Abstract
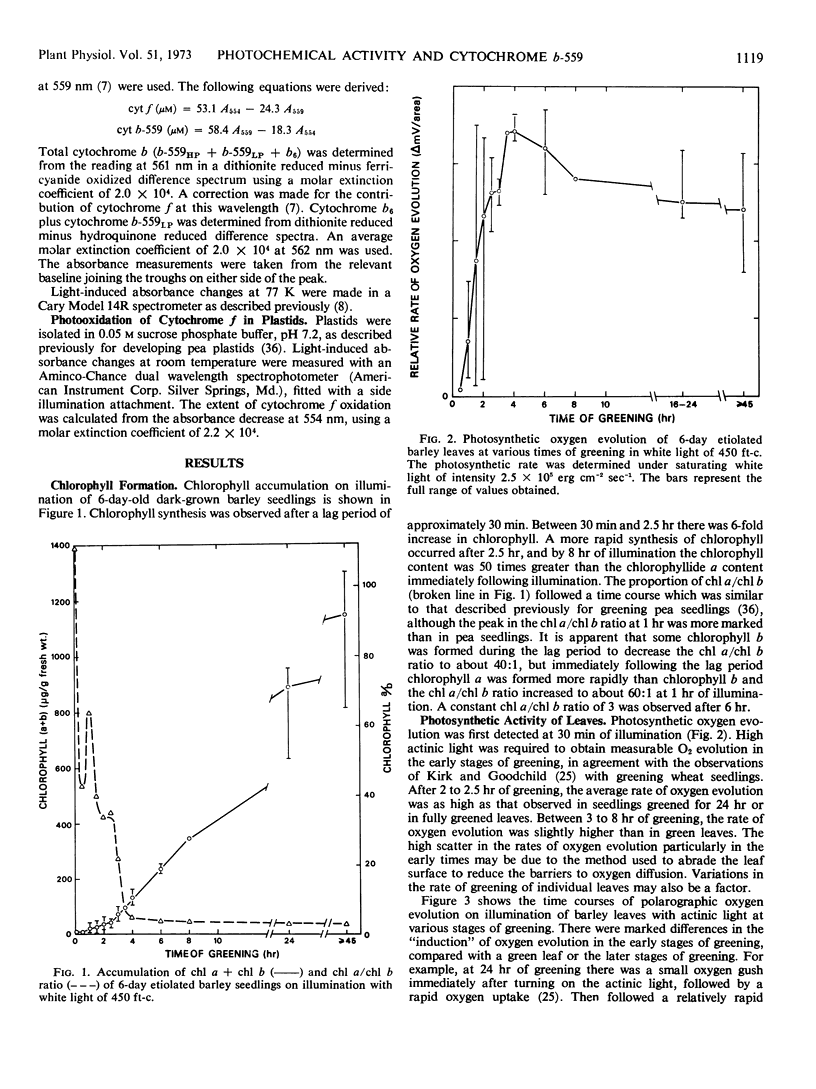
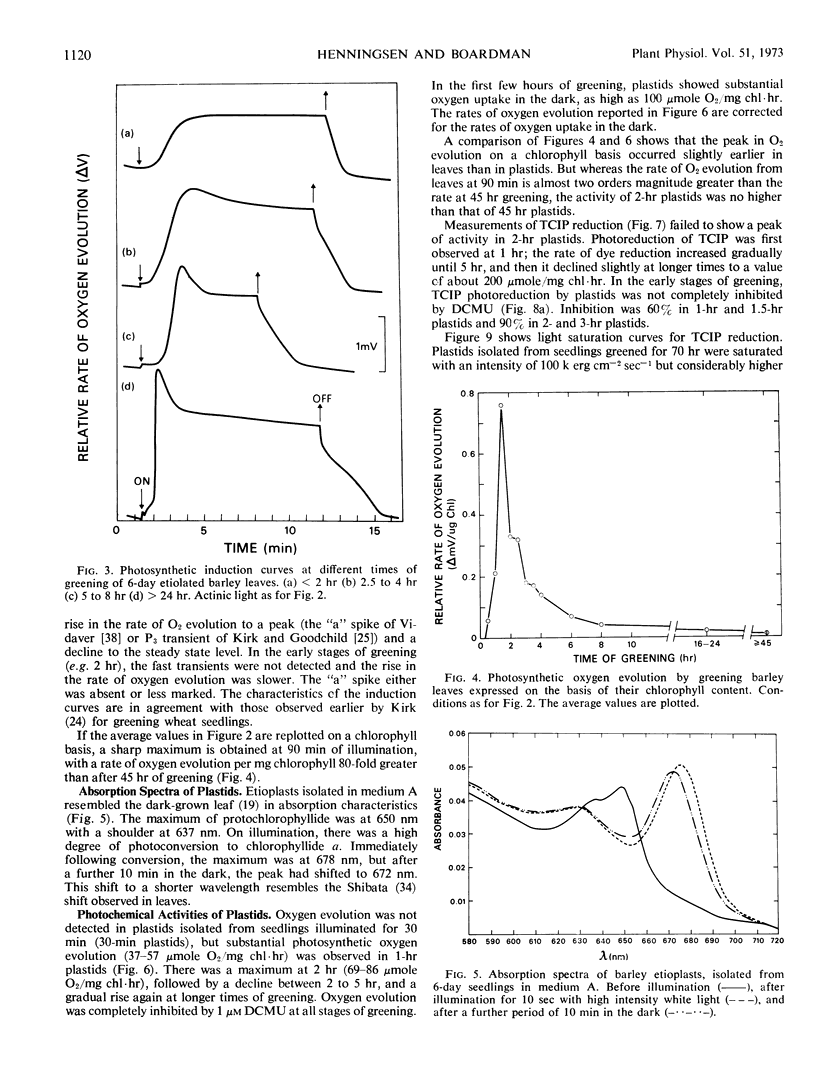
The development of photochemical activity during the greening of dark-grown barley seedlings (Hordeum vulgare L. cv. Svalöfs Bonus) was studied in relation to the formation of the high potential form of cytochrome b-559 (cytochrome b-559HP). Photosynthetic oxygen evolution from leaves was detected at 30 minutes of illumination. The rate of oxygen evolution per gram fresh weight of leaf was as high at 2 to 2.5 hours of greening as at 24 hours or in fully greened leaves. On a chlorophyll basis, the photosynthetic rate at 90 minutes of greening was 80-fold greater than the rate at 45 hours. It is concluded that the majority of photosynthetic units are functional at an early stage of greening, and that chlorophyll synthesis during greening serves to increase the size of the units.
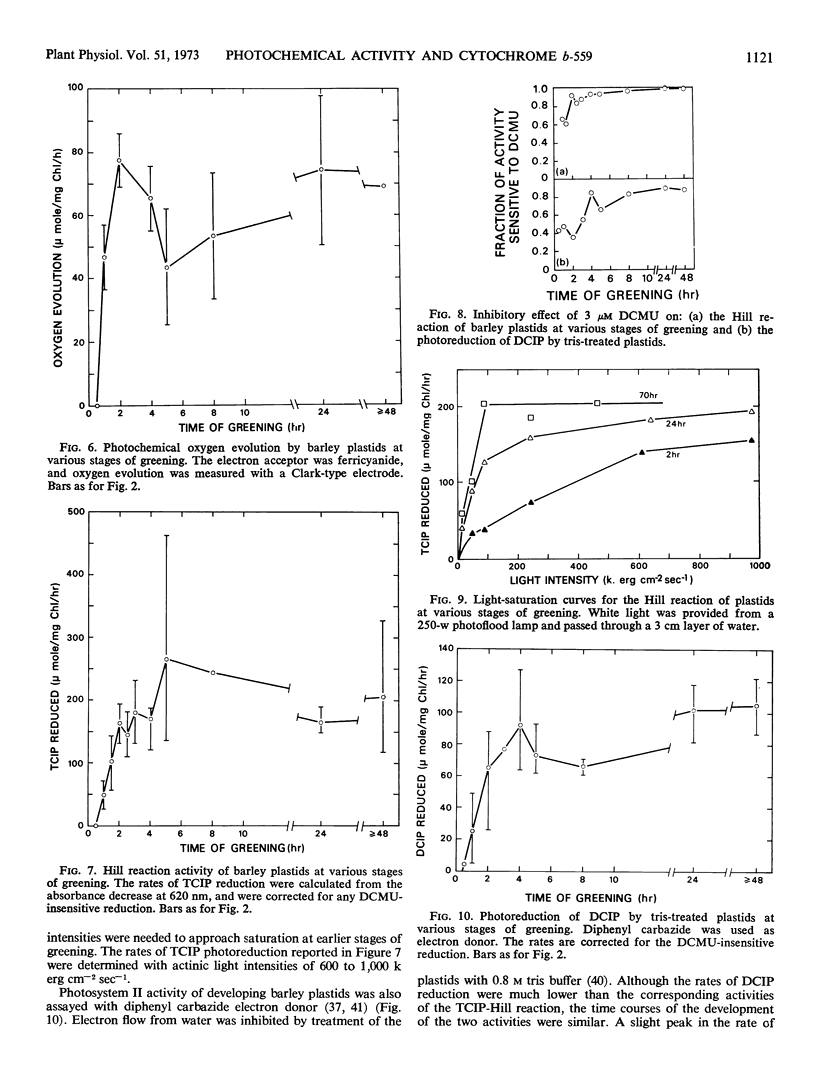
Plastids showed substantial photochemical oxygen evolution after a seedling greening time of 1 hour. However, a comparison of the relative activity of leaves and plastids at 2 hours and 24 hours of greening suggests that there was some inactivation of greening plastids during isolation. Appreciable photosystem I activity was observed as early as 15 minutes of greening.
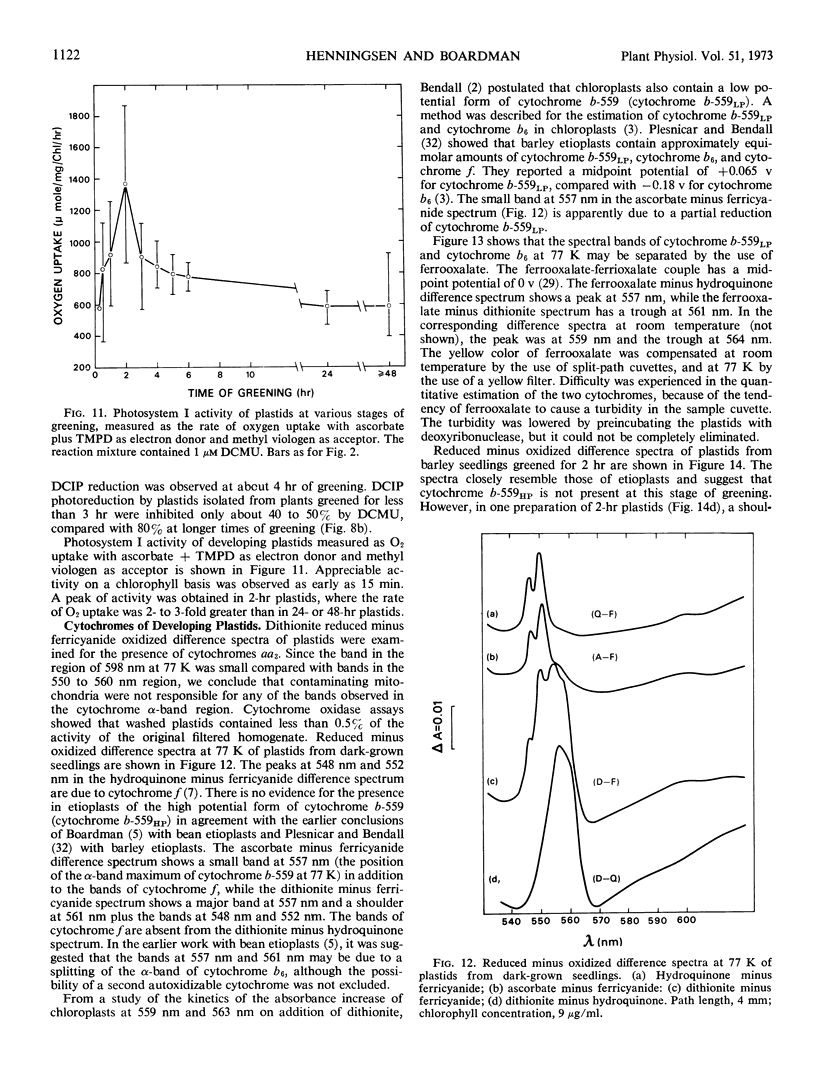
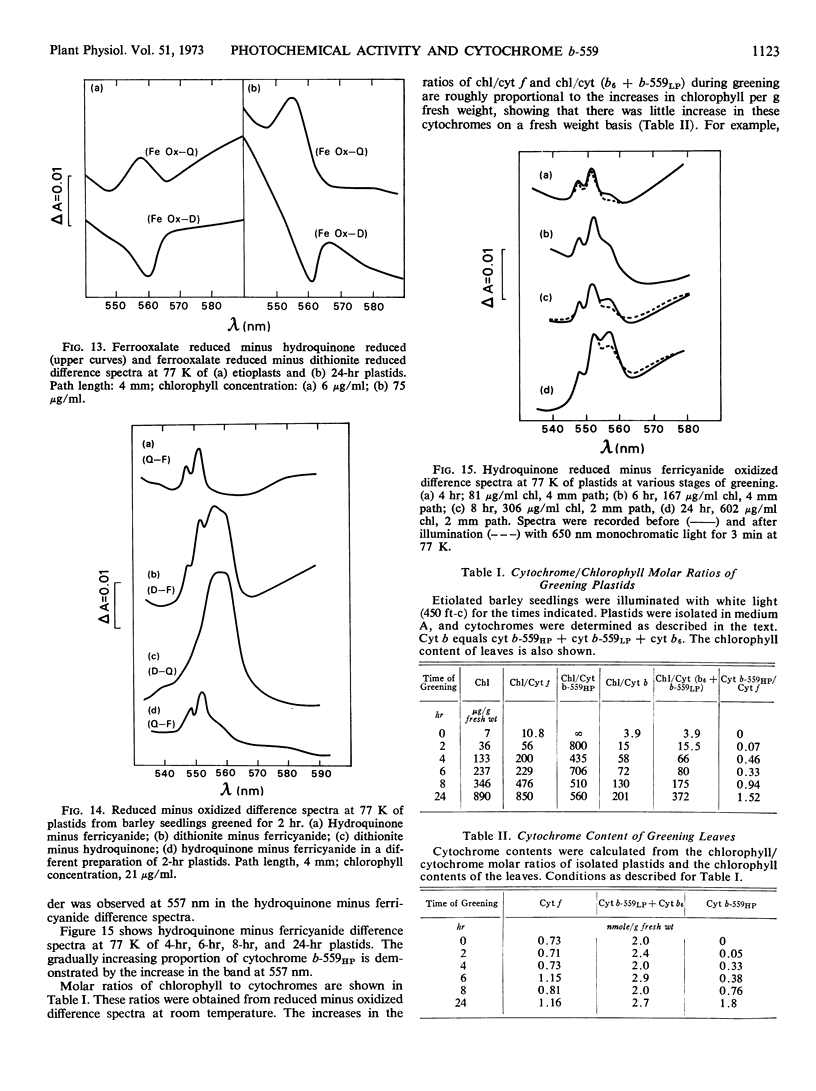
The synthesis of cytochrome b-559HP during greening does not correlate with the onset of oxygen evolution. Cytochrome b-559HP was absent from etioplasts and in most preparations of 2-hour plastids. The average amount of cytochrome b-559HP at 2 hours of greening was well below the level needed to provide 1 molecule of the carrier for each functional photosynthetic chain. The results suggest that cytochrome b-559HP is not essential for oxygen evolution. Cytochrome f, cytochrome b6, and the low potential form of cytochrome b-559 were present in the etioplast. There was little increase in the levels of these cytochromes during 24 hours of greening.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Bendall D. S. Oxidation-reduction potentials of cytochromes in chloroplasts from higher plants. Biochem J. 1968 Sep;109(3):46P–47P. doi: 10.1042/bj1090046pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall D. S., Sofrová D. Reactions at 77 degrees K in photosystem 2 of green plants. Biochim Biophys Acta. 1971 Jun 15;234(3):371–380. doi: 10.1016/0005-2728(71)90204-0. [DOI] [PubMed] [Google Scholar]
- Boardman N. K., Anderson J. M. Fractionation of the photochemical systems of photosynthesis. II. Cytochrome and carotenoid contents of particles isolated from spinach chloroplasts. Biochim Biophys Acta. 1967 Jul 5;143(1):187–203. doi: 10.1016/0005-2728(67)90120-x. [DOI] [PubMed] [Google Scholar]
- Boardman N. K., Anderson J. M., Hiller R. G. Photooxidation of cytochromes in leaves and chloroplasts at liquid-nitrogen temperature. Biochim Biophys Acta. 1971 Apr 6;234(1):126–136. doi: 10.1016/0005-2728(71)90137-x. [DOI] [PubMed] [Google Scholar]
- Boardman N. K., Highkin H. R. Studies on a barley mutant lacking chlorophyll b. I. Photochemical activity of isolated chloroplasts. Biochim Biophys Acta. 1966 Oct 10;126(2):189–199. doi: 10.1016/0926-6585(66)90054-9. [DOI] [PubMed] [Google Scholar]
- Boardman N. K. Photochemical properties of a photosystem II subchloroplast fragment. Biochim Biophys Acta. 1972 Dec 14;283(3):469–482. doi: 10.1016/0005-2728(72)90263-0. [DOI] [PubMed] [Google Scholar]
- Boardman N. K., Thorne S. W. Sensitive fluorescence method for the determination of chlorophyll a-chlorophyll b ratios. Biochim Biophys Acta. 1971 Nov 2;253(1):222–231. doi: 10.1016/0005-2728(71)90248-9. [DOI] [PubMed] [Google Scholar]
- Cox R. P., Bendall D. S. The effects on cytochrome b-559HP and P546 of treatments that inhibit oxygen evolution by chloroplasts. Biochim Biophys Acta. 1972;283(1):124–135. doi: 10.1016/0005-2728(72)90104-1. [DOI] [PubMed] [Google Scholar]
- Erixon K., Butler W. L. The relationship between Q, C- 550 and cytochrome b 559 in photoreactions at -196 degrees in chloroplasts. Biochim Biophys Acta. 1971 Jun 15;234(3):381–389. doi: 10.1016/0005-2728(71)90205-2. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Chance B., Devault D. Low temperature photo-induced reactions in green leaves and chloroplasts. Biochim Biophys Acta. 1971 Jan 12;226(1):103–112. doi: 10.1016/0005-2728(71)90182-4. [DOI] [PubMed] [Google Scholar]
- Forti G., Bertolè M. L., Zanetti G. Purification and properties of cytochrome f from parsley leaves. Biochim Biophys Acta. 1965 Sep 27;109(1):33–40. doi: 10.1016/0926-6585(65)90087-7. [DOI] [PubMed] [Google Scholar]
- Henningsen K. W., Boynton J. E. Macromolecular physiology of plastids. VII. The effect of a brief illumination on plastids of dark-grown barley leaves. J Cell Sci. 1969 Nov;5(3):757–793. doi: 10.1242/jcs.5.3.757. [DOI] [PubMed] [Google Scholar]
- Hiller R. G., Anderson J. M., Boardman N. K. Photooxidation of cytochrome b-559 in leaves and chloroplasts at room temperature. Biochim Biophys Acta. 1971 Sep 7;245(2):439–452. doi: 10.1016/0005-2728(71)90161-7. [DOI] [PubMed] [Google Scholar]
- Hiller R. G., Boardman N. K. Light driven redox changes of cytochrome f and the development of photosystems I and II during greening of bean leaves. Biochim Biophys Acta. 1971 Dec 7;253(2):449–458. doi: 10.1016/0005-2728(71)90048-x. [DOI] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. LIGHT-INDUCED OXIDATION OF A CHLOROPLAST B-TYPE CYTOCHROME AT -189 degrees C. Proc Natl Acad Sci U S A. 1969 Jul;63(3):956–962. doi: 10.1073/pnas.63.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. Spectral evidence for a new photoreactive component of the oxygen-evolving system in photosynthesis. Proc Natl Acad Sci U S A. 1969 Jul;63(3):963–969. doi: 10.1073/pnas.63.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze-Karow H., Butler W. L. The development of photophosphorylation and photosynthesis in greening bean leaves. Plant Physiol. 1971 Nov;48(5):621–625. doi: 10.1104/pp.48.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. H. The Development of Chlorophyll and Oxygen-evolving Power in Etiolated Barley Leaves When Illuminated. Plant Physiol. 1954 Mar;29(2):143–148. doi: 10.1104/pp.29.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne S. W., Boardman N. K. Formation of chlorophyll B, and the fluorescence properties and photochemical activities of isolated plastids from greening pea seedlings. Plant Physiol. 1971 Feb;47(2):252–261. doi: 10.1104/pp.47.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon L. P., Shaw E. R. Photoreduction of 2,6-dichlorophenolindophenol by diphenylcarbazide: a photosystem 2 reaction catalyzed by tris-washed chloroplasts and subchloroplast fragments. Plant Physiol. 1969 Nov;44(11):1645–1649. doi: 10.1104/pp.44.11.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Inhibition of the Hill Reaction by Tris and Restoration by Electron Donation to Photosystem II. Plant Physiol. 1969 Mar;44(3):435–438. doi: 10.1104/pp.44.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photoreduction and photophosphorylation with tris-washed chloroplasts. Plant Physiol. 1968 Dec;43(12):1978–1986. doi: 10.1104/pp.43.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


