Abstract
Purpose
Despite the great attention focused on cup positioning in primary total hip arthroplasty (PTHA), it is surprising to find so few studies that have dealt with cup placement. A common thwarting problem for correct cup placement during PTHA is the existence of osteophytes, which obscure the anatomical landmarks. In this study we aimed to evaluate the morphology of acetabular osteophyte formation in patients with osteoarthritis.
Method
We evaluated 276 patients with hip complaints, using plain X-rays and CT scans.
Results
Of these patients, 57 underwent surgery. We developed a staging system for central osteophytes in hip osteoarthritis based on the radiographic and anatomical findings of our patients.
Conclusion
We recommend routine use of CT scans for patients scheduled for PTHA in order to assess the stage of osteophyte before surgery and, thus, reduce the risk of failure resulting from the interrupted acetabular landmarks.
Introduction
Despite the striking number of studies elaborating the cup position, inclination (abduction) and anteversion in primary total hip arthroplasty (PTHA) [1], it is surprising how few papers have considered the crucial question of cup placement in terms of inward, outward, upward, and downward placement during the operation [2]. As for the former case, Lewinnek et al. [3] have defined the safe zone of cup setting angle as 40° ± 10° in abduction and 15° ± 10° in anteversion [1].
The transverse acetabular ligament and tear drop are the references for placing the acetabular component and reaming, because the distance between centre of the acetabulum and tear drop shows little variation [4, 5]. However, a major challenge for identifying these anatomical references is posed by osteophytes, which may develop from the borders of the joint (marginal osteophytes) or from the subchondral area of the cartilaginous surface of the joint (central osteophytes) [6]. In the hip joint, the marginal osteophytes may form laterally or medially (around fovea and the medial margin of acetabular rim). The central and medial osteophytes can cause the buttressing effect medial to the head of femur, thus resulting in its lateralisation in the acetabular fossa and hip incongruency [6–8]. The central and inferomedial osteohpytes may easily be neglected in simple hip X-rays due to the fact that anatomically, they are more likely to lie inside the joint.
The development of medial and central osteophytes lateralises the head of femur and yields a double tear drop view in pre-operative radiographs, and also covers the fossa during surgery. These changes hinder the correct placement of the acetabular cup and reduce the joint stability [8].
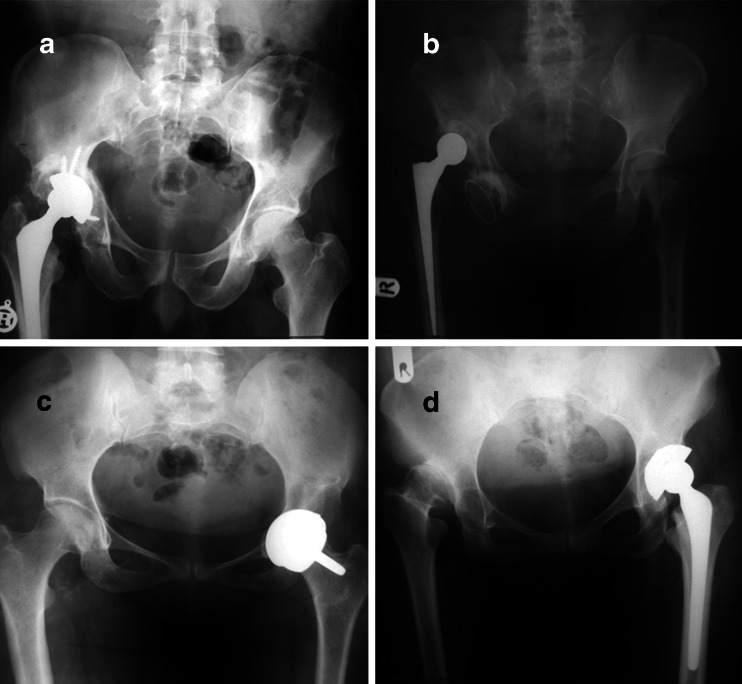
Despite successes in total hip arthroplasty, the optimal fixation method for the acetabular component has yet to be defined [9]. Due to its potential complications [10], acetabular revision is considered the most difficult aspect of hip total replacement, especially for patients with severe acetabular deficiencies and poor bone quality [11]. In an unpublished study conducted by one of the authors, 286 patients undergoing PTHA in our hospital were reviewed retrospectively over a period of 20 years. Among these patients, 12 were found to have cup placement lateral to or higher than the true acetabular wall, all of whom had been operated upon by senior residents of orthopaedics (Fig. 1). Early and rapid identification of osteoarthritic changes not only contributes to early diagnosis for hip pain, but also plays an important role in long-term management of the disease (Fig. 1).
Fig. 1.
A serious complication of primary total hip arthroplasty (PTHA): upward (a), downward (b), inward (c), and outward (d) lateralisation of cup
To reduce the risk of treatment failure resulting from the distorted acetabular landmarks, in this study, we aimed to evaluate the morphology of acetabular osteophyte formation in patients with osteoarthritis (OA). More specifically, we aimed to account for the importance of identifying the landmark for proper placement of the cup—the cup must be exactly at the centre of the real acetabulum; the fovea—in addition to being mindful of proper positioning of the cup.
Method
Two hundred and seventy six (n = 276) patients, 125 men and 151 women, with an average age of 49 years (range 19–72) were included in the study. Patients were eligible to participate in the study if they were referred to our clinic with a history of hip pain, restriction of hip motion, hip deformity, and were skeletally mature. Having obtained the patient’s informed consent, their medical charts were reviewed including a search for any history of previous hip disease. Patients with degenerative changes in the centre of the inferomedial rim of acetabulum underwent CT scanning. The CT images were evaluated by two orthopaedic surgeons looking for different patterns of central osteophytes. Ultimately, the aetiology of articular degenerative changes was classified as either primary (in patients without previous history of hip diseases and with centre-edge angle >20° and acetabular roof obliquity <15°) or secondary, and the frequency of each one was determined. Ultimately, 57 patients underwent total hip arthroplasty (THA) and the radiographic findings were compared to the gross appearance of the lesion in order to yield a comprehensive description of central osteophytes. The appropriate approach to each type of osteophyte during surgery was proposed.
Results
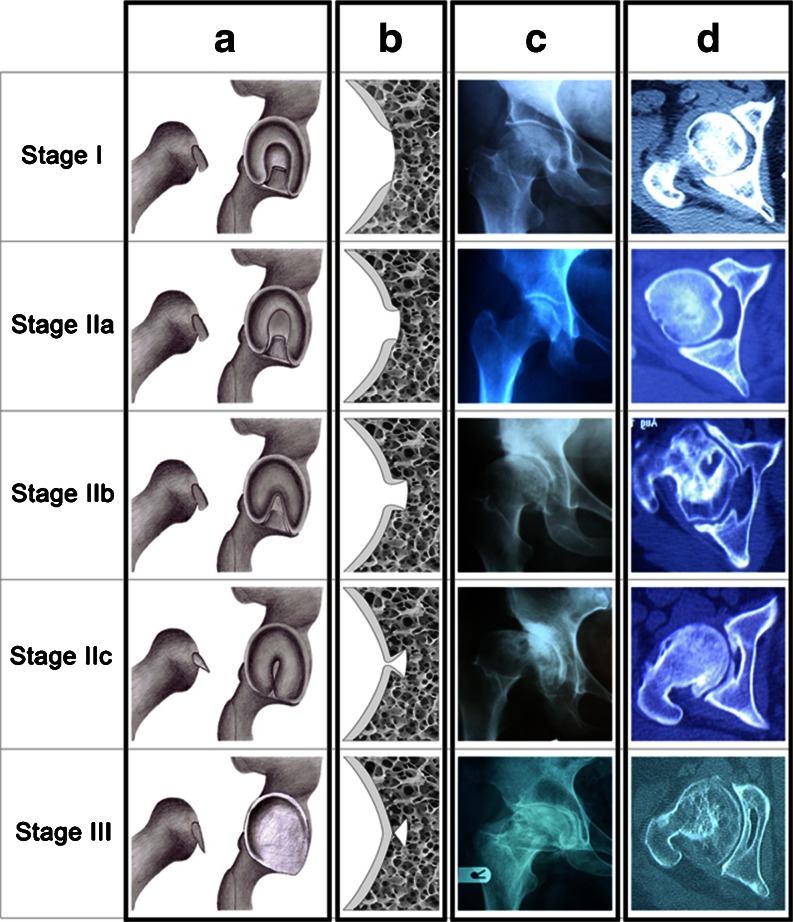
Among 276 patients in our study, the most frequent aetiologies of osteoarthritis included developmental dysplasia of hip (DDH, 122 cases, 44.2 %), avascular necrosis (AVN, 85 cases, 30.8 %), previous infection (20 cases, 7.2 %), previous trauma other than acetabular fracture (19 cases, 6.9 %), Legg-Calvé-Perthes disease (LCPD, 16 cases, 5.8 %), osseous dysplasia (four cases, 1.4 %), following poliomyelitis (two cases, 0.7 %), and primary or idiopathic types (eight cases, 2.9 %). Among these, 36 cases had bilateral hip osteoarthritis. Except for twin sisters with epiphyseal dysplasia, all other cases had numerous and asymmetrical involvement with two cases (5.5 %) of neonatal infection, 12 cases (32 %) of AVN of head of femur, and 20 cases (55.5 %) of DDH. Evaluation of the CT scans by two orthopaedic surgeons revealed different stages of central acetabular osteophytes in our patients; thus, we propose the following staging system based on our observations (Table 1; Fig. 2):
-
Stage I:
This stage represents the normal acetabulum; the maximum distance between foveal rim and its floor is 5 mm, and the cartilaginous tissue forms a sloping ramp facing upward, which joins the foveal rim to the centre of the foveal floor. In our series, the most common aetiology of patients in this stage is AVN.
-
Stage IIa:
In this stage, acetabular sclerosis occurs, particularly around the fovea (in a horse-shoe pattern) thus increasing the foveal depth to 5–8 mm. The cartilaginous tissue forms a wall perpendicular to the foveal floor. AVN and DDH were the first and second common aetiologies of patients in this stage.
-
Stage IIb:
The peri-foveal sclerosis has progressed further, forming an umbrella-like ceiling over the fovea. However, there is a circular orifice of ten millimetres or more in diameter which remains to provide access to the foveal floor. All types of background conditions were observed for this stage.
-
Stage IIc:
The sclerosis is quite advanced now, the edges almost closing on each other. However, the foveal fossa may be still found through a small orifice, or the remnants of fibro-fatty tissues, or the degenerated remnants of ligamentum teres. A sharp tool, such as a haemostat, may be used to reveal the fovea. Post-traumatic followed by DDH-related osteoarthritis of the hip were the most frequent entities in this stage.
-
Stage III:
In this stage, once the acetabulum is exposed, its floor will be observed as shallow and homogenous, unlike the radiological findings. The acetabulum slopes up mildly towards its proximal and distal ends, forming an osseous rim. This rim usually lies below the transverse ligament, thus rendering the identification of obturator foramen more difficult. On CT-scans, it seems as if there are two walls present in the acetabular floor. DDH and LCP were the most frequent aetiologies of OA for patients in this group (Fig. 2; Table 1).
Table 1.
Our proposed system of staging for central osteophytes in osteoarthritis (OA) of the hip, alongside the most common aetiologies associated with each one
| Stage | Foveal height | Foveal floor visibility | Most common aetiology |
|---|---|---|---|
| I | <5 mm | Yes | AVN |
| IIa | 5–8 mm | Yes | AVN, DDH |
| IIb | >8 mm | Yes | Miscellaneous |
| IIc | >8 mm | No | Trauma, DDH |
| III | >8 mm | No | DDH, LCP |
AVN avascular necrosis, DDH developmental dysplasia of hip, LCP Legg-Calvé-Perthes disease
Fig. 2.
Our proposed system of staging for central osteophytes in OA of the hip. a Schematic view presenting the position of caput femoris and acetabulum. b Schematic view of the depth of acetabular fossa. c X-ray radiographies of hip joint. d CT scans of hip joint
The patients’ CT scans were reviewed by a surgeon and a radiologist outside the research group and the percentage error was compared between CT images and the pictures taken during surgery. The percentage error for stage I was 3 %, for stages II A and II B approximately 2 %, and for stage II C it was 1.3 %. Percentage error for stage III was negligible.
Discussion
Our study classifies central osteohpytes of the acetabulum based on radiographic findings compared to the gross appearance of the acetabulum during THA surgery. Various methods have been proposed for classification of hip osteoarthritis. In 1957 Kellgren-Lawrence initiated a scaling system for osteoarthritis (Table 2) [12]. This system is considered the gold standard in epidemiological studies; however, there are certain limitations and exceptions, which justify the adoption of newer methods [13, 14]. The Altman and Goften classification of hip osteoarthritis considers other aspects such as articular erosion and site of degenerative changes [6, 15]. Nowadays, the development of digital modalities for evaluation of radiological views has provided modern computer-aided methods for classification of hip osteoarthritis [16, 17].
Table 2.
Kellgren-Lawrence criteria for osteoarthritis of the hip
| Grade | Definition |
|---|---|
| 0 | No change |
| 1 | Definite osteophytes only |
| 2 | JSN only (defined as an MJS of 2.5 mm) |
| 3 | Presence of two of the following: |
| • JSN | |
| • Osteophytes | |
| • Subchondral bone sclerosis (of 5 mm) | |
| • Cyst formation | |
| 4 | Presence of three of the following: |
| • JSN | |
| • Osteophytes | |
| • Subchondral bone sclerosis (of 5 mm) | |
| • Cyst formation | |
| 5 | Same as grade 4, but with deformity of the femoral head or total hip replacement due to osteoarthritis verified by recommended views |
JSN joint space narrowing, MJS minimal joint space
Although some studies maintain that the medial wall formed by acetabular osteophytes does not get in the way of surgery and thus suggest against removing them [18], the necessity of removing osteophytes during PTHA has been suggested in previous studies [4, 5, 19, 20]. Yet others have suggested the feasibility of defining a new plane of reference as an alternative to the anterior pelvic plane (APP), which is often used during computer-assisted orthopaedic surgery [10].
In our years of practicing orthopaedics, we have confronted many cases of PTHA, which have failed early after surgery. Having central osteophytes regardless of their size could be a reason for misplacing the acetabular cup. Although many of these failures may be due to malpositioning of the cup (anteversion and inclination/abduction), suboptimal placement of the cup also play an important role, which tend to be ignored. Lateral, upward or downward positioning of the cup in relation to the acetabular wall results in an increased joint reaction force, which aggravates the rate of wear, thus bringing about failure of the arthroplasty [21]. Although, the transverse ligament and the cotyloid notch have been deemed the points of reference for reaming during the operation [4]; however, our findings suggest that visualising the fovea during surgery, at least in those with advanced osteoarthritic changes, may serve as an important point of reference, compared to the ligament, in terms of reducing the risk of premature joint failure. An intra-operative X-ray or fluoroscopy is highly valued in checking the position of the reamers and the cup. We need to be sure that the wall is the real acetabular wall and not the osteoarthritic wall. For this reason we have used CT scans preoperatively. Therefore, in order to tackle the problem of central osteophytes systematically, we recommend all the patients nominated for PTHA undergo a pre-operative hip CT scan. We, then propose a staging system to guide the approach to the osteophytes, identified before surgery. The classification system that is proposed is an option that can inform one of deformed and obscured acetabulum.
In stage I, which is indeed the natural condition of the joint, the surgery may be performed as described in literature [22]. In stage II, the procedure is very much the same with one important remark—all the osteophytes must be removed in order to make the foveal fossa appear for subsequent placing of the cup. In lower stages, this may be easily achieved through curettage of the fossa. In stage IIc, where the orifice may not be easily found, using a Ronguer followed by extensive curettage may prove helpful.
Stage III poses the greatest problem for the surgeon, particularly the young, inexperienced surgeon. Primarily, it is essential to identify the existence of the medial wall, and secondly, a special technique must be used to remove it. We propose performing the following steps in sequential order: curettage, followed by Ronguer, and last but not least, perpendicular reaming of the fossa, beginning with the smallest reamer size (38 or 40) and progressing to larger sizes. Once the true acetabular wall appears, the surgery may resume as directed in the literature.
Conclusion
Considering the detrimental role of osteophytes in PTHA, adapting a systematic approach for osteophytes treatment is well justified by reason. Therefore, we recommend performing a thorough radiological review of the hip in the patient scheduled for surgery, in order to identify the possible osteophytes. This prepares the surgeon for what to expect at the operation, and reduces the risk of misplacing the cup. A pre-operative CT scan is not a substitute for skillful and accurate surgical technique. Obtaining intraoperative imaging is of much more value. Although, it does not have to be done routinely, however, it may be beneficial in advanced hypertrophic OA, where the true position of the acetabulum is uncertain. Although CT scans require far more radiation than other imaging modalities and cost much more than X-rays, errors in treatment could also be harmful and costly and result in additional X-rays and poor treatment outcome. This is particularly true of DDH cases that are more prone to development of stage III central osteophytes.
It is noteworthy that our study focuses primarily on central osteophytes, while peripheral osteophytes may also play a role in failure of PTHA. Thus, it seems sensible to have studies designed to deal with peripheral osteophytes in the future. Additionally, performing the hip arthroplasty from the anterior approach with the patient supine affords he ability to have good fluoroscopic images intra-operatively and assure good position of the cup. This requires an expensive operating table and good image intensifier which are not available in all OR settings. However, we recommend this for future studies.
Acknowledgements
The authors are thankful to Drs. Mohsen Bazargan, Hadi Okhovatpour, Amir Mehrvarz Serkesheh, Majid Boreiri and Masoomeh Sadeghian, NS for their insightful comments and suggestions. Dr. Yazdanshenas’ contribution was supported in part by “National Institutes of Health (NIH)-NIMHD” grant U54MD007598 (formerly U54RR026138).
Contributor Information
Firooz Madadi, Phone: +98-21-22678533, FAX: +98-21-22678544, Email: fmedadi@yahoo.com.
Hamed Yazdanshenas, Email: hamedyzdanshenas@cdrewu.edu, Email: fmedadi@sbmu.ac.ir.
Firoozeh Madadi, Email: fmadadi33@gmail.com.
Shahrzad Bazargan-Hejazi, Email: shahrzadbazargan@cdrewu.edu, Email: shahrzadb@ucla.edu.
References
- 1.Chandran P, Azzabi M, Lister A, Andrews M, Stone MH. Acetabular version and long posterior wall cup in total hip replacement. Hip Int. 2008;18(1):11–16. doi: 10.1177/112070000801800103. [DOI] [PubMed] [Google Scholar]
- 2.Kumar MA, Shetty MS, Kiran KG, Kini AR. Validation of navigation assisted cup placement in total hip arthroplasty. Int Orthop. 2012;36(1):17–22. doi: 10.1007/s00264-011-1268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 4.Hartzband MA. Posterolateral mini-incision total hip arthroplasty. Oper Tech Orthop. 2006;16(2):93–101. doi: 10.1053/j.oto.2006.03.005. [DOI] [Google Scholar]
- 5.Bernasek TL, Haidukewych GJ, Gustke KA, Hill O, Levering M. Total hip arthroplasty requiring subtrochanteric osteotomy for developmental hip dysplasia: 5- to 14-year results. J Arthroplast. 2007;22:145–150. doi: 10.1016/j.arth.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Altman RD. Musculoskeletal manifestations of Paget’s disease of bone. Arthritis Rheum. 1980;23:1121–1127. doi: 10.1002/art.1780231008. [DOI] [PubMed] [Google Scholar]
- 7.Ajani JA, Barthel JS, Bentrem DJ, D’Amico TA, Das P, Denlinger CS, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9(8):830–887. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 8.Aaker GD, Gracia L, Myung JS, Borcherding V, Banfelder JR, D’Amico DJ, et al. Three-dimensional reconstruction and analysis of vitreomacular traction: quantification of cyst volume and vitreoretinal interface area. Arch Ophthalmol. 2011;129(6):809–811. doi: 10.1001/archophthalmol.2011.123. [DOI] [PubMed] [Google Scholar]
- 9.Pakvis D, van Hellemondt G, de Visser E, Jacobs W, Spruit M. Is there evidence for a superior method of socket fixation in hip arthroplasty? A systematic review. Int Orthop. 2011;35(8):1109–1118. doi: 10.1007/s00264-011-1234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hausselle J, Moreau PE, Wessely L, de Thomasson E, Assi A, Parratte S, et al. Intra- and extra-articular planes of reference for use in total hip arthroplasty: a preliminary study. Int Orthop. 2012;36(8):1567–1573. doi: 10.1007/s00264-012-1516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulido L, Rachala SR, Cabanela ME. Cementless acetabular revision: past, present, and future. Revision total hip arthroplasty: the acetabular side using cementless implants. Int Orthop. 2011;35(2):289–298. doi: 10.1007/s00264-010-1198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellgren JHLJ. Radiologic assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spector TD, Cooper C. Radiographic assessment of osteoarthritis in population studies: whither Kellgren and Lawrence? Osteoarthr Cartil. 1993;1:203–206. doi: 10.1016/S1063-4584(05)80325-5. [DOI] [PubMed] [Google Scholar]
- 14.Hart DJ, Spector TD. Radiographic criteria for epidemiologic studies of osteoarthritis. J Rheumatol Suppl. 1995;43:46–48. [PubMed] [Google Scholar]
- 15.Goften JP (1983) A classification of osteoarthritis of the hip and its relevance to pathogenesis. J Rheumatol 10 (suppl 9):65–66
- 16.Boniatis I, Costaridou L, Cavouras D, et al. Assessing hip osteoarthritis severity utilizing a probabilistic neural network based classification scheme. Med Eng Phys. 2007;29:227–237. doi: 10.1016/j.medengphy.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Boniatis I, Costaridou L, Cavouras D, et al. Osteoarthritis severity of the hip by computer-aided grading of radiographic images. Med Biol Eng Comput. 2006;44:793–803. doi: 10.1007/s11517-006-0096-3. [DOI] [PubMed] [Google Scholar]
- 18.Shyamalan G, Ghosh K (2008) Primary arthroscopic stabilization for a first-time anterior dislocation of the shoulder. J Bone Joint Surg Am 90:2550; author reply 2550–2551 [PubMed]
- 19.Gonzalez Della Valle A, Comba F, Taveras N, Salvati EA. The utility and precision of analogue and digital preoperative planning for total hip arthroplasty. Int Orthop. 2008;32:289–294. doi: 10.1007/s00264-006-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung WK, Liu D, Foo LS. Mini-incision total hip replacement–surgical technique and early results. J Orthop Surg (Hong Kong) 2004;12:19–24. doi: 10.1177/230949900401200105. [DOI] [PubMed] [Google Scholar]
- 21.Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11:89–99. doi: 10.5435/00124635-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Wasielewski RC, Cooperstein LA, Kruger MP, Rubash HE. Acetabular anatomy and the transacetabular fixation of screws in total hip arthroplasty. J Bone Joint Surg Am. 1990;72:501–508. [PubMed] [Google Scholar]