Abstract
Rationale
Many studies have reported medication effects on alcohol cue-elicited brain activation or associations between such activation and subsequent drinking. However, few have combined the methodological rigor of a randomized clinical trial (RCT) with follow-up assessments to determine whether cue-elicited activation predicts relapse during treatment, the crux of alcoholism.
Objectives
This study analyzed functional magnetic resonance imaging (fMRI) data from 48 alcohol-dependent subjects enrolled in a six-week RCT of an investigational pharmacotherapy.
Methods
Subjects were randomized, based on their level of alcohol withdrawal (AW) at study entry, to receive either a combination of gabapentin (up to 1200 mg for 39 days) and flumazenil infusions (two days) or two placebos. Midway through the RCT, subjects were administered an fMRI alcohol cue reactivity task.
Results
There were no main effects of medication or initial AW status on cue-elicited activation, but these factors interacted, such that the gabapentin-flumazenil/higher AW and placebo/lower AW groups, which had previously been shown to have relatively reduced drinking, demonstrated greater dorsal anterior cingulate cortex (dACC) activation to alcohol cues. Further analysis suggested that this finding represented differences in task-related deactivation and was associated with greater control over alcohol-related thoughts. Among study completers, regardless of medication or AW status, greater left dorsolateral prefrontal cortex (DLPFC) activation predicted more post-scan heavy drinking.
Conclusions
These data suggest that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.
Keywords: alcoholism, anticonvulsant, craving, neuroimaging, relapse
Introduction
Among individuals with alcohol dependence, functional magnetic resonance imaging (fMRI) studies have consistently demonstrated that alcohol-related stimuli (cues) elicit activation in brain regions associated with reward processing, including ventral (VS) and dorsal (DS) striatum, medial and dorsolateral prefrontal cortex (mPFC and DLPFC), orbitofrontal cortex (OFC), anterior and posterior cingulate cortex (ACC and PCC), precuneus, and insula (see Schacht et al., 2012, for review). Medications that reduce drinking among alcoholics have also been shown to reduce alcohol cue-elicited activation in a variety of brain areas. An acute dose of amisulpride, an atypical dopamine antagonist, has been reported to reduce thalamic activation (Hermann et al., 2006). We previously reported that both naltrexone, a μ-opioid receptor antagonist, and aripiprazole, an atypical dopamine partial agonist, reduced VS activation (Myrick et al., 2008, 2010) (although a follow-up study replicated the naltrexone finding only among individuals who carried a specific combination of genetic variants (Schacht et al., in press)). In the original naltrexone study, naltrexone, either alone or in combination with ondansetron, also reduced DS, mPFC, and OFC activation. Recently, Langosch and colleagues (2012) reported that acamprosate, an N-methyl-D-aspartate modulator, had no effects on cue-elicited activation. However, with the exception of the negative acamprosate study, previous research has focused on acute or sub-acute treatment paradigms among non-treatment-seeking subjects. In these studies, little follow-up has been conducted to determine subjects’ subsequent drinking behavior and how it might relate to the imaging data.
Irrespective of medication, several studies have reported prospective associations between cue-elicited brain activation and subsequent drinking behavior outside of the scanner. Among detoxified, treatment-seeking adult alcoholics, mPFC activation has been reported to be greater among those who subsequently relapse, while VS and midbrain activation have been reported to be greater among those who maintain abstinence (Beck et al., 2012, Grüsser et al., 2004). VS and thalamic activation elicited by affectively positive (non-alcohol-related) visual stimuli have also been negatively associated with the subsequent frequency and amount of alcohol intake (Heinz et al., 2007). Structural imaging studies have suggested volume reductions in mPFC, OFC, and parieto-occipital areas among adult alcoholics who relapse to heavy drinking after treatment (Beck et al., 2012, Cardenas et al., 2011, Rando et al., 2011), and cortical perfusion in many of these areas is also reduced among these individuals (Durazzo et al., 2010). However, these studies have all employed treatment-as-usual paradigms, in which subjects were recruited from addiction clinics where they received individually varying treatment before or after the scan that sometimes included medication and was not placebo controlled.
Only one previous neuroimaging study of medication effects on alcohol cue reactivity has combined imaging with the methodological rigor and detailed follow-up of a randomized clinical trial (RCT) (Langosch et al., 2012). In response to this gap in the literature, we incorporated an established fMRI cue reactivity paradigm (Myrick et al., 2004, 2008, 2010, Schacht et al., 2011a, in press) into a six-week double-blind RCT of a combination of two γ–aminobutyric acid (GABA)- and glutamatergic medications, gabapentin (GBP) and flumazenil (FMZ), among treatment-seeking alcoholic adults. We have previously shown that GBP, a GABA analogue that impedes excitatory calcium currents (Hendrich et al., 2008) and may reduce glial glutamate production (Bonnet et al., 1999), effectively reduces alcohol withdrawal (AW) symptoms (Myrick et al., 2009), and, in combination with naltrexone, reduces drinking more than naltrexone monotherapy, particularly among individuals who experience AW at study entry or who have a history of inpatient alcohol detoxification (Anton et al., 2011). Since AW is associated with reduced GABA tone and increased glutamatergic signaling (Cagetti et al., 2003, Tsai et al., 1998), GBP is likely to be more effective among individuals with higher AW. In combination with FMZ, an antagonist at the GABAA receptor benzodiazepine site, GBP may shift the subunit composition of GABAA receptors to an arrangement that is more responsive to GABA signaling (Biggio et al., 2007, Sanna et al., 2003). Accordingly, our RCT demonstrated, as we had hypothesized, that a combination of oral GBP and two FMZ infusions interacted with subjects’ AW severity at study entry in its effect on drinking: subjects who received these medications and who had higher initial AW drank less, as did subjects who received placebos but had lower initial AW (Anton et al., 2009).
The current study, which was part of the previously published RCT (Anton et al., 2009), had two goals: 1) to analyze the effects of a GBP/FMZ combination, subjects’ initial AW severity, and the interaction of these factors on alcohol cue-elicited brain activation; and 2) to analyze associations between cue-elicited activation and drinking during the RCT. We predicted that the neuroimaging data would be consistent with the RCT outcomes, such that subjects treated with GBP/FMZ who had higher initial AW and those treated with placebos who had lower initial AW would demonstrate reduced cue-elicited activation in brain areas that had previously displayed susceptibility to pharmacological suppression of such activation. Further, we predicted that greater cue-elicited activation of one or more of these areas would be associated with an increased likelihood of drinking during the weeks after the scan.
Materials and methods
Overview
Subjects were randomized, based on their initial AW severity, to either a combination of oral GBP for 39 days (six weeks) and FMZ infusions on the first two days (the Prometa protocol; Hythiam, Inc., Los Angeles, CA), or an oral placebo and placebo infusions on the same time course. During the second or third week of treatment (mean scan day = day 15; SD = 2.5 days), subjects were administered an alcohol cue reactivity task during an fMRI scan. All procedures were approved by the Institutional Review Board for Human Research at the Medical University of South Carolina.
Subjects
Subjects were recruited through media advertisements. Inclusion criteria were 1) age 18–70; 2) DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, revised 4th edition; APA, 2000) Alcohol Dependence diagnosis, as assessed by the Structured Clinical Interview for DSM-IV-TR (SCID; First et al., 2002); 3) minimum of five drinks per day on 70% of days in the month before screening; and 4) last drink no more than 72 hours before medication randomization. Exclusion criteria were: 1) any other Axis I diagnosis, as assessed by SCID; 2) past-month use of any substance except marijuana or nicotine, as assessed by self-report and urine drug screen (UDS); 3) history of seizures or delirium tremens during AW, as assessed by self report; and 4) use of any psychoactive medication, including benzodiazepines, in the previous two weeks, or use of zolpidem or zaleplon more than three times in the previous two weeks. Although recent marijuana use was not exclusionary, individuals were required to have a negative UDS for Δ9-tetrahydrocannabinol (cut-off: 50 ng/mL) before beginning medication.
Of the 60 individuals who met these criteria and participated in the larger trial, four subjects had MRI contraindications and three more dropped out of the study before the scan session. Of the 53 individuals who were scanned, three were subsequently excluded from analysis for excessive head motion (see below), one was excluded because she was not scanned until day 28, and one was excluded because he was court-ordered to alcohol treatment (a protocol violation), yielding a final sample of 48. Following the scan session, two subjects dropped out before their next (week 3) appointment, and three more dropped out between the week 3 and week 4 appointments, leaving 43 subjects with complete drinking data for the six-week trial. The AW and medication groups for these subsets of subjects are listed in Table 1, and demographic and drinking variables for the 48 scanned subjects are listed in Table 2.
Table 1.
Subject flowchart.
| Lower AW | Higher AW | ||||
|---|---|---|---|---|---|
| Total N | Placebos | GBP/FMZ | Placebos | GBP/FMZ | |
| Original sample | 60 | 18 | 26 | 9 | 7 |
| Scanned | 53 | 16 | 24 | 7 | 6 |
| Usable scan data | 48 | 14 | 22 | 6 | 6 |
| Complete drinking data | 43 | 13 | 19 | 5 | 6 |
Table 2.
Demographic and drinking data for subjects with usable scan data.
| Lower AW | Higher AW | Test for difference | |||
|---|---|---|---|---|---|
| Placebos | GBP/FMZ | Placebos | GBP/FMZ | ||
| N | 14 | 22 | 6 | 6 | |
| Age | 47.9 (11.2) | 44.0 (12.2) | 53.0 (6.4) | 48.0 (4.8) | n.s. |
| Gender (% female) | 21.4 | 22.7 | 33.3 | 16.7 | n.s. |
| Education (years) | 15.0 (2.8) | 14.1 (2.2) | 15.3 (2.1) | 13.0 (2.4) | n.s. |
| ADS | 12.4 (6.0) | 13.4 (5.2) | 13.0 (3.5) | 18.5 (7.1) | n.s. |
| OCDS (baseline) | 13.9 (4.8) | 15.0 (4.1) | 21.2 (4.9) | 19.3 (6.0) | Higher AW > Lower, p = .001 |
| Heavy drinking days, % | |||||
| Baseline (past 90 days) | 91.7 (9.6) | 86.8 (14.8) | 90.4 (18.9) | 92.4 (17.0) | n.s. |
| Pre-scan | 5.4 (6.4) | 13.3 (16.5) | 2.2 (3.5) | 1.2 (2.9) | Lower AW > Higher, p = .001 |
| Post-scan* | 10.6 (19.7) | 23.1 (25.5) | 8.4 (13.0) | 4.6 (9.7) | Lower AW > Higher, p = .04 |
All figures except Ns are means (standard deviations). Pre- and post-scan drinking data represent the percentage of heavy drinking days for each subject before vs. after that subject’s scan. ADS, Alcohol Dependence Scale; OCDS, Obsessive Compulsive Drinking Scale; n.s., not significant.
Excludes participants who dropped out after the scan (N = 5; 1 from low AW/placebo group, 3 from low AW/GBP/FMZ group, and 1 from higher AW/GBP/FMZ group).
Assessment and randomization
Inclusion and exclusion criteria were initially assessed by phone, after which prospective subjects were invited to the laboratory for further assessment, where, before assessment began, they provided informed consent. Assessments included the SCID, the Alcohol Dependence Scale (ADS; Skinner & Allen, 1982) and the Obsessive Compulsive Drinking Scale (OCDS; Anton et al., 1996). Eligible subjects were then scheduled for their first treatment appointment (day 1), and instructed to abstain from alcohol the night before the appointment. On day 1, subjects were administered the Clinical Institute Withdrawal Assessment for Alcohol-Revised (CIWA-Ar) (Sullivan et al., 1989), a clinician-administered rating scale for AW symptoms (range: 0–67). To ensure equal distribution of individuals with higher AW between medication groups, CIWA-Ar scores were subsequently used as an urn variable for medication randomization. Urn randomization assigns subjects to treatment groups randomly, with the exception that the assignment is biased toward balance in the various covariate (urn variable) combinations (Stout et al., 1994). Subjects with CIWA-Ar scores ≥7 (higher AW group) were considered to have clinically significant AW, and were approximately evenly randomized to active medications vs. placebos, as were subjects with CIWA-Ar scores <7 (lower AW group). As the medication combination assessed here is designed for outpatient administration (Urschel et al., 2007), the cut-off of 7 was chosen to reflect the level of AW severity typically seen in outpatient alcohol treatment settings, rather than the cut-off of 10 originally proposed for inpatient alcohol detoxification (Sullivan et al., 1989).
Medications
The treatment procedure, including detailed time-courses of the FMZ infusions and GBP titration and taper, is fully described in Anton et al., 2009. Briefly, on day 1, subjects were administered either FMZ (2 mg) or sterile saline intravenously. Before leaving the laboratory, subjects were provided with GBP (300 mg) or identically packaged placebo, and were instructed to take the medication orally at bedtime. Subjects returned to the laboratory on day 2, and the infusion procedure was repeated. After the infusions were complete, all subjects returned to the laboratory weekly for six appointments, during which their drinking was assessed with the Timeline Follow-back (TLFB; Sobell & Sobell, 1992) and they received a manualized therapy session (combined behavioral intervention; Miller, 2004). At the week 1, 3, and 6 visits, subjects were also administered the OCDS again. GBP was increased to 1200 mg by day 4 (day 1: 300 mg; day 2: 600 mg; day 3: 900 mg), and was tapered from days 31 to 39.
Alcohol cue reactivity task
At the scan session, subjects were breathalyzed to ensure that they had not recently consumed alcohol and were again administered the CIWA-Ar. No subject had a breath alcohol level > 0 or a CIWA-Ar score > 3. Subsequently, subjects were positioned supine in the scanner and administered a 720-s-long alcohol cue reactivity task consisting of 24 pseudorandomly interspersed blocks of alcoholic beverage images (ALC), non-alcoholic beverage images (BEV), blurred versions of both of these types of images that served as visual controls, and a fixation cross (REST). Each 24-s-long block was composed of five individual pictures, each displayed for 4.8 s, and was followed by a 6-s washout period intended to allow the hemodynamic response from the previous block to decline before the next was presented. Images were selected from a normative set (Stritzke et al., 2004), supplemented with images from advertisements, and matched for intensity, color, and complexity. Alcohol image blocks were equally distributed between images of beer, wine, and liquor. In past versions of this task (Myrick et al., 2004, 2008, 2010; Schacht et al., 2011a, in press), subjects were administered a sip of alcohol immediately before the scan. However, because subjects in this study were engaged in treatment, no alcohol was administered.
Image acquisition and pre-processing
Functional images were acquired with a 3T Philips (Amsterdam, Netherlands) Interra scanner, using a gradient echo, echo-planar imaging scan sequence. Image acquisition parameters were: repetition/echo time = 1853/30 ms; 386 volumes; flip angle = 90°; field of view = 208 mm; matrix = 64 x 64; voxel size = 3.25 x 3.25 mm; 36 contiguous 3.0-mm-thick transverse slices. Using Statistical Parametric Mapping 8 software (Wellcome Trust Centre for Neuroimaging, London), implemented in MATLAB 7.9 (Mathworks, Natick, MA), images were realigned to the first volume, registered to the Montreal Neurological Institute (MNI) 152-subject average EPI template, resampled to 3 mm isotropic voxel size, and spatially smoothed with an 8 mm anisotropic Gaussian kernel. Three subjects had > 2 mm of translational or 2° of rotational movement during the scan and were excluded from further analysis. Images were also spatially whitened with a global autoregressive filter to account for temporal correlations between spatially adjacent voxels. To eliminate low-frequency noise in the blood-oxygen-level-dependent (BOLD) signal, a high-pass filter with a period of 240 s was used. A boxcar function representing stimulus presentation and duration times was then convolved with a canonical hemodynamic response function to create a basis function for general linear modeling (GLM). To eliminate task-correlated motion, for each subject, the six motion parameters from the realignment were also included in this first-level model.
Statistical analysis
To examine effects of medication, initial AW severity, and their interaction, a contrast image of the difference in activation between alcoholic and non-alcoholic image blocks (ALC-BEV) was obtained for each subject, and these images were entered into a second-level whole-brain GLM that included medication group (GBP/FMZ vs. placebos), AW group (higher vs. lower), and their interaction. To avoid over-fitting the second-level models, only age and the number of treatment days before the scan were included as covariates. However, a model in which age and gender were covaried was also tested; its results were not significantly different from those reported below.
To examine the relationship between ALC-BEV activation and drinking after the scan, for each subject, the percentage of days after the scan on which that subject drank heavily (percent heavy drinking days [%HDD]: > 5 drinks in a day for men or 4 for women), as assessed by TLFB, was subsequently entered as a covariate in a whole-brain GLM that also included medication group, AW group, and the interaction of these factors. To account for the effect of recent drinking behavior (i.e., to determine activation related specifically to future drinking), %HDD during the treatment days before the scan was also covaried, as were age and the number of treatment days before the scan.
All analyses were corrected for multiple comparisons with a voxelwise height threshold of p < .001, an extent threshold of 15 voxels, and a family-wise error (FWE) cluster-corrected whole-brain threshold of p < .05. Anatomic localization of significant clusters was performed with the Talairach Daemon database (Lancaster et al., 2000). To follow up significant interactions and further interrogate associations between brain activation and drinking, the timecourse of the BOLD signal was extracted from all of the voxels in significant clusters and entered as the dependent variable (DV) in a hierarchical linear model (HLM), using HLM v. 6.0.8 (Scientific Software International, Inc., Skokie, IL). After entering the cue reactivity task stimuli and their order, onset, and duration of presentation were entered as first-level independent variables, these models yielded an estimate of ALC-BEV activation in each significant cluster for each subject. These estimates were then used to produce group averages and scatter plots.
Results
Behavioral data
There were no significant differences in medication or AW group assignment between subjects randomized to medication and those who were scanned (χ2(3, N = 60) = 1.42, p = .70), those with usable scans (χ2(3, N = 53) = 1.55, p = .67), or those with usable scans who completed the study (χ2(3, N = 48) = 1.86, p = .60). Among subjects with usable scans, there were no significant differences between medication or AW groups in demographic variables, alcohol dependence severity, or pre-treatment %HDD, but the higher AW group had significantly higher OCDS scores than the lower AW group (t(46) = −5.69, p = .001) (see Table 2). The higher AW group also had significantly less %HDD than the lower AW group before (t(43.2) = −3.42, p = .001, equal variances not assumed) and after (t(37.4) = −2.19, p = .04, equal variances not assumed) the scan. More detailed behavioral data for the full sample are presented in Anton et al., 2009 and Schacht et al., 2011b.
Main effect of ALC-BEV activation
Across all subjects, regardless of initial AW status or medication, ALC images elicited greater activation than BEV images in a variety of brain areas, including mPFC, superior frontal gyrus (SFG), bilateral insula, PCC, and ACC (see Figure 1 and Table 3).
Figure 1.

Main effect of ALC-BEV activation. Across all subjects, alcohol cues, relative to neutral beverage cues, elicited greater activation in medial prefrontal cortex (mPFC), superior frontal gyrus (SFG), insula, and anterior (ACC) and posterior (PCC) cingulate cortices. Colors correspond to the value of the t statistic for the ALC-BEV contrast.
Table 3.
Local maxima for main effect of ALC-BEV activation.
| Cluster size (voxels) | Corrected p | Anatomy | Tmax | x | y | z |
|---|---|---|---|---|---|---|
| 3154 | < .001 | L superior frontal gyrus | 8.19 | −6 | 24 | 57 |
| L medial frontal gyrus | 7.80 | −6 | 48 | 12 | ||
| R cingulate gyrus | 7.14 | 3 | 30 | 24 | ||
| 206 | < .001 | L insula | 6.90 | −45 | 12 | −6 |
| L precentral gyrus | 5.09 | −54 | 18 | 3 | ||
| 115 | .006 | L angular gyrus | 5.43 | −57 | −60 | 39 |
| L middle temporal gyrus | 5.15 | −51 | −72 | 33 | ||
| R inferior parietal lobule | 3.71 | −48 | −66 | 48 | ||
| 114 | .006 | R insula | 4.65 | 45 | 12 | −9 |
| R claustrum | 4.65 | 33 | 15 | −15 |
Voxelwise threshold p < .001 uncorrected; extent threshold k = 15; cluster-corrected p < .05 family-wise error. Tmax, value of the T statistic for each local maximum. Coordinates are in MNI space.
Effects of alcohol withdrawal and medications
There were no main effects of initial AW status or medication, but there was a significant interaction between these factors, such that subjects with higher AW who received GBP/FMZ and those with lower AW who received placebos demonstrated greater cue-elicited activation, relative to the other groups, in a cluster that encompassed dorsal ACC (dACC) (FWE-corrected cluster probability of p = .012; 99 voxels; local maxima at [−3, 39, 18] and [6, 33, 9]) (see Figure 2). All simple effects for this interaction were significant (i.e., in the GBP/FMZ group, higher AW subjects had significantly greater activation, while in the placebo group, lower subjects AW had greater activation; and among higher AW subjects, those who received GBP/FMZ had greater activation, while among lower AW subjects, those who received placebos had greater activation). Because the cluster of interest encompassed dACC, an area that is susceptible to task-related deactivation, ALC-REST and BEV-REST activation were also analyzed within this cluster. Across all subjects, there was greater activation in the REST condition than either the ALC or BEV conditions, and the magnitude of deactivation for these contrasts differed significantly by AW and medication groups (see Figure 2). Relative to the other groups, the higher AW/GBP/FMZ and lower AW/placebos had both significantly less ALC-REST deactivation (F(1, 42) = 14.24, p < .001) and significantly greater BEV-REST deactivation (F(1, 42) = 4.29, p = .045).
Figure 2.

Interaction of medication and initial AW status on the a) ALC-BEV, b) ALC-REST, and c) BEV-REST contrasts. Subjects with higher AW who received GBP/FMZ and those with lower AW who received placebos (the groups with relatively reduced drinking) demonstrated greater ALC-BEV activation in dorsal anterior cingulate cortex (dACC). This region demonstrated deactivation for both the ALC-REST and BEV-REST contrasts, but the magnitude of the deactivation was greater for BEV-REST than for ALC-REST in the groups with relatively reduced drinking. Colors correspond to the value of the t statistic for the ALC-BEV interaction. Bars display values for each contrast (arbitrary units) from dACC for each treatment group (± standard error).
Because the groups with greater ALC-BEV activation and BEV-REST deactivation and less ALC-REST deactivation in dACC were the same ones with reduced drinking throughout the trial, we speculated that this region might underlie resistance to cue-elicited craving. To further explore this possibility, we examined the correlation between dACC ALC-BEV activation and subjects’ OCDS scores from week 3 (i.e., the visit closest in time to the scan session). The correlation between dACC activation and total OCDS score approached significance (r(45) = −0.27, p = .08). Given this trend, we further evaluated the three empirically derived factors of the OCDS (Roberts et al., 1999) after removing the items related to alcohol consumption (which was already known to differ between treatment groups). This revealed a significant inverse correlation between dACC activation and the Resistance/Control Impairment factor (r(45) = −0.31, p = .04), such that subjects with greater activation (i.e., less deactivation to alcohol cues) reported significantly more control over drinking-related thoughts and impulses. The correlation between dACC activation and the OCDS Obsession factor approached significance (r(45) = −0.28, p = .06), such that subjects with greater activation reported less frequent and less impactful drinking-related thoughts and drives, while the correlation with the Interference factor was not significant (r(45) = −0.19, p = .21).
Association with drinking
Controlling for initial AW status, medication, and their interaction, as well as for pre-scan %HDD, age, and the number of treatment days before the scan, post-scan %HDD was positively correlated with ALC-BEV activation in a cluster that encompassed the left middle frontal and precentral gyri (i.e., DLPFC) (FWE-corrected cluster probability of p = .03; 78 voxels; local maxima at [−36, 6, 30] and [−39, 9, 42]) (see Figure 3), such that subjects with greater activation in this cluster had greater post-scan %HDD. The partial correlation between DLPFC activation and post-scan %HDD was r(40) = 0.63, and did not significantly differ by medication or AW group. Across all subjects, the ALC-REST contrast was positive in this cluster (t(42) = 4.95, p < .001), while the BEV-REST contrast was negative (t(42) = −2.91, p = .006). Both contrasts correlated with post-scan heavy drinking, but in opposite directions: ALC-REST activation was positively associated with post-scan drinking (r(40) = 0.44, p = .003), while BEV-rest activation was negatively associated with post-scan drinking (r(40) = −0.38, p = .01).
Figure 3.
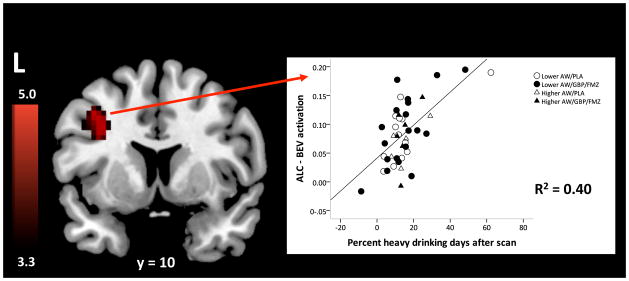
Correlation between ALC-BEV activation and percent heavy drinking days (%HDD) after the scan. Subjects with greater activation in left dorsolateral prefrontal cortex (DLPFC) had higher post-scan %HDD. Colors correspond to the value of the t statistic testing the value of the correlation against 0. The scatter plot displays individual ALC-BEV values (arbitrary units) from DLPFC and post-scan %HDD values; the latter can be negative because they represent predicted residual values, controlling for pre-scan %HDD.
Discussion
These data add to the nascent literature regarding medication effects on alcohol cue-elicited brain activation and the relationship between this activation and drinking behavior. Regarding the absence of main effects of medication or AW status, the former is consistent with a recent report that another GABA- and glutamatergic medication, acamprosate, did not affect alcohol cue-elicited activation (Langosch et al., 2012). No previous studies have examined AW effects on cue-elicited activation, although repeated alcohol detoxifications have been associated with alterations in cortical modulation of emotional perception (O’Daly et al., 2012). The hypothesis that cue-elicited activation would be attenuated in the treatment groups with relatively reduced drinking in the RCT (i.e., the higher AW/GBP/FMZ and lower AW/placebo groups) was not supported; in fact, these groups displayed greater dACC activation, which was associated with greater control over drinking-related thoughts. Furthermore, across all subjects who completed the RCT, irrespective of AW status and/or medication, greater left DLPFC activation was prospectively associated with more frequent heavy drinking.
The finding of greater ALC-BEV activation (reflecting attenuated ALC-REST deactivation and greater BEV-REST deactivation) in dACC among groups with reduced drinking raises several issues. ALC-BEV dACC activation was modestly associated with greater control over drinking-related thoughts, and could thus reflect an adaptive response that contributed to these groups’ better treatment outcome. Dorsal ACC has been implicated in cognitive, rather than affective, information processing (Bush et al., 2000), and is believed to underlie cognitive control (Paus, 2001) and response inhibition, or the suppression of a prepotent behavior (Garavan et al., 2002, Matthews et al., 2004). These functions are compelling because, upon exposure to factors that increase motivation to drink, abstinent alcoholics who intend to stay sober may mobilize coping skills, including attempts to inhibit craving (Monti et al., 2000). It should be noted that all subjects in the current study received six sessions of combined behavioral intervention (Miller, 2004), which explicitly addresses resistance to craving and other cognitive coping strategies. In addition, gabapentin has been reported to reduce cue-elicited alcohol craving (Mason et al., 2009), and smokers who are instructed to resist cigarette craving during cigarette cue exposure display increased cue-elicited dACC activation (Brody et al., 2007). Further, heavy drinkers demonstrate greater dACC activation during restrained choices in a delay-discounting task (Claus et al., 2011). However, since between-group differences in dACC ALC-BEV activation in the current study reflected discrepant task-related deactivation, it is also possible that these differences represent another phenomenon. Dorsal ACC is a component of the default mode network, a group of functionally connected regions that demonstrate deactivation during task performance and activation at rest (Fox et al., 2005). Among substance-dependent individuals, enhanced cue-elicited dACC deactivation has been positively associated with substance use (Goldstein et al., 2009), and the strength of resting state functional connectivity between dACC and the ventral striatum has been positively associated with dependence severity (Hong et al., 2009). Thus, attenuated deactivation of dACC during ALC stimuli, relative to rest, may indicate relative recovery of normal function in this area. The association between such preservation and greater cognitive control, while exploratory, is consistent with this theoretical construct and should be explored in future research.
The correlation between heavy drinking after the scan and cue-elicited DLPFC activation might be interpreted in light of the purported function of this region. DLPFC has reciprocal projections to the striatum and other subcortical nuclei, and is believed to underlie selective attention and the maintenance of information in working memory (Petrides, 2005). In the processing of reward-related stimuli, it may integrate reward histories with contextual information about reward likelihood (Park et al., 2010). Alcohol cue-elicited DLPFC activation has been correlated with in vivo alcohol craving (see Schacht et al., 2012, for review), and disruption of DLPFC activity with transcranial direct current stimulation reduces craving (Boggio et al., 2008). Across many substances of abuse, cue-elicited DLPFC activation has been found most frequently in non-treatment-seeking individuals, suggesting that such activation may be modulated by perceived drug use opportunity, and may represent the generation and maintenance of drug-seeking behavioral goals (Wilson et al., 2004). The association between DLPFC activation and relapse drinking among treatment-seeking subjects in this study supports this putative function in alcohol cue processing.
The only previous studies to note an association between alcohol cue-elicited activation and prospective risk of relapse reported greater mPFC activation among relapsers (Beck et al., 2012, Grüsser et al., 2004). Two factors may account for the fact that the current study did not replicate this finding. First, subjects in the prior studies had undergone inpatient alcohol detoxification and had been abstinent for three to seven weeks before imaging; most subjects in the current study drank heavily up until medication randomization, and some had additional heavy drinking days between randomization and the scan. Thus, cue-elicited mPFC activation may be associated with relapse risk after treatment, while DLPFC activation may be associated with such risk during treatment. Further, Beck et al. (2012) defined relapse as at least one heavy drinking day during three months of follow-up. When we defined relapse in this manner (i.e., at least one heavy drinking day after the scan), we found no differences between relapsers and non-relapsers in mPFC, DLPFC, or elsewhere (data not shown), suggesting that continuous definitions of relapse may reveal associations obscured by dichotomization of this variable.
This study had several important strengths and limitations. It represents one of the first attempts to incorporate a functional neuroimaging paradigm into an RCT, and thus illustrates some challenges in integrating these kinds of research. As is often true of both kinds of research, the sample size was relatively small, especially in the higher AW group, in which women (N = 3, of whom only one received GBP/FMZ) were particularly under-represented. Although the sample was representative of the relatively low prevalence of clinically significant AW, particularly among women (Caetano et al., 1998), future research in this area should aim for a larger number of individuals with higher AW. Further, subjects who dropped out after the scan (N = 5) were excluded from the heavy drinking association analysis; while these subjects might have relapsed to their pre-scan drinking behavior, we did not know their reasons for dropping out. Finally, while most (70%) of the remaining subjects had at least one post-scan heavy drinking day, the distribution of post-scan %HDD was right-skewed. Logarithmic transformations of this variable intended to produce more symmetric distributions did not change the association between post-scan %HDD and DLPFC activation.
An additional caveat is that, unlike previous iterations of our alcohol cue reactivity task, subjects were not administered any alcohol before visual stimuli were presented, as they were actively engaged in treatment. This change might explain the lack of cue-elicited VS activation among the placebo group (data not shown), which we had previously reported in 5 of the 6 studies that employed this task among non-treatment-seeking alcoholics, and might thus have reduced the ability to detect differences in VS activation between treatment groups. Some theories of DA signaling in VS hold that it represents a reward prediction signal (Schultz et al., 1997); thus, to the extent that the BOLD signal in VS represents DA signaling, its absence here may reflect alterations in reward prediction when alcohol-related pictures were not accompanied by the taste of alcohol. Given recent reports of lower cue-elicited and alcohol-induced VS activation among heavier drinkers, relative to lighter drinkers (Gilman et al., 2012, Vollstädt-Klein et al., 2010), it is also possible that activation in this area was not observed because subjects in this study were heavy-drinking, treatment-seeking alcoholics. Some theories of addiction posit a down-regulation of DA function after chronic heavy alcohol use, especially after alcohol withdrawal (Koob et al., 1998), leading to hyporesponsivity to alcohol cues in brain areas with heavy DA innervation. Only direct comparisons between early- and late-stage alcoholics will completely resolve this issue.
Conclusions
This study combined functional neuroimaging with an RCT of an investigational pharmacotherapy for alcohol dependence. Considered alone, neither a GBP/FMZ combination nor subjects’ initial AW status affected alcohol cue-elicited brain activation. However, these factors interacted in their effects, such that subjects who received GBP/FMZ and had higher initial AW and those who received placebos and had lower AW —the same groups that had less subsequent drinking—demonstrated greater cue-elicited dACC activation, which reflected differences in deactivation between alcohol and beverage stimuli. This finding could suggest greater resistance to cue-elicited craving among these groups. Regardless of medication or initial AW status, DLPFC activation was positively associated with post-scan heavy drinking. These results need replication, and the association between these areas of cue-elicited activation and relapse potential require further exploration. Overall, the integration of functional neuroimaging paradigms into clinical research offers a unique opportunity to better understand treatment effects on the brain and the predictive value of neuroimaging data for treatment success.
Acknowledgments
Funding: This work was conducted under an unrestricted grant from Hythiam, Inc. This funding source had no involvement in the study design, in the collection, analysis, and interpretation of data, in the writing of the paper, or in the decision to submit for publication. Drs. Schacht and Anton were supported by grants from the National Institute on Alcohol Abuse and Alcoholism (T32 AA007474 and K05 AA017435).
Footnotes
Portions of this work were presented as a poster at the 34th Anuual Meeting of the Research Society on Alcoholism (June, 2011, Atlanta, GA).
References
- Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–31. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- Anton RF, Myrick H, Baros AM, Latham PK, Randall PK, Wright TM, Stewart SH, Waid R, Malcolm R. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol. 2009;29:334–42. doi: 10.1097/JCP.0b013e3181aba6a4. [DOI] [PubMed] [Google Scholar]
- Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR, Randall PK. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2011.10101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69:842–52. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Biggio F, Gorini G, Caria S, Murru L, Sanna E, Follesa P. Flumazenil selectively prevents the increase in alpha(4)-subunit gene expression and an associated change in GABA(A) receptor function induced by ethanol withdrawal. J Neurochem. 2007;102:657–66. doi: 10.1111/j.1471-4159.2007.04512.x. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A, Fregni F. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 2008;92:55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Banger M, Leweke FM, Maschke M, Kowalski T, Gastpar M. Treatment of alcohol withdrawal syndrome with gabapentin. Pharmacopsychiatry. 1999;32:107–9. doi: 10.1055/s-2007-979203. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Caetano R, Clark CL, Greenfield TK. Prevalence, trends, and incidence of alcohol withdrawal symptoms: analysis of general population and clinical samples. Alcohol Health Res World. 1998;22:73–9. [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol Psychiatry. 2011;70:561–7. doi: 10.1016/j.biopsych.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Kiehl KA, Hutchison KE. Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcohol Clin Exp Res. 2011;35:1209–19. doi: 10.1111/j.1530-0277.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Mon A, Meyerhoff DJ. Cortical perfusion in alcohol-dependent individuals during short-term abstinence: relationships to resumption of hazardous drinking after treatment. Alcohol. 2010;44:201–10. doi: 10.1016/j.alcohol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17:1820–9. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37:467–77. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A. 2009;106:9453–8. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grüsser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res. 2007;31:1138–47. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105:3628–33. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res. 2006;30:1349–54. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–41. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langosch JM, Spiegelhalder K, Jahnke K, Feige B, Regen W, Kiemen A, Hennig J, Olbrich HM. The Impact of Acamprosate on Cue Reactivity in Alcohol Dependent Individuals: A Functional Magnetic Resonance Imaging Study. J Clin Psychopharmacol. 2012 doi: 10.1097/JCP.0b013e318267b586. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol. 2009;14:73–83. doi: 10.1111/j.1369-1600.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. NeuroImage. 2004;22:1151–6. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Miller WR. Combined Behavioral Intervention Manual. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004. [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction. 2000;95(Suppl 2):S229–36. doi: 10.1080/09652140050111799. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–75. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Randall PK, Boyle E, Anton RF, Becker HC, Randall CL. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33:1582–8. doi: 10.1111/j.1530-0277.2009.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Li X, Randall PK, Henderson S, Voronin K, Anton RF. The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J Clin Psychopharmacol. 2010;30:365–72. doi: 10.1097/JCP.0b013e3181e75cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37:2267–76. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–53. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–95. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry. 2011;168:183–92. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JS, Anton RF, Latham PK, Moak DH. Factor structure and predictive validity of the Obsessive Compulsive Drinking Scale. Alcohol Clin Exp Res. 1999;23:1484–91. [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Spiga S, Follesa P, Biggio G. Changes in GABA(A) receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J Neurosci. 2003;23:11711–24. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Stability of fMRI striatal response to alcohol cues: A hierarchical linear modeling approach. NeuroImage. 2011a;56:61–8. doi: 10.1016/j.neuroimage.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Voronin KE, Randall PK, Li X, Henderson S, Myrick H. Interacting effects of naltrexone and OPRM1 and DAT1 variation on the neural response to alcohol cues. Neuropsychopharmacology. doi: 10.1038/npp.2012.195. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Randall PK, Waid LR, Baros AM, Latham PK, Wright TM, Myrick H, Anton RF. Neurocognitive performance, alcohol withdrawal, and effects of a combination of flumazenil and gabapentin in alcohol dependence. Alcohol Clin Exp Res. 2011b;35 doi: 10.1111/j.1530-0277.2011.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen JP, Litten RZ, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–5. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Stritzke WG, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: advances in reliability, specificity, and validity. Psychol Addict Behav. 2004;18:148–59. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Ragan P, Chang R, Chen S, Linnoila VM, Coyle JT. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry. 1998;155:726–32. doi: 10.1176/ajp.155.6.726. [DOI] [PubMed] [Google Scholar]
- Urschel HC, Hanselka LL, Gromov I, White L, Baron M. Open-label study of a proprietary treatment program targeting type A gamma-aminobutyric acid receptor dysregulation in methamphetamine dependence. Mayo Clin Proc. 2007;82:1170–8. doi: 10.4065/82.10.1170. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010 doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–4. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]


