Abstract
Purpose
Our aim was to elucidate the pooled outcome of the CementLess Spotorno (CLS) system in total hip arthroplasty (THA).
Methods
We compared the outcome of clinical inventor studies, independent clinical studies, and worldwide register data. The main endpoints for analysis were revision rates.
Results
Twenty clinical studies were evaluated and, with one exception, overall found revision rates largely in line with register data. Revision rates (revisions per 100 observed component years) range from 0.15 (inventor study) to 0.28 (independent studies) and 0.43 (register datasets).
Conclusion
Data of journal publications and register datasets using the CLS system do not differ significantly with respect to revision rates. Only the initial inventor study reports a revision rate three times lower than in pooled worldwide register datasets.
Introduction
The outcome and revision rate of the CementLess Spotorno (CLS) system (Zimmer, Warsaw, IN) are of high practical relevance to surgeons and other decision-makers in the health care system. Investigations undertaken in the course of an EFORT-EAR project have shown that the revision rates of clinical studies published in peer-reviewed journals may deviate from register data to a statistically significant and clinically relevant extent in about 50 % of the implant systems under examination [1–4]. Thus, it seems reasonable to evaluate data from clinical studies carefully and from a critical distance by using an objective procedure. Great care should be exercised in drawing analogies and generalising conclusions from sample-based clinical studies as long as systematic problems in the established scientific procedures cannot be excluded.
The hip stem developed by Prof. Lorenzo Spotorno in 1983 was first implanted in 1985 and has subsequently become one of the most successful cementless endoprostheses [5, 6]. Implants following the same philosophy are currently offered by various manufacturers, with the CLS stem by Zimmer (Warsaw, IN) being the most commonly used.
The revision rate is one of the most important indicators for the long-term success of total hip arthroplasty. Two main data sources are available for assessment: clinical sample-based follow-up studies and worldwide register data. While studies try to draw conclusions from the results of a sample to the general population, the datasets of high-value registers comprise all cases which occurred in a certain country or area. They can therefore serve as reference data for the reproducibility of the results of sample-based studies. In due consideration of the quality of register data and clinical studies, it thus makes sense to undertake a final evaluation of the revision rate of the CLS system.
The aim of this study was to further elucidate the outcome of the CLS system and therefore evaluate its comparative pooled revision rate in the published literature and worldwide arthroplasty registers.
Materials and methods
We conducted a web-based literature research in Medline using the keywords “(Spotorno)” AND “(hip arthroplasty)”. The subsequent manual literature research was complemented by a direct request for literature from the implant manufacturer. To be considered in our evaluation, a publication had to fulfil the following inclusion criteria: (1) the implant must be clearly identified, (2) data on the revision rate must either be mentioned in the text or unambiguously calculable from the data contained, (3) “revisions for any reason” are compliant with the standardised definitions in arthroplasty register reports, (4) study in English (as the primary language for scientific publications) or German (as the authors native language) and published in Medline-listed, peer-reviewed journals. We excluded case reports, reviews, and previous meta-analyses from this analysis.
Twenty publications were identified as meeting the inclusion criteria and further analysed in full text [5–24]. We compared clinical follow-up studies with datasets from arthroplasty registers and distinguished between results from inventor studies, independent studies (not from the inventor) and register datasets. The included annual arthroplasty register reports were accessible via the EFORT portal [25].
Data concerning the CLS Spotorno stem are available in the register reports from Australia and Sweden. The Australian report also contains data on implant combinations. An analysis of the register reports publishing implant-specific distributions shows that about one third of all surgeries performed are isolated cup revisions [3]. These data were adjusted accordingly.
The main criterion for evaluation was the parameter 'revision rate'. Comparative assessment was based on a variation of this indicator: revisions per 100 observed component years. It was used pursuant to the guidelines of the Australian National Joint Replacement Registry [26].
The concept of revisions per 100 observed component years basically summarises the individual years after surgery, all patients as “observed component years”, during which they are at risk for revision (no. of cases x average follow-up period). This value is then compared to the number of revisions observed in the same cohort. Thus, the number of cases and the follow-up period of any publication can be considered with respect to its impact on the results. Owing to the higher number of observed component years, larger studies and longer follow-up periods are given higher weight in the calculation. The advantage of this procedure is that different studies and data sources can be compared directly using a single value. A value of one revision per 100 observed component years corresponds to a revision rate of 5 % at five years or a revision rate of 10 % at ten years in conventional follow-up studies.
As secondary endpoint for revision clinical outcome scores were analysed. Since the Merle d’Aubigné and Harris hip score were mentioned in a considerable number of articles, these scores were chosen for evaluation [5–8, 10–23].
The included journal publications were analysed with respect to the source of publication, authors, geographical region, number of cases, and follow-up period. By definition, any publication naming Prof. Spotorno as author or co-author [6] was rated as a publication by the development team and therefore no longer considered independent.
All data were pooled in a standardised way for all data sources. For each parameter, with the exception of follow-up times, exact values were required for inclusion in the study. If no specific follow-up times, but mere follow-up periods were given, a linear distribution of cases was assumed.
Statistical significance was determined through 95 % confidence intervals (CI) calculated using the Circulator v4 Excel-based software program of the University of Adelaide. Given the variability in basic data and study designs of the studies included, no further statistical evaluations were performed.
Results
The cumulative number of cases published in clinical studies is approximately 9,200, while registers include about 2,200 cases. Due to the slightly longer average follow up in registers, the ratio of data from clinical studies to data from registers is approximately three to one.
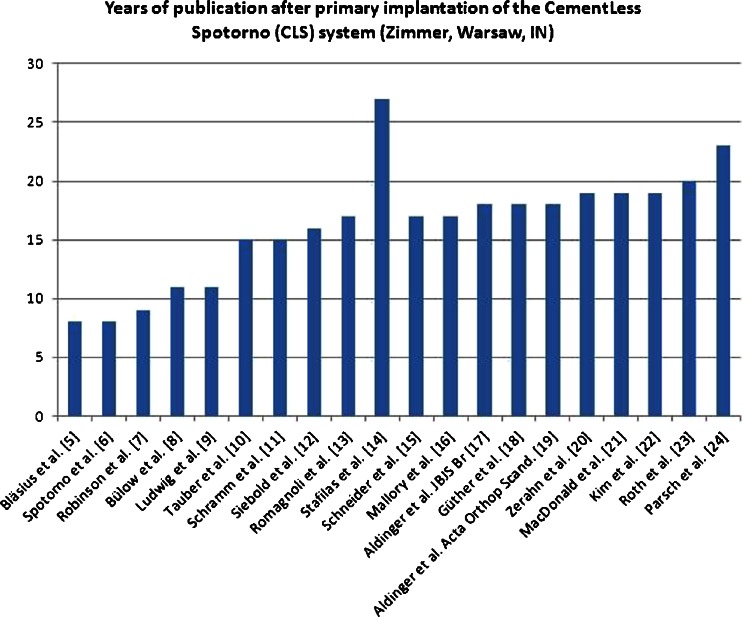
The first studies were published in 1993, which was eight years after the product had been brought on the market [6, 7], and included a publication by the developer [6] reporting excellent results, and data from a multi-centre analysis [7] with short-term results and an average follow up of two and a half years. The majority of studies were published between the years 2000 and 2005, that is more than 15 after market launch and after the product was already established. The years of publication of the included clinical studies [5–24] are illustrated in Fig. 1.
Fig. 1.
Years passed after primary implantation (in 1985) of the CementLess Spotorno (CLS) system (Zimmer, Warsaw, IN) in total hip arthroplasty (THA) before publication of 20 clinical studies evaluating its revision rate [5–24]. The first articles were published eight years after primary implantation
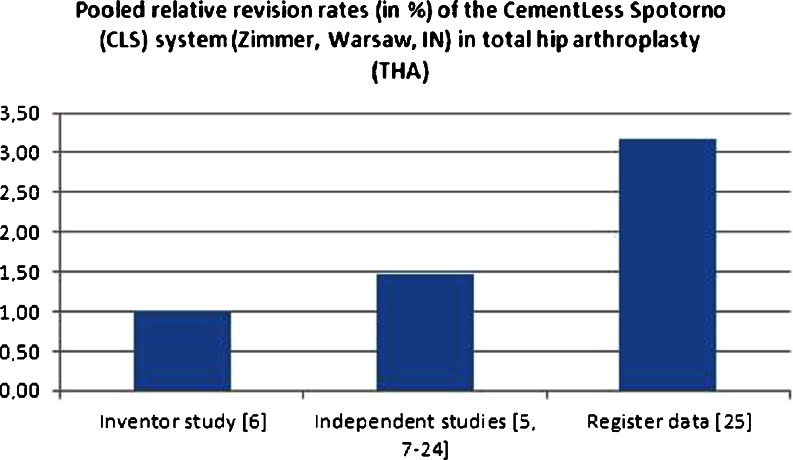
The revision rates reported by individual studies were very low for the first few years, whereas an increase in revision rates was observed from the ninth postoperative year. The pooled relative revision rates of the CementLess Spotorno (CLS) system (Zimmer, Warsaw, IN) in total hip arthroplasty (THA) of one inventor study [6], 19 independent studies [5, 7–24] and worldwide arthroplasty registers [25] are illustrated in Fig. 2.
Fig. 2.
Pooled relative revision rates of the CementLess Spotorno (CLS) system (Zimmer, Warsaw, IN) in total hip arthroplasty (THA) of one inventor study [6], 19 independent studies [5, 7–24] and worldwide arthroplasty register datasets [25] in percent
A structured comparative analysis of the data shows that the average revision rates published in clinical studies is largely in line with the values from registers, as is shown in Table 1.
Table 1.
Revision rates of the CementLess Spotorno (CLS) system (Zimmer, Warsaw, IN) in total hip arthroplasty (THA) evaluated in clinical studies (one inventor study [6], 19 independent studies [5, 7–24]) and worldwide arthroplasty register datasets [25]
| Type of study | Number of articles | Follow-up period | Revision rate (%) | Revisions (n) | Primaries (n) | Observed component years | Revisions per 100 observed component years | CI | Ratio difference to register average |
|---|---|---|---|---|---|---|---|---|---|
| Independent studies [5, 7–24] | 19 | 5.49 | 1.46 | 130 | 8,933 | 46,311.54 | 0.28 | 0.24–0.33 | |
| Inventor study [6] | 1 | 6.83 | 1.00 | 3 | 300 | 2,049.99 | 0.15 | 0.05–0.43 | 2.94 |
| Clinical studies total [5–24] | 20 | 5.24 | 1.44 | 133 | 9,233 | 48,361.53 | 0.28 | 0.23–0.33 | 1.56 |
| Register data [25] | 7.39 | 3.18 | 72 | 2,265 | 16,738.00 | 0.43 | 0.34–0.54 |
Only the developer’s initial publication [6] reports a revision rate that is lower by a factor of three compared to the results from registers as a benchmark for average patient care.
Discussion
The primary aim of the present study was to conduct a structured analysis of the revision rate of the CLS system, with the secondary aim to compare its performance with other implants, on the one hand, and, on the other hand, identify potential systemic problems that may be associated with the published data.
Structured comparative analysis of the data has shown that the average revision rates published in clinical studies are largely consistent with the comparative values from registers. Only the initial study published by the developer reports a lower revision rate than the independent clinical studies and registers.
It is remarkable that eight years had passed since product launch until the first publications of revision rates appeared. Sufficient data to assess the implant were only available after 15 years, at a time when the implant had already been well established on the market worldwide.
The initial publication by the developer reports very good outcomes. Considering the small number of studies and cases published, this could have had an effect on the opinions of experts in the field within the first 15 years after the product had been introduced. However, in a critical analysis of the data currently available this factor is not easily detectable. Only about 3 % of published cases for the Spotorno stem were actually published by the developer. This does not apply to other implants and publications from the United States, where developers dominate the publications about their products in a considerable number of implants and publish revision rates which are irreproducible in average patient treatment [1–4, 27].
The published revision rates for the Spotorno stem are generally low during the first few years, which suggests good primary stability and safe use even in the hands of less experienced surgeons.
In registers the average revision rates of the Spotorno stem are even better than the average benchmark of all total hip arthroplasty systems calculated independent of the product and based on worldwide average values [27]. The CLS system can therefore be regarded as an implant with above-average performance.
The published datasets are self-consistent, and no relevant confounders have been identified. Even though the results published by the developer [6] differ from the outcome achieved in average patient care, the differences are not statistically significant and can be explained by high personal expertise and the special circumstances typically occurring in the process of product development [27, 28]. Analyses of publications by implant developers should generally take into account that they are not representative of the average surgeon in all aspects [27, 28].
Longtime preoccupation with a particular topic naturally leads to exceptional personal expertise; implants and instruments are developed against a specific background and adjusted to personal preferences. Intensive follow-up especially during the initial phase of use in the patient allows for early implementation of comprehensive quality improvement measures. Apart from this, it should not be ignored that personal and financial interests may also be an issue.
Even the slightly better average results of independent clinical studies can be explained by the fact that the majority are conducted in large and specialised centres, which is not to be rated as a systematic confounder. The published outcomes of the Spotorno stem show the usual dispersion of individual results about the mean.
The cementless Spotorno stem can generally be considered a good and safe implant that is likely to ensure very good long-term results even when used by surgeons with an average number of cases.
As every meta-analysis, the present study has the limitation that it depends on the quality of the primary data included. In addition, pooled datasets from arthroplasty registers are relatively small in comparison to the pooled clinical data and therefore not equal for comparative analysis. Next, we did not state that this analysis was performed according to the PRISMA criteria, as we did not present a trial flow of study identification. However, we would like to underline the significant benefit that this is the first study in the literature to determine the revision rate of the CLS system comparing clinical studies and worldwide arthroplasty register datasets. Therefore, this study is most likely to reflect the actual revision rate of this implant.
Conclusion
Data of journal publications and register datasets of total hip arthroplasty using the CementLess Spotorno (CLS) system do not differ significantly with respect to revision rates. Only the initial study presented by the developer reports a revision rate that is three times lower than in pooled worldwide register datasets.
References
- 1.Labek G, on behalf of the QoLA Study Group. Quality of publications regarding the outcome of revision rate after arthroplasty—interim report of the QoLA Project presented at the EFORT Congress 2010 in Madrid. http://www.ear.efort.org/. Accessed 26 February 2013
- 2.Graves S. The value of arthroplasty registry data. Acta Orthop. 2010;81(1):8–9. doi: 10.3109/17453671003667184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labek G, Frischhhut S, Schlichtherle R, Williams A, Thaler M. Outcome of the cementless Taperloc stem: a comprehensive literature review including arthroplasty register data. Acta Orthop. 2011;82(2):143–148. doi: 10.3109/17453674.2011.570668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labek G, Sekyra K, Pawelka W, Janda W, Stöckl B. Outcome and reproducibility of data concerning the Oxford unicompartmental knee arthroplasty: a structured literature review including arthroplasty register data. Acta Orthop. 2011;82(2):131–135. doi: 10.3109/17453674.2011.566134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bläsius K, Cotta H, Schneider U, Thomsen M. CLS multicenter study—8-year results. Z Orthop Ihre Grenzgeb. 1993;131(6):547–552. doi: 10.1055/s-2008-1040069. [DOI] [PubMed] [Google Scholar]
- 6.Spotorno L, Romagnoli S, Ivaldo N, Grappiolo G, Bibbiani E, Blaha DJ, Guen TA. The CLS-system theoretical concept and results. Acta Orthop Belg. 1993;59(Suppl 1):144–148. [PubMed] [Google Scholar]
- 7.Robinson RP, Lovell TP, Green TM. Hip arthroplasty using the cementless CLS stem. A 2–4 year experience. J Arthroplasty. 1994;9(2):177–192. doi: 10.1016/0883-5403(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 8.Bülow JU, Scheller G, Arnold P, Synatschke M, Jani L. Follow-up (6–9 years) results of the uncemented CLS Spotorno stem. Arch Orthop Trauma Surg. 1996;115(3–4):190–194. doi: 10.1007/BF00434551. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig FJ, Melzer C, Backofen D. Criteria for radiologic evaluation of cement-free hip endoprotheses exemplified by the Spotorno shaft. Unfallchirurg. 1996;99(1):750–757. doi: 10.1007/s001130050051. [DOI] [PubMed] [Google Scholar]
- 10.Tauber C, Kidron A. Total hip arthroplasty revision using the press-fit CLS Spotorno cementless stem. Twenty-four hips followed between 1987 and 1998. Arch Orthop Trauma Surg. 2000;120(3–4):209–211. doi: 10.1007/s004020050046. [DOI] [PubMed] [Google Scholar]
- 11.Schramm M, Keck F, Hohmann D, Pitto RP. Total hip arthroplasty using an uncemented femoral component with taper design: outcome at 10-year follow-up. Arch Orthop Trauma Surg. 2000;120(7–8):407–412. doi: 10.1007/PL00013771. [DOI] [PubMed] [Google Scholar]
- 12.Siebold R, Scheller G, Schreiner U, Jani L. Long-term results with the cement-free Spotorno CLS shaft. Orthopade. 2001;30(5):317–322. doi: 10.1007/s001320050614. [DOI] [PubMed] [Google Scholar]
- 13.Romagnoli S. Press-fit hip arthroplasty: a European alternative. J Arthroplasty. 2002;17(4 Suppl 1):108–112. doi: 10.1054/arth.2002.32689. [DOI] [PubMed] [Google Scholar]
- 14.Stafilas K, Kitsoulis P, Zaharis K, Xenakis T. Total hip arthroplasty with uncemented CLS Spotorno Stem for dysplastic or congenitally dislocated hips in adults. A long-term follow-up study. J Bone Joint Surg. 2003;85-B(SUPP III):225. [Google Scholar]
- 15.Schneider U, Breusch SJ, Thomsen M, Wirtz DC, Lukoschek M. Influence of implant position of a hip prosthesis on alignment exemplified by the CLS shaft. Unfallchirurg. 2002;105(1):31–35. doi: 10.1007/s113-002-8162-6. [DOI] [PubMed] [Google Scholar]
- 16.Mallory TH, Lombardi AV, Leith JR, Fujita H, Hartman JF, Capps SG, Kefauver CA, Adams JB, Christian G. Why a taper? J Bone Joint Surg Am. 2002;84-A(Suppl 2):81–89. doi: 10.2106/00004623-200200002-00010. [DOI] [PubMed] [Google Scholar]
- 17.Aldinger PR, Breusch SJ, Lukoschek M, Mau H, Ewerbeck V, Thomsen M. A ten- to 15-year follow-up of the cementless spotorno stem. J Bone Joint Surg Br. 2003;85(2):209–214. doi: 10.1302/0301-620X.85B2.13216. [DOI] [PubMed] [Google Scholar]
- 18.Güther D, Pap G, Bamert P, Eggli S. Long-term results with the cementless CLS stem in hip replacement. Z Orthop Ihre Grenzgeb. 2003;141(3):309–315. doi: 10.1055/s-2003-40080. [DOI] [PubMed] [Google Scholar]
- 19.Aldinger PR, Thomsen M, Mau H, Ewerbeck V, Breusch SJ. Cementless Spotorno tapered titanium stems: excellent 10–15-year survival in 141 young patients. Acta Orthop Scand. 2003;74(3):253–258. doi: 10.1080/00016470310014157. [DOI] [PubMed] [Google Scholar]
- 20.Zerahn B, Lausten GS, Kanstrup IL. Prospective comparison of differences in bone mineral density adjacent to two biomechanically different types of cementless femoral stems. Int Orthop. 2004;28(3):146–150. doi: 10.1007/s00264-003-0534-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonald A, Mutimer J, Ross A (2004) 10-years results of cementless total hip arthroplasty in young patients using the CLS Spotorno stem and Morscher cup. J Bone Joint Surg Br 86-B(SUPP III)
- 22.Kim SY, Kyung HS, Ihn JC, Cho MR, Koo KH, Kim CY. Cementless Metasul metal-on-metal total hip arthroplasty in patients less than fifty years old. J Bone Joint Surg Am. 2004;86-A(11):2475–2481. doi: 10.2106/00004623-200411000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Roth A, Richartz G, Sander K, Sachse A, Fuhrmann R, Wagner A, Venbrocks RA. Periprosthetic bone loss after total hip endoproshesis. Dependence on the type of prosthesis and preoperative bone configuration. Orthopade. 2005;34(4):334–344. doi: 10.1007/s00132-005-0773-1. [DOI] [PubMed] [Google Scholar]
- 24.Parsch D, Jung AW, Thomsen M, Ewerbeck V, Aldinger PR. Good survival of uncemented tapered stems for failed intertrochanteric osteotomy: a mean 16 year follow-up study in 45 patients. Arch Orthop Trauma Surg. 2008;128(10):1081–1085. doi: 10.1007/s00402-007-0444-2. [DOI] [PubMed] [Google Scholar]
- 25.Anonymous. EFORT-portal. http://www.ear.efort.org/registers.aspx. Accessed 26 February 2013.
- 26.Anonymous. Annual Report 2004–2010. AOA-National Joint Replacement Registry. http://www.dmac.adelaide.edu.au/aoanjrr/publications.jsp?section=reports2010. Accessed 26 February 2013.
- 27.Labek G, Thaler M, Janda W, Agreiter M, Stöckl B. Revision rates after total joint replacements: cumulative results from worldwide joint register datasets. J Bone Joint Surg Br. 2011;93(3):293–297. doi: 10.1302/0301-620X.93B3.25467. [DOI] [PubMed] [Google Scholar]
- 28.Sadoghi P, Schröder C, Fottner A, Steinbrück A, Betz O, Müller PE, Jansson V, Hölzer A. Application and survival curve of total hip arthroplasties: a systematic comparative analysis using worldwide hip arthroplasty registers. Int Orthop. 2012;36(11):2197–2203. doi: 10.1007/s00264-012-1614-6. [DOI] [PMC free article] [PubMed] [Google Scholar]