Abstract
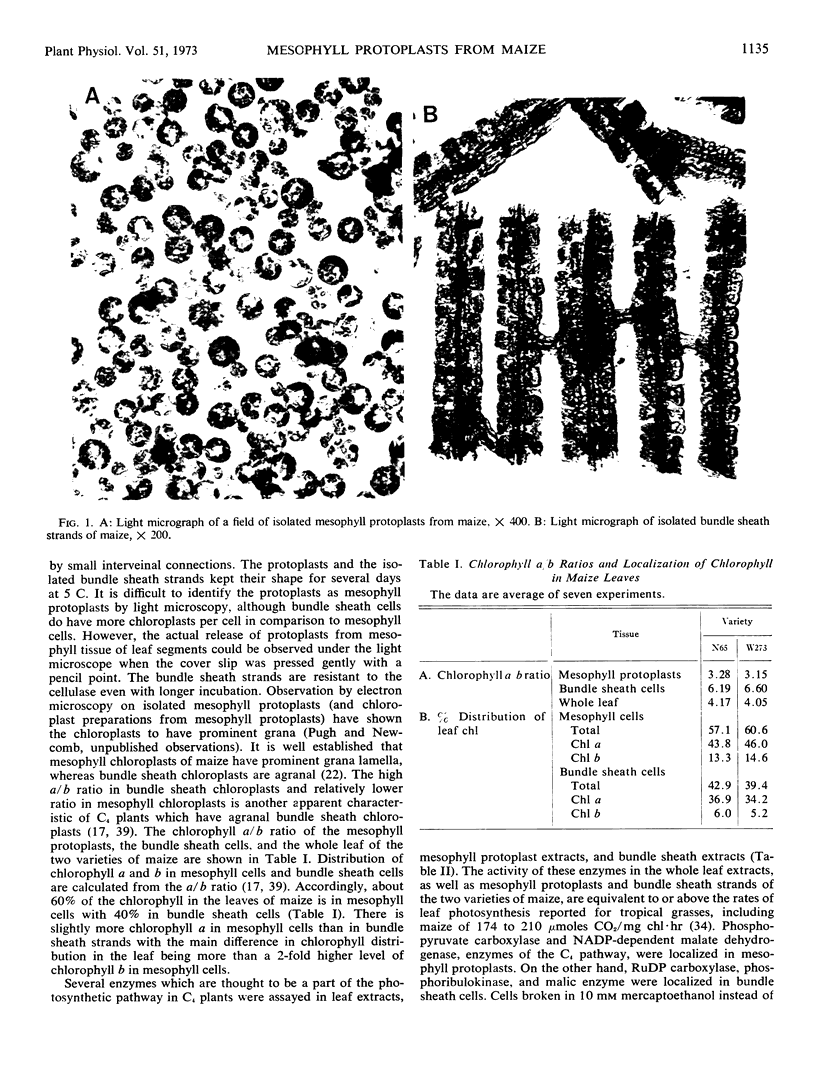
Mesophyll protoplasts and bundle sheath strands of maize (Zea mays L.) leaves have been isolated by enzymatic digestion with cellulase. Mesophyll protoplasts, enzymatically released from maize leaf segments, were further purified by use of a polyethylene glycol-dextran liquid-liquid two phase system. Bundle sheath strands released from the leaf segments were isolated using filtration techniques. Light and electron microscopy show separation of the mesophyll cell protoplasts from bundle sheath strands. Two varieties of maize isolated mesophyll protoplasts had chlorophyll a/b ratios of 3.1 and 3.3, whereas isolated bundle sheath strands had chlorophyll a/b ratios of 6.2 and 6.6. Based on the chlorophyll a/b ratios in mesophyll protoplasts, bundle sheath cells, and whole leaf extracts, approximately 60% of the chlorophyll in the maize leaves would be in mesophyll cells and 40% in bundle sheath cells. The purity of the preparations was also evident from the exclusive localization of phosphopyruvate carboxylase (EC 4.1.1.31) and NADP-dependent malate dehydrogenase (EC 1.1.1) in mesophyll cells and ribulose 1,5-diphosphate carboxylase (EC 4.1.1.39), phosphoribulokinase (EC 2.7.1.19), and “malic enzyme” (EC 1.1.1.40) in bundle sheath cells. NADP-glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.13) was found in both mesophyll and bundle sheath cells, while ribose 5-phosphate isomerase (EC 5.3.1.6) was primarily found in bundle sheath cells. In comparison to the enzyme activities in the whole leaf extract, there was about 90% recovery of the mesophyll enzymes and 65% recovery of the bundle sheath enzymes in the cellular preparations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., JANG R. Purification and properties of phosphoribo-isomerase from alfalfa. J Biol Chem. 1954 Aug;209(2):847–855. [PubMed] [Google Scholar]
- Carlson P. S., Smith H. H., Dearing R. D. Parasexual interspecific plant hybridization. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2292–2294. doi: 10.1073/pnas.69.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet R., Oglen W. L. Oxygen inhibits maize bundle sheath photosynthesis. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2062–2066. doi: 10.1016/0006-291x(72)90759-0. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Black C. C. Isolation of Mesophyll Cells and Bundle Sheath Cells from Digitaria sanguinalis (L.) Scop. Leaves and a Scanning Microscopy Study of the Internal Leaf Cell Morphology. Plant Physiol. 1971 Jan;47(1):149–156. doi: 10.1104/pp.47.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Gutierrez M. Metabolic Activities in Extracts of Mesophyll and Bundle Sheath Cells of Panicum miliaceum (L.) in Relation to the C(4) Dicarboxylic Acid Pathway of Photosynthesis. Plant Physiol. 1972 Dec;50(6):728–732. doi: 10.1104/pp.50.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Lee S. S., Chen T. M., Black C. C. Carboxylation reactions and photosynthesis of carbon compounds in isolated mesophyll and bundle sheath cells of Digitaria sanguinalis (L.) Scop. Biochem Biophys Res Commun. 1970 May 11;39(3):389–395. doi: 10.1016/0006-291x(70)90589-9. [DOI] [PubMed] [Google Scholar]
- GREGORY D. W., COCKING E. C. THE LARGE-SCALE ISOLATION OF PROTOPLASTS FROM IMMATURE TOMATO FRUIT. J Cell Biol. 1965 Jan;24:143–146. doi: 10.1083/jcb.24.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Microbody enzymes and carboxylases in sequential extracts from c(4) and c(3) leaves. Plant Physiol. 1972 Aug;50(2):242–248. doi: 10.1104/pp.50.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., von Garnier R., Gibbs M. Effect of photosynthesis, photosynthetic inhibitors and oxygen on the activity of ribulose 5-phosphate kinase. Biochem Biophys Res Commun. 1970;39(6):1140–1144. doi: 10.1016/0006-291x(70)90678-9. [DOI] [PubMed] [Google Scholar]
- Osmond C. B., Harris B. Photorespiration during C 4 photosynthesis. Biochim Biophys Acta. 1971 May 11;234(2):270–282. doi: 10.1016/0005-2728(71)90082-x. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P. The distribution of carbonic anhydrase and ribulose diphosphate carboxylase in maize leaves. Plant Physiol. 1972 Sep;50(3):336–340. doi: 10.1104/pp.50.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D., Goodchild D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 Sep;114(3):489–498. doi: 10.1042/bj1140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]



