Abstract
Purpose
Diabetes mellitus is an important aetiological factor in intervertebral disc degeneration. The disappearance of notochordal cells in the nucleus pulposus is thought to be the starting point for intervertebral disc degeneration. A cellular effect of diabetes mellitus on apoptosis of notochordal cells and intervertebral disc degeneration has been recently reported. However, how the duration and severity of diabetes mellitus affects viability of notochordal cells and intervertebral disc degeneration is still unknown .
Methods
Rat notochordal cells were isolated, cultured, and placed in either 10 % foetal bovine serum (FBS) (normal control) or 10 % FBS plus three different high glucose concentrations (0.1 M, 0.2 M, and 0.4 M) (experimental conditions) for one, three, five and seven days, respectively. We identified and quantified the degree of proliferation and apoptosis, caspase activities, and cleavages of Bid and cytochrome-c. In addition, we examined the cells for expression of matrix metalloproteinases (MMPs) and their tissue inhibitors of metalloproteinases (TIMPs).
Results
Each three high glucose concentrations significantly decreased proliferation and increased apoptosis of notochordal cells from culture days one to seven in a dose-dependent manner. Compared with those of 10 % FBS, caspase-9 and -3 activities and cleavage of Bid and cytochrome-c were significantly increased in each three high glucose concentrations, accompanied by increased expression of MMP-1, -2, -3, -7, -9, and -13 and TIMP-1 and -2.
Conclusions
High glucose concentration significantly decreased proliferation and increased apoptosis of notochordal cells via the intrinsic pathway with dose- and time-dependent effects. We also found that expression of MMPs and TIMPs was increased with dose- and time-dependent effects. Therefore, these results suggest that aggressive glucose control from an early stage of diabetes mellitus should be recommended to prevent or limit intervertebral disc degeneration.
Introduction
Diabetes mellitus is a systemic disease that causes premature degeneration in virtually every organ, including the eyes, heart, kidneys, blood vessels, central and peripheral nervous systems, and joints [1–5]. Diabetes mellitus is an important aetiological factor in premature intervertebral disc degeneration [6–8]. There is a high risk of degenerative disc diseases in patients with diabetes at a younger age than in the nondiabetic population [9–11]. In addition, diabetic patients with degenerative disc diseases have a relatively unsatisfactory surgical outcome compared to nondiabetic patients. Therefore, understanding the mechanism by which diabetes mellitus affects intervertebral disc degeneration is the first step in designing therapeutic modalities to delay or prevent intervertebral disc degeneration caused by diabetes mellitus.
Notochordal cells form the notochord, which in turn contributes to the formation and maintenance of the nucleus pulposus of the intervertebral disc. After birth, the majority of notochordal cells gradually disappear by apoptosis [12–15]. In humans, notochordal cells are very rarely present after the age of ten years, and the nucleus pulposus transforms with time into a fibrocartilaginous nucleus pulposus. With this transition, intervertebral disc degeneration begins [16]. Therefore, the disappearance of notochordal cells by apoptosis in the nucleus pulposus is thought to be the starting point of intervertebral disc degeneration. A previous study reported that non-diabetic rat notochordal cells undergo Fas type 2-mediated intrinsic (mitochondrial) pathway, and a regulated negative balance of notochordal cell proliferation against apoptosis is likely to involve the disappearance of notochordal cells [17].
Won et al. recently reported a cellular effect of diabetes mellitus on apoptosis of notochordal cells and intervertebral disc degeneration [18]. In their study using aged-matched diabetic and nondiabetic rats, Won et al. suggested that diabetes mellitus is associated with premature, excessive apoptosis of notochordal cells of the nucleus pulposus, accelerating the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus. The premature phenotypic change of the nucleus pulposus in diabetic rats increased the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), resulting in rapid intervertebral disc degeneration and fibrosis. However, how the duration and severity of diabetes mellitus affects the intervertebral disc degeneration including viability, apoptotic pathway, expression of MMPs and TIMPs of notochordal cells is still unknown. Therefore, we performed a study to investigate these questions.
Materials and methods
Cell culture and treatment with three different high glucose concentrations
All lumbar intervertebral discs (L1-6) were harvested from four-week-old male Sprague Dawley rats (Orient Bio., Korea) immediately after they were killed. We carefully dissected the discs under a microscope to obtain only the gelatinous notochordal nucleus pulposus (NP) tissue, and the harvested NP tissue was pooled in [alpha]-minimum essential medium ([alpha]-MEM; Gibco BRL, Grand Island, NY, USA). The notochordal cells were released from the NP tissue in HBSS (Hyclone, Ottawa, Ontario, Canada) with 0.02 % pronase (Sigma, St. Louis, MO) by vigorous pipetting. Notochordal cells were cultured in a complete medium [alpha]-MEM supplemented with 10 % foetal bovine serum (Hyclone), and 1 % penicillin-streptomycin (Gibco BRL) at 37 °C in a humidified atmosphere (95 % air–5 % CO2). After the cells grew to confluence, they were split once (passage 1) and grew to confluence again. For use in the experiments, the cells were then trypsinised, washed, and plated on six- or 48-well culture plates. When notochordal cells reached 80 to 90 % confluence, the notochordal cells were placed in either foetal bovine serum (FBS) (normal control) of 10 % FBS plus three different high glucose concentrations (0.1 M, 0.2 M, and 0.4 M) (experimental conditions) for one, three, five and seven days. This study was approved by the Institution’s Animal Care and Use Committee.
Evaluation of cell proliferation
For the MTS (CellTiter 96® Aqueous, Promega, Madison, WI) assay method, 40 μl of (5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) in normal control and experimental conditions medium was added to each well. After incubation for two hours at 37 °C in a humidified, 5 % CO2 atmosphere with MTS, the absorbance at 490 nm was then recorded using an ELISA plate reader. Each point represents the mean ± SD of six replicates.
Evaluation of cell apoptosis
For evaluation of apoptosis, notochordal cells were fixed with 4 % paraform aldehyde (PFA) (Fluka-Sigma Aldrich) in PBS for 15 minutes on ice and incubated with 0.1 % PBS-Triton-X-100 (PBS-T) at room temperature for 20 minutes. After washing, the cells were stained with 4′, 6-diamidino-2-phenylindole (DAPI) staining (VECTASHILED, VECTR, Burlingame, CA, USA). Apoptosis of notochordal cells was determined under the fluorescence microscopy (Olympus, Melville, NY, USA) based on morphologic changes, such as chromatic condensation and nuclear fragmentation. Two pathologists who were unaware of the clinical date were responsible for determining the apoptosis of notochordal cells. Total and apoptotic cells were counted three times in six high-power fields (x400) from each of three sections of specimen and summed. The average of total and apoptotic cells per specimen was then obtained. Finally, the percentage of apoptotic cells compared with total cells was calculated as apoptotic index (%).
Western blot analysis
For western blot analysis, antibodies to MMP-2, -3, -9, and -13 (Abcam Plc, Cambridge, UK), MMP-1 (Lifespan Providence, RI, USA), MMP-7 (Calbiochem, Dasrmstadt, Germany), Caspase-8, Bid, TIMP-1 and -2 (SantaCruz Biotechnology, CA, USA), caspase-3, -9, cytochrome-c, Akt, Phospho-p38 MAPK and poly (ADP) ribose polymerase (PARP) (Cell Signaling Technology Inc., MA, USA) were purchased. In addition, antibody for loading control ß-actin was obtained from SIGMA (Sigma, St. Louis, MO, USA).
Expressions of MMP-1, -2, -3, -7, -9, and -13 and TIMP-1 and -2 were determined by western blot analysis according to the manufacturer’s instructions. ß-actin was used as an internal control for protein-loading. The cells were washed with ice-cold phosphate-buffered saline solution and lysed in PRO-PREP protein extraction solution (iNtRON Biotechnology, Inc., Korea). The cell lysates were centrifuged at 12,000 g for 15 minutes, and protein concentrations were measured with the Bicinchoninic Acid (BCA)(Thermo scientific) method. Samples (50–70 μg of protein) were electrophoresed on 10 % to 15 % SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and transferred to a nitrocellulose membrane. The membranes were incubated with primary antibodies, followed by second antibodies of HRP (horseradish peroxidase)-linked IgG (immunoglobulin G) (Bio-Rad, Hercules, CA, USA), and immunereactive bands were visualised with the western blotting luminol reagent (Santa Cruz Biotechnology, CA, USA). Blots were quantified using Imaging Densitometer GF670 and Molecular Analyst software (Bio-Rad, Hercules, CA, USA) three times in each sample, and the average of three densities was used as the final density. The value of the density was presented as the mean ± SD (arbitrary units).
Statistical analysis
All experiments were independently conducted three times, and the results were expressed as the mean and standard deviation of the values derived with the three tests. Statistical analysis was done with the paired-samples and independent-samples t test. P < 0.05 was considered to be the level of significance.
Results
Effect of high glucose concentration on proliferation of notochordal cells
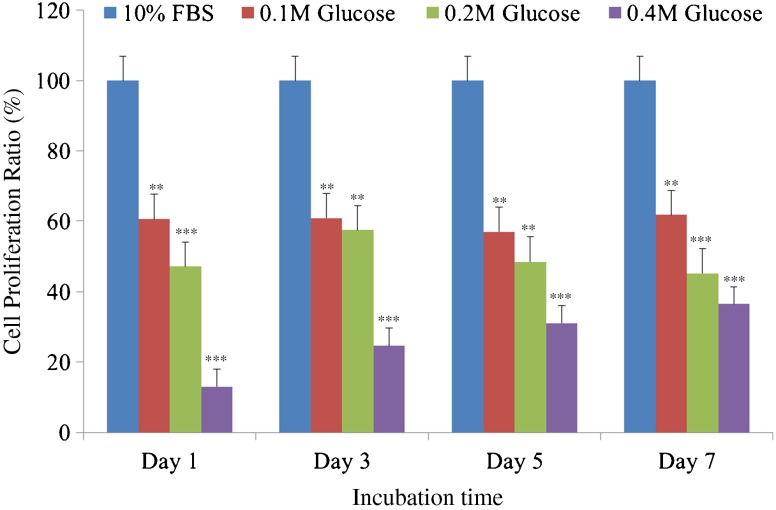
Each three different high glucose concentrations significantly decreased proliferation of notochordal cells from culture days one to seven with time- and dose-dependent manner (Fig. 1).
Fig. 1.
Each of three high glucose concentrations significantly suppress proliferation of notochordal cells from culture days 1 to 7 with dose- and time-dependent effects compared with those of 10 % foetal bovine serum. ***p < 0.001; **p < 0.01
Effect of high glucose concentration on apoptosis of notochordal cells
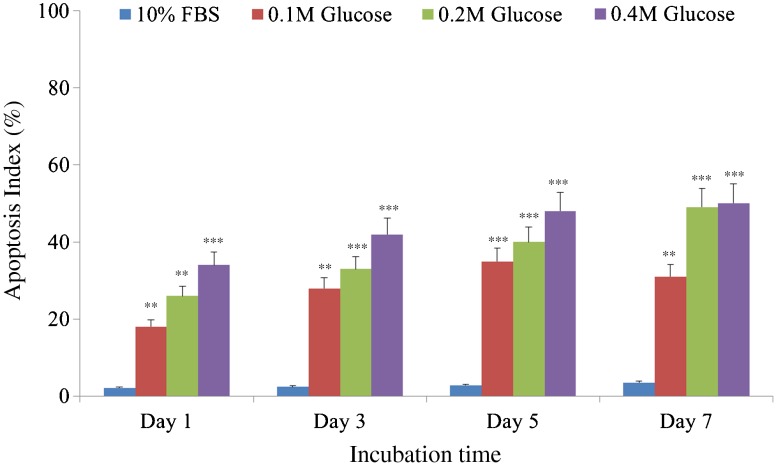
DAPI staining demonstrated that notochordal cells underwent apoptosis under the three different high glucose concentrations in culture day one (Fig. 2a), day three (Fig. 2b), day five (Fig. 2c), and day seven (Fig. 2d). Figure 3 shows that each different high glucose concentration significantly increased apoptosis of notochordal cells from culture days one to seven with time- and dose-dependent manner.
Fig. 2.
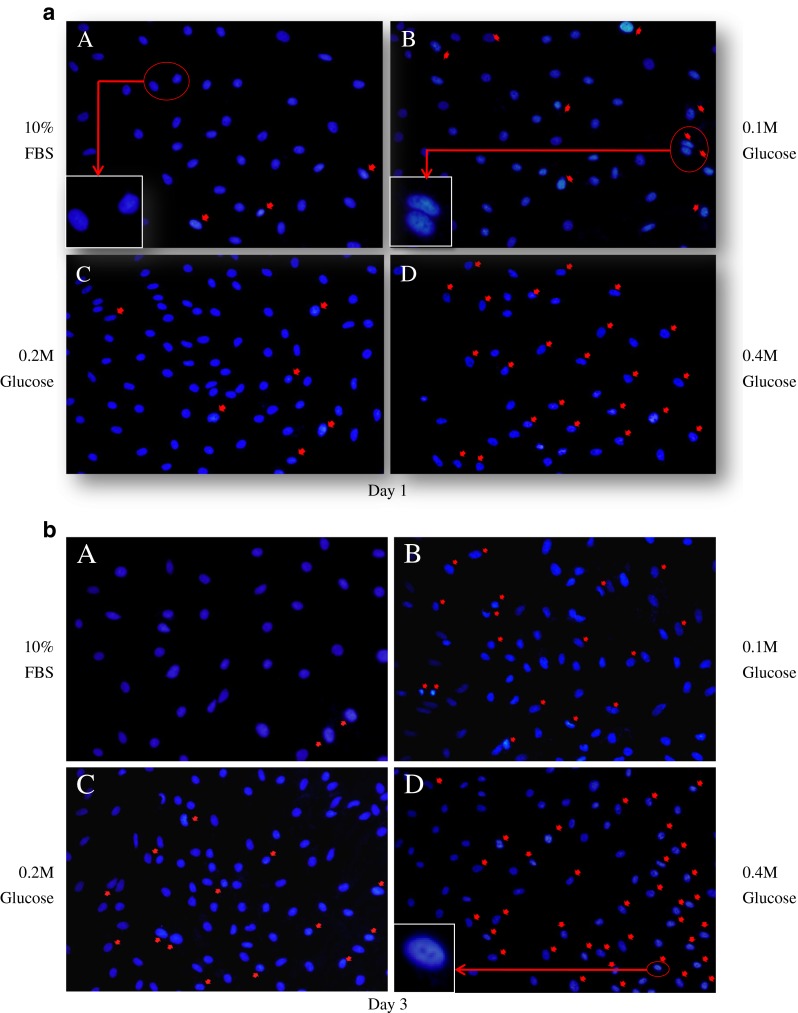
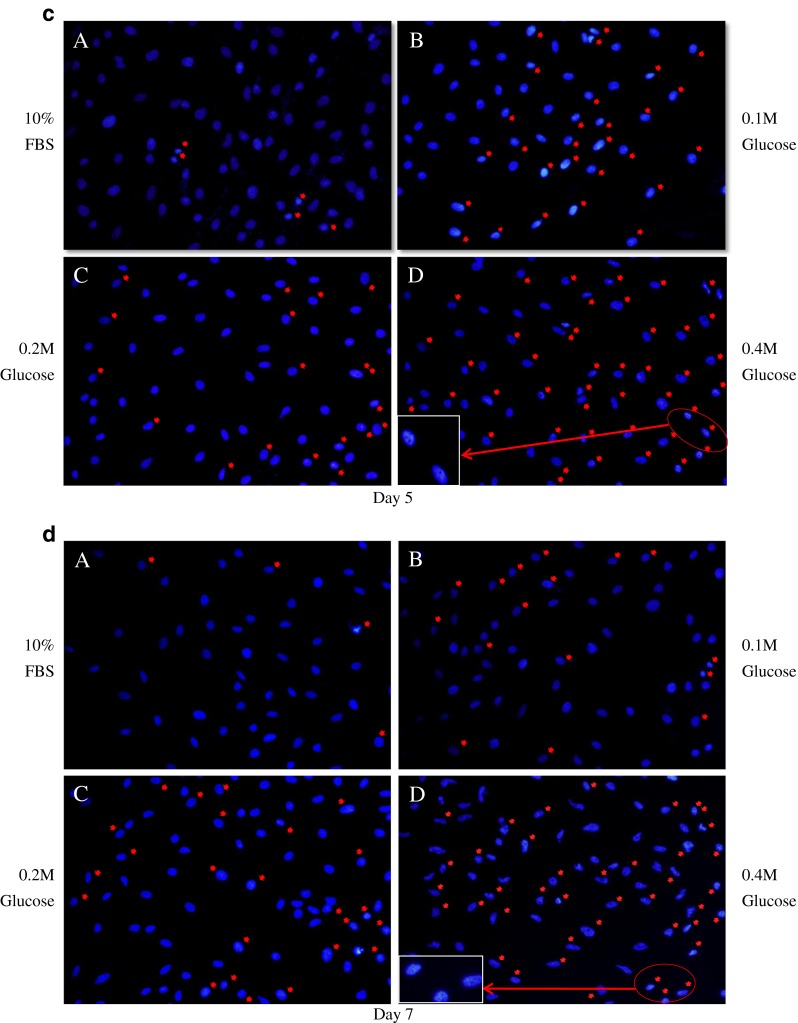
4′, 6-diamidino-2-phenylindole (DAPI) staining demonstrated that notochordal cells underwent apoptosis under each of the three different high glucose concentrations in culture day 1 (a), day 3 (b), day 5 (c), and day 7 (d). Apoptosis of notochordal cells was determined by morphological changes, such as chromatic condensation and nuclear fragmentation (red arrows)
Fig. 3.
Each of the three different high glucose concentrations significantly increased apoptosis of notochordal cells from culture days 1 to 7 in a time- and dose-dependent manner. ***p < 0.001; **p < 0.01
Apoptotic pathway of notochordal cells under high glucose concentration
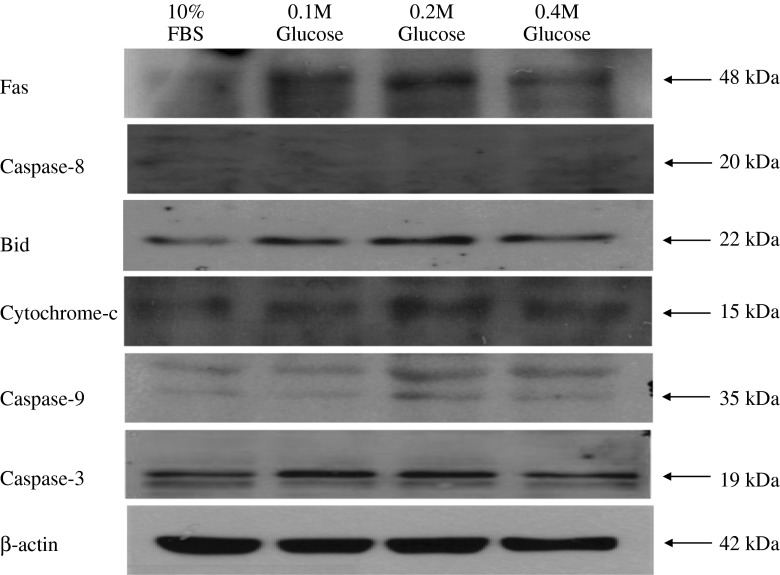
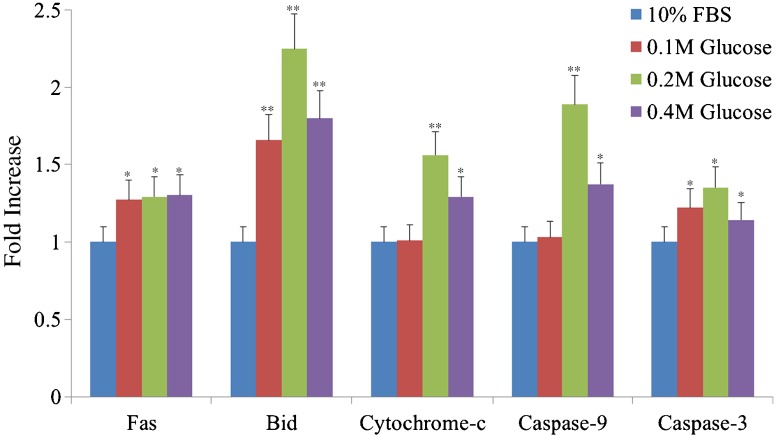
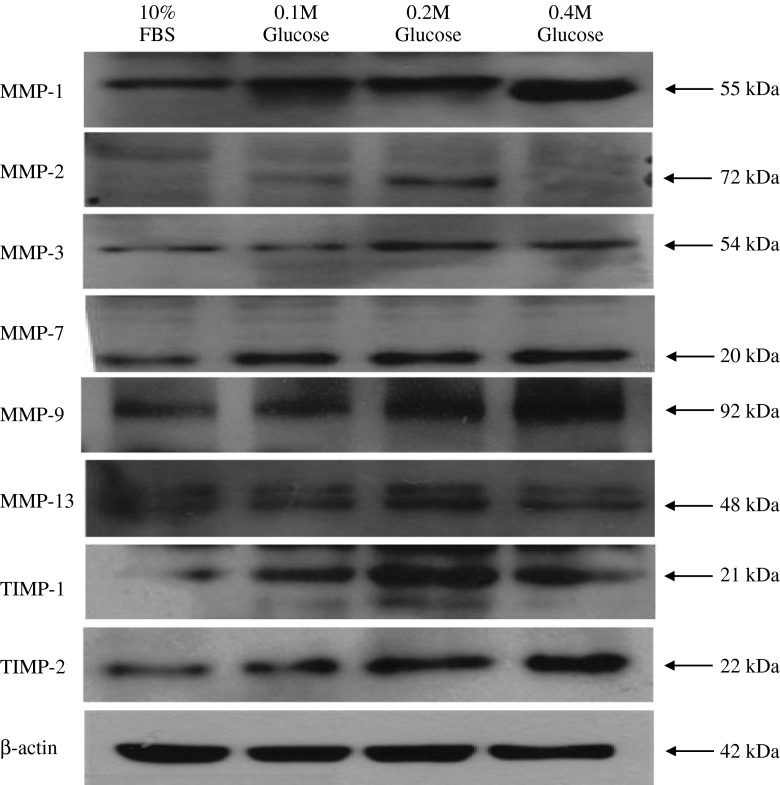
Western blot analysis showed an increased expression of Fas, the proteins associated with the intrinsic (mitochondrial) pathway of apoptosis (Bid, cytochrome-c, caspase-9), and the final executioner of apoptosis (caspase-3) in notochordal cells treated with each different high glucose concentration. However, the protein associated with the extrinsic pathway (caspase-8) was not identified in notochordal cells treated with each different high glucose concentration (Fig. 4). The degree of expression of Fas, Bid, cytochrome-c, caspase-3, and caspase-3 was significantly increased in each the three different high glucose concentrations compared with those of 10 % FBS (Fig. 5).
Fig. 4.
Western blot analysis shows an increased expression of Fas, the proteins associated with the intrinsic (mitochondrial) pathway of apoptosis (Bid, cytochrome-c, caspase-9), and the final executioner of apoptosis (caspase-3) in notochordal cells treated with each of the three different high glucose concentrations. However, the protein associated with the extrinsic pathway (caspase-8) was not identified in notochordal cells treated with each of the different high glucose concentrations
Fig. 5.
The degree of expression of Fas, Bid, cytochrome-c, caspase-3, and caspase-3 was significantly increased in each of the three different high glucose concentrations compared with those of 10 % FBS. **p < 0.01; *p < 0.05
Effect of high glucose concentration on expression of MMPs and TIMPs
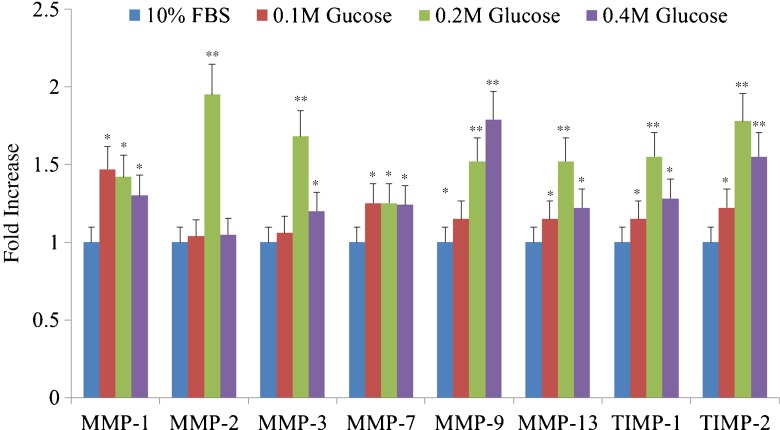
Western blot analysis showed an increased expression of MMP-1, -2, -3, -7, -9, and -13 and TIMP-1 and -2 in notochordal cells treated with each different high glucose concentration (Fig. 6). The degree of expression of MMP-1, -2, -3, -7, and -13 and TIMP-1 and -2 was significantly increased in each of the three different high glucose concentrations compared with those of 10 % FBS (Fig. 7).
Fig. 6.
Western blot analysis shows an increased expression of MMP-1, -2, -3, -7, -9, and -13 and TIMP-1 and -2 in notochordal cells treated with each of the three different high glucose concentration
Fig. 7.
The degree of expression of MMP-1, -2, -3, -7, and -13 and TIMP-1 and -2 was significantly increased in each of the three different high glucose concentrations compared with those of 10 % FBS. **p < 0.01; *p < 0.05
Discussion
Notochordal cells have a very unusual lifespan. The notochordal cells reside in the notochordal nucleus pulposus and produce gel-like vacuole-rich nucleus pulposus tissue until they disappear at some point during the individual’s lifetime [12–15]. As notochordal cells disappear by apoptosis, chondrocytes migrating from cartilage-endplates gradually replace notochordal cells and change the gelatinous notochordal nucleus pulposus into a fibrocartilaginous nucleus pulposus [16]. With progress of this chronological transition, intervertebral disc degeneration starts. Therefore, the disappearance of notochordal cells from the nucleus pulposus is thought to be the starting point of intervertebral disc degeneration, which may occur at different ages even in the same species.
Fas induces apoptosis in Fas-bearing cells on binding of its ligand (FasL) via two major pathways including extrinsic (death receptor) and intrinsic (mitochondrial) pathways [19–22]. Caspases are a family of cysteine proteases that cleave their substrates after aspartic acid residues. The caspases involved in apoptosis are divided into two subfamilies, the initiator (caspase 2, 8, 9, and 10) and executioner caspases (caspase 3, 6, and 7). In the extrinsic (death receptor) pathway, caspase-8 directly activates caspase-3, an executioner of apoptosis, whereas in the intrinsic (mitochondrial) pathway, caspase-3 is activated by caspase-9 via mitochondria such as cleavage of Bid and cytochrome-c. It has been reported that in non-diabetic rat, notochordal cells undergo Fas-mediated apoptosis via the type 2 intrinsic (mitochondrial) pathway and a regulated negative balance of notochordal cell proliferation against apoptosis is likely to involve the disappearance of notochordal cells in the nucleus pulposus [17].
Our study demonstrates that each of three high glucose concentrations significantly decreased proliferation and increased apoptosis of notochordal cells from culture on days one to seven in a dose-dependent manner compared with 10 % FBS normal control. In addition, Fas, caspase-9 and -3 activities and cleavage of Bid and cytochrome-c were significantly increased in each of three high glucose concentrations. These findings suggest that diabetes mellitus enhances apoptosis of notochordal cells via the Fas type 2-mediated intrinsic (mitochondrial) pathway, which occurs in a non-diabetic condition, with dose- and time-dependent effects and decreases proliferation of notochordal cells. As a result, an enhanced regulated negative balance of notochordal cell proliferation against apoptosis seems to cause more rapid disappearance of notochordal cells in diabetic nucleus pulposus compared to non-diabetic. This results in an accelerated transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus, which leads to early intervertebral disc degeneration.
MMPs are a family of zinc-dependent enzymes that degrade extracellular matrix components [23, 24]. It is known that increased expression of MMPs is responsible for intervertebral disc degeneration and disc herniation [25, 26]. The activity of MMPs can be affected by several factors, especially TIMPs. The TIMPs bind strongly, but noncovalently, to activated MMPs. They are coexpressed with MMPs and contribute to the regulation of their activity so that increases in TIMP levels reduce MMP activity. However, contradictory results regarding the delicate balance between MMP and TIMP expression have been reported in several pathological conditions, such as abdominal aortic aneurysms, chronic liver injury, and osteoarthritis [27–29].
According to Won et al., the expression of MMPs and TIMPs was significantly increased in the nucleus pulposus of diabetic rats compared with non-diabetic rats of the same ages [18]. Our study also demonstrates that each three high glucose concentration significantly increased the expression of MMP-1, -2, -3, -7, -9, and -13 and TIMP-1 and -2 in notochordal cells from culture days one to seven in a dose-dependent manner compared with 10 % FBS normal control. These findings suggest the possibility of a cycle wherein the presence of denatured extracellular matrix components leads to increased MMP production, in turn generating more denatured extracellular matrix components. The profound destruction of extracellular matrix components in intervertebral disc degeneration may stimulate further increased expression of TIMPs, resulting in rapid intervertebral disc degeneration and fibrosis.
There were some limitations to our study. First, while the in vitro cell culture system is a well-accepted study model, what occurs in notochordal cells in vivo is different from that which occurs in notochordal cells in vitro. Second, we cultured the notochordal cells in three different high glucose concentrations (0.1 M, 0.2 M, and 0.4 M) for one, three, five, and seven days. However, we do not know how these experimental culture conditions reflect the degree and duration of diabetes mellitus in vivo. Therefore, our results should not be viewed as a definitive explanation of what occurs in patients with diabetes mellitus. Despite some limitations, now that we have a better understanding of the degenerative process that occurs with diabetes, we can design further experiments to determine if therapeutic modalities—including modulation of serum glucose levels, and employing inhibitors of MMPs—can alter the chronology of events.
In conclusion, our study demonstrates that high glucose concentration significantly decreased proliferation and increased apoptosis of notochordal cells via the intrinsic pathway with dose- and time-dependent effects. We also found that expression of MMPs and TIMPs was increased with dose- and time-dependent effects. Therefore, aggressive glucose control from an early stage of diabetes mellitus should be recommended to prevent or limit intervertebral disc degeneration.
References
- 1.Burner TW, Rosenthal AK. Diabetes and rheumatic diseases. Curr Opin Rheumatol. 2009;21:50–54. doi: 10.1097/BOR.0b013e32831bc0c4. [DOI] [PubMed] [Google Scholar]
- 2.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.RES.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 3.Mishra R, Emancipator SN, Kern T, Simonson MS. High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int. 2005;67:82–93. doi: 10.1111/j.1523-1755.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 4.Rizk NN, Rafols J, Dunbar JC. Cerebral ischemia induced apoptosis and necrosis in normal and diabetic rats. Brain Res. 2005;1053:1–9. doi: 10.1016/j.brainres.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan S, Stevens M, Wiley JW. Diabetic peripheral neuropathy. Evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 2000;49:1932–1938. doi: 10.2337/diabetes.49.11.1932. [DOI] [PubMed] [Google Scholar]
- 6.Lotan R, Oron A, Anekstein Y, Shalmon E, Mirovsky Y. Lumbar stenosis and systemic diseases. Is there any relevance? J Spinal Disord Tech. 2008;21:247–251. doi: 10.1097/BSD.0b013e31813707af. [DOI] [PubMed] [Google Scholar]
- 7.Robinson D, Mirovsky Y, Halperin N, Evron Z, Nevo Z. Changes in proteoglycans of intervertebral disc in diabetic patients: a possible cause of increased back pain. Spine. 1998;23:849–855. doi: 10.1097/00007632-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ziv I, Moskowitz RW, Kraise I, Adler JH, Maroudas A. Physicochemical properties of the aging and diabetic sand rat intervertebral disc. J Orthop Res. 1992;10:205–210. doi: 10.1002/jor.1100100207. [DOI] [PubMed] [Google Scholar]
- 9.Mobbs RJ, Newcombe RL, Chandran KN. Lumbar discectomy and the diabetic patient: incidence and outcome. J Clin Neurosci. 2001;8:10–13. doi: 10.1054/jocn.2000.0682. [DOI] [PubMed] [Google Scholar]
- 10.Sakellaridis N. The influence of diabetes mellitus on lumbar intervertebral disk herniation. Surg Neurol. 2006;66:152–154. doi: 10.1016/j.surneu.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Simpson JM, Silveri CP, Balderston RA, Simeone FA, An HS. The results of operations on the lumbar spine in patients who have diabetes mellitus. J Bone Joint Surg Am. 1993;75:1823–1829. doi: 10.2106/00004623-199312000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Park JB, Lee CK, Koh JS, Lee JK, Park EY, Riew KD. Overexpressions of nerve growth factor and its tropomyosinrelated kinase A receptor on chordoma cells. Spine (Phila Pa 1976) 2007;32:1969–1973. doi: 10.1097/BRS.0b013e318133fbb5. [DOI] [PubMed] [Google Scholar]
- 13.Suhl KH, Park JB, Park EY, Rhee SK. Effect of nerve growth factor and its transforming tyrosine kinase protein and low-affinity nerve growth factor receptors on apoptosis of notochordal cells. Int Orthop. 2012;36:1747–1753. doi: 10.1007/s00264-012-1586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14:359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe HJ, Putschar WG, Vickery AL. Role of the notochord in human intervertebral disk. I. Fetus and infant. Clin Orthop Relat Res. 1965;39:205–222. [PubMed] [Google Scholar]
- 16.Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine (Phila Pa 1976) 2003;28:982–990. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- 17.Kim KW, Kim YS, Ha KY, Woo YK, Park JB, Park WS, An HS. An autocrine or paracrine Fas mediated counterattack: a potential mechanism for apoptosis of notochordal cells in intact rat nucleus pulposus. Spine (Phila Pa 1976) 2005;30:1247–1251. doi: 10.1097/01.brs.0000164256.72241.75. [DOI] [PubMed] [Google Scholar]
- 18.Won HY, Park JB, Park EY, Riew KD. Effect of hyperglycemia on apoptosis of notochordal cells and intervertebral disc degeneration in diabetic rats. J Neurosurg Spine. 2009;11:741–748. doi: 10.3171/2009.6.SPINE09198. [DOI] [PubMed] [Google Scholar]
- 19.Reichmann E. The biological role of the Fas/FasL system during tumor formation and progression. Semin Cancer Biol. 2002;12:309–315. doi: 10.1016/S1044-579X(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 20.Park JB, Kim KW, Han CW, Chang H. Expression of Fas receptor on disc cells in herniated lumbar disc tissue. Spine (Phila Pa 1976) 2001;26:142–146. doi: 10.1097/00007632-200101150-00006. [DOI] [PubMed] [Google Scholar]
- 21.Krammer PH. CD95 (APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/S0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- 22.Park JB, Lee JK, Park EY, Riew KD. Fas/FasL interaction of nucleus pulposus and cancer cells with the activation of caspases. Int Orthop. 2008;32:835–840. doi: 10.1007/s00264-007-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 24.Johnson LL, Dyer R, Hupe DJ. Matrix metalloproteinases. Curr Opin Chem Biol. 1998;2:466–471. doi: 10.1016/S1367-5931(98)80122-1. [DOI] [PubMed] [Google Scholar]
- 25.Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652–655. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 26.Crean JK, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22:2877–2884. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 27.Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113:443–453. doi: 10.1007/s004180000150. [DOI] [PubMed] [Google Scholar]
- 28.Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis of osteoarthritis. Ann Rheum Dis. 2000;59:455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JB, Kong CG, Suhl KH, Chang ED, Riew KD. The increased expression of matrix metalloproteinases associated with elastin degradation and fibrosis of the ligamentum flavum in patients with lumbar spinal stenosis. Clin Orthop Surg. 2009;1:81–89. doi: 10.4055/cios.2009.1.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]