Abstract
Atrial fibrillation (AF) is the most-common sustained arrhythmia observed in clinical practice, but response to therapy is highly variable between patients. Current drug therapies to suppress AF are incompletely and unpredictably effective and carry substantial risk of proarrhythmia and noncardiac toxicities. The limited success of therapy for AF is partially the result of heterogeneity of the underlying substrate, interindividual differences in disease mechanisms, and our inability to predict response to therapies in individual patients. In this Review, we discuss the evidence that variability in response to drug therapy is also conditioned by the underlying genetic substrate for AF. Increased susceptibility to AF is mediated through diverse genetic mechanisms, including modulation of the atrial action-potential duration, conduction slowing, and impaired cell-to-cell communication, as well as novel mechanisms, such as regulation of signalling proteins important in the pathogenesis of AF. However, the translation of genetic data to the care of the patients with AF has been limited because of poor understanding of the underlying mechanisms associated with common AF-susceptibility loci, a dearth of prospective, adequately powered studies, and the challenges associated with determining efficacy of antiarrhythmic drugs. What is apparent, however, is the need for appropriately designed, genotype-directed clinical trials.
Introduction
Atrial fibrillation (AF) is the most-common sustained arrhythmia observed in clinical practice. The condition affects 25% of people aged >40 years during their lifetime and is associated with considerable morbidity and an approximately twofold increase in mortality.1–3 Given the clinical and genetic heterogeneity of AF,4 this arrhythmia is likely to represent the final common phenotype of multiple diverse pathways. Most patients with symptomatic AF are initially managed with antiarrhythmic drugs; failure of such therapy leads to consideration of catheter-based ablation in selected patients. Considerable variation exists in how patients with AF are managed,5 which is likely to be a result of several factors, including the lack of reliably effective therapies for maintaining sinus rhythm, the absence of mechanism-based therapies, and toxicities associated with antiarrhythmic drugs. Furthermore, randomized controlled trials in which rate-control and rhythm-control strategies have been compared suggest that the two approaches are equivalent in terms of survival, and indicate against maintaining sinus rhythm with current antiarrhythmic drugs, especially in patients with minimally symptomatic AF.6–8 Nevertheless, a rhythm-control approach is necessary in many patients because of intolerable symptoms associated with AF, and might be appropriate for broader use given that survival of patients in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial9 was improved if sinus rhythm was restored and maintained.
Without the ability to target therapy to the underlying AF mechanisms in an individual patient, the current strategies for trials of antiarrhythmic drug therapy primarily focus not on efficacy, but on empirical approaches to limiting adverse effects of the available membrane-active drugs. Few evidence-based data are available to assist physicians in selecting the antiarrhythmic drug most likely to be effective in a particular patient. The authors of the 2006 ACC/AHA/ESC guidelines recommend a rate-control strategy for all asymptomatic patients.10 The failure of antiarrhythmic drugs to show superiority over a rate-control strategy in a large population of patients with AF might not only reflect underlying heterogeneity of AF, but also our inability to subcategorize AF by underlying initiating or perpetuating mechanisms. Given that the current success rate for antiarrhythmic drugs in AF is approximately 50% over 6–12 months,11 continued research aimed at refining antiarrhythmic drug therapy that is targeted to mechanisms is a critical unmet need.
Our incomplete understanding of the complex pathophysiology of AF is one reason for the lack of effective therapies for this common arrhythmia. One approach to unravelling the underlying mechanisms of AF is through the identification of the genes responsible for the disease, and an initial strategy is to study families with AF. Extending such knowledge into large populations has the long-term attraction of potentially allowing the selection of therapies that are mechanism-based and tailored to individual patients. In this Review, we discuss genetic mechanisms of AF and the implications that this emerging understanding has on patient response to pharmacological therapies for this condition.
Genetic basis of AF
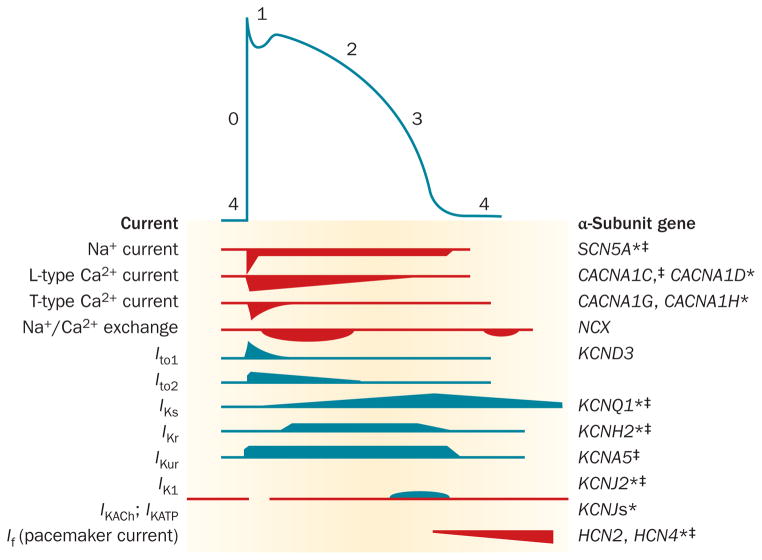
The shape of the cardiomyocyte action potential is highly variable and dependent on whether the cell is located in the specialized conduction system of the heart or within the atria or ventricles. The central role in cardiac electrogenesis of genes that encode ion channels is emphasized when one considers that gain-of-function and loss-of-function ion-channel variants can increase susceptibility to AF.12 The genes encoding the major currents of the atrial action potential and those that have been identified to have an important role in AF susceptibility are illustrated in Figure 1.
Figure 1.
The relationship between ionic currents and the duration of the atrial action potential. The action potential is initiated by a rapid influx of Na+ ions (phase 0), followed by early (phases 1 and 2) and late (phase 3) stages of repolarization, before returning to the resting membrane potential (phase 4). Repolarization is controlled by a balance between inward (red) and outward (blue) currents. The genes encoding the major currents of the atrial action potential are shown. *Function-modifying subunit. ‡Mutation in this gene associated with atrial fibrillation.
Heritability
Evidence for a genetic contribution to the development of AF was first provided in 1943 by Wolff, when three brothers with a rare autosomal-dominant form of AF were identified.13 Since then, many epidemiological studies have confirmed the heritability of AF, especially AF occurring in the absence of recognized heart disease (‘lone’ AF).4,14–19 Specific, rare AF risk alleles have been identified through linkage and candidate-gene approaches in AF kindreds. Subsequently, common AF-susceptibility single nucleotide polymorphisms (SNPs) have been identified in the general population through genome-wide association studies (GWAS).
Linkage studies
Various AF loci and genes with large effect sizes in AF kindreds have been identified in positional cloning and linkage analyses (Table 1). The first AF locus was discovered in 1997;20 over the past decade, four additional loci have been identified.21–24 Given that large AF kindreds are rare, identifying an endophenotype (an intermediate phenotype that cosegregates with the poorly penetrant phenotype) might help to discover novel AF genes and loci. In 2008, we identified a large family from Middle Tennessee, USA with familial AF and, using a prolonged signal-averaged P-wave duration as an endophenotype for AF, we were able to localize a novel AF locus on chromosome 5p15.12
Table 1.
Genes implicated in AF susceptibility by positional cloning and candidate-gene approaches
| Gene | Mode of inheritance | Effect on function | Physiological effect | Associated phenotypes |
|---|---|---|---|---|
| KCNQ125,30,54,120–126 | Autosomal dominant | Gain-of-function | ↓ Atrial APD | Prolonged QT interval |
| KCNE1, KCNE240,127,128 | Autosomal dominant | Gain-of-function | ↓ Atrial APD | Frequent premature atrial contractions |
| KCNE540,128 | Autosomal dominant | Gain-of-function | ↓ Atrial APD | None |
| KCNJ239,129,130 | Autosomal dominant | Gain-of-function | ↓ Atrial APD | None |
| GJA556,63,131 | Somatic mutations Isolated lone AF cases | ↓ Electrical cell-to-cell coupling | Regions of heterogeneous conduction | None |
| KCNA541,49–51 | Autosomal dominant | Loss-of-function | ↑ Atrial APD, EADs, TA | None |
|
SCN5A,132–136 SCN1B/2B/3B137–141 |
Autosomal dominant Autosomal dominant |
Gain-of-function Loss-of-function |
↑ Atrial APD EADs, TA | Hypertrophic cardiomyopathy and dilated cardiomyopathy |
| SCN10A142 | Autosomal dominant | Enhanced late INa | ↑ APD, EADs, TA | Slow ventricular rates |
| CACNA1C,143 CACNB2144 | Autosomal dominant | Loss-of-function | ↑ APD, EADs, TA | None |
| NPPA26,29,120,145,146 | Autosomal dominant | Gain-of-function | ↓ Atrial APD | Atrial myopathy |
| NUP155147 | Autosomal recessive | Loss-of-function of the nuclear protein transport (heat shock protein 70) | ↓ Trafficking of Ca2+-handling proteins and ion channels | Sudden cardiac death |
Abbreviations: AF, atrial fibrillation; APD, action-potential duration; EAD, early afterdepolarization; TA, triggered activity.
The first gene (KCNQ1) to be linked with familial AF was identified in 2003.25 KCNQ1 encodes the delayed-rectifier cardiac potassium channel (IKs). This discovery led investigators to screen other cardiac genes as candidates in the pathogenesis of AF. The first atrial gene implicated in AF not to encode an ion channel was natriuretic peptide precursor A (NPPA), which encodes atrial natriuretic peptide (ANP).26 Various rare, mostly ‘private’ genetic variants affecting only a single kindred that encode diverse ion-channel and signalling proteins have been found to increase the risk of developing AF through distinct genetic mechanisms. This diversity is likely to contribute to the genetic heterogeneity of AF and the differential response to therapies. The extent to which genetic variants, or combinations of genetic variants,27,28 with variable penetrance,29 determine susceptibility to AF is an area of active investigation.
Candidate-gene studies
The identification of KCNQ1 as an AF-susceptibility gene led investigators to consider genes encoding other potassium and cardiac ion channels as candidates, and several rare variants have now been identified in AF probands and their families using this approach. In 2007, investigators examined 50 families with a history of AF and identified a single mutation (R14C) in KCNQ1 in one family.30 The R14C mutation had no direct effect on KCNQ1 or KCNE1 current amplitudes in cultured cells at baseline, but upon exposure to hypotonic solution, the mutant channels exhibited a marked increase in current amplitude compared with wild-type channels.30 Interestingly, only those patients with left atrial dilatation developed AF. We have shown that the risk of developing AF markedly increases (odds ratio [OR] 12–26) when a rare AF variant interacts with common AF risk alleles at the 4q25 locus.29 Taken together, these data support the idea of a ‘two-hit’ hypothesis—the combination of a genetic variant with additional risk factors, such as left atrial dilatation or other genomic variants, is important in AF pathogenesis (Figure 2).30
Figure 2.
The ‘two-hit’ hypothesis states that a combination of a genetic (blue) and an acquired (green) risk factor is required for the development of atrial fibrillation.
Association studies
Most patients with AF have one or more identifiable risk factors, such as hypertension or structural heart disease; however, many patients with these risk factors do not develop AF. Thus, a working hypothesis is that genetic determinants increase AF susceptibility in some individuals with other identifiable risk factors (genetic or acquired)—a basis for the ‘two-hit’ hypothesis. In early genetic association studies, patients with nonfamilial AF were compared with controls and, typically, a small number of variants in candidate genes previously implicated in AF pathogenesis were tested. Subsequently, the GWAS paradigm of surveying the whole genome in an unbiased fashion has been used successfully to identify new genomic loci contributing to AF susceptibility. In 2007, a locus on chromosome 4q25 was identified in a GWAS as being strongly associated with prevalent AF with modest size effects (relative risk [RR] 1.39–1.72).31 The locus is in an intergenic region, but the closest gene is the paired-like homeodomain 2 (PITX2) gene, which is increasingly recognized as being critical for cardiac development. This association has now been replicated in four large populations with ambulatory AF,32 and in individuals with AF after cardiac surgery.33 Subsequently, investigators associated with deCODE genetics, Inc. and the CHARGE (Cohorts for Heart and Ageing Research in Genomic Epidemiology) Consortium identified two additional AF-susceptibility loci on chromosomes 16q2234,35 and 1q2136 using a similar approach (Table 2). A meta-analysis of GWAS of AF confirmed these three SNPs and identified and replicated six additional novel AF-susceptibility alleles (Table 3).37 The identified loci not only implicate transcription factors important in cardiopulmonary development, but also cardiac ion channels and cell-signalling molecules in modulating susceptibility to AF.
Table 2.
| SNP | Locus | Closest gene | Functional effect | Minor/major allele | MAF (%) | Relative risk, 95% CI | P value |
|---|---|---|---|---|---|---|---|
| rs2200733 rs10033464 |
4q25 4q25 |
PITX2 PITX2 |
Development of pulmonary vein myocardial sleeve | C/T G/C |
13.1 25.8 |
1.71, 1.54–2.21 1.42, 1.13–1.77 |
6.1 × 10−41 3.1 × 10−11 |
|
| |||||||
| rs7193343 | 16q22 | ZFHX3 | Unknown | T/C | 17.6 | 1.25, 1.17–1.30 | 1.8 × 10−15 |
|
| |||||||
| rs13376333 | 1q21 | KCNN3 | Calcium-activated potassium channel | T/C | 29.5 | 1.56, 1.38–1.77 | 6.3 × 10−12 |
Abbreviations: AF, atrial fibrillation; MAF, minor-allele frequency in white individuals; SNP, single nucleotide polymorphism.
Table 3.
Summary of genome-wide association study meta-analysis identifying six additional, novel AF loci37
| SNP | Locus | Closest gene | Protein/functional effect | Minor/major allele | MAF (%) | Relative risk, 95% CI | Meta P value |
|---|---|---|---|---|---|---|---|
| rs3903239 | 1q24 | PRRX1 | Development of great vessels | G/A | 44.7 | 1.14, 1.10–1.18 | 9.1 × 10−11 |
| rs3807989 | 7q31 | CAV1 | Atrial signal transduction protein | A/G | 40.4 | 0.88, 0.84–0.91 | 9.6 × 10−11 |
| rs10821415 | 9q22 | C9ORF3 | Unknown | A/C | 42.4 | 1.13, 1.08–1.18 | 7.9 × 10−9 |
| rs10824026 | 10q22 | SYNPO2L | Cardiac protein that localizes to the Z disc | G/A | 15.8 | 0.85, 0.81–0.90 | 1.7 × 10−8 |
| rs1152591 | 14q23 | SYNE2 | Structural protein of the cardiac sarcomere | A/G | 47.6 | 1.13, 1.09–1.18 | 6.2 × 10−10 |
| rs7164883 | 15q24 | HCN4 | Ion channel (If) involved in cardiac pacemaking | G/A | 16.0 | 1.16, 1.10–1.22 | 1.3 × 10−8 |
Abbreviations: AF, atrial fibrillation; MAF, minor-allele frequency in white individuals; SNP, single nucleotide polymorphism.
Insights into genetic mechanisms of AF
Data from linkage and candidate-gene studies
One conceptual model proposed for AF pathogenesis describes reduced atrial refractory period as a substrate for re-entrant arrhythmias.38 This model is supported by reports of gain-of-function mutations in genes encoding subunits of cardiac ion channels responsible for generating IKs (KCNQ1 and KCNE2) and IK1 (KCNJ2); these mutations are predicted to decrease atrial action-potential duration and, therefore, refractoriness.25,39,40 We have identified a novel KCNA5 deletion in a kindred with earlyonset lone familial AF.41 This mutation disrupts a prolinerich motif involved in tyrosine-kinase regulation of the ultra-rapid potassium current (IKur), reduces wild-type current, but renders the channel kinase-resistant. The precise mechanism for AF in this kindred is not certain, and might involve gain-of-function or loss-of-function of IKur (discussed further below) but, importantly, this study established the tyrosine-kinase signalling pathway as a potential therapeutic target in AF.42
In 2008, a frameshift mutation in the NPPA gene was identified in a large family with multiple members affected by AF.26 The mutant ANP caused significant shortening of the monophasic action-potential duration and the effective refractory period in a rat isolated whole-heart Langendorff model.26 Such understanding provides a therapeutic rationale for prolonging the atrial refractory period, but this approach is not universally effective and can lead to proarrhythmia in some patients with AF.10,38 Additional potential mechanisms by which the circulating mutant ANP might cause AF relates to the role of ANP in modulating the immune system and its proinflammatory effects.43–45 Prolonged exposure to the mutant ANP can also lead to electrical and structural remodelling of the atria with resultant atrial fibrosis and conduction slowing, which creates an ‘atrial cardiomyopathic’ re-entrant substrate for AF.46 Support for this mechanism comes from a study in which an autosomal-recessive mutation in NPPA resulted in massive atrial dilatation associated with atrial standstill and the need for a pacemaker in multiple members of an affected family.47
To investigate this mechanism further, we generated a transgenic mouse that overexpressed the human mutant ANP.48 A triple FLAG-tag was fused in-frame with the 3′ end of either the human wild-type NPPA (WT–NPPA–FLAG) cDNA or the mutant NPPA peptide containing the COOH-terminal 12-amino-acid extension (mut–NPPA–FLAG) isolated from individuals with familial AF.26 In vitro assays showed that that the FLAG-tag did not diminish NPPA biological activity. Transoesophageal pacing at 16 weeks induced more and longer-lasting episodes of AF in the mut–NPPA–FLAG mice than in the WT–NPPA–FLAG mice (incidence of AF: 62.5 ± 5.6% versus 30.4 ± 5.7%, P <0.05; total time in AF: 19.1 ± 2.7 s versus 5.3 ± 1.4 s, P <0.05).48 Even more-compelling results were observed in telemetry-monitored NPPA mice when they were challenged with isoproterenol: 67% of the mutant NPPA mice developed AF that persisted for 20 min, whereas wild-type mice remained in sinus rhythm throughout the duration of the isoproterenol infusion.48
Prolongation or shortening of ventricular repolarization (assessed by the QT interval) predisposes to arrhythmias. Similarly, a second major mechanism by which genetic variants can increase susceptibility to AF is by lengthening of the atrial action-potential duration. One study showed that a nonsense mutation in KCNA5 that encodes Kv1.5, a voltage-gated potassium channel expressed in human atrium, translated into action-potential prolongation and early afterdepolarizations in human atrial myocytes. 49 These data also predicted increased vulnerability to stress-induced triggered activity, and carriers of this KCNA5 variant were prone to develop AF when challenged with isoproterenol.49 This postulated mechanism for increased susceptibility to AF is supported by two studies in which investigators discovered loss-of-function mutations in KCNA5 in patients with lone AF.50,51 Therefore, AF-associated mutations are likely to trigger AF by multiple mechanisms other than shortening of the atrial action-potential duration.52,53 The high prevalence of early-onset AF in patients with the congenital long QT syndrome also supports a similar mechanism for AF in these patients.54,55
A third mechanism by which rare ion-channel and signalling-molecule variants might increase susceptibility to AF is through abnormal and heterogeneous disturbance of cell-to-cell impulse propagation. Connexins are proteins that have an important role in electrical coupling between atrial myocytes. Investigators discovered somatic mutations in GJA5, which encodes gap junction α5 protein (also known as connexin-40), in atrial tissue, but not in lymphocytes from patients with lone AF.56 Gap junctions are critical for the intercellular communication between cells, so disruption can lead to heterogeneity of cardiac conduction and increased susceptibility to AF. Furthermore, common polymorphisms in the promoter region of GJA5 have also been associated with AF. The frequency of a common connexin-40 promoter haplotype (−44A, +71G) was significantly higher in individuals with AF than in controls, and functional studies showed that the promoter haplotype was associated with reduced luciferase activity, which is indicative of cardiac conduction heterogeneity.57 The same group demonstrated that the promoter haplotype was associated with decreased activity of two transcription factors: Sp1 and GATA-4.58 Germline mutations have been identified in GJA5 in patients with lone AF, and impairment of cell-to-cell communication has been confirmed in functional studies.59–62 Collectively, these data suggest that rare genetic variants in connexin-40 modulate expression of this gap-junction protein, with reduced expression causing impaired electrical cell-to-cell communication and creating conduction heterogeneity and, therefore, a substrate for AF maintenance. Whereas the early genetic-association studies suggested that the connexin-40 gene promoter –G haplotype was associated with risk of AF, a subsequent study suggests that the –A allele is associated with the arrhythmia.63
The critical role of PITX2 in the development of the pulmonary myocardium has led investigators to examine other developmental genes important for atrial differentiation and cardiac development. A novel interaction was identified between AF and a rare variant (Q76E) within the coding region of gremlin-2 (GREM2; an antagonist of bone morphogenetic protein), which increases its inhibitory activity and cardiac development.64 The Q76E variant was originally identified in the 1000 Genomes Project,65 but its minor allele frequency was significantly higher in our lone AF cohort (0.03% versus 0.5%; P = 0.03).64 Furthermore, functional modelling in zebrafish revealed that, through regulation of bone morphogenetic protein signalling, GREM2 is required for cardiac laterality and atrial differentiation during embryonic development.64 Gremlin-2 overactivity results in a slowed cardiac contraction rate in zebrafish, and induction of previously identified AF-candidate genes encoding ANP, connexin-40, and sarcolipin in mouse differentiated embryonic stem cells.64 Using live heart imaging in zebrafish overexpressing wild-type or variant GREM2, we found abnormal contraction velocity specifically in atrial cardiomyocytes.64 These results implicate, for the first time, regulators of bone morphogenetic protein signalling in human AF, providing mechanistic insights into the pathogenesis of the disease and identifying potential new therapeutic targets.
Positional cloning and candidate-gene approaches have provided novel insights into the genetic mechanisms of AF, but how applicable these mechanisms are to the more-common forms of AF seen in clinical practice is unclear. The identified variants are all rare and most are ‘private’ to a particular kindred.
Data from GWAS
Variants in pulmonary myocardial development
SNPs located in the intergenic region at chromosome 4q25 have been consistently identified in GWAS as being strongly associated with AF.31 The precise mechanism by which these noncoding SNPs modulate AF risk remains unclear, but investigators have discovered multiple susceptibility alleles at this locus that are associated with an increased risk of AF, and demonstrated localization of regulatory elements.66 The chromosome 4q25 locus is located in a genomic ‘desert’; the closest gene (PITX2) is critical for early cardiac development, left-to-right asymmetry, and suppression of a default pathway for sinus node development in the pulmonary vein myocardium.31,67,68 Given that the contemporary approach to treating symptomatic paroxysmal AF entails catheter ablation of pulmonary vein triggers, PITX2C (the cardiac isoform of PITX2) seems to be a logical candidate gene regulated by chromosome 4q25 AF risk alleles. Not only have transgenic mouse models of Pitx2 and Pitx2c shown that these genes are essential for left-to-right asymmetry, but these mice also display increased susceptibility to AF.67–70
In a meta-analysis of data from GWAS of AF,37 one important, novel association with AF was detected on chromosome 1q24 in PRRX1, which encodes a homeodomain transcription factor, also highly expressed in the developing heart. In a Prrx1 knockout mouse model, foetal pulmonary vasculature development was impaired.71 Given that the pulmonary vein myocardial sleeve is targeted during AF ablation,68,72 the functional studies of PITX2 and PRRX1 collectively suggest that one common mechanism by which AF-susceptibility alleles increase AF risk is by modulation of pulmonary myocardial development or function.
Variants modulating cardiac ion channels
PITX2 and PRRX1 are likely to be important in the normal development of the pulmonary myocardium, but the precise mechanism by which they and other AF loci increase risk has not yet been fully elucidated. In transgenic mouse models of Pitx2 suppression, both upregulation of Kcnq1 and differential distribution and expression of the inward-rectifier potassium current IK1 were observed.69,70,73,74
Additional AF-susceptibility loci encoding cardiac ion channels or protein modifiers include the small conductance calcium-activated potassium channel gene KCNN3 on chromosome 1q21;36 the potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel gene HCN4 on chromosome 15q24, which has been linked with sinus node dysfunction (HCN4 protein is the predominant cardiac pacemaker channel in the sinoatrial node);76,77 and the caveolin-1 gene CAV1 on chromosome 7q31, which encodes a cellular membrane protein selectively expressed in the atria and involved in signal transduction.75 Cav1 knockout in mice has been associated with dilated cardiomyopathy.78 Importantly, the caveolin-1 protein colocalizes with, and negatively regulates the activity of, KCNH2 protein, a potassium channel involved in cardiac repolarization. Indeed, KCNH2 has been associated with AF in a candidate-gene association study.79
In the first GWAS, common AF variants were discovered on chromosome 4q25 that associated with a modest effect size (RR 1.4–1.7), whereas loci with smaller effects have been identified in subsequent GWAS (OR 1.1–1.2; Tables 2 and 3).31 In 2009, two separate groups identified common risk alleles on chromosome 16q22 that associated with prevalent and incident AF (OR 1.1–1.2).34,35 Both SNPs are close to the gene that encodes the zinc finger homeobox protein 3 (ZFHX3). Similarly to PITX2, ZFHX3 (also known as AT motif binding-factor 1) is a transcription factor that regulates skeletal muscle and neuronal development, with variable expression in many tissues, including the heart.80–83 Interestingly, ZFHX3 regulates the transcription of the POU1F1 gene (encoding POU class 1 homeobox 1), which not only facilitates DNA binding, but also modulates transcriptional activity of PITX2.84 ZFHX3 might also mediate its effect on the risk of AF by modulating oxidative stress, as discussed below.
Variants modulating atrial fibrosis
SNPs associated with AF might have a role in atrial structural remodelling processes (Table 3). For example, the role of PITX2 in cardiac development raises the possibility that its dysfunction could cause atrial structural remodelling.70 Atrial-specific reduced Pitx2c expression results in atrial enlargement in mice. Pitx2c+/− mice have structurally normal hearts, but microarray analysis has shown differential expression of genes involved in Wnt signalling, a key fibrosis signalling pathway.69 In gene-expression studies, the greatest enrichment was in collagen and other extracellular-matrix genes.69
ZFHX3, a tumour-suppressor gene, promotes survival of neurons by inducing the expression of platelet-derived growth factor receptor (PDGFR)-β and protecting against oxidative stress.85 The gene associates with runt-related transcription factor 3 (RUNX3) and translocates in response to transforming growth factor (TGF)-β signalling, an important mediator of fibrosis.86,87 Given that inflammation and oxidative stress are important in the pathogenesis of AF,88 ZFHX3 might increase susceptibility to AF by modulating these pathways.
CAV1 is strongly implicated in myocardial fibrosis, and variants in this gene have been associated with pulmonary hypertension.89 TGF-β receptors are internalized by caveolin-1-associated lipid rafts to inhibit TGF-β signalling. 90 PRRX1, a paired homeobox transcriptional coactivator that induces genes involved in growth and differentiation, is required for normal lung development, ultimately regulates PDGFR, and is linked to pulmonary and liver fibrosis.91,92 C9ORF3 shows increased expression in normal and scleroderma fibroblasts stimulated with TGF-β.93
SYNPO2L encodes a cytoskeletal, heart-enriched, actin-associated protein. Knock-down of this gene in zebrafish caused aberrant cardiac and skeletal muscle development and function.94 SYNE2 encodes nesprin-2 that, with nesprin-1, forms a network in muscle linking the nucleo-skeleton to nuclear membrane structures and the actin cytoskeleton. α-Catenin interacts with nesprin-2 and emerin to regulate Wnt signalling-dependent transcription, a pathway implicated in fibrosis in the heart, kidney, and lung.95,96 Taken together, considerable evidence suggests that many common AF-susceptibility variants have the potential to modulate atrial fibrosis. Additionally, all these risk variants are likely to mediate their effect not only by regulating atrial conduction slowing, but also by modulating electrical remodelling processes that promote AF, such as shortening of the effective refractory period.
Clinical application of genetic data
Elucidating the underlying genetic mechanisms of AF in an individual patient not only allows a mechanism-based approach to treatment, but might also allow tailored therapy with improved efficacy and a reduced risk of adverse effects. The rare ion-channel and other variants that have been identified in patients with AF have obvious (but, as yet, untested) therapeutic implications. Patients with AF who harbour rare, characterized, loss-of-function, potassium-channel variants are likely to benefit from a drug that blocks potassium channels, such as sotalol. By contrast, sodium-channel blockers would be ineffective and possibly proarrhythmic in patients with rare variants in SCN5A or its β-subunits.
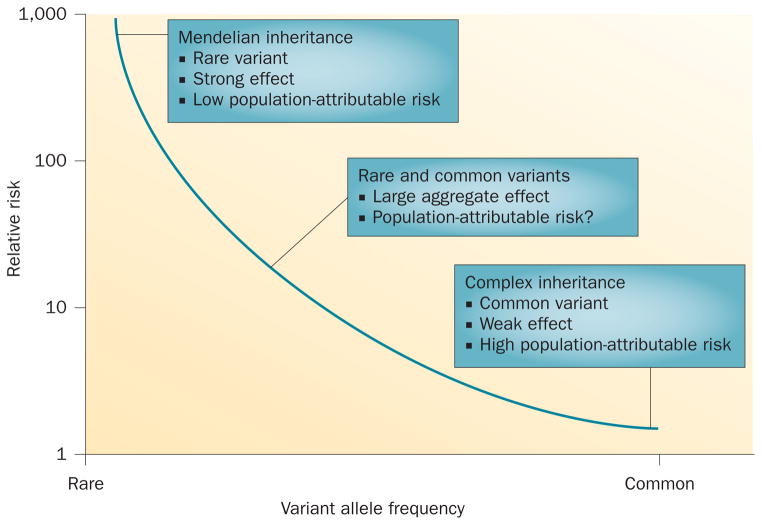
AF variants in genes encoding cardiac ion channels are associated with large effect sizes (Figure 3), but are rare97 and occur mostly in individuals with early-onset AF. Therefore, genotype-directed therapy would not be widely applicable to most patients with the arrhythmia. Conversely, common genetic variants identified by GWAS are likely to have a much greater aggregate effect, especially if combinations of common and rare AF variants modulate AF risk (Figure 3), as our initial studies suggest.29 However, genomic predictors of response to therapies for AF have been examined in very few studies, often limited by their retrospective design and small sample size. When combined with the challenges of defining therapeutic efficacy, few results have been independently validated.98 Furthermore, limited understanding about underlying genetic mechanisms also remains a major barrier to a therapeutic strategy grounded on genotype. As discussed above, the effect sizes associated with common AF-susceptibility variants are only modest (OR 1.1–1.7) and probably insufficient to contemplate therapy that is genotype-driven.98
Figure 3.
Allele frequencies and risk in families and populations. The combination of rare and common variants of atrial fibrillation susceptibility genes might have a large aggregate risk for the development of this arrhythmia.
Variability in response to pharmacological therapy is universally accepted in clinical medicine. Recognizing that genetic factors can have an important role in modulating drug responses, the National Institutes of Health formed the Pharmacogenomics Research Network in 2000.99,100 The goals of this network are to examine the role of genetic factors in modulating response to drugs and to determine whether this information can be used to improve the prescription of available drugs and identify novel therapeutic pathways. An important requirement to conduct pharmacogenomic studies is the recruitment of a large number of patients with well-characterized drug-response phenotypes. One project within the network is to establish a DNA repository for the large Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial,101 in which two major approaches for the management of AF—ablation and drugs to maintain sinus rhythm—will be compared. CABANA gene is a repository for appropriately consented DNA samples from patients in the CABANA trial, which, together with well-studied drug-response phenotypes, will allow investigators to address questions such as which patients are most likely to respond to, or develop complications with, ablation or drug therapy.
Assessing response to drug therapy
Much progress has been made in our understanding of the genetic basis of AF, but the translation of this genetic knowledge to the care of patients has so far been limited. Reasons for this include the poorly defined relationship between the response to drug therapies (especially antiarrhythmic drugs) and genetic variation, the lack of randomized clinical trials with patients selected for pharmacological therapies on the basis of their genotype, and limited knowledge about genetic predictors of drug response in general. Several common AF-susceptibility alleles have been identified and validated in GWAS, but limited data exist regarding their modulatory effect on response to pharmacological and ablation therapies.
One of the major reasons why studies to evaluate the response to pharmacological therapies, especially antiarrhythmic drugs, have been difficult to perform relates to the lack of well-defined end points to measure efficacy of these drugs. Owing to historical limitations in noninvasive ambulatory monitoring technology and a lack of reliable algorithms to identify and provide quantitative summaries for specific arrhythmias, AF burden has generally not been used as a clinical or study end point. One widely used, symptom-based metric is ‘time to first symptomatic AF episode’, but this measure correlates poorly with frequency of symptomatic episodes.102 The unreliability of symptoms to estimate AF burden and to identify patients with or without AF has been revealed not only in trials of pacemakers, but also in patients without implanted devices.
Technological advances that allow the continuous monitoring of cardiac rhythm have now made possible the rigorous definition of AF burden as a surrogate end point for AF therapy.103,104 AF burden can now be measured using novel, full-disclosure, ambulatory monitoring devices that can store up to 30 days of continuous electrocardiogram tracings. The arrhythmia algorithm analyses consecutive 3.5 s intervals and can discriminate between complex supraventricular rhythms including AF and atrial flutter. Furthermore, these devices not only transmit continuously in real-time through a mobile telephone network, but also provide remote access to quantitative statistical reports including measurement of AF and atrial flutter burden. AF burden measured in this way might be a more-robust end point for AF therapy than previous measures because it is less subject to investigator bias and does not have the sampling error associated with episodic monitoring or reliance on patient symptoms.105–107 Furthermore, AF burden is favoured to become the metric of choice to assess not only response to antiarrhythmic drugs, but also monitoring after AF-ablation therapy.108 Implantable recording devices do exist, but are impractical for research studies given all the inherent factors accompanying an elective, invasive procedure. Novel, noninvasive methods for long-term monitoring might hold an answer to this issue.
Genetic variants and therapy response
Despite overall advances in treatments for arrhythmias, therapeutic options for most patients with AF have remained largely unchanged. Targeting pulmonary vein triggers with radiofrequency ablation has been successful for paroxysmal AF, but the success rate for persistent AF has remained poor and, for this group of patients, pharmacological therapies remain the mainstay of treatment. Advances in our understanding of the molecular mechanisms of AF support the idea that variability in the response to drug therapy might reflect differences in underlying disease mechanisms (Table 4).
Table 4.
Common genetic polymorphisms that modulate the response to therapies for AF
| Gene or SNP | Study design | Primary end point | Results | Replication? |
|---|---|---|---|---|
| Rhythm-control therapy | ||||
| Angiotensin-converting enzyme I/D | Response to conventional antiarrhythmic drugs98 | Adequate response defined as ≥75% reduction in symptomatic AF burden | Lone AF and DD or ID genotypes are highly significant predictors of failure of antiarrhythmic-drug therapy | No |
| β1-Adrenergic receptor polymorphisms (G389R, S49G) | Impact of genotypes on antiarrhythmic-drug action of flecainide in patients with AF110 | Resting heart rate and success of flecainide-induced cardioversion | β1-Adrenergic receptor Arg389Arg genotype is associated with increased flecainide potency (OR 3.30, 95% CI 1.34–8.13, P= 0.003) and increased heart rate during AF | Yes |
| 4q25: rs2200733, rs10033464 16q22: rs7193343 1q21: rs13376333 |
Response to antiarrhythmic drugs modulated by three common AF loci109 | Successful rhythm control if remained taking same antiarrhythmic drug with ≥75% reduction in symptomatic AF burden | rs10033464 independently predicts AF recurrence (OR 3.27, 95% CI 1.7–6.0, P<0.001) | Yes |
| 4q25: rs2200733, rs10033464 | Response to ablation therapy111,112 | Early and late recurrence of AF after ablation | Any variant allele increases early (OR 1.99, 95% CI 1.04–3.84, P = 0.039) and late (OR 4.18, 95% CI 1.32–12.66, P=0.011) AF recurrence | Yes |
| 4q25: rs2200733, rs10033464 16q22: rs7193343 1q21: rs13376333 |
Evaluate whether AF after successful direct-current cardioversion is modulated by common AF loci113 | AF recurrence after successful direct-current cardioversion | Any common SNP (rs2200733, rs10033464) at the 4q25 locus independently predicts AF recurrence (HR 2.1, 95% CI 1.21–3.30, P=0.008) | No |
| Rate-control therapy | ||||
| β1-Adrenergic receptor polymorphisms (G389R, S49G) | Impact of genotypes on ventricular rate-control therapy114,110 | ‘Responders’ displayed adequate ventricular rate control (assessed using AFFIRM criteria*) | G389R more likely to respond favourably to rate-control therapy than R389R (OR 1.42, 95% CI 1.00–2.03, P<0.05) | Yes |
AFFIRM criteria: average heart rate at rest ≤80 bpm and maximum heart rate during a 6 min walking test ≤110 bpm, or average heart rate during 24 h Holter monitoring ≤100 bpm.
Abbreviations: AF, atrial fibrillation; SNP, single nucleotide polymorphism.
The underlying mechanisms by which common variants on chromosomes 1q21, 4q25, and 16q22 increase susceptibility to AF are poorly defined, but they might also modulate the response to antiarrhythmic drugs. One study showed that carrying the reference 4q25 genotype was independently associated with an improved response to class I or II antiarrhythmic drugs (OR 4.7) with 83% of patients maintaining sinus rhythm.109 This association was validated in a replication cohort (72% achieved successful rhythm control).109 These findings are intriguing and might pave the way for a randomized, genotype-directed trial. Another study showed that a β1-adrenergic receptor polymorphism (Arg389Gly) was associated with flecainide efficacy and increased heart rate during AF.110 As discussed below, we have confirmed the finding that homozygotes for the 389Arg variant have an increased ventricular rate during AF, and that the Arg389Arg genotype requires intensified β-blockade treatment for rate control.111
Ablation therapies
Common AF-susceptibility alleles might be used to identify genetic subtypes of AF with differential responses to ablation therapy. In 2010, Husser et al. evaluated whether the common AF-susceptibility SNPs on the chromosome 4q25 locus modulated the response to catheter ablation.112 They reported that 21% of patients had a late recurrence of AF and no clinical or echocardiographic parameters predicted the response. However, the presence of one of the 4q25 variant SNPs did associate with AF recurrence after catheter ablation (OR 4.1). This finding was replicated in a large cohort of patients with ‘typical’ AF (associated with known risk factors), where the overall recurrence rate was 53% over an 18-month period.113 The presence of the 4q25 SNP risk allele predicted a 24% shorter recurrence-free time (survival time ratio 0.76) than did the presence of the wild-type SNP.113 The ability to risk stratify individuals on the basis of preprocedural characteristics, such as genotype, is highly desirable given that the procedure can be associated with increased morbidity and, with some genetic variants, increased mortality.
Individuals carrying the common AF-susceptibility alleles on chromosome 4q25 might belong to a genetic subgroup of AF with common underlying genetic mechanisms. If so, these SNPs might also be independent predictors of AF recurrence after direct-current cardioversion. Genetic predictors of AF recurrence at the three loci (1q21, 4q25, and 16q22) were prospectively evaluated after successful direct-current cardioversion. In 208 patients who underwent electrical cardioversion, a common polymorphism on chromosome 4q25 was an independent predictor of AF recurrence after restoration of sinus rhythm, which occurred in 67% of the patients during follow-up (median 60 days; hazard ratio 2.1).114 A potential role for stratification by genotype is indicated.
Drug therapies
Rate-control therapy is considered to be the first choice for many patients with AF, especially those with an increased risk of AF recurrence or adverse effects from antiarrhythmic drugs. However, identification of genetic or clinical predictors of adequate response to rate-control therapy has been challenging. A nonsynonymous SNP (Arg389Gly) in the β1-adrenergic receptor is known to modulate the outcome of β-blockade in heart failure, which indicates its involvement in other cardiac phenotypes. Therefore, we evaluated the impact of clinical factors and two common β1-adrenergic receptor polymorphisms (Arg389Gly and Ser49Gly) on response to ventricular rate-control therapy in patients with AF.111 We studied 543 patients prospectively enrolled in the Vanderbilt AF registry and managed with rate-control therapy. A ‘responder’ displayed adequate ventricular rate-control assessed by the AFFIRM criteria.115 A ‘nonresponder’ displayed uncontrolled ventricular rates necessitating a change in therapy and, in our cohort, 54.3% were responders. Baseline clinical characteristics were similar in responders and nonresponders, except for mean resting heart rate (76 ± 20 bpm versus 70 ± 15 bpm; P <0.01) and smoking (5.8% versus 1.2%; P <0.01). Multiple clinical variables (such as age, sex, and hypertension) failed to predict response to rate-control therapy. By contrast, the Arg389Gly variant was significantly associated with adequate rate control (OR 1.44).111 This association persisted after correction for multiple clinical factors (OR 1.42).111 Therefore, this common β1-adrenergic receptor polymorphism modulates the response to rate-control therapy in patients with AF.
We also performed a GWAS to evaluate genetic determinants of rate-control therapy.116 Patients with AF who responded effectively were compared with those who responded ineffectively to ventricular rate-control therapy. ‘Cases’ were defined as those patients whose ventricular rates were not adequately controlled with three or more atrioventricular nodal drugs necessitating atrioventricular nodal ablation and pacemaker implantation (n = 95). ‘Controls’ were defined as those patients in whom the ventricular rates met the AFFIRM rate-control efficacy criteria with two or fewer atrioventricular nodal blocking agents (n = 192). Genotype association with failure to respond to three or more atrioventricular nodal blockers was assessed after correction for age and sex. Loci with multiple SNPs at or near genome-wide significance were identified within three genes: MYO7A on chromosome 11 (P = 5.29 × 10−6), SOX5 on chromosome 12 (P = 5.48 × 10−6), and LANCL2 on chromosome 7 (P = 2.87 × 10−5). SOX5 is a transcription factor involved in the regulation of embryonic development and cell fate. Importantly, this gene is expressed in the heart, and data from GWAS have implicated SNPs in SOX5 as modulators of the PR interval.117 A preliminary analysis of a replication cohort validates this locus as an important modulator of rate-control therapy in patients with AF. Identification of genomic predictors of the response to rate-control therapy in patients with AF will not only identify novel genes that modulate atrioventricular nodal conduction, but also support drug development for the management of patients with AF.
Implications for clinical practice
Positional cloning and candidate-gene approaches have identified rare variants with large effects, whereas common AF-susceptibility loci with smaller effects have been identified in GWAS, but these approaches collectively explain only a small fraction of the heritability of AF (Figure 3).118,119 This shortfall raises the possibility that the development of AF is determined by combinations of rare and common AF-susceptibility variants.29 As discussed above, the risk of developing AF markedly increases (OR 12–26) when a rare AF variant combines with a common risk allele at the chromosome 4q25 locus.29
The direct application of genetic data to the care of patients with AF has, so far, been limited. In addition to those discussed above, other possible explanations for this shortcoming include the small effect sizes associated with the AF-susceptibility alleles, small numbers in some studies, and lack of replication to date. Interestingly, when the three AF risk alleles on chromosomes 1q21, 4q25, and 16q22 were examined for their effect on modulating response to antiarrhythmic drugs, ablation therapy, and direct-current cardioversion, the effect sizes were considerably greater than those seen in prediction of AF itself in the general population.112–114 A reluctance to translate these genetic data to the bedside might also result from the inherent limitations of retrospective studies and the lack of prospective, randomized clinical trials to validate these common AF risk alleles as important modulators of the response to AF therapies.
Future directions
The field of AF genomics has moved spectacularly fast—the first GWAS data were reported in 2007, and all the variants with modest effect sizes identified in other GWAS, and the vast majority of rarer variants with larger effect sizes, have been reported subsequently. Consideration of how these data might be deployed in clinical practice is very appealing, but substantial barriers must be overcome before such a vision can become a reality. Firstly, in an era of unprecedented discovery, deployment of known markers might be premature when novel ones remain to be discovered. The latest, very large meta-analysis probably means that most of the genes in which common variation drives AF susceptibility have now been identified. Therefore, assessment of how combinations of clinical and genomic factors predict the development of AF might be timely. Specifically, to what extent does genomic variation add to ordinary predictors, such as hypertension or family history? Studies to address this issue might be feasible in large population cohorts. In this regard, reports of effect sizes of combinations of variants require both replication as well as the discovery of novel, rare variants in kindreds.
Secondly, reports published to date of the way in which genomic variation predicts drug effects should be considered preliminary and in need of replication. Thirdly, after variants that predict drug effects have been replicated, appropriately designed trials to evaluate their effect size and potential impact on choice of therapy will need to be conducted. The design could be a randomized clinical trial, but alternative designs (such as adaptive trials) could be considered to maximize sample size.
Conclusions
Great progress has been made in understanding the genetic basis of AF, with important insights being made into the underlying genetic mechanisms, but the direct application of this knowledge to the care of patients with AF associated with known risk factors has, so far, not occurred. This barrier might relate to the challenges associated with defining the efficacy of pharmacological therapies, but is also a result of the retrospective and observational nature of the studies demonstrating common AF-susceptibility alleles that modulate the response to antiarrhythmic drugs, catheter ablation, and direct-current cardioversion. However, an emerging body of evidence clearly indicates that the underlying substrate for AF in individuals who are carriers of the common chromosome 4q25 SNPs respond to pharmacological and other therapies differently from individuals without these SNPs. These patients might constitute a genetic subgroup of AF, but the only definitive way to determine whether these SNPs modulate the response to therapies is to perform appropriately designed, genotype-driven clinical trials.
Key points.
The incidence of atrial fibrillation (AF) is rising, with approximately 16 million of the US population projected to develop AF by 2050
Limitations of current therapies for AF have spurred research into understanding the genetic basis of this arrhythmia
Positional cloning and candidate-gene approaches have linked rare genetic variants in ion channels, gap-junction proteins, and signalling molecules with the development of AF
Genome-wide association studies have identified nine commonly occurring AF-susceptibility loci associated with cardiopulmonary development, cardiac ion channels, and cell-signalling molecules that might be important in the pathogenesis of AF
Both common and rare genetic variants have provided insights into AF genetic mechanisms, but the direct impact of this understanding on the management of patients has been limited
Exploiting the genetic mechanisms of AF to prescribe personalized therapy is an important goal, but clinical trials are needed to determine whether genotype-directed treatment of AF is viable
Acknowledgments
This work was supported by NIH grants (U19 HL65962 and R01 HL092217), and an AHA Established Investigator Award (0940116N).
Footnotes
Competing interests
D. Darbar declares associations with the following organizations: the AHA and the NIH. D. M. Roden declares an association with the following company: Clinical Data, Inc. See the article online for full details of the relationships.
Author contributions
Both authors researched data for the article and contributed substantially to discussions of its content. D. Darbar wrote the manuscript, and both authors reviewed/edited it before submission.
References
- 1.Lloyd-Jones DM, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Darbar D, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185–2192. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 5.Piccini JP, et al. Pharmacotherapy in Medicare beneficiaries with atrial fibrillation. Heart Rhythm. 2012;9:1403–1408. doi: 10.1016/j.hrthm.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyse DG, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 7.Van Gelder IC, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 8.Opolski G, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) study. Chest. 2004;126:476–486. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 9.Corley SD, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 10.Fuster V, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to revise the 2001 guidelines for the management of patients with atrial fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin EJ, et al. Prevention of atrial fibrillation: report from a national Heart, Lung, and Blood Institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darbar D. Genetics of atrial fibrillation: rare mutations, common polymorphisms, and clinical relevance. Heart Rhythm. 2008;5:483–486. doi: 10.1016/j.hrthm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff L. Familial auricular fibrillation. N Engl J Med. 1943;229:396–398. [Google Scholar]
- 14.Ellinor PT, Yoerger DM, Ruskin JN, Macrae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 15.Arnar DO, et al. Familial aggregation of atrial fibrillation in Iceland. Eur Heart J. 2006;27:708–712. doi: 10.1093/eurheartj/ehi727. [DOI] [PubMed] [Google Scholar]
- 16.Christophersen IE, et al. Familial aggregation of atrial fibrillation: a study in Danish twins. Circ Arrhythm Electrophysiol. 2009;2:378–383. doi: 10.1161/CIRCEP.108.786665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox CS, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 18.Marcus GM, et al. A first-degree family history in lone atrial fibrillation patients. Heart Rhythm. 2008;5:826–830. doi: 10.1016/j.hrthm.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubitz SA, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304:2263–2269. doi: 10.1001/jama.2010.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brugada R, et al. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–911. doi: 10.1056/NEJM199703273361302. [DOI] [PubMed] [Google Scholar]
- 21.Ellinor PT, Shin JT, Moore RK, Yoerger DM, MacRae CA. Locus for atrial fibrillation maps to chromosome 6q14-16. Circulation. 2003;107:2880–2883. doi: 10.1161/01.CIR.0000077910.80718.49. [DOI] [PubMed] [Google Scholar]
- 22.Schott JJ, Probst V, Mabo P. A new locus for atrial fibrillation maps to chromosome 20q12-13. Circulation. 2004;110:1245. [Google Scholar]
- 23.Oberti C, et al. Genome-wide linkage scan identifies a novel genetic locus on chromosome 5p13 for neonatal atrial fibrillation associated with sudden death and variable cardiomyopathy. Circulation. 2004;110:3753–3759. doi: 10.1161/01.CIR.0000150333.87176.C7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volders PG, et al. Mapping a novel locus for familial atrial fibrillation on chromosome 10p11-q21. Heart Rhythm. 2007;4:469–475. doi: 10.1016/j.hrthm.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Chen YH, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 26.Hodgson-Zingman DM, et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–165. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann SA, et al. Epistatic effects of potassium channel variation on cardiac repolarization and atrial fibrillation risk. J Am Coll Cardiol. 2012;59:1017–1025. doi: 10.1016/j.jacc.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 28.Darbar D, Parvez B, Abraham R. Repolarization recipes for atrial fibrillation: beyond single channel variants. J Am Coll Cardiol. 2012;59:1026–1028. doi: 10.1016/j.jacc.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie MD, et al. Chromosome 4q25 variants are genetic modifiers of rare ion channel mutations associated with familial atrial fibrillation. J Am Coll Cardiol. 2012;60:1173–1181. doi: 10.1016/j.jacc.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otway R, et al. Stretch-sensitive KCNQ1 mutation: a link between genetic and environmental factors in the pathogenesis of atrial fibrillation? J Am Coll Cardiol. 2007;49:578–586. doi: 10.1016/j.jacc.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 31.Gudbjartsson DF, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;488:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 32.Kaab S, et al. Large scale replication and meta-analysis of variants on chromosome 4q25 associated with atrial fibrillation. Eur Heart J. 2009;30:813–819. doi: 10.1093/eurheartj/ehn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Body SC, et al. Variation in the 4q25 chromosomal locus predicts atrial fibrillation after coronary artery bypass graft surgery. Circ Cardiovasc Genet. 2009;2:499–506. doi: 10.1161/CIRCGENETICS.109.849075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gudbjartsson DF, et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;8:876–878. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamin EJ, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellinor PT, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellinor PT, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 39.Xia M, et al. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332:1012–1019. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, et al. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75:899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang T, Yang P, Roden DM, Darbar D. Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart Rhythm. 2010;7:1246–1252. doi: 10.1016/j.hrthm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinlapawittayatorn K, Deschenes I. Alteration of tyrosine kinase signaling: another player in the arrhythmogenesis of atrial fibrillation? Heart Rhythm. 2010;7:1253–1254. doi: 10.1016/j.hrthm.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Chung MK, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 44.Vollmar AM. The role of atrial natriuretic peptide in the immune system. Peptides. 2005;26:1086–1094. doi: 10.1016/j.peptides.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 45.Roberts JD, Gollob MH. Impact of genetic discoveries on the classification of lone atrial fibrillation. J Am Coll Cardiol. 2010;55:705–712. doi: 10.1016/j.jacc.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 47.Disertori M, et al. Autosomal recessive atrial dilated cardiomyopathy with standstill evolution associated with mutation of natriuretic peptide precursor A. Circ Cardiovasc Genet. 2013;6:27–36. doi: 10.1161/CIRCGENETICS.112.963520. [DOI] [PubMed] [Google Scholar]
- 48.Savio-Galimberti E, et al. NPPA overexpression in mice increases susceptibility to atrial fibrillation [abstract] Circulation. 2012;126:A19074. [Google Scholar]
- 49.Olson TM, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, et al. Novel KCNA5 loss-of-function mutations responsible for atrial fibrillation. J Hum Genet. 2009;54:277–283. doi: 10.1038/jhg.2009.26. [DOI] [PubMed] [Google Scholar]
- 51.Christophersen IE, et al. Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Eur Heart J. doi: 10.1093/eurheartj/ehs442. http://dx.doi.org/10.1093/eurheartj/ehs442. [DOI] [PubMed]
- 52.Satoh T, Zipes DP. Cesium-induced atrial tachycardia degenerating into atrial fibrillation in dogs: atrial torsades de pointes? J Cardiovasc Electrophysiol. 1998;9:970–975. doi: 10.1111/j.1540-8167.1998.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 53.Ehrlich JR, Zicha S, Coutu P, Hebert TE, Nattel S. Atrial fibrillation-associated minK38G/S. polymorphism modulates delayed rectifier current and membrane localization. Cardiovasc Res. 2005;67:520–528. doi: 10.1016/j.cardiores.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Johnson JN, et al. Prevalence of early-onset atrial fibrillation in congenital long QT syndrome. Heart Rhythm. 2008;5:704–709. doi: 10.1016/j.hrthm.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemoine MD, et al. Arrhythmogenic left atrial cellular electrophysiology in a murine genetic long QT syndrome model. Cardiovasc Res. 2011;92:67–74. doi: 10.1093/cvr/cvr166. [DOI] [PubMed] [Google Scholar]
- 56.Gollob MH, et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354:2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- 57.Firouzi M, et al. Association of human connexin 40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circ Res. 2004;95:e29–e33. doi: 10.1161/01.RES.0000141134.64811.0a. [DOI] [PubMed] [Google Scholar]
- 58.Firouzi M, et al. The human Cx40 promoter polymorphism −44G→A differentially affects transcriptional regulation by Sp1 and GATA4. Biochim Biophys Acta. 2006;1759:491–496. doi: 10.1016/j.bbaexp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Yang YQ, et al. Novel connexin40 missense mutations in patients with familial atrial fibrillation. Europace. 2010;12:1421–1427. doi: 10.1093/europace/euq274. [DOI] [PubMed] [Google Scholar]
- 60.Yang YQ, et al. Connexin40 nonsense mutation in familial atrial fibrillation. Int J Mol Med. 2010;26:605–610. doi: 10.3892/ijmm_00000505. [DOI] [PubMed] [Google Scholar]
- 61.Gu JY, Xu JH, Yu H, Yang YQ. Novel GATA5 loss-of-function mutations underlie familial atrial fibrillation. Clinics (Sao Paulo) 2012;67:1393–1399. doi: 10.6061/clinics/2012(12)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun Y, et al. Novel germline GJA5/connexin40 mutations associated with lone atrial fibrillation impair gap junctional intercellular communication. Hum Mutat. 2013;34:603–609. doi: 10.1002/humu.22278. [DOI] [PubMed] [Google Scholar]
- 63.Christophersen IE, et al. Rare variants in GJA5 are associated with early-onset lone atrial fibrillation. Can J Cardiol. 2013;29:111–116. doi: 10.1016/j.cjca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Muller II, et al. Use of a zebrafish model demonstrates that atrial fibrillation-associated gene GREM2 regulates cardiac laterality, cardiomyocyte differentiation and atrial rhythm. Dis Model Mech. 2013;6:332–341. doi: 10.1242/dmm.010488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lubitz SA, et al. Independent susceptibility markers for atrial fibrillation on chromosome 4q25. Circulation. 122:976–984. doi: 10.1161/CIRCULATIONAHA.109.886440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mommersteeg MT, et al. Pitx2c and Nkx2–5 are required for the formation and identity of the pulmonary myocardium. Circ Res. 2007;101:902–909. doi: 10.1161/CIRCRESAHA.107.161182. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, et al. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc Natl Acad Sci USA. 2010;107:9753–9758. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirchhof P, et al. PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ Cardiovasc Genet. 2011;4:123–133. doi: 10.1161/CIRCGENETICS.110.958058. [DOI] [PubMed] [Google Scholar]
- 70.Chinchilla A, et al. PITX2 insufficiency leads to atrial electrical and structural remodeling linked to arrhythmogenesis. Circ Cardiovasc Genet. 2011;4:269–279. doi: 10.1161/CIRCGENETICS.110.958116. [DOI] [PubMed] [Google Scholar]
- 71.Ihida-Stansbury K, et al. Paired-related homeobox gene Prx1 is required for pulmonary vascular development. Circ Res. 2004;94:1507–1514. doi: 10.1161/01.RES.0000130656.72424.20. [DOI] [PubMed] [Google Scholar]
- 72.Haissaguerre M, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 73.Pandit SV, et al. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voigt N, et al. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:472–480. doi: 10.1161/CIRCEP.110.954636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volonte D, McTiernan CF, Drab M, Kasper M, Galbiati F. Caveolin-1 and caveolin-3 form heterooligomeric complexes in atrial cardiac myocytes that are required for doxorubicin-induced apoptosis. Am J Physiol Heart Circ Physiol. 2008;294:H392–H401. doi: 10.1152/ajpheart.01039.2007. [DOI] [PubMed] [Google Scholar]
- 76.Stieber J, et al. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA. 2003;100:15235–15240. doi: 10.1073/pnas.2434235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nof E, et al. Point mutation in the HCN4 cardiac ion channel pore affecting synthesis, trafficking, and functional expression is associated with familial asymptomatic sinus bradycardia. Circulation. 2007;116:463–470. doi: 10.1161/CIRCULATIONAHA.107.706887. [DOI] [PubMed] [Google Scholar]
- 78.Zhao YY, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sinner MF, et al. The non-synonymous coding IKr-channel variant KCNH2-K897T is associated with atrial fibrillation: results from a systematic candidate gene-based analysis of KCNH2 (HERG) Eur Heart J. 2008;29:907–914. doi: 10.1093/eurheartj/ehm619. [DOI] [PubMed] [Google Scholar]
- 80.Berry FB, et al. Positive and negative regulation of myogenic differentiation of C2C12 cells by isoforms of the multiple homeodomain zinc finger transcription factor ATBF1. J Biol Chem. 2001;276:25057–25065. doi: 10.1074/jbc.M010378200. [DOI] [PubMed] [Google Scholar]
- 81.Sun X, et al. Frequent somatic mutations of the transcription factor ATBF1 in human prostate cancer. Nat Genet. 2005;37:407–412. doi: 10.1038/ng1528. [DOI] [PubMed] [Google Scholar]
- 82.Burgner D, et al. A genome-wide association study identifies novel and functionally related susceptibility loci for Kawasaki disease. PLoS Genet. 2009;5:e1000319. doi: 10.1371/journal.pgen.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lubitz SA, et al. Genetics of atrial fibrillation: implications for future research directions and personalized medicine. Circ Arrhythm Electrophysiol. 2010;3:291–299. doi: 10.1161/CIRCEP.110.942441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qi Y, et al. Atbf1 is required for the Pit1 gene early activation. Proc Natl Acad Sci USA. 2008;105:2481–2486. doi: 10.1073/pnas.0712196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim TS, et al. The ZFHX3 (ATBF1) transcription factor induces PDGFRB, which activates ATM in the cytoplasm to protect cerebellar neurons from oxidative stress. Dis Model Mech. 2010;3:752–762. doi: 10.1242/dmm.004689. [DOI] [PubMed] [Google Scholar]
- 86.Verheule S, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-β1. Circ Res. 2004;94:1458–1465. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mabuchi M, et al. Tumor suppressor, AT motif binding factor 1 (ATBF1), translocates to the nucleus with runt domain transcription factor 3 (RUNX3) in response to TGF-β signal transduction. Biochem Biophys Res Commun. 2010;398:321–325. doi: 10.1016/j.bbrc.2010.06.090. [DOI] [PubMed] [Google Scholar]
- 88.Li J, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. doi: 10.1016/j.hrthm.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Austin ED, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5:336–343. doi: 10.1161/CIRCGENETICS.111.961888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Del Galdo F, Lisanti MP, Jimenez SA. Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2008;20:713–719. doi: 10.1097/bor.0b013e3283103d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cohen RI, et al. Ghrelin receptor expression in lymphocytes isolated from adult cystic fibrosis patients. Respiration. 2009;79:141–146. doi: 10.1159/000254486. [DOI] [PubMed] [Google Scholar]
- 92.Fritz D, Stefanovic B. RNA-binding protein RBMS3 is expressed in activated hepatic stellate cells and liver fibrosis and increases expression of transcription factor Prx1. J Mol Biol. 2007;371:585–595. doi: 10.1016/j.jmb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.reSOLVE. Wound Healing and Fibrosis-related Genes. 2013 [online], http://www.resolve-whfg.appspot.com/array/Sargent2010/
- 94.Beqqali A, et al. CHAP is a newly identified Z-disc protein essential for heart and skeletal muscle function. J Cell Sci. 2010;123:1141–1150. doi: 10.1242/jcs.063859. [DOI] [PubMed] [Google Scholar]
- 95.Homer RJ, Herzog EL. Recent advances in pulmonary fibrosis: implications for scleroderma. Curr Opin Rheumatol. 2010;22:683–689. doi: 10.1097/BOR.0b013e32833ddcc9. [DOI] [PubMed] [Google Scholar]
- 96.He W, et al. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ellinor PT, et al. Mutations in the long QT gene, KCNQ1, are an uncommon cause of atrial fibrillation. Heart. 2004;90:1487–1488. doi: 10.1136/hrt.2003.027227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Darbar D, Motsinger AA, Ritchie MD, Gainer JV, Roden DM. Polymorphism modulates symptomatic response to antiarrhythmic drug therapy in patients with lone atrial fibrillation. Heart Rhythm. 2007;4:743–749. doi: 10.1016/j.hrthm.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roden DM, et al. Pharmacogenomics: challenges and opportunities. Ann Intern Med. 2006;145:749–757. doi: 10.7326/0003-4819-145-10-200611210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giacomini KM, et al. The pharmacogenetics research network: from SNP discovery to clinical drug response. Clin Pharmacol Ther. 2007;81:328–345. doi: 10.1038/sj.clpt.6100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.US National Library of Medicine. ClinicalTrials.gov. 2013 [online], http://clinicaltrials.gov/ct2/show/NCT00911508.
- 102.Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol. 2000;4:369–382. doi: 10.1023/a:1009823001707. [DOI] [PubMed] [Google Scholar]
- 103.Friedman PA, et al. Atrial therapies reduce atrial arrhythmia burden in defibrillator patients. Circulation. 2001;104:1023–1028. doi: 10.1161/hc3401.095039. [DOI] [PubMed] [Google Scholar]
- 104.Israel CW, Grönefeld G, Ehrlich JR, Li YG, Hohnloser SH. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43:47–52. doi: 10.1016/j.jacc.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 105.Euler DE, Friedman PA. Atrial arrhythmia burden as an endpoint in clinical trials: is it the best surrogate? Lessons from a multicenter defibrillator trial. Card Electrophysiol Rev. 2003;7:355–358. doi: 10.1023/B:CEPR.0000023138.85821.63. [DOI] [PubMed] [Google Scholar]
- 106.Charitos EI, et al. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation. 2012;126:806–814. doi: 10.1161/CIRCULATIONAHA.112.098079. [DOI] [PubMed] [Google Scholar]
- 107.Hindricks G, Piorkowski C. Atrial fibrillation monitoring: mathematics meets real life. Circulation. 2012;126:791–792. doi: 10.1161/CIRCULATIONAHA.112.124735. [DOI] [PubMed] [Google Scholar]
- 108.Calkins H, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696.e621. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 109.Parvez B, et al. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol. 2012;60:539–545. doi: 10.1016/j.jacc.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nia AM, et al. β1-Adrenoceptor polymorphism predicts flecainide action in patients with atrial fibrillation. PLoS ONE. 2010;5:e11421. doi: 10.1371/journal.pone.0011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parvez B, et al. A common β1-adrenergic receptor polymorphism predicts favorable response to rate-control therapy in atrial fibrillation. J Am Coll Cardiol. 2012;59:49–56. doi: 10.1016/j.jacc.2011.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2010;55:747–753. doi: 10.1016/j.jacc.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 113.Shoemaker BM, et al. Common atrial fibrillation risk alleles at 4q25 predict recurrence after catheter-based atrial fibrillation ablation. Heart Rhythm. 2013;10:394–400. doi: 10.1016/j.hrthm.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parvez B, et al. Common genetic polymorphisms at 4q25 locus predicts predicts atrial fibrillation after successful cardioversion. Heart Rhythm. doi: 10.1016/j.hrthm.2013.02.018. http://dx.doi.org/10.1016/j.hrthm.2013.02.018. [DOI] [PMC free article] [PubMed]
- 115.Olshansky B, et al. The atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study: approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 2004;43:1201–1208. doi: 10.1016/j.jacc.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 116.Muhammad R, et al. Genome-wide association analysis to identify genomic modulators of rate control therapy in patients with atrial fibrillation [abstract] Circulation. 2011;124:A12933. doi: 10.1016/j.amjcard.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pfeufer A, et al. Genome-wide association study of PR interval. Nat Genet. 2010;42:153–159. doi: 10.1038/ng.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manolio TA, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Parvez B, Darbar D. The “missing” link in atrial fibrillation heritability. J Electrocardiol. 2011;44:641–644. doi: 10.1016/j.jelectrocard.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abraham RL, Yang T, Blair M, Roden DM, Darbar D. Augmented potassium current is a shared phenotype for two genetic defects associated with familial atrial fibrillation. J Mol Cell Cardiol. 2010;48:181–190. doi: 10.1016/j.yjmcc.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hong K, et al. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc Res. 2005;68:433–440. doi: 10.1016/j.cardiores.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 122.Lundby A, Ravn LS, Svendsen JH, Olesen SP, Schmitt N. KCNQ1 mutation Q147R is associated with atrial fibrillation and prolonged QT interval. Heart Rhythm. 2007;4:1532–1541. doi: 10.1016/j.hrthm.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 123.Bartos DC, et al. A KCNQ1 mutation causes a high penetrance for familial atrial fibrillation. J Cardiovasc Electrophysiol. doi: 10.1111/jce.12068. http://dx.doi.org/10.1111/jce.12068. [DOI] [PMC free article] [PubMed]
- 124.Henrion U, et al. Overlapping cardiac phenotype associated with a familial mutation in the voltage sensor of the KCNQ1 channel. Cell Physiol Biochem. 2012;29:809–818. doi: 10.1159/000178470. [DOI] [PubMed] [Google Scholar]
- 125.Bartos DC, et al. R231C mutation in KCNQ1 causes long QT syndrome type 1 and familial atrial fibrillation. Heart Rhythm. 2011;8:48–55. doi: 10.1016/j.hrthm.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Das S, et al. Mutation in the S3 segment of KCNQ1 results in familial lone atrial fibrillation. Heart Rhythm. 2009;6:1146–1153. doi: 10.1016/j.hrthm.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olesen MS, et al. Mutations in the potassium channel subunit KCNE1 are associated with early-onset familial atrial fibrillation. BMC Med Genet. 2012;13:24. doi: 10.1186/1471-2350-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ravn LS, et al. Gain of function in IKs secondary to a mutation in KCNE5 associated with atrial fibrillation. Heart Rhythm. 2008;5:427–435. doi: 10.1016/j.hrthm.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kharche S, et al. Atrial proarrhythmia due to increased inward rectifier current (IK1) arising from KCNJ2 mutation—a simulation study. Prog Biophys Mol Biol. 2008;98:186–197. doi: 10.1016/j.pbiomolbio.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 130.Hattori T, et al. A novel gain-of-function KCNJ2 mutation associated with short-QT syndrome impairs inward rectification of Kir2.1 currents. Cardiovasc Res. 2012;93:666–673. doi: 10.1093/cvr/cvr329. [DOI] [PubMed] [Google Scholar]
- 131.Shi HF, et al. Prevalence and spectrum of GJA5 mutations associated with lone atrial fibrillation. Mol Med Rep. 2013;7:767–774. doi: 10.3892/mmr.2012.1252. [DOI] [PubMed] [Google Scholar]
- 132.Laitinen-Forsblom PJ, et al. SCN5A mutation associated with cardiac conduction defect and atrial arrhythmias. J Cardiovasc Electrophysiol. 2006;17:480–485. doi: 10.1111/j.1540-8167.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 133.Ellinor PT, et al. Cardiac sodium channel mutation in atrial fibrillation. Heart Rhythm. 2008;5:99–105. doi: 10.1016/j.hrthm.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 134.Darbar D, et al. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008;117:1927–1935. doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Makiyama T, et al. A novel SCN5A gain-of-function mutation M1875T associated with familial atrial fibrillation. J Am Coll Cardiol. 2008;52:1326–1334. doi: 10.1016/j.jacc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 136.Li Q, et al. Gain-of-function mutation of Nav1.5 in atrial fibrillation enhances cellular excitability and lowers the threshold for action potential firing. Biochem Biophys Res Commun. 2009;380:132–137. doi: 10.1016/j.bbrc.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 137.Watanabe H, et al. Mutations in sodium channel β1- and β2-subunits associated with atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:268–275. doi: 10.1161/CIRCEP.108.779181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang P, et al. Functional dominant-negative mutation of sodium channel subunit gene SCN3B associated with atrial fibrillation in a Chinese GeneID population. Biochem Biophys Res Commun. 2010;398:98–104. doi: 10.1016/j.bbrc.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Olesen MS, et al. Mutations in sodium channel β-subunit SCN3B are associated with early-onset lone atrial fibrillation. Cardiovasc Res. 2010;89:786–793. doi: 10.1093/cvr/cvq348. [DOI] [PubMed] [Google Scholar]
- 140.Olesen MS, Holst AG, Svendsen JH, Haunso S, Tfelt-Hansen J. SCN1Bb R214Q found in 3 patients: 1 with Brugada syndrome and 2 with lone atrial fibrillation. Heart Rhythm. 2012;9:770–773. doi: 10.1016/j.hrthm.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 141.Olesen MS, et al. High prevalence of long QT syndrome-associated SCN5A variants in patients with early-onset lone atrial fibrillation. Circ Cardiovasc Genet. 2012;5:450–459. doi: 10.1161/CIRCGENETICS.111.962597. [DOI] [PubMed] [Google Scholar]
- 142.Muhammad R, et al. Rare SCN10A variants modulate ventricular response rates during atrial fibrillation [abstract] Circulation. 2011;124:A13007. [Google Scholar]
- 143.Splawski I, et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA. 2005;102:8089–8968. doi: 10.1073/pnas.0502506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Antzelevitch C, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ren X, et al. Identification of NPPA variants associated with atrial fibrillation in a Chinese GeneID population. Clin Chim Acta. 2010;411:481–485. doi: 10.1016/j.cca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 146.Roberts JD, et al. Evaluation of nonsynonymous NPPA single nucleotide polymorphisms in atrial fibrillation. Europace. 2010;12:1078–1083. doi: 10.1093/europace/euq161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang X, et al. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135:1017–1027. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]