Abstract
Purpose
The purpose of this study was to examine the effect of subperiosteal injection of chondroinductive growth factors on the histological and biomechanical outcome of autologous osteoperiosteal grafts.
Methods
Thirty six standardised osteochondral defects were created in the trochlear groove of 18 Göttinger Minipigs and evaluated after six, 12 and 52 weeks. Defects were treated with press-fit implantation of autologous osteoperiosteal cylindrical block-grafts with or without subperiosteal injection of a chondroinductive growth factor mixture (GFM).
Results
Histomorphological analysis showed complete osseointegration of all grafts from six weeks. The periosteum remained in place in 35 of 36 cases. Fibrocartilagineous repair tissue formation occurred at the cambium layer with a maximum at 12 weeks in both groups. Histomorphological grading and biomechanical testing showed highest values at 12 weeks, with signs of tissue degradation at one year. There was no significant difference between both groups.
Conclusion
Transplantation of autologous osteoperiosteal grafts is an effective method to restore subchondral bone defects, but not the overlying cartilage as the repair tissue deteriorates in the long term. Subperiosteal growth factors injection did not stimulate tissue differentiation on a biomechanical and histomorphological level.
Introduction
Surgical treatment of deep osteochondral defects is still a challenging problem. Autologous chondrocyte transplantation (ACT) was introduced in the treatment of full thickness articular cartilage defects and shows good clinical outcome after ten to 20 years [1]. Concerning deep osteochondral defects, the transplantation of autologous osteochondral grafts (mosaicplasty) has been popular but has variable clinical results [2–4]. It is the only technique to cover defects with genuine articular cartilage and to restore the cancellous bone. But donor-site morbidity may compromise the clinical outcome [2, 5, 6]. Artificial scaffolds recently have shown less favourable results for the treatment of osteochondral defect in the knee [7, 8].
The chondrogenic potential of periosteum is well documented in vitro [9] and in vivo [10] and was linked to periosteal precursor cells in the cambium layer [11]. Free autogenous periosteal grafts have been shown to restore cartilage defects [12]. Various growth factors including BMPs [13] are chondrogenic and in vitro experiments have shown that growth factors can stimulate and sustain periosteal chondrogenesis [14, 15]. Additionally, subperiosteal injection of TGF-β1 has effectively increased periosteal cartilage formation in vitro [16] and in vivo [17].
We have shown earlier that native periosteum-covered bone grafts improved bone and cartilage repair in a larger animal model [18]. We have reported on the molecular character of osteoperiosteal grafts treated with subperiosteal injection of the chondroinductive growth factor mixture [19] where Col2a1 expression was stimulated by GFM treatment after six weeks. In this paper we postulate that subperiosteal injection of chondroinductive growth factors may stimulate the histomorphological and biomechanical properties of periosteum derived articular cartilage repair tissue when autologous osteoperiosteal grafts are transplanted into critical size osteochondral defects in a large animal model.
Materials and methods
Study design and surgical procedure
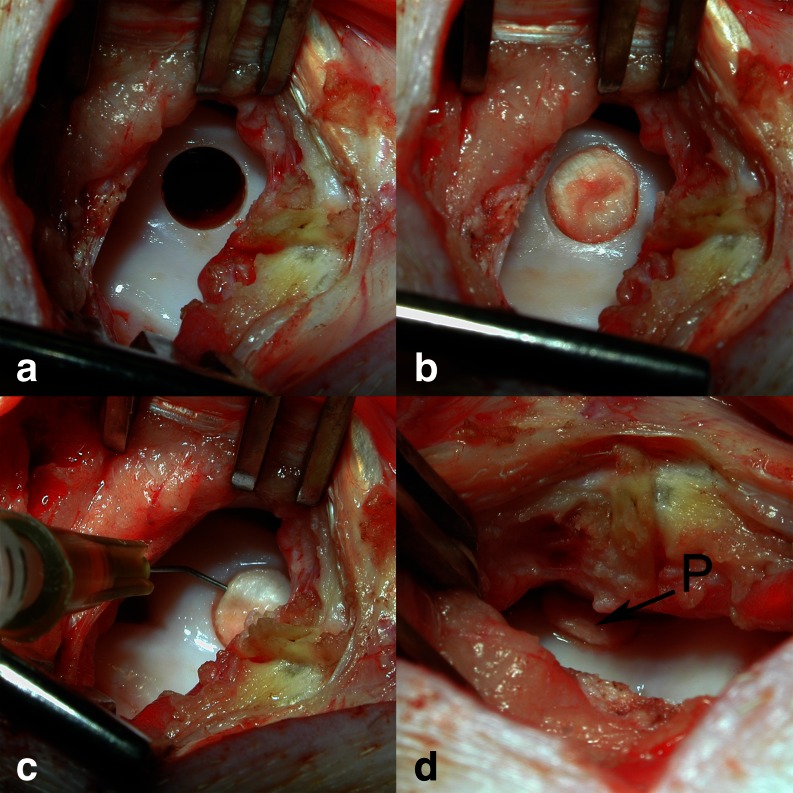
The surgical procedure was performed under general anaesthesia as described earlier [19]. A critical size osteochondral defect [20] was created in the centre of the high weight bearing medial facet of the femoral trochlear groove [21] (6.3 × 10 mm, Fig. 1a) in both knee joints of 18 skeletally mature Göttingen Minipigs (Ellegaard Minipig, Dallmose, Denmark, mean age 30 months). Each defect was treated by press-fit implantation of an autologous osteoperiosteal bone plug, harvested from the ipsilateral proximal tibia (Fig. 1b). The attached periosteum was randomly either left untreated (group P = control) or treated with subperiosteal injection when the osteoperiosteal block graft was in place (Fig. 1c) of a chondrogenic and osteogenic growth factor mixture (group P + GF = periosteum + growth factors) in order to stimulate mesenchymal derived neo-chondrogenesis and tissue differentiation. Donor-site defects in the tibia were filled with commercially available bone substitutes to prevent stress-fracture [22]. After surgery, all animals were kept in cages and allowed free exercise and weight bearing. The animals were euthanised at six (n = 6), 12 (n = 6) and 52 weeks (n = 6) follow up by intravenous injection of a barbiturate overdose (Narcobarbital, Eunarcon©, Parke-Davis, Germany). All procedures were performed according to the German animal welfare act dated 25th May 1998 and the experimental design was approved by the animal rights protection authorities in Baden-Württemberg, Germany (AZ.: 35-9185.81/148/00).
Fig. 1.
Operative procedure of creating standardised osteochondral defect in the medial facet of the trochlear groove. a Osteochondral defect of exact width (6.3 mm) and depth (10 mm) prior to treatment. b Perfectly implanted autologous osteoperiosteal graft; the periosteum was flush with the surrounding cartilage. c Application of approximately 125 μl of the liquid growth factor mixture (GFM) into the periosteum bone interface, in order to stimulate tissue differentiation. d The growth factor augmented periosteum (P)
Protein mixture/growth factors
A highly purified growth factor mixture (GFM) extracted from dematerialised bovine bone [23] was provided by Centerpulse Biologics (Winterthur, Switzerland). GFM contains proteins of the BMP superfamily such as BMP 2, 3, 4, 6, 7, TGF-β1, -2, -3, aFGF-1, osteocalcin and osteonectin [23, 24]. Cell culture experiments have proven a dose-dependent chondrogenic induction potential on a murine stem cell line [25] and collagen 2a1 expression in bone marrow derived stem cells without molecular signs of chondrocyte hypertrophy [19]. The freeze-dried powder of the GFM was dissolved in 2 mM HCl to a final concentration of 0.5 mg/ml. Aliquots of 300 μl were stored at 4 °C until use. A fine, angulated needle (Microlance© 3, 26 G5/8; Becton Dickinson, Germany) and a 1 ml syringe (Plastipack©, Becton Dickinson, Germany) were used to inject a total volume of 125 μl of the growth factor mixture into the periosteum (Fig. 1c). As a result the periosteum was augmented (Fig. 1d). Knees were flexed several times before closing the wound in layers.
Tissue processing, biomechanical testing and histology
After necropsy, both limbs were separated and the knee joint capsule was opened for digital imaging of the defect area under moist conditions (NaCl 0,9 %, Braun, Melsungen, Germany). To assess the biomechanical quality of the repair tissue, a non-destructive indentation test was applied as described elsewhere [26]. After biomechanical assessment, the defect area was sawn into two pieces, whereby one piece contained 60 % of the total defect area and was fixed in 4 % buffered formalin (pH = 7.4) for 48 hours. The remaining 40 % of each sample was frozen in liquid nitrogen and stored at –80 °C for detailed molecular characterisation. These results were published elsewhere [19]. Specimens were decalcified (0.5 M EDTA), embedded in paraffin and sectioned (5–7 μm) in the sagittal plane to include the centre of the defect and the adjacent cartilage. Sections were stained with toluidin-blue and safranin O/fast green. Qualitative histomorphological evaluation and semi-quantitative scoring of the repair tissue was carried out by a blinded observer according to the ICRS II histological scoring system [27].
Statistical analysis
The descriptive analyses include the percent data and the arithmetic mean ± the standard deviation (AM ± SD) for the interval scale level variables. The question of whether there is a significant difference between the two outcome groups was examined in a second step by bivariate chi-square tests (nominal and ordinal variables) and ANOVA (metric variables) was used to calculate significance. The tests were carried out as two-sided tests with significance limit p < 0.05 without alpha-adjustment.
Results
There were no wound complications and all animals could be euthanised as planned. Donor-site defects at the medial aspect of the proximal tibia did not show any morbidity related to graft harvesting [22]. All knees were free of any signs of inflammation, infection, synovial adhesions or arthrofibrosis. Macroscopic evaluation revealed (six weeks) periosteal flap loosening (group P) in one animal; thus in total, 35 defects were evaluated. Gross appearance of the defect side revealed that four of the evaluated 35 cases were not transplanted level with the surrounding subchondral bone plate. In these cases we found severe disruption and ulceration of the retro patellar cartilage. No graft hypertrophy was seen.
Biomechanical testing
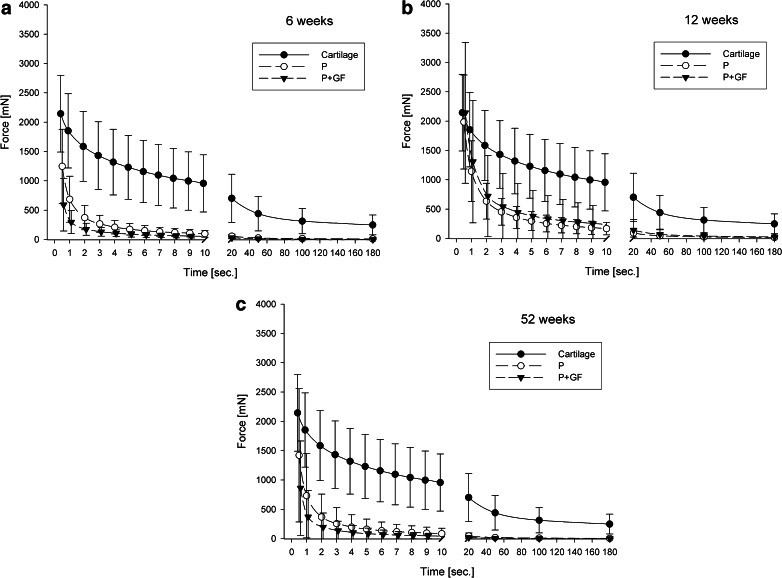
Both groups (P and P+GF) showed a much weaker compressive stiffness compared to healthy articular cartilage at six, 12 and 52 weeks follow up (Fig. 2a–c). Although the maximal axial compressive stiffness of both groups was seen at 12 weeks after surgery (Fig. 2b), the repair tissue became softer at one-year follow up (Fig. 2c). Growth factor treatment did not improve the biomechanical properties of the repair tissue at any time point.
Fig. 2.
Axial reaction forces as a function of time after stress-relaxation testing at 6 (a), 12 (b) and 52 (c) weeks follow up. All data are shown as mean ± standard deviation. First values represent maximal axial reaction force after imposing a displacement of 20 % of total tissue thickness with a given speed of 50 mm/min. The following values represent the time history of the mean values during the first 10 and at 20, 50, 100, 180 s
Semi-quantitative histomorphological grading
Total score and individual parameter values for both groups are summarised in Table 1. At 6, 12, and 52 weeks mean score values were similar in both groups. Compared to six-weeks follow-up mean total score values increased at 12 weeks, indicating repair tissue maturation. At 52-weeks follow up mean overall score values declined to values comparable at six weeks follow up in both groups. Lowest values in the subscore “surface architecture” and “matrix staining” were reached at 52 weeks, indicating tissue degeneration by surface fibrillation and loss of glycosaminoglycans of the extracellular matrix in both treatment groups. There were no statistically significant differences between both groups and any time.
Table 1.
Histological scoring of repair tissue at 6, 12 and 52 weeks follow up. According to the ICRS II scoring system [27]. All values are given as the mean and standard deviation for each parameter
| Score parameter | 6 weeks | 12 weeks | 52 weeks | |||||
|---|---|---|---|---|---|---|---|---|
| P | P + GF | P | P + GF | P | P + GF | |||
| 1 | Tissue morphology | 0–100 | 12.6 ± 10.1 | 7.2 ± 3.8 | 9.2 ± 6.1 | 15.5 ± 18.9 | 1.7 ± 1.4 | 2.7 ± 3.9 |
| 2 | Matrix staining | 0–100 | 9.2 ± 7.5 | 7.7 ± 6.9 | 11.3 ± 10.6 | 14.0 ± 14.5 | 3.5 ± 1.9 | 2.3 ± 3.2 |
| 3 | Cell morphology | 0–100 | 36.2 ± 27.9 | 17.7 ± 8.8 | 32.8 ± 19.0 | 35.0 ± 34.2 | 45.2 ± 24.7 | 34.2 ± 19.9 |
| 4 | Chondrocyte Clustering | 0–100 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| 5 | Surface architecture | 0–100 | 65.8 ± 25.4 | 82.5 ± 25.4 | 94.5 ± 4.2 | 77.7 ± 17.1 | 65.3 ± 16.3 | 60.0 ± 38.1 |
| 6 | Basal integration | 0–100 | 95.6 ± 9.8 | 90.5 ± 21.4 | 99.5 ± 1.2 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| 7 | Formation of a tidemark | 0–100 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 20.0 ± 36.3 | 5.0 ± 12.2 |
| 8 | Subchondral bone abnormalities | 0–100 | 77.0 ± 11.6 | 78.3 ± 14.6 | 94.7 ± 6.1 | 97.8 ± 3.5 | 92.5 ± 6.1 | 96.7 ± 8.2 |
| 9 | Inflammation | 0–100 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| 10 | Abnormal calcification/ossification | 0–100 | 100 ± 0.0 | 100 ± 0.0 | 86.7 ± 20.8 | 91.3 ± 16.8 | 97.5 ± 6.1 | 89.2 ± 13.6 |
| 11 | Vascularisation | 0–100 | 90.2 ± 13.4 | 85.2 ± 10.0 | 74.8 ± 25.5 | 76.0 ± 26.9 | 95.8 ± 6.6 | 96.7 ± 5.2 |
| 12 | Superficial assessment | 0–100 | 28.2 ± 14.6 | 43.0 ± 15.8 | 38.3 ± 17.3 | 28.3 ± 23.8 | 25.8 ± 12.0 | 25.3 ± 17.3 |
| 13 | Mid/Deep zone assessment | 0–100 | 29.4 ± 20.9 | 20.2 ± 12.2 | 39.0 ± 18.8 | 30.8 ± 33.6 | 19.2 ± 11.1 | 21.3 ± 5.0 |
| 14 | Overall assessment | 0–100 | 29.0 ± 15.0 | 22.0 ± 13.6 | 33.2 ± 14.6 | 29.3 ± 28.6 | 16.7 ± 7.5 | 20.5 ± 10.5 |
| Total | Mean | 0–100 | 55.2 ± 5.7 | 53.9 ± 5.5 | 58.1 ± 8.2 | 56.8 ± 11.2 | 55.9 ± 6.9 | 53.8 ± 5.6 |
P control group, P + GF periosteum + growth factors group
Qualitative histomorphological evaluation
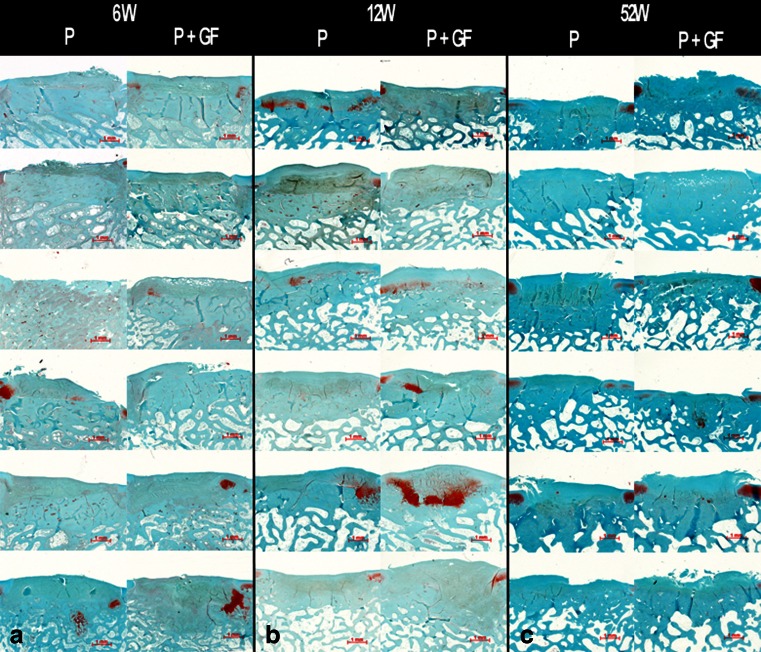
From six weeks on all defects were completely filled with repair tissue (Fig. 3a) and the graft with periosteum was easy to distinguish. The periosteal repair tissue was integrated on both sides to the adjacent cartilage and the surface of the graft was smooth and regular. The character of the repair tissue was immature with mainly fibrous and some fibrocartilaginous tissue. In the cambium layer few signs of neo-chondrogenesis were evident. By 12 weeks, complete filling of the cartilage defect with repair tissue was found in both groups. Compared to six weeks, the repair tissue maturated with more intense neo-chondrogenesis in both groups (Fig. 3b). If present, randomly scattered chondrocytes were found in well defined lacunae surrounded by a very intensively stained, glycosaminoglycan rich extracellular matrix (Fig. 4). By 52 weeks, the architecture of the surface started to exhibit signs of fibrillation and became irregular (Fig. 3c). The amount of neo-chondrogenesis at the cambium layer decreased in both groups similarly. The predominant character of the tissue was fibrocartilaginous with a loss of safranin O positive stain intensity, indicating a decrease of the glycosaminoglycan content of the extracellular matrix. No hypertrophy of repair tissue was evident throughout evaluation.
Fig. 3.
Histological sections of all specimens at 6 (a), 12 (b) and 52 (c) weeks. Safranin O/Fast green; original magnification 20-fold. Left column untreated control group (P). Right column growth factor treated group (periosteum + growth factors)
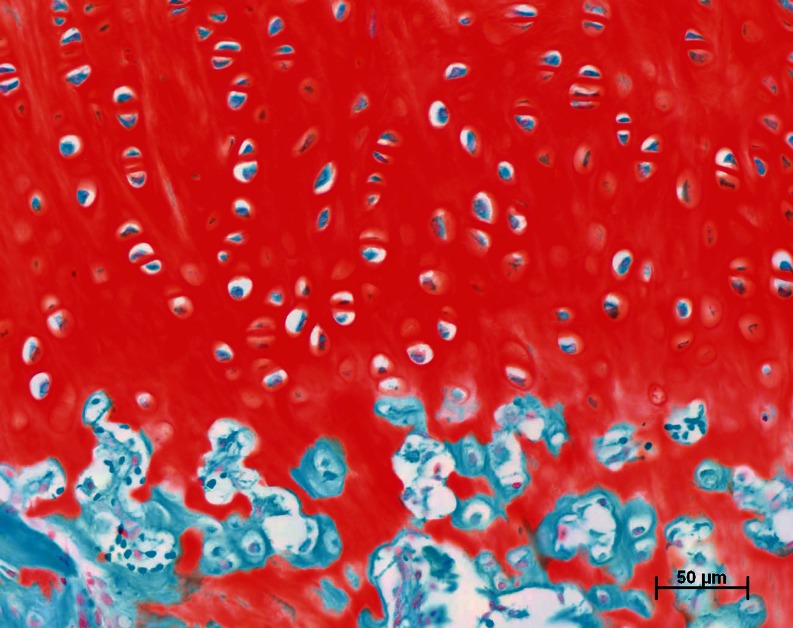
Fig. 4.
Detail of Fig. 3b with highest amount of periosteal neo-chondrogenesis 12 weeks after growth factor treatment (periosteum + growth factors). Safranin O/fast green; original magnification × 200-fold
Osseous integration of the corticocancellous bone plug started at six weeks with microcallus formation and enchondral ossification at both upper edges of the transplant (Fig. 3a). Contact bone healing occurred with a complete restoration of the bony defect. From 12 weeks on, the subchondral bone plate of the osteoperiosteal graft was remodelled into a more trabecular pattern. No osteolysis or cystic lesions were observed in either group and the graft was completely integrated in the native trabecular bone (Fig. 3b). After 12 and 52 weeks one to three defects in both groups showed signs of ossification but limited to the juxtacortical repair tissue with no hypertrophic bone formation. The subchondral bone showed no pathological changes at one year (Fig 3c).
Discussion
Histological data of various animal experiments have demonstrated that the intrinsic biological capacity to restore osteochondral defects extending deep into the subchondral bone is limited [18, 28]. Even if macroscopic coverage of the defect with fibrocartilaginous repair tissue was attained, cross-sectional histology revealed severe disruption of the subchondral bone integrity with formation of intraosseous cystic lesions. Additionally, biomechanical overload was present at the border of such osteochondral defects and promoted a degenerative osteoarthritic process at the adjacent articular cartilage [28, 29]. It is not surprising that there is concern among clinicians about the fate of deep osteochondral defects. The only protocol to treat such defects with genuine articular cartilage in one procedure is the transplantation of autologous osteochondral grafts. Applying this technique implies the creation of donor-site defects, which can be responsible for new clinical symptoms, even if the donor-site is assumed to be minor weight bearing [30]. Other techniques include cancellous bone grafts supplemented by either periosteum [12] or autologous bone graft with chondrocyte implantation [31].
Osteoperiosteal transplants for the restoration of the joint surface
Histo-anatomically, periosteum consists of two different layers, termed the stratum fibrosum and stratum osteogenicum also known as the cambium layer. In vitro experiments have shown the importance of the cambium layer for neo-chondrogenesis and linked this ability to the presence of mesenchymal progenitor cells [11]. Mechanical, e.g. CPM [32], and biochemical stimuli, e.g. growth factors of the TGF-beta superfamily [14], were able to support neochondrogenesis in periosteum [9]. Additionally, subperiosteal injection of TGF-β1 has effectively increased cellularity and periosteal cartilage formation in vitro [16] and enhanced the cartilage repair tissue quality in a rabbit model [17]. Our study has shown that transplantation of autologous periosteum covered bone plugs into the mechanical environment of a joint provides complete restoration of the cancellous bone defect but only a temporary restoration of the joint surface. The attached periosteum on top of the graft was not delaminated during joint motion and remained in place up to one year postoperatively. By transplanting osteoperiosteal plugs, the integrity of the subchondral cancellous bone was restored up to the original tidemark and no cystic lesions could be found at any time. These findings are contrary to those reported by van Susante et al. [33]. They found that autologous osteoperiosteal grafts did not incorporate into osteochondral defects (medial femoral condyle) in a goat model with osteoclastic resorption host bone collapse. The authors concluded that restoration of the joint surface by osteoperiosteal grafts was insufficient. An explanation for these poor results may be the use of a different operation technique. Van Susante et al. [33] used sharp chisels for donor and recipient site preparation. Such instruments destroy the micro-architecture of the trabecular graft bone and cannot ensure contact bone healing. By way of contrast, we used diamond-coated instruments allowing highly precise bone preparation, preserving the trabecular architecture with a press-fit of 0.1 mm [34]. Our results underline that this technique allows contact bone healing with full osseous integration of osteoperiosteal grafts.
Intraperiosteal neo-chondrogenesis with some cartilagineous repair tissue formation took place at the level of the cambium layer in either group. It became visible from six weeks onwards and reached its peak at 12 weeks follow up. In any event, the overall character of the periosteal repair tissue was fibrocartilaginous. It is well known that fibrocartilage is incapable of withstanding mechanical load during joint function and may undergo degenerative change over time. We found a noticeable degree of surface fibrillation in both groups after 52 weeks combined with a loss of glycosaminoglycan content. At the same time mechanical stiffness of the repair tissue decreased after reaching a peak at 12 weeks follow up in both groups. We conclude that even though complete restoration of the joint surface with fibrous or fibrocartilagineous repair tissue was present up to one year, the repair tissue induced by osteoperiosteal grafting is likely to deteriorate and disrupt over time.
Growth factor treatment
The concept of injecting substances into and underneath periosteum physiologically attached to its host bone is a new approach to influence tissue differentiation after osteoperiosteal grafting. The effect of growth factor treatment on cell and tissue differentiation is dose and time dependent. The applied technique of single dose injection could only provide a transient local growth factor concentration. Miura et al. could see a stimulatory effect on chondrogenesis in periosteal explants even after short exposure to TGF-ß1 [14]. Additionally, single dose subperiosteal injection of TGF-β1 has effectively increased cellularity and periosteal cartilage formation in vitro [16] and increased osteochondral repair tissue quality in vivo after subperiosteal injection seven days prior to surgery in a rabbit model [17]. On the other hand it is known that TGF-ß1 might induce a hypertrophic chondrocyte phenotype ex vivo [35] and also might induce arthrofibrosis [36]. In earlier published data looking at the molecular aspects of the GFM treatment we were able to show that hypertrophic markers were found equally in both treatment groups after 52 weeks, indicating no negative effect of GFM on cell hypertrophy in this setting [19].
We used a growth factor mixture that contained proteins of the BMP and TGF-β superfamily [23, 24]. Its dose-dependent effect on cartilage formation [19, 25] has already been experimentally shown. A limited amount of neo-chondrogenesis was evident in both groups reaching its peak at 12 weeks with no effect of growth factor treatment on the histomorphological and biomechanical tissue characteristics. The time duration of the presence of the growth factors in our setup might play a relevant role in the stimulating effect on neochondrogenesis. A short exposure with a single shot bolus was not sufficient to effectively induce and sustain chondrogenic differentiation of the periosteal precursor cells. Earlier studies have shown that the chondrogenic effect is dependent on the substance type, the amount and time interval between injection and tissue harvest [16]. This indicates that a more effective stimulus on neo-chondrogenesis in periosteum may be reached by using recombinant growth factors such as TGF-β1 or OP-1 [14, 37].
In summary, this study represents the first investigation to evaluate the effects of intraperiosteal injection of growth factors on tissue differentiation in autologous osteoperiosteal grafting at the time of surgery. Our results demonstrate the feasibility of such a procedure, but do not show a positive effect of GFM on the intrinsic capacity of periosteum for neo-chondrogenesis. Clinical use of native autologous osteoperiosteal grafting has been reported for the filling of donor-site defects after mosaicplasty or for primary treatment of osteochondral defects [12]. Artificial “off-the-shelf” implants, recently showed a catastrophic clinical outcome with a revision rate up to 70 % [7, 8]. Our data support the use of autologous protocols as autologous periosteum-bone grafts can provide a complete restoration of the subchondral cancellous bone defect. The cartilage repair tissue is only of an inferior fibrocartilaginous character and will deteriorate in the long term. Therefore the use of autologous osteoperiosteal grafts for the restoration of chondral defects cannot be recommended.
Acknowledgments
This work was funded by Centerpulse Biologics, Winterthur, Switzerland and the Research Fund of the Orthopaedic Department of the University of Heidelberg. The authors thank Dr. vet. H. Lorenz, R. Föhr, K. Goetzke, and M. Daniels for their excellent work and J.-F. Clémence for his support during the experimental procedures.
Footnotes
This work was supported by Centerpulse Biologics, Inc., Winterthur, Switzerland.
Contributor Information
Tobias Gotterbarm, Email: Tobias.Gotterbarm@med.uni-heidelberg.de.
Steffen J. Breusch, Email: steffen.breusch@gmail.com
Simona Berardi Vilei, Email: sberardivilei@bluewin.ch.
Pierre Mainil-Varlet, Email: pm@aginko.com.
Wiltrud Richter, Email: Wiltrud.Richter@med.uni-heidelberg.de.
Martin Jung, Phone: +49-89-206082320, FAX: +49-89-206082333, Email: martin.jung@ocm-muenchen.de.
References
- 1.Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 2.Hangody L, Vasarhelyi G, Hangody LR, Sukosd Z, Tibay G, Bartha L, Bodo G. Autologous osteochondral grafting–technique and long-term results. Injury. 2008;39(Suppl 1):S32–S39. doi: 10.1016/j.injury.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94:504–509. doi: 10.1302/0301-620X.94B4.27495. [DOI] [PubMed] [Google Scholar]
- 4.Krych AJ, Harnly HW, Rodeo SA, Williams RJ., 3rd Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94:971–978. doi: 10.2106/JBJS.K.00815. [DOI] [PubMed] [Google Scholar]
- 5.Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 6.Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: a prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Joshi N, Reverte-Vinaixa M, Diaz-Ferreiro EW, Dominguez-Oronoz R. Synthetic resorbable scaffolds for the treatment of isolated patellofemoral cartilage defects in young patients: magnetic resonance imaging and clinical evaluation. Am J Sports Med. 2012;40:1289–1295. doi: 10.1177/0363546512441585. [DOI] [PubMed] [Google Scholar]
- 8.Dhollander AA, Liekens K, Almqvist KF, Verdonk R, Lambrecht S, Elewaut D, Verbruggen G, Verdonk PC. A pilot study of the use of an osteochondral scaffold plug for cartilage repair in the knee and how to deal with early clinical failures. Arthroscopy. 2012;28:225–233. doi: 10.1016/j.arthro.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Tarng YW, Huang BF, Su FC. A novel recirculating flow-perfusion bioreactor for periosteal chondrogenesis. Int Orthop. 2012;36:863–868. doi: 10.1007/s00264-011-1291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Driscoll SW, Fitzsimmons JS (2001) The role of periosteum in cartilage repair. Clin Orthop:S190–207 [DOI] [PubMed]
- 11.Ito Y, Fitzsimmons JS, Sanyal A, Mello MA, Mukherjee N, O’Driscoll SW. Localization of chondrocyte precursors in periosteum. Osteoarthr Cartil. 2001;9:215–223. doi: 10.1053/joca.2000.0378. [DOI] [PubMed] [Google Scholar]
- 12.Korkala O, Kuokkanen H. Autogenous osteoperiosteal grafts in the reconstruction of full-thickness joint surface defects. Int Orthop. 1991;15:233–237. doi: 10.1007/BF00192300. [DOI] [PubMed] [Google Scholar]
- 13.Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S. Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop. 2002;26:131–136. doi: 10.1007/s00264-002-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura Y, Parvizi J, Fitzsimmons JS, O’Driscoll SW. Brief exposure to high-dose transforming growth factor-beta1 enhances periosteal chondrogenesis in vitro: a preliminary report. J Bone Joint Surg Am. 2002;84-A:793–799. doi: 10.2106/00004623-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, O’Driscoll SW. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthr Cartil. 2003;11:55–64. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- 16.Reinholz GG, Fitzsimmons JS, Casper ME, Ruesink TJ, Chung HW, Schagemann JC, O’Driscoll SW. Rejuvenation of periosteal chondrogenesis using local growth factor injection. Osteoarthr Cartil. 2009;17:723–734. doi: 10.1016/j.joca.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivos-Meza A, Fitzsimmons JS, Casper ME, Chen Q, An KN, Ruesink TJ, O’Driscoll SW, Reinholz GG. Pretreatment of periosteum with TGF-beta1 in situ enhances the quality of osteochondral tissue regenerated from transplanted periosteal grafts in adult rabbits. Osteoarthr Cartil. 2010;18:1183–1191. doi: 10.1016/j.joca.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotterbarm T, Reitzel T, Schneider U, Voss HJ, Stofft E, Breusch SJ. Integration of periosteum covered autogenous bone grafts with and without autologous chondrocytes. An animal experiment using the Gottinger minipig. Orthopäde. 2003;32:65–73. doi: 10.1007/s00132-002-0396-8. [DOI] [PubMed] [Google Scholar]
- 19.Jung M, Gotterbarm T, Gruettgen A, Vilei SB, Breusch S, Richter W. Molecular characterization of spontaneous and growth-factor-augmented chondrogenesis in periosteum-bone tissue transferred into a joint. Histochem Cell Biol. 2005;123:447–456. doi: 10.1007/s00418-005-0775-4. [DOI] [PubMed] [Google Scholar]
- 20.Gotterbarm T, Breusch SJ, Schneider U, Jung M. The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: retrospective analysis of 180 defects. Lab Anim. 2008;42:71–82. doi: 10.1258/la.2007.06029e. [DOI] [PubMed] [Google Scholar]
- 21.Jung M, Breusch S, Daecke W, Gotterbarm T. The effect of defect localization on spontaneous repair of osteochondral defects in a Gottingen minipig model: a retrospective analysis of the medial patellar groove versus the medial femoral condyle. Lab Anim. 2009;43:191–197. doi: 10.1258/la.2008.007149. [DOI] [PubMed] [Google Scholar]
- 22.Spies CK, Schnurer S, Gotterbarm T, Breusch S. The efficacy of Biobon and Ostim within metaphyseal defects using the Gottinger Minipig. Arch Orthop Trauma Surg. 2009;129:979–988. doi: 10.1007/s00402-008-0705-8. [DOI] [PubMed] [Google Scholar]
- 23.Boden SD, Grob D, Damien C. Ne-Osteo bone growth factor for posterolateral lumbar spine fusion: results from a nonhuman primate study and a prospective human clinical pilot study. Spine. 2004;29:504–514. doi: 10.1097/01.BRS.0000101446.26071.EB. [DOI] [PubMed] [Google Scholar]
- 24.Damien CJ, Grob D, Boden SD, Benedict JJ. Purified bovine BMP extract and collagen for spine arthrodesis: preclinical safety and efficacy. Spine. 2002;27:S50–S58. doi: 10.1097/00007632-200208151-00012. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson BL, Fantle KS, Benedict JJ, Huffer WE, Gutierrez-Hartmann A. Combination of osteoinductive bone proteins differentiates mesenchymal C3H/10 T1/2 cells specifically to the cartilage lineage. J Cell Biochem. 1997;65:325–339. doi: 10.1002/(SICI)1097-4644(19970601)65:3<325::AID-JCB3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 26.Gotterbarm T, Richter W, Jung M, Berardi Vilei S, Mainil-Varlet P, Yamashita T, Breusch SJ. An in vivo study of a growth-factor enhanced, cell free, two-layered collagen-tricalcium phosphate in deep osteochondral defects. Biomaterials. 2006;27:3387–3395. doi: 10.1016/j.biomaterials.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38:880–890. doi: 10.1177/0363546509359068. [DOI] [PubMed] [Google Scholar]
- 28.Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J Bone Joint Surg Am. 2001;83-A:53–64. doi: 10.2106/00004623-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Brown TD, Pope DF, Hale JE, Buckwalter JA, Brand RA. Effects of osteochondral defect size on cartilage contact stress. J Orthop Res. 1991;9:559–567. doi: 10.1002/jor.1100090412. [DOI] [PubMed] [Google Scholar]
- 30.Feczko P, Hangody L, Varga J, Bartha L, Dioszegi Z, Bodo G, Kendik Z, Modis L. Experimental results of donor site filling for autologous osteochondral mosaicplasty. Arthroscopy. 2003;19:755–761. doi: 10.1016/S0749-8063(03)00402-X. [DOI] [PubMed] [Google Scholar]
- 31.Steinhagen J, Bruns J, Deuretzbacher G, Ruether W, Fuerst M, Niggemeyer O. Treatment of osteochondritis dissecans of the femoral condyle with autologous bone grafts and matrix-supported autologous chondrocytes. Int Orthop. 2010;34:819–825. doi: 10.1007/s00264-009-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Driscoll SW, Salter RB. The induction of neochondrogenesis in free intra-articular periosteal autografts under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1984;66:1248–1257. [PubMed] [Google Scholar]
- 33.van Susante JL, Wymenga AB, Buma P. Potential healing benefit of an osteoperiosteal bone plug from the proximal tibia on a mosaicplasty donor-site defect in the knee. An experimental study in the goat. Arch Orthop Trauma Surg. 2003;123:466–470. doi: 10.1007/s00402-003-0577-x. [DOI] [PubMed] [Google Scholar]
- 34.Draenert K, Draenert Y. A new procedure for bone biopsies and cartilage and bone transplantation. Sandorama. 1988;III–IV:33–40. [Google Scholar]
- 35.Narcisi R, Quarto R, Ulivi V, Muraglia A, Molfetta L, Giannoni P. TGF beta-1 administration during ex vivo expansion of human articular chondrocytes in a serum-free medium redirects the cell phenotype toward hypertrophy. J Cell Physiol. 2012;227:3282–3290. doi: 10.1002/jcp.24024. [DOI] [PubMed] [Google Scholar]
- 36.Watson RS, Gouze E, Levings PP, Bush ML, Kay JD, Jorgensen MS, Dacanay EA, Reith JW, Wright TW, Ghivizzani SC. Gene delivery of TGF-beta1 induces arthrofibrosis and chondrometaplasia of synovium in vivo. Lab Invest. 2010;90:1615–1627. doi: 10.1038/labinvest.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruber R, Mayer C, Bobacz K, Krauth MT, Graninger W, Luyten FP, Erlacher L. Effects of cartilage-derived morphogenetic proteins and osteogenic protein-1 on osteochondrogenic differentiation of periosteum-derived cells. Endocrinology. 2001;142:2087–2094. doi: 10.1210/en.142.5.2087. [DOI] [PubMed] [Google Scholar]