TO THE EDITOR
The biochemical diagnosis of Krabbe disease (KD), a lysosomal storage disease with deficiency of galactosylceramidase (GALC), can be carried out by determination of the residual enzymatic activity in a variety of biological samples such as fibroblasts, dried blood spots, leukocyte pellets and even amniotic fluid cells. However, the correlation between the clinical phenotype of affected individuals and the level of GALC activity is difficult to determine. Many late onset KD cases have GALC activities similar to early onset cases and even low GALC activity may be found in some asymptomatic individuals1. This lack of correlation may be relevant at the time of treating and following up patients.
Galactosylsphingosine (psychosine) is a neurotoxic sphingolipid degraded by GALC. Predictably, affected individuals show progressive accumulation of psychosine in nervous tissue. The accumulation of this sphingolipid has been proposed to trigger neuropathogenic mechanisms leading to the neurological phenotype in KD 2, 3 and consequently, may be a better endpoint to correlate with clinical phenotype.
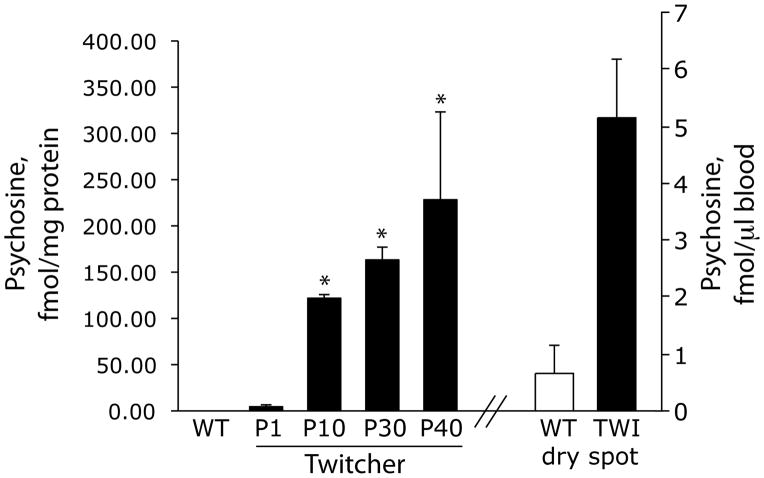
Blood analyses, including the use of dried blood spots, are routine procedures in neonatology. We examined if psychosine was present in detectable quantities in the blood of the Twitcher mouse, a natural occurring murine model of KD. Blood (~150 microliter per animal) was collected at various postnatal ages. Psychosine was analyzed from methanol/chloroform extracts purified on a cation exchanger column. Aliquots from each extract were passed through a Waters XTerra C18 analytical column and then through an Applied Biosystems PI 4000 triple quadrupole mass spectrometer4. Results were expressed as mean fmol psychosine/mg protein from 3 samples per group. Figure 1 shows that psychosine is detectable (at low levels) in blood samples from newborn mutants but becomes statistically significant after ten days of postnatal (P) age. Psychosine levels were essentially at trace levels in all control samples. Blood levels of psychosine reach their maximum at P40. At this age, Twitcher mice develop the full array of neurological symptoms with ataxic movements, strong twitching and muscle wasting and eventually full paralysis and death. Residual GALC activity was measured in triplicate aliquots of each sample (20 μg protein) by incubation with N-lissamine rhodaminyl-6- aminohexanoylgalactosyl ceramide with results expressed as mean nmol/h/mg protein from 3 individual samples per group. GALC activity ranged between 0.059–0.294 nmol/h/mg in mutant white blood cells irrespective of age. GALC activity in wild type blood cells was ~4 nmol/h/mg. No significant correlation was determined between the GALC activity in mutant blood and the clinical phenotype at any age. However, psychosine increases in the mutant blood paralleled the progression of the neurological disease.
Figure 1. Psychosine is present in GALC deficient blood.
Blood was collected at 1,10,30 and 40 postnatal (P) days from Twitcher mice (3 animals per time point) and wild type littermates (3 animals) in non-heparinized tubes. Serum was separated and blood cells were extracted and processed for tandem mass spectrometry. Results are expressed as fmol of psychosine per mg of protein. Blood was also collected from P20 mutant and control animals (3 animals per group) and spotted on filter paper strips and dried. Psychosine was determined and expressed as fmol of psychosine per μl of spotted blood.
To test whether this method may be applied on dried blood spots, blood (50 μl) from 20-day-old mice was spotted and dried onto 1.5×1.5 cm Whatman qualitative filter strips. Dried blood spots were then maintained frozen for about a week before psychosine quantification. Our experience with quantifying psychosine shows that this is a very stable compound (we have successfully quantified psychosine in several decade-old autopsied brain material). Using these filters as starting material, we were able to statistically identify mutants, which had ~10 fold increase in psychosine respect control samples, (Figure 1).
These results suggest that measuring circulating blood levels of psychosine in KD patients may serve as an endpoint for correlation with their neurological phenotype. Further, because human myelination starts during the last trimester of pregnancy, KD babies are likely to be born with detectable psychosine in their blood, facilitating their identification in dried blood spot samples in reference centers for screening of metabolic inherited diseases. Likewise, the procedure described herein may be of more rapid application to follow up levels of correction in KD cases subjected to hematopoietic replacement5.
Acknowledgments
This study was funded with federal funds to ERB (NIH 1R01NS065808).
References
- 1.Wenger DA, Riccardi VM. Possible misdiagnosis of Krabbe disease. J Pediatr. 1976;88(1):76–79. doi: 10.1016/s0022-3476(76)80732-9. [DOI] [PubMed] [Google Scholar]
- 2.White AB, Givogri MI, Lopez-Rosas A, et al. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J Neurosci. 2009;29(19):6068–6077. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castelvetri LC, Givogri MI, Zhu H, et al. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta Neuropathol. 2011;122(1):35–48. doi: 10.1007/s00401-011-0814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galbiati F, Basso V, Cantuti L, et al. Autonomic denervation of lymphoid organs leads to epigenetic immune atrophy in a mouse model of Krabbe disease. J Neurosci. 2007;27(50):13730–13738. doi: 10.1523/JNEUROSCI.3379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffner PK, Caggana M, Orsini JJ, et al. Newborn screening for Krabbe disease: the New York State model. Pediatr Neurol. 2009;40(4):245–252. doi: 10.1016/j.pediatrneurol.2008.11.010. [DOI] [PubMed] [Google Scholar]