Summary
CD4+Foxp3+ regulatory T cells (Tregs) are required for maintenance of self-tolerance, as demonstrated by profound autoimmunity in mice and humans with inactivating Foxp3 mutations. Recent studies demonstrate that Tregs are anatomically compartmentalized within secondary lymphoid organs based on their TCR repertoire and specific organ-protective function; however, whether this reflects differential homing or in situ selection is not known. Here, using Foxp3-GFP reporter mice, we have examined the ability of polyclonal Tregs from cervical LN to return to their site-of-origin following adoptive transfer to non-lymphopenic congenic recipients. We find that bulk cervical LN Tregs do not home directly to cervical LN but, rather, accumulate site-specifically over time following transfer. Site-specific enrichment is both more rapid and more pronounced among a population of recently activated (CD69+) Tregs. These data suggest that compartmentalization of Tregs within secondary lymphoid organs may be governed by antigen recognition and implicate CD69 as a potential marker of recently activated Tregs recognizing local-expressed antigens.
Keywords: Regulatory T cells, T cell receptor, lymph nodes, cell trafficking, cellular immunology
Introduction
CD4+Foxp3+ regulatory T cells (Tregs) play a non-redundant role in preventing naturally occurring autoreactive lymphocytes from causing spontaneous autoimmune disease. This is underscored by the widespread fatal autoimmunity in mice and humans with inactivating Foxp3 mutations [1]. Although Treg defects have been implicated in various spontaneous autoimmune diseases, identification of defects within specific Treg subsets and their role in autoimmunity requires a more comprehensive understanding of how Tregs are anatomically organized. Further, better understanding how Tregs distribute throughout secondary lymphoid organs following adoptive transfer is particularly important as cellular adoptive therapies become a reality [2].
Tregs are distributed anatomically with distinct TCR repertoires within different secondary lymphoid organs [3] and display enriched organ-protective function within organ-draining LN [4]. However, whether and how this is achieved following adoptive transfer is unknown. Specifically, do adoptively transferred Tregs home directly to their site-of-origin, or do they initially distribute non-preferentially with subsequent site-specific accumulation? In this study, through a series of adoptive transfers of Tregs into non-lymphopenic congenic hosts, we show that Tregs from cervical LN (cervLN) do not directly or exclusively home to cervLN following transfer but, instead, accumulate site-specifically over weeks following transfer. Moreover, we show that a sizeable proportion of cervLN Tregs are recently activated (CD69+) at steady-state and demonstrate earlier site-specific accumulation compared to bulk cervLN donor Tregs and greater site-specific accumulation compared to either CD69− or bulk cervLN donor Tregs, suggesting this process may be dependent on antigen recognition. Our work thus supports a role for antigen in establishing and maintaining specific Treg distribution and identifies CD69+ cervLN Tregs as a useful population for studies to further elucidate molecular mechanisms of the site-specific accumulation of Tregs following transfer.
Results and Discussion
CervLN donor Tregs accumulate site-specifically over time following adoptive transfer
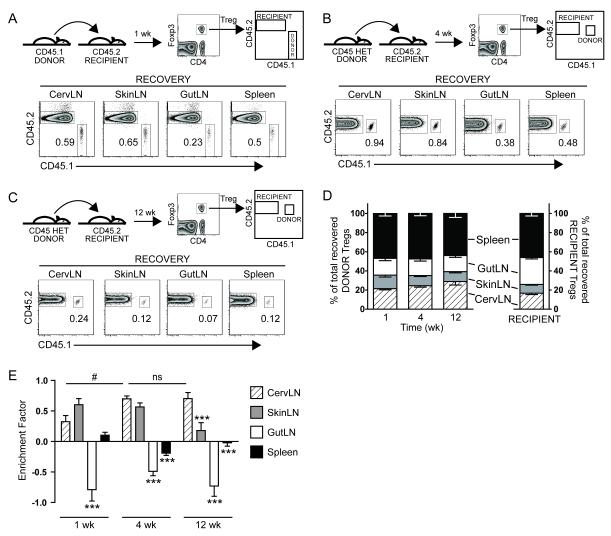
To evaluate whether Tregs home directly and exclusively to their site-of-origin following adoptive transfer, congenically marked Tregs purified from cervLN of Foxp3-GFP donor mice were transferred to non-lymphopenic congenic Foxp3-GFP recipient mice. The presence of donor Tregs within different secondary lymphoid organs was then assessed by flow cytometry at serial time points (Fig. 1). We chose to focus on cervLN Tregs for several reasons: 1) cervLN Tregs have been shown to be compartmentalized in both TCR repertoire and organ-protective function studies [3, 4]; 2) cervLN Tregs represent a larger population than skin-draining LN (skinLN) Tregs and thus require fewer donor mice for adequate donor cell numbers (Table 1); and 3) cervLN donor Tregs are more readily recoverable following adoptive transfer to non-lymphopenic recipients compared to gut-draining LN (gutLN) donor Tregs (unpublished observations). The relatively small donor Treg populations recovered (Fig. 1A–C) are comparable to previous reports of Treg transfers to non-lymphopenic recipients [5, 6]. At 1, 4, and 12 wk following transfer, donor Tregs were recovered from each location, including cervLN, skinLN, gutLN, and spleen (Fig. 1A–C, and Supporting Information Fig. 1). Thus, adoptively transferred Tregs do not home directly or exclusively to their site-of-origin.
Figure 1.
CervLN Tregs accumulate site-specifically over time following adoptive transfer. FACS-purified cervLN Tregs from Foxp3-GFP reporter mice were transferred into congenic Foxp3-GFP recipient mice. At 1 (A), 4 (B), and 12 wk (C) following transfer secondary lymphoid organs were harvested and analyzed by flow cytometry for the presence of congenically-marked donor Tregs. Schema with representative details of specific congenic markers along with gating strategies (first gated on Tregs then on specific donor/recipient populations) are shown (above) and representative flow plots from an individual recipient are shown (below) for each time point. Numbers indicate % of all Tregs within the donor gate. (D) Graph represents mean ± SEM of the % of total donor (left) or recipient (right) Tregs recovered at indicated sites and time points. (E) Enrichment factors were calculated as log2 [(% of total donor Tregs recovered at site x) / (% of total recipient Tregs recovered at site x)] for each site in each individual mouse. The graph represents mean ± SEM of enrichment factors for indicated sites and time points. Statistical analysis by two-way repeated measures ANOVA demonstrates significant difference between recovery sites (p<0.0001) and significant interaction between recovery site and time (p=0.0033) with specific p values from Bonferroni post-test as shown (*** p<0.001 compared to recovery from cervLN at same time point; # p<0.05 comparing cervLN recovery between indicated time points). Data in (D) and (E) are pooled from independent experiments as follows: 3 recipients in 2 experiments (1 wk), 11 recipients in 4 experiments (4 wk), and 7 recipients in 3 experiments (12 wk). cervLN, cervical LN; gutLN, gut-draining LN; HET, CD45.1+CD45.2+ heterozygous; ns, not significant; skinLN, skin-draining LN.
Table 1.
Anatomical LN Groups.
| Group | Abbreviation | LNa | Total # cells (×106)b |
CD4+Foxp3+ (% of total cells)b |
CD4+Foxp3+ (×105)b |
|---|---|---|---|---|---|
| Cervical | cervLN | Mandibularc, accessory mandibularc, superficial parotid |
8.75±0.625 | 5.24±0.36 | 4.56±0.4 (***) |
| Skin- draining |
skinLN | Proper axillary, accessory axillaryd, subiliace, popliteal |
5.16±0.664 | 4.77±0.47 | 2.34±0.25 (***) |
| Gut- draining |
gutLN | Jejunalf, colic, pancreaticoduodenalg, gastric |
13.7±2.72 | 5.39±0.24 | 7.05±1.1 |
| Spleen | – | – | 41.1±3.98 | 3.04±0.16 | 12.6±1.5 |
Live cell counts were determined by Trypan blue exclusion, the percentage (%) CD4+Foxp3+ cells was determined by flow cytometry, and the number of CD4+Foxp3+ cells was calculated from the former two. Data are shown as the mean ± SEM of 8 individual mice pooled from 3 independent experiments.
p=0.0005 by two-tailed paired t test comparing cervLN to skinLN.
Commonly referred to collectively as superficial cervical LN
Commonly referred to as the brachial LN
Commonly referred to as the inguinal LN
Commonly referred to as the mesenteric LN
Commonly referred to as the pancreatic LN
To determine if Tregs would accumulate within the cervLN over time, we first determined their relative distribution at each time point post-transfer (Fig. 1D). Over time, there is an increasing trend in the proportion of donor Tregs recovered within the cervLN (Fig. 1D). Not surprisingly, many donor Tregs were recovered from sites with greater total cell numbers such as spleen and gutLN (Fig. 1D and Table 1). To account for this, we calculated enrichment factors which normalized the relative distributions of donor Tregs to that of recipient Tregs for each site within each individual mouse. Specifically, we calculated enrichment factors as log2 of the ratio: % of all recovered donor Tregs that are recovered from a particular site divided by the % of all recovered recipient Tregs recovered from that site. Enrichment factors >0 suggest relative enrichment of donor cells at that site, whereas values <0 suggest a relative exclusion from that site. At all time points following transfer, cervLN donor Tregs are relatively excluded from gutLN (Fig. 1E). This is consistent with the recent finding that pooled LN and spleen-derived Tregs are recovered preferentially within non-gutLN following adoptive transfer [6] and suggests decreased access to, or decreased retention or survival within gutLN. Initially there is a trend toward preferential accumulation within skinLN (Fig. 1E). By 4 wk post-transfer, maximal enrichment within cervLN is achieved; however, non-site-specific enrichment within skinLN remains high at this time. Thus, at 4 wk post-transfer bulk cervLN Tregs have not preferentially accumulated site-specifically. At 12 wk post-transfer, maximal site-specific (i.e., cervLN) enrichment is maintained whereas non-site-specific enrichment has markedly diminished. These data demonstrate that over weeks following adoptive transfer, donor Tregs accumulate preferentially (though not exclusively) within their site-of-origin.
One possibility is that cervLN are unique and promote the preferential accumulation of any transferred Tregs regardless of origin. To evaluate this we transferred Tregs isolated from either skinLN or gutLN to congenic recipients as above. Over time, adoptively transferred donor Tregs from skinLN and gutLN do not accumulate within cervLN to the same extent as do adoptively transferred Tregs from cervLN (Supporting Information Fig. 2). Thus, preferential accumulation of Tregs within cervLN following transfer is specific to cervLN donor Tregs.
To determine whether similar site-specific accumulation is a property of conventional CD4+Foxp3− T cells (Tconv), we performed transfer studies with Tconv isolated from gutLN and non-gutLN. Neither gutLN nor non-gutLN Tconv accumulated site-specifically (Supporting Information Fig. 3) suggesting that site-specific accumulation may be a property specific to Tregs.
Recently activated Tregs from cervLN demonstrate enhanced site-specific enrichment
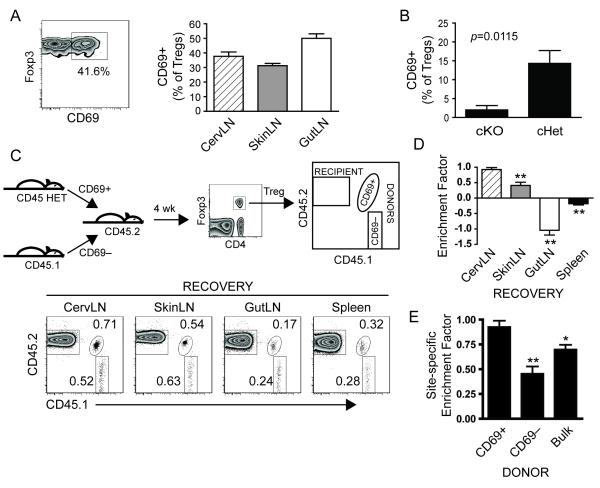
Given the distinct Treg TCR repertoires within different secondary lymphoid organs [3], we hypothesized that recently activated Tregs would demonstrate enhanced site-specific accumulation following adoptive transfer. CD69 is a cell surface marker rapidly upregulated following TCR activation [7] but which also may be upregulated by lymphocytes in the presence of inflammatory cues such as type I interferons [8]. In unmanipulated Foxp3-GFP reporter mice 30–60% of Tregs within each LN group were CD69+ (Fig. 2A). To evaluate the requirement for TCR signaling in CD69 expression by Tregs at steady-state, we analyzed Tregs from mice in which the key TCR signaling adaptor molecule SLP-76 was conditionally deleted [9]. For these experiments, we used mice lacking either one (conditional heterozygous; cHet) or both (conditional knockout; cKO) copies of SLP-76. Following conditional deletion of SLP-76, the CD69+ Treg population in cervLN of cKO mice was greatly diminished demonstrating a requirement for SLP-76-mediated signaling in maintaining the population of CD69+ Tregs at steady-state (Fig. 2B). This suggests CD69 expression by Tregs may be TCR-mediated, though we have not excluded the possibility that other signals, such as via integrins, may play a role. We therefore used CD69 as a marker of activation to address our hypothesis that the recently activated Treg subset would demonstrate enhanced site-specific accumulation compared to the non-activated Treg subset in adoptive transfer experiments. CervLN Tregs congenically marked based on CD69 expression were co-transferred to recipient mice of a third congenic specificity (Fig. 2C) such that all three populations (CD69+ donor Tregs, CD69− donor Tregs, and recipient Tregs) were readily identifiable. While donor Tregs (both CD69+ and CD69−) were again recovered at all locations (Fig. 2C and Supporting Information Fig. 2), the CD69+ donor Tregs accumulated site-specifically at 4 wk post-transfer (Fig. 2D). Notably, this site-specific accumulation (defined as accumulation preferentially within site-of-origin compared to other sites) occurred earlier compared to that of bulk cervLN donor Tregs. Enrichment within cervLN was greater than enrichment within each non-cervLN site at 4 wk post-transfer for CD69+ donor Tregs (Fig. 2D), whereas similar greater enrichment than each non-cervLN site was not achieved by bulk donor Tregs until 12 wk post-transfer (Fig. 1E). Moreover, the CD69+ cervLN Tregs clearly demonstrated enhanced site-specific accumulation compared to the CD69− or bulk cervLN donor Tregs at 4 wk post-transfer (Fig. 2E). This was specific to recovery at cervLN as recovery at non-cervLN sites was not dominated by CD69+ donor cells (Supporting Information Fig. 5). These data suggest that the site-specific accumulation of Tregs may be governed, at least in part, by local SLP-76-mediated activation signals such as via TCR, presumably reflecting antigen-dependence.
Figure 2.
Recently activated cervLN Tregs demonstrate enhanced site-specific accumulation. (A) LN from unmanipulated Foxp3-GFP mice were harvested and analyzed by flow cytometry for expression of cell surface CD69. Representative plot (left) gated on Tregs from cervLN is shown. Number indicates % of Tregs within the indicated CD69+ gate. Graph (right) shows mean ± SEM of % CD69+ Tregs from LN as indicated from 4 samples in 2 independent experiments. Each sample is composed of lymph nodes pooled (by anatomical site) from 10-15 mice per lymph node group. (B) SLP-76 cKO or cHet mice were treated with tamoxifen to induce conditional SLP-76 deletion resulting in no copies (cKO) or one copy (cHet) of SLP-76 within YFP+ cells. FACS-purified YFP+ cells were analyzed for CD69 expression on Tregs. Graph shows mean ± SEM of % CD69+ Tregs from cervLN of 4 cKO and 3 cHet mice pooled from 2 independent experiments. Statistical analysis was by two-tailed unpaired t test. (C–E) CD69+ and CD69− Tregs from cervLN in (A) were purified by FACS from congenically marked donors and transferred into recipient mice of a third congenic specificity as shown in representative schematic (C). After 4 wk, secondary lymphoid organs were harvested and analyzed by flow cytometry. (C) Representative donor/recipient schematic along with Treg gating and identification of each donor or recipient Treg population is shown (top). Representative plots from one recipient are shown with numbers indicating % of Tregs within each donor gate. (D) Graph represents mean ± SEM of enrichment factors (calculated as in Fig. 1E) for CD69+ donor cervLN Tregs from 7 recipients in 2 independent experiments. Statistical analysis by one-way repeated measures ANOVA demonstrated significant difference (p<0.0001) with specific p values from Dunnett’s post-test as indicated (** p<0.01 compared to recovery from cervLN). (E) Graph represents mean ± SEM of site-specific enrichment factor (i.e., enrichment factor for cervLN donor Tregs recovered at cervLN) for CD69+ and CD69− donor Tregs from 7 recipients in 2 independent experiments or from bulk cervLN donor Tregs from 4 wk time point in Fig. 1E. Statistical analysis was by one-way ANOVA with p=0.0001 and specific p values from Dunnett’s post-test as indicated (* p<0.05, ** p<0.01 compared to CD69+ donor group). cervLN, cervical LN; cHet, SLP-76 conditional heterozygous; cKO, SLP-76 conditional knockout; gutLN, gut-draining LN; skinLN, skin-draining LN.
This site-specific accumulation is likely due to a combination of local proliferation, survival, and retention. In a TCR transgenic system, antigen-specific Tregs proliferated preferentially in LN draining their cognate antigen [5]. Retention within secondary lymphoid organs has recently been demonstrated to result from CD69-mediated downregulation of sphingosine 1 phosphate receptor 1 (S1P1), a receptor required for lymphocyte egress from LN [8, 10]. Our data show that TCR-mediated signals may be required for the expression of CD69 on a subset of Tregs at steady-state, and others have demonstrated downregulation of S1P1 by Tregs following TCR stimulation [11]. Thus, site-specific retention mediated by downregulation of S1P1 following TCR stimulation may play a key role in site-specific accumulation following transfer by increasing Treg dwell time within LN in which their cognate antigen is presented. The recovery of either bulk or CD69+ donor cervLN Tregs from all sites at each time point strongly argues against site-directed LN homing as a major factor in establishing Treg distribution within specific LN. Similarly, as of yet, no specific homing receptors which distinguish one LN versus another have been identified. Given the finding that Tregs are present within both lymphoid and non-lymphoid tissues at steady-state [12], however, we cannot exclude the possible contribution of non-lymphoid tissue-specific homing followed by recirculation to the draining LN.
Concluding Remarks
This study highlights a potential key role for antigen recognition in establishing and maintaining the compartmentalization of Tregs within different secondary lymphoid organs. Our data identify CD69+ cervLN Tregs as a readily abundant population of Tregs which accumulate site-specifically over time following adoptive transfer making them an ideal population for studies to further elucidate the molecular mechanisms of the compartmentalization of Tregs within secondary lymphoid organs. Further understanding these mechanisms, such as identification of Treg-specific self-antigens, will provide tools to develop more efficient Treg-based cellular therapies aimed at directing in vitro expanded Tregs to particular lymphoid compartments to treat organ-specific autoimmunity.
Materials and Methods
Mice
Foxp3-GFP knockin reporter mice on the C57BL/6 background (CD45.2) [13] were bred with CD45.1 congenic C57BL/6 mice (B6.SJL-Ptprca Pepcb/BoyJ, The Jackson Laboratory) to generate CD45.1+, CD45.2+, and CD45.1+CD45.2+ heterozygous (CD45 HET) Foxp3-GFP reporter congenic strains. Tamoxifen inducible SLP-76 conditional knockout (cKO; SLP-76F/nullR26RyfpCreT2) and conditional heterozygous (cHet; SLP-76F/+R26RyfpCreT2) mice have been previously described [9]. For induced SLP-76 deletion, mice were treated with tamoxifen (Sigma), 200 μg/g/day by oral gavage for 5 days, then LN were harvested 6–14 days after the last tamoxifen dose. Mice were maintained at the University of Pennsylvania under specific pathogen free conditions and used in accordance with University of Pennsylvania Animal Care and Use Committee guidelines.
Cell sorting and adoptive transfer
CervLN, skinLN, or gutLN (Table 1) were isolated from multiple congenic Foxp3-GFP mice. Single cell suspensions were treated with ACK lysing buffer (Lonza) to lyse RBC and labeled with Alexa Fluor 700-conjugated anti-CD4 (eBioscience) with or without allophycocyanin-conjugated anti-CD69 (BD) monoclonal antibodies. Treg (CD4+Foxp3+) or Tconv (CD4+Foxp3−) were purified by FACS on a FACSAria (BD). For some experiments, Treg were further sorted based on CD69 expression. For co-transfer experiments, CD69+ and CD69− Treg from different congenic Foxp3-GFP donor mice were combined prior to transfer. Cells in sterile PBS were transferred by retro-orbital injection. For SLP-76 experiments, YFP+ cells (SLP−/− for cKO or SLP+/− for cHet) were purified on a FACSAria prior to surface and intracellular staining.
Flow cytometry
Single cell suspensions from secondary lymphoid organs (Table 1) were labeled with fluorochrome conjugated monoclonal antibodies specific for cell surface molecules CD4 (Alexa Fluor 700), CD45.1 (eFluor450, eBioscience), CD45.2 (allophycocyanin, BD), with or without CD69 (PerCP-Cy5.5, BD), or appropriate isotype controls. For some experiments, cells were then fixed and permeabilized with a Foxp3 intracellular staining kit (eBioscience) according to manufacturer’s protocol and stained with allophycocyanin-conjugated anti-Foxp3 (eBioscience) monoclonal antibody. Data were collected on a FACSCanto II (BD) or FACSCalibur (BD) and analyzed using FlowJo software (version 7.6.1, TreeStar Software, Inc). For transfer study analyses, a minimum of 1.5×106 singlet events within the lymphocyte gate were collected.
Calculation of distribution % and enrichment factor
Donor Treg distribution % was calculated as the absolute number of donor Tregs recovered within a particular site at a given time point divided by the total number of donor Tregs recovered at all sites at that time point x 100%. Recipient Treg distribution % was similarly calculated in each experiment but data were pooled together from different time points given the observation that these recipient distribution % did not differ between different time points.
Enrichment was defined as [(# donor Tregs recovered at site x)/(total # donor Tregs recovered at all sites)] / [(# recipient Tregs recovered at site x)/(total # recipient Tregs recovered at all sites)]. In other words, enrichment represents the fold-change of the fraction of all recovered donor Tregs which were recovered at site x compared to the fraction of all recovered recipient Tregs recovered at that site. Similar enrichment calculations were performed with Tconv. To transform these exponentially growing ratios to a linear function, the enrichment factor was defined as log2[enrichment].
Statistics
Statistical analyses were performed with Prism version 4.00 (GraphPad Software). For comparison of two groups, unpaired two-tailed Student’s t test was used. For comparison of multiple groups, repeated measures one-way ANOVA with Dunnett’s post-test was used. For two-dimensional analyses, repeated measures two-way ANOVA with Bonferroni post-test was used. Repeated measures ANOVA were used to account for cells isolated from different locations within the same recipient mouse. Statistical significance was defined as p < 0.05.
Supplementary Material
Acknowledgments
We thank Ryan Wychowanec and the University of Pennsylvania Flow Cytometry and Cell Sorting facility for technical assistance, Martha Jordan and Gary Koretzky for critical reading of the manuscript, and Adeeb Rahman, Tao Zou, Matthew Riese, and members of the Turka, Koretzky, and Behrens labs for assistance and discussion. This work was supported by NIH grants T32 DK07006-35 (S.M.L.), AI37691 and AI41521 (L.A.T.), and by an Arthritis Foundation Postdoctoral Fellowship (S.M.L.).
Abbreviations
- cHet
conditional heterozygous
- cKO
conditional knockout
- cervLN
cervical LN
- gutLN
gut-draining LN
- skinLN
skin-draining LN
Footnotes
Conflicts of Interest: The authors have no financial or commercial conflicts of interest.
References
- 1.Sakaguchi S, Wing K, Miyara M. Regulatory T cells - a brief history and perspective. Eur. J. Immunol. 2007;37(Suppl 1):S116–123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Lu L, Jiang S. Regulatory T cells: customizing for the clinic. Sci. Transl. Med. 2011;3:83ps19. doi: 10.1126/scitranslmed.3001819. [DOI] [PubMed] [Google Scholar]
- 3.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J. Exp. Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheeler KM, Samy ET, Tung KS. Cutting edge: normal regional lymphnode enrichment of antigen-specific regulatory T cells with autoimmune disease-suppressive capacity. J. Immunol. 2009;183:7635–7638. doi: 10.4049/jimmunol.0804251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous Activation of Autoreactive CD4+ CD25+ Regulatory T Cells in the Steady State. J. Exp. Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fohse L, Suffner J, Suhre K, Wahl B, Lindner C, Lee CW, Schmitz S, et al. High TCR diversity ensures optimal function andhomeostasis of Foxp3(+) regulatory Tcells. Eur. J. Immunol. 2011;41:3101–3113. doi: 10.1002/eji.201141986. [DOI] [PubMed] [Google Scholar]
- 7.Testi R, D’Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol. Today. 1994;15:479–483. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 8.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 9.Wu GF, Corbo E, Schmidt M, Smith-Garvin JE, Riese MJ, Jordan MS, Laufer TM, et al. Conditional deletion of SLP-76 in mature T cells abrogates peripheral immune responses. Eur. J. Immunol. 2011;41:2064–2073. doi: 10.1002/eji.201040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 14.Vandenbroeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J. Immunol. Methods. 2006;312:12–19. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Hummel KP, Richardson FL, Fekete E. Anatomy. In: Green EL, editor. Biology of the Laboratory Mouse. 2nd Edn Dover Publications; New York: 1968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.