Abstract
Purpose
Two-stage revision represents the gold standard in the treatment of infected total knee arthroplasty. Different techniques have been proposed, mostly not preserving range of motion. An articulated antibiotic-loaded cement spacer made in association with two unicompartmental implants has been used as an alternative to a static spacer in an effort to retain as much movement as possible between the stages in young, high-demand patients with preserved ROM.
Methods
We evaluated nine consecutive patients with a mean age of 66.5 years. The second stage was performed after lab tests returned to normal and culture proved negative. Mean follow-up was 4.6 years.
Results
Mean ROM from a preoperative value of 105.6° was 103.5° after the first stage, and improved to 110.0° after the definitive implant. Mean Knee Society score was 27.6 preoperatively improving to 86.4 points postoperatively. WOMAC score showed that six patients were very satisfied with the overall result of their reimplanted knee, three subjects were somewhat satisfied. No recurrence of infection, no significant radiolucent lines or osteolysis were recorded at clinical and radiological follow-up and the patients were satisfied with the outcome.
Conclusions
Results indicated that this technique may ensure the advantages of a static spacer, but allow a greater ROM and better functional recovery. It may be considered as a viable option in selected cases even though the higher costs of two unicompartmental implants should be considered in the light of other aspects, such as prolonged hospital stay and rehabilitation in revision of infected total knee arthroplasty.
Introduction
Infection is one of the most common causes of failure in total knee arthroplasty (TKA), with an incidence ranging from 1 % to 23 % [1–4]: appropriate antibiotic strategies, operating room improvements, and medical care protocols have over the last decades decreased the rate of this complication [5, 6]. Treatment of infected TKA still represents one of the most challenging and expensive procedures in modern orthopaedics [7, 8]. Different variables affect clinical results including timing of onset of infection after primary implant, patients’ age and health status, and virulence of the infecting agent [1–3].
Medical or surgical treatments often in combination have been proposed, such as antibiotics administration, irrigation and debridement, resection arthroplasty, one-stage or two-stage revision, sterilisation of removed components, knee fusion, and amputation [9–19].
A two-stage procedure is considered the gold standard, associated with satisfactory functional results in more than 90 % of cases [20–22]. However, two procedures and related costs, disability during the interval between stages, and postoperative loss of range of motion (ROM) even after prolonged rehabilitation protocols represent significative shortcomings of this technique.
Several spacers have been proposed to manage the interval between the two steps, including static, monoblock spacers, or antibiotic-loaded beads [20, 23, 24]: both fail to preserve range of motion, may induce bone loss, stiffness, and instability, thus articulating spacers have been proposed to avoid these complications [16, 19, 21, 23]. Considering the poor results with a first generation techniques, a new concept of articulating spacers was introduced. Initially cemented, its final evolution was represented by metal prosthesis with antibiotics, popularised as “prosthesis antibiotics loaded acrylic cement” (PROSTALAC®, DePuy Orthopaedics, Warsaw, IN) [18]. ROM preservation and limitation of intra-articular adhesions were achieved, despite higher costs in relation to static spacers.
The purpose of this study was a modification of the original technique consisting of an articulated antibiotic-loaded cement spacer assembled with two unicompartmental implants to be used in selected cases of infected TKA in order to preserve ROM and soft tissue integrity in young, high-demand patients: our hypothesis was to ensure efficacy in the treatment of infection, preserve bone at the final stage, maintaining an adequate ROM before final reimplantation, and not significantly increase costs.
Materials and methods
At the authors’ institution, between January 2004 and September 2007, nine patients (six women, three men) were treated with “double uni” articulated antibiotic-impregnated cement spacer for a two-stage procedure in infected TKA. The mean age was 66.5 years (range: 59–71) at the time of initial TKA. The reason for primary replacement was osteoarthritis in all cases; one patient had undergone TKA after a previous high tibial osteotomy. The mean interval from primary TKA to first stage revision was 15.5 months (range: 5–32).
Inclusion criteria were: identification of the infecting organism, adequate ROM (over 90° without flexion contractures above 5°) at the time of first stage procedure, substantial soft tissues preservation, and preserved bone stock without large defects. Patients were excluded in case of flexion contractures over five degrees, limited ROM (under 90°), severe soft tissues compromise or skin necrosis, and a low level of activity. The diagnosis of infection was made on the basis of clinical examination, increased leucocyte count, erythrocyte sedimentation rate (ESR), C-reactive protein (CPR), radiographs, bone scan, knee aspiration, and microbiology cultures of tissue and fluid specimens at the time of surgery. Bone stock was assessed preoperatively on X-rays based on the criteria of Anderson Orthopaedic Research Institute (AORI) bone defect classification [25], and confirmed intraoperatively after infected components removal.
All knees were exposed through previous scars, and after removal of the infected implant, thorough irrigation and debridement were performed. Femur and tibia spacers were prepared in such a fashion as to preserve bone stock as much as possible. Double uni articulated spacer was created with StageOne® medical grade silicone knee moulds (Biomet, Warsaw, IN), available in four different sizes, resembling the geometry of femoral and tibial surfaces. Two 40-g packages of Palacos cement (Zimmer, Warsaw, IN), each loaded with 2 g of vancomycin and 1 g of imipenem–cilastatin were used for each mould.
Two little windows were produced before cement filling in each femoral mould to allow positioning of the unicompartmental femoral components during hardening of cement. Tibial inserts were assembled in the cement at the proper distance for femoral congruence (Fig. 1). Oxford III® unicompartmental implants (Biomet, Warsaw, IN) were used in all cases.
Fig. 1.
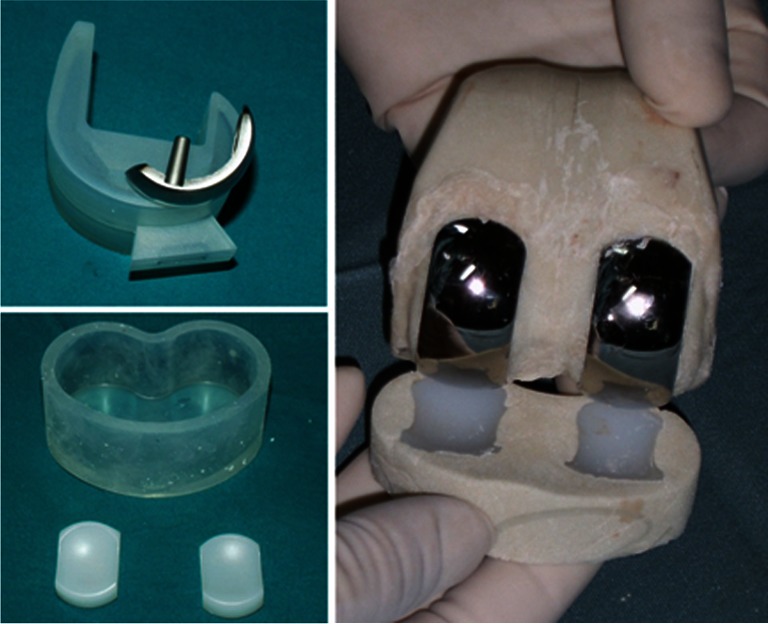
Double uni articulating cement spacer is assembled with preformed moulds in association with unicompartmental knee components
In one patient, with bone loss in both the femoral and tibial (both AORI 2a) metaphyses after components removal, a rod made of cement, over a long screw, was added to the femoral and tibial spacers to ensure sufficient stability. The cementing technique of the double uni spacer was performed much as for a standard knee implant: however, light pressurisation was applied to reduce cement penetration into the host bone. This was done to prevent bone loss and to simplify removal at the second surgical step (Fig. 2).
Fig. 2.
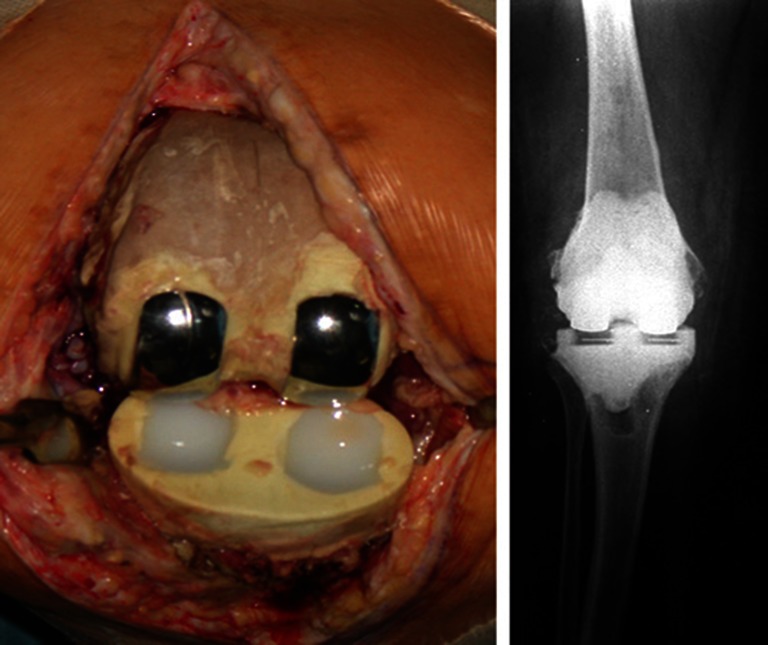
Double uni spacer positioning and postoperative radiographic aspect
Continuous passive motion (CPM), the use of two crutches, and partial weight-bearing were immediately instituted. If tolerated, complete weight-bearing with the devices was subsequently permitted. All patients were maintained on an antibiotic regimen as recommended by the infectious disease consultants for three to seven weeks depending on each case, with a mean period of 4.2 weeks. The eradication of infection was suggested by the return of inflammatory markers to normal (checked every three weeks) and a negative knee aspiration. This was done to avoid relying upon serological testing alone [26, 27]. During the second stage, two or three specimens of synovial fluid and intra-articular tissue were taken for microbiological analysis. The Double uni articulating cement spacers were then removed, followed by debridement, and reimplantation with a posterior stabilised primary prosthesis with extension uncemented stems and tibial wedges in three cases (Genesis II®, Smith & Nephew, Memphis, TN); another revision implant with same characteristics in five cases (Legion®, Smith & Nephew, Memphis, TN); or with a highly constrained implant in one case (RHK®, Zimmer, Warsaw, IN).
Patients were discharged from the hospital seven to eleven days after surgery and instructed to maintain and gradually increase as much as possible knee flexion during activities of daily living (Fig. 3): a protocol with daily muscular strengthening exercises was prescribed (stationary bike, stepper).
Fig. 3.
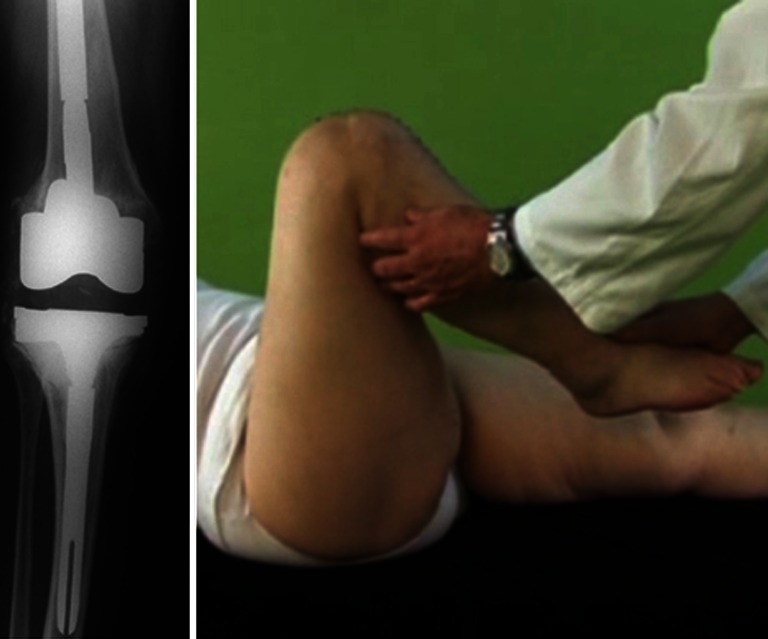
X-rays and ROM assessment after two-stage procedure with Double uni spacer
All patients were evaluated clinically at follow-up with the ROM, measured pre and postoperatively using a goniometer. The results were based upon Knee Society clinical rating score (KSS) [28]: an excellent outcome scored between 85 and 100 points, good was between 70 and 84 points, fair between 60 and 69 points, and poor below 60. Subjective patient satisfaction was assessed using Western Ontario questionnaire (WOMAC) [29]. Radiological evaluation was conducted with standing bilateral anteroposterior, lateral, and patellar views, including implant alignment (determining a perpendicular line to the anatomical long axis of the femur and tibia), noting the presence of radiolucent lines and osteolysis, according to the criteria of Knee Society [30].
Follow-up evaluation was performed at one, two, three, and six months after final implantation and then subsequently at yearly intervals.
A cost evaluation for each patient was performed including the calculation of mean factors (antibiotic loaded spacer, two unicompartmental implants, revision implant with wedges/stems, physical therapy, and rehabilitation protocol), and was then compared to a standard two-stage procedure without unicompartmental components, as generally conducted at the Authors’ Institution.
A statistical study was performed with Wilcoxon signed rank test to compare preoperative versus postoperative values in non-parametric values as KSS score and ROM. A p value of less than or equal to 0.05 was taken to indicate a statistically significant difference.
Results
The infecting agent was identified in all cases (Staphylococcus Epidermidis in three cases, Staphylococcus Aureus in the remainder, five of these Methicillin resistant). A sinus tract infection was present in two patients. Antibiotics used for the treatment varied depending upon conditions: teicoplanin 800 mgs/day, vancomycin 2 gs/day, linezolid 1200 mgs/day, amikacin 1 g/day, and sulfamethoxazole/trimethoprim 1600 mgs + 320 mgs/day, with periodic evaluation of hepatic and renal function. Amikacin was also used as part of an institutional antibiotic protocol since local specific risk for Gram-negative organisms.
No complications occurred during the first stage, or during preparation of double uni spacers. The soft tissues were compliant in all cases, presenting minimal adhesions, and allowing excellent exposure. Bone defects at the time of removal of infected components are indicated in Table 1. The evaluation of infection serological markers after the first stage confirmed the eradication of infection at the time of the second step in all cases: all aspirations were negative. The second stage was performed after a mean interval of 8.3 weeks (range: seven to ten) from first procedure. All spacers were stable, and easily removed by gentle tapping with a mallet during second stage. No complications or significant bone defects with respect to those found after first stages were noted after the second procedure. Bone loss management is also noted on Table 1.
Table 1.
Data concerning demographics, agent of infection, preoperative ROM and AORI evaluation for bone loss after removal of infected components, type of implant for final revision, choice of stems and wedges, and postoperative ROM
| Patient | Sex/Age | Agent of infection | Preop ROM | AORI bone loss (after infected components removal) | Revision implant | Stems length | Management of bone loss | Postop ROM |
|---|---|---|---|---|---|---|---|---|
| #1 | ♀ 64 y | Staphylococcus epidermidis | 5°–100° | -F 1 | Legion S & N | Mid-tibial, mid-femoral | Femoral distal medial 5 mm | 0°–105° |
| #2 | ♂ 59 y | Staphylococcus aureus Met. R. | 0°–115° | – | Genesis II S & N | Mid-tibial | – | 0°–115° |
| #3 | ♀ 65 y | Staphylococcus aureus Met. R. | 0°–105° | – | Genesis II S & N | Mid-tibial | – | 0°–110° |
| #4 | ♂ 70 y | Staphylococcus epidermidis | 0°–105° | T 1 F 1 | Legion S & N | Long tibial, long femoral | Tibial medial 5 mm | 0°–105° |
| #5 | ♀ 70 y | Staphylococcus aureus | 5°–110° | – | RHK Zimmer | Long tibial, long femoral | – | 0°–105° |
| #6 | ♀ 69 y | Staphylococcus epidermidis | 0°–95° | T 2a F 2a | Legion S & N | Offset mid-tibial, mid-Femoral | Tibial medial 5 mm Femoral distal medial 5 mm | 0°–105° |
| #7 | ♀ 65 y | Staphylococcus aureus Met. R. | 5°–120° | T 1 | Genesis II S & N | Midtibial | Tibial medial 5 mm | 0°–125° |
| #8 | ♂ 71 y | Staphylococcus aureus | 0°–100° | T 2a | Legion S & N | Long tibial | Tibial medial 5 mm | 0°–105° |
| #9 | ♀ 66 y | Staphylococcus aureus Met. R. | 0°–115° | -F 2a | Legion S & N | Offset midtibial Offset mid-femoral | -Femoral distal medial & posterior 5 mm | 0°–115° |
No patient had an infection recurrence after completing treatment. All wounds healed completely without complications. The mean follow-up was 4.6 years (range: four to seven).
Mean ROM at the time of first procedure was of 105.6° (range: 95°–115°), and no contractures over five degrees were present. Mean ROM after the first stage was substantially maintained with a mean value of 103.5° (range: 98°–108°). After the final procedure, ROM significatively improved to 110.0° without residual flexion contractures (range: 105°–125°; p < 0.001).
The mean preoperative Knee Society score was 27.6 (range: 19–48); at follow-up, the score improved to 86.4 points (range: 74–97; p < 0.001).
The WOMAC score showed that six patients were very satisfied with the overall result of their reimplanted knee, three subjects were somewhat satisfied.
Radiographs showed all implants to be in valgus alignment after the second stage with a mean femorotibial angle of 4.1° ± 1.8°.
No significant radiolucent lines or osteolysis were revealed at radiographic follow-up; three patients presented a 1-mm-wide radiolucent line on the medial tibial side in the AP view, both under a medial wedge, however with no progression at subsequent radiographic follow-up.
To date, all patients are free from infection, generally satisfied, and have maintained adequate ability in daily or professional activities.
The resulting costs are reported in Table 2.
Table 2.
Data concerning mean costs of standard vs Double Uni procedure
| Standard procedure | Double uni procedure | ||
|---|---|---|---|
| MEAN COSTS (€) | Stage 1 (Implant removal) | 1.300 | 1.300 |
| Spacer | 700 | 2.500 | |
| Stage 2 (Revision) | 13.800 | 13.800 | |
| Revision implant | 4.330 | 3.910 | |
| Wedges | 610 | 390 | |
| Stems | 1.650 | 1.250 | |
| Rehabilitative protocol | 950 | 800 | |
| Total | 23.340 | 23.950 | |
Discussion
Two-stage revision with an antibiotic-impregnated cement spacer remains the gold standard for patients with infected knee arthroplasty: the goal is to allow the continuous intra-articular delivery of antibiotics, and to avoid ligament retraction. A spacer is also intended to be a stable insert able to maintain adequate ligament length and ROM, and to allow full weight-bearing, usually restricted with static blocks [16, 21, 31, 32]. Complications such as spacer migration, ligament retraction, stiffness, patellar or quadriceps tendon rupture, tibial tubercle avulsion, and bone loss may significantly be minimised [16, 17, 20, 22, 31]. Our new concept for the spacer allows objective improvements: In static blocks, ROM after the two-stage procedure is reduced, and consequently poorer clinical outcome is expected. Emerson et al. obtained a mean ROM of 94° with monoblock insert compared with 108° for an articulating spacer [24]. Fehring et al. reported in a limited number of cases, 98° for the block compared with 105° for the articulating spacer with low significance, but finding no functional advantage of the articulated over the static: moreover, after first stage, patients were immobilised for ten days, and subsequently discouraged from moving their knees postoperatively until the definitive implant [23].
There are concerns about the use of a spacer made of foreign material in a joint space, even if a temporary cemented block might have a theoretical advantage over another made of metal and plastic parts, viable substrate for bacterial glycocalyx adhesion. However, Kendall et al. reported that Staphylococcus (S. aureus and S. epidermidis) may be found in vitro on antibiotic-impregnated polymethylmethacrylate disks after 96-hours incubation, concluding that the surface of antibiotic-impregnated bone cement is still a suitable substrate for bacterial adherence and growth even in the presence of antibiotics [33]. The same author reported other findings in vivo on 23 patients treated with an antibiotic spacer: no bacterial adherence was identified on the retrieved spacer at the time of reimplantation [34]. Hofmann et al. and Meek et al. used metal and plastic implants in their series with a low infection rate [16, 18], probably related to high doses of antibiotics in the cement. In a recent study, Johnson et al. reported comparable reinfection rates, KSS score, and postoperative ROM in a retrospective analysis of two groups patients treated with dynamic or static spacer [35].
Revision of an infected TKA represents an expensive procedure, related to hospitalisation, surgery, prolonged use of antibiotics, bone loss after the first stage, long rehabilitation period between and after the stages. In addition, there are significant social costs. Modern articulated spacers with or without metal implants are an additional expense, but they may limit the costs of therapy protocols and improve activities of daily living and work ability.
The proposed spacer is a dynamic implant, made with antibiotic-loaded cement as has been previously reported, but with a novel feature, represented by the two unicompartmental prostheses. The rationale is to allow a better sliding compared to cement on cement surfaces, preserving the ROM of the knee, which can be limited when using standard dynamic spacers. Costs related to the use of these unicompartmental implants are surely higher than conventional spacers, however, may be compensated by several positive aspects. With an improved functional ability between stages, the tendency to be bone sparing (as no significant widening of previous defects was recorded after spacer removal in this series) related to a better “tribology”, the need for a less constrained and complex revision implant, and the reduction of the rehabilitation protocol after the definitive implant with respect to our experience with the classic two-stage procedure are the benefits of this procedure. Efficacy in terms of infection control and ease of spacer removal are in line with the results reported in the literature. The slight, but not significant, reduction of the mean ROM after first stage was probably related to patients’ perception of a not definitively fixed implant. After reimplantation, ROM improved to mean values higher than before infected TKA removal. Finally, the undoubted drawback related to the initial high costs may be substantially tolerated in selected, young, and strongly motivated patients, with highly functional (even if infected) TKAs.
Several shortcomings are to be considered. A small number of patients with heterogeneous characteristics, and absence of a control group may have biased our outcomes, limiting the possibility of an adequate power analysis.
Initial results have been encouraging, confirming that selection of patients is crucial. This novel technique is a viable option in specific cases, and especially in patients with high activity level.
Acknowledgments
The authors state that they have no conflict of interests.
Conflict of interest statement
The authors state that:
- The paper is original and not under publication of other journal in any language
- The clinical protocol was approved by the Local Ethical Committee of the Institution, and all patients gave their informed consent to the procedures and follow-up
- All the authors exclude any form of external or internal funding, having no professional or financial affiliations that may be perceived to have altered the paper and its content
- They all contributed to the ideation, preparation, and writing of the manuscript.
References
- 1.Insall JN. Infection of total knee arthroplasty. Instr Course Lect. 1986;35:319–324. [PubMed] [Google Scholar]
- 2.Rand JA, Fitzgerald RH., Jr Diagnosis and management of the infected total knee arthroplasty. Orthop Clin North Am. 1989;20:201–210. [PubMed] [Google Scholar]
- 3.Rorabeck CH. Session IV: salvage of the infected total knee replacement. Infection: the problem. Clin Orthop. 2002;404:113–115. doi: 10.1097/00003086-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72:878–883. [PubMed] [Google Scholar]
- 5.Williams DN, Gustilo RB. The use of preventive antibiotics in orthopaedic surgery. Clin Orthop. 1984;190:83–88. [PubMed] [Google Scholar]
- 6.Fitzgerald RH., Jr Total hip arthroplasty sepsis: prevention and diagnosis. Orthop Clin North Am. 1992;23:259–264. [PubMed] [Google Scholar]
- 7.Barrack RL. Economics of the infected total knee replacement. Orthopedics. 1996;19:780–782. doi: 10.3928/0147-7447-19960901-21. [DOI] [PubMed] [Google Scholar]
- 8.Sculco TP. The economic impact of infected total joint arthroplasty. AAOS Instr Course Lect. 1993;42:340–351. [PubMed] [Google Scholar]
- 9.Hanssen AD, Rand JA, Osmon DR. Treatment of the infected total knee arthroplasty with insertion of another prosthesis. The effect of antibiotic-impregnated bone cement. Clin Orthop Relat Res. 1994;309:44–55. [PubMed] [Google Scholar]
- 10.Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and debridement. Clin Orthop Relat Res. 1991;273:113–118. [PubMed] [Google Scholar]
- 11.Marsh PK, Cotler JM. Management of an anaerobic infection in a prosthetic knee with long-term antibiotics alone: a case report. Clin Orthop. 1981;155:133–135. [PubMed] [Google Scholar]
- 12.Burger RR, Basch T, Hopson CN. Implant salvage in infected total knee arthroplasty. Clin Orthop. 1991;273:105–112. [PubMed] [Google Scholar]
- 13.Schoifet JD, Morrey BF. Treatment of infection after total knee arthroplasty by debridement with retention of the components. J Bone Joint Surg Am. 1990;72:1383–1390. [PubMed] [Google Scholar]
- 14.Goksan SB, Freeman MA. One-stage reimplantation for infected total knee arthroplasty. J Bone Joint Surg Br. 1992;74:78–82. doi: 10.1302/0301-620X.74B1.1732271. [DOI] [PubMed] [Google Scholar]
- 15.Teeny SM, Dorr L, Murata G, Conaty P. Treatment of infected total knee arthroplasty. Irrigation and debridement versus two-stage reimplantation. J Arthroplasty. 1990;5:35–39. doi: 10.1016/S0883-5403(06)80007-0. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Treatment of infected total knee arthroplasty using an articulating spacer. Clin Orthop. 1995;321:45–54. [PubMed] [Google Scholar]
- 17.McMaster WC. Technique for intraoperative construction of PMMA spacer in total knee revision. Am J Orthop. 1995;24:178–180. [PubMed] [Google Scholar]
- 18.Meek RM, Masri BA, Dunlop D, Garbuz DS, Greidanus NV, McGraw R, Duncan CP. Patient satisfaction and functional status after treatment of infection at the site of a total knee arthroplasty with use of a PROSTALAC articulating spacer. J Bone Joint Surg Am. 2003;85:1888–1892. doi: 10.1302/0301-620X.85B8.14214. [DOI] [PubMed] [Google Scholar]
- 19.Trezies A, Parish E, Dixon P, Cross M. The use of an articulating spacer in the management of infected total knee arthroplasties. J Arthroplasty. 2006;21:702–704. doi: 10.1016/j.arth.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 20.Goldman RT, Scuderi GR, Insall JN. Two stage reimplantation for infected total knee replacement. Clin Orthop. 1996;331:118–124. doi: 10.1097/00003086-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Gusso MI, Capone A, Civinini R, Scoccianti G. The spacer block technique in revision of total knee arthroplasty with septic loosening. Chir Organ Mov. 1995;80:21–27. [PubMed] [Google Scholar]
- 22.Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Two-stage reimplantation for the salvage of total knee arthroplasty complicated by infection: Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272–278. [PubMed] [Google Scholar]
- 23.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Emerson RH, Jr, Muncie M, Tarbox TR, Higgins LL. Comparison of a static with a mobile spacer in total knee infection. Clin Orthop Relat Res. 2002;404:132–138. doi: 10.1097/00003086-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Engh GA, Ammeen DJ. Classification and preoperative radiographic evaluation: knee. Orthop Clin N Am. 1998;29:205–217. doi: 10.1016/S0030-5898(05)70319-9. [DOI] [PubMed] [Google Scholar]
- 26.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection. What is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;467:1699–1705. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2010;35:1621–1626. doi: 10.1007/s00264-010-1175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the knee society clinical rating system. Clin Orthop. 1989;248:13–14. [PubMed] [Google Scholar]
- 29.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip of knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 30.Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 31.Calton TF, Fehring TK, Griffin WL. Bone loss associated with the use of spacer blocks in infected total knee arthroplasty. Clin Orthop Relat Res. 1997;345:148–154. doi: 10.1097/00003086-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Park SJ, Song EK, Seon JK, Yoon TR, Park GH. Comparison of static and mobile antibiotic-impregnated cement spacers for the treatment of infected total knee arthroplasty. Int Orthop. 2010;34:1181–1186. doi: 10.1007/s00264-009-0907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendall RW, Duncan CP, Smith JA, Ngui-Yen JH. Persistence of bacteria on antibiotic loaded acrylic depots. Clin Orthop. 1996;329:273–280. doi: 10.1097/00003086-199608000-00034. [DOI] [PubMed] [Google Scholar]
- 34.Kendall RW, Duncan CP, Beauchamp CP. Bacterial growth on antibiotic-loaded acrylic cement. J Arthroplasty. 1990;10:817–822. doi: 10.1016/S0883-5403(05)80081-6. [DOI] [PubMed] [Google Scholar]
- 35.Johnson AJ, Sayeed SA, Naziri Q, Khanuja HS, Mont MA. Minimizing dynamic knee spacer complications in infected revision arthroplasty. Clin Orthop Relat Res. 2012;470:220–227. doi: 10.1007/s11999-011-2095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


