who on average, were at low CVD risk. Although this is the largest report, to date, describing CVD risk among treatment-naïve HIV-infected individuals, it still is relatively small and underpowered for detecting modest risk factor associations with our CVD risk markers, especially considering the high intra-individual and/or measurement variability of some of the markers we studied. Also, this was a cross-sectional analysis; longitudinal follow-up, which is in progress, may be even more informative.
Given these limitations, the absence of significant associations between inflammation and the arterial measurements in this study does not exclude a role for inflammation and immune activation as contributors to CVD risk in patients with HIV. The association between increasing interleukin-6 levels and the prevalence of carotid artery lesions was the only independent association between any inflammatory marker or adipocytokine with a vascular marker that we evaluated. The meaning of this finding is unclear; however, higher interleukin-6 levels have been linked to increased mortality in individuals with HIV. It is possible that other unmeasured markers of inflammation or immune activation may be more strongly associated with vascular disease. In recent studies, CD4 and CD8 T-cell activation and CD8 T-cell senescence were associated with increased carotid artery lesion prevalence and increased carotid arterial stiffness in HIV-infected individuals [18,29].
Finally, this study sheds insight into the interaction between brachial artery size and FMD. FMD is lower in individuals with larger arteries; however, it is unclear if this is merely a mathematical function of brachial artery size being in the denominator of the formula for calculating FMD, or if it represents pathophysiology related to larger patients having risk factors associated with increasing body size and adiposity. Contemporaneous measurement of body composition helped us show that lean body mass was associated much more strongly with brachial artery size than any fat depot or adiposity measure. Lean body mass was associated with height and weight, indicating that the association between increased brachial artery size and CVD risk is not due to adiposity; arteries are larger in bigger people with greater muscle mass.
Subjects in this study reflect contemporary patients with HIV initiating their first ART regimen. They have notably less advanced HIV disease than in historical cohorts, even more recent ART-naïve cohorts such as in ACTG Study A5224 s [30]. Contemporary patients starting ART have higher CD4 cell counts and a shorter duration of HIV infection, but also have a different burden of traditional risk factors than historical cohorts. They tend to be younger, have lower (albeit still excessive) rates of smoking, and lower triglycerides, but they are heavier and have higher BMIs. The changing demographics and CVD risk burden of HIV-infected patients initiating ART further complicates efforts to understand predictors of CVD risk in patients with HIV.
Conclusions
In a large, contemporary cohort of HIV-infected ART-naive individuals, ultrasonographic measures of CVD risk were more strongly associated with traditional risk factors than CD4 cell count, viral replication, and inflammatory markers. Increasing age, body size, and lipoprotein measures were most consistently associated with CIMT and brachial artery FMD. Efforts to prevent excessive weight gain and improve lipoproteins are likely to reduce CVD risk in HIV-infected ART-naïve patients.
Acknowledgments
Principal Contributions of Each Author
JH Stein – conception, design, obtained funding, conduct of study, data analysis, draft of manuscript, critical revision of manuscript
TB Brown – design, conduct of study, critical revision of manuscript
HJ Ribaudo - design, conduct of study, data analysis, critical revision of manuscript
Yun Chen - conduct of study, data analysis, critical revision of manuscript
Mingzhu Yan - conduct of study, critical revision of manuscript
Elizabeth Lauer-Brodell - conduct of study, critical revision of manuscript
GA McComsey - design, conduct of study, critical revision of manuscript
MP Dube – conduct of study, critical revision of manuscript
RL Murphy - conduct of study, critical revision of manuscript
HN Hodis – design, conduct of study, critical revision of manuscript
JS Currier - conception, design, obtained funding, conduct of study, data analysis, critical revision of manuscript
Financial Support: The project described was supported by Award Number U01AI068636 from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH), and the National Institute of Dental and Craniofacial Research (NIDCR). This research also was supported by NIH grants HL095132, HL095126, AI 068636, AI068634, AI69471 and AI56933 from the National Heart, Lung, and Blood Institute and the National Institute of Allergy and Infectious Diseases.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
The following ACTUs participated in this study: 103 -Beth Israel Deaconess Medical Center ACTG CRS 6; 107 - Brigham and Women’s Hospital ACTG CRS 5; 201 - Johns Hopkins Adult AIDS CRS 11; 401 - NY University HIV/AIDS CRS 11; 601 - UCLA CARE Center CRS 8; 603 - Harbor-UCLA Med. Ctr. CRS 24; 801 - UCSF AIDS CRS 4; 1001 – University of Pittsburgh CRS 4; 1101 – University of Rochester ACTG CRS 4;1108 - AIDS Care CRS 8;1201 - USC CRS 30; 1401 - University of Washington AIDS CRS 18; 1601 - Duke University Medical Cener Adult CRS 3;2101 - Washington U CRS 23; 2301 - Ohio State University AIDS CRS 9; 2401 - Univ. of Cincinnati CRS 28; 2501 - Case Western Reserve CRS 12; 2503 -MetroHealth CRS 1; 2701 - Northwestern University CRS 23; 2702 - Rush University Medical Center ACTG CRS 8; 3201 - UNC AIDS CRS 15; 3652 - Vanderbilt Therapeutics CRS 17; 5802 - Ponce de Leon Center CRS 3; 6101 - University of Colorado Hospital CRS 40; 31473 - Houston AIDS Research Team CRS 10;31477 -New Jersey Medical School - Adult Clinical Research Ctr. CRS 9.
The assistance of the ACTG Statistical and Data Analysis Center and the ACTG Optimization of Antiretroviral Therapy Committee, as well as the clinical trials support from Social and Scientific Systems, Inc. are appreciated.
Footnotes
Conflicts of interest
Conflicts of Interest and Source of Funding: Regarding the content of this manuscript, there are no conflicts of interest to disclose. The disclosures of each author are reported separately.
References
- 1.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Sabin CA, Friis-Moller N, Reiss P, El Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 3.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 4.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–1748. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 6.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 7.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 8.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 9.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive post-menopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 10.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, et al. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 11.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 13.Hodis H, Mack W, LaBree L, Selzer R, Liu C, Liu C, et al. The role of carotid arterial intima-medial thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 15.Selzer RH, Hodis HN, Kwong-Fu H, Mack WJ, Lee PL, Liu CR, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 16.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan RC, Kingsley LA, Gange SJ, Benning L, Jacobson LP, Lazar J, et al. Low CD4RT-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodarzi MO, Taylor KD, Guo X, Quinones MJ, Cui J, Li Y, et al. Association of the diabetes gene calpain-10 with subclinical atherosclerosis: the Mexican-American Coronary Artery Disease Study. Diabetes. 2005;54:1228–1232. doi: 10.2337/diabetes.54.4.1228. [DOI] [PubMed] [Google Scholar]
- 20.Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dube MP, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Char-bonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 22.Lazzaretti RK, Kuhmmer RD, Sprinz E, Polancyk CA, Ribeiro JP. Dietary Intervention Prevents Dyslipidemia Associated with Highly Active Antiretroviral Therapy in HIV-1-Infected Individuals: A Randomized Trial. J Am Card Cardiol. 2012 doi: 10.1016/j.jacc.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 23.Currier JS, Kendall MA, Zackin R, Henry WK, Alston-Smith B, Torriani FJ, et al. Carotid artery intima-media thickness and HIV infection: traditional risk factors overshadow impact of protease inhibitor exposure. AIDS. 2005;19:927–933. doi: 10.1097/01.aids.0000171406.53737.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Currier JS, Kendall MA, Henry WK, Alston-Smith B, Torriani FJ, Tebas P, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS. 2007;21:1137–1145. doi: 10.1097/QAD.0b013e32811ebf79. [DOI] [PubMed] [Google Scholar]
- 25.Seaberg EC, Benning L, Sharrett AR, Lazar JM, Hodis HN, Mack WJ, et al. Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke. 2010;41:2163–2170. doi: 10.1161/STROKEAHA.110.583856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strategic Timing of Antiretroviral Treatment (START) 2012 http://clinicaltrials.gov/ct2/show/NCT00867048.
- 27.Baker JV, Henry WK, Patel P, Bush TJ, Conley LJ, Mack WJ, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clin Infect Dis. 2011;53:826–835. doi: 10.1093/cid/cir497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13:177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Melbourne K, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. AIDS. 2012;26:1371–1385. doi: 10.1097/QAD.0b013e328354f4fb. [DOI] [PMC free article] [PubMed] [Google Scholar]


