Abstract
High circulating glucose has been associated with increased risk of breast cancer. There may also be a link between serum glucose and prognosis in women treated for breast cancer. We assessed the effect of peridiagnostic fasting blood glucose and body mass index (BMI) on long-term breast cancer prognosis.
We retrospectively investigated 1261 women diagnosed and treated for stage I-III breast cancer at the National Cancer Institute, Milan, in 1996, 1999, and 2000. Data on blood tests and follow-up were obtained by linking electronic archives, with follow-up to end of 2009. Multivariate Cox modelling estimated hazard ratios (HR) with 95% confidence intervals (CI) for distant metastasis, recurrence and death (all causes) in relation to categorized peridiagnostic fasting blood glucose and BMI. Mediation analysis investigated whether blood glucose mediated the BMI-breast cancer prognosis association.
The risks of distant metastasis were significantly higher for all other quintiles compared to the lowest glucose quintile (reference <87 mg/dL) (respective HRs: 1.99 95%CI 1.23-3.24, 1.85 95%CI 1.14-3.0, 1.73 95%CI 1.07-2.8, and 1.91 95%CI 1.15-3.17). The risk of recurrence was significantly higher for all other glucose quintiles compared to the first. The risk of death was significantly higher than reference in the second, fourth and fifth quintiles. Women with BMI ≥25 kg/m2 had significantly greater risks of recurrence and distant metastasis than those with BMI <25 kg/m2, irrespective of blood glucose. The increased risks remained invariant over a median follow-up of 9.5 years. Mediation analysis indicated that glucose and BMI had independent effects on breast cancer prognosis.
Peridiagnostic high fasting glucose and obesity predict worsened short- and long-term outcomes in breast cancer patients. Maintaining healthy blood glucose levels and normal weight may improve prognosis.
Keywords: fasting glucose, breast cancer, BMI, prognosis
Introduction
Breast cancer is the commonest cancer in women. A role of high circulating glucose in carcinogenesis was first suggested by Warburg in the 1920s [1]. In 1962 it was reported that two psychotic patients given insulin (with induction of hypoglycaemic coma) experienced complete remission of metastatic breast cancer [2]. In 1977 Carroll reported an ecological correlation between pro-capita sugar intake and breast cancer mortality [3]. In 2002, the prospective ORDET study found that high fasting glucose was a risk factor for breast cancer, further supported by a second ORDET study with longer follow-up [4,5].
By contrast Holmes et al. [6] found no association between glycaemic index or glycaemic load, and breast cancer risk. A study on the Swedish National Diabetes Register cohort also found no relation between diabetes and cancer [7].
The original observation of Warburg, that high glucose favours the selection of malignant cell clones resistant to hypoxia, in which energy is produced mainly by glycolysis, is one biological mechanism linking cancer development with high circulating glucose [8,9]. Enhanced glycolysis and glucose uptake characterizes most tumour cells [1]. Glucose metabolism may also promote malignant change through the generation of free radicals that damage both DNA and the enzymes involved in DNA repair and processing [10,11]. It has also been found that levels of glucose transporters are elevated in most human cancers, including breast cancer [12,13].
As regards breast cancer prognosis, evidence of a link to circulating glucose levels is more limited. A study on cultured breast cancer cells found reduced chemotherapy-induced cell death in cells subjected to high glucose concentrations, suggesting that hyperglycaemia confers resistance to chemotherapy [14]. A dietary intervention study in breast cancer patients found that the risk of recurrence was significantly higher in patients with metabolic syndrome – one of whose main characteristics is elevated blood glucose [15].The HEAL cohort study also provided some indication that dietary glycaemic load might be related to breast cancer prognosis [16], while an observational study on 46 cancer patients, eight with breast cancer, found significantly lower average blood glucose in patients in remission [17]. Finally, a recent cohort study on non-diabetic breast cancer patients found an association between blood glucose and distant metastasis but only for the first five years after diagnosis [18].
The aim of the present study was to investigate the relationship between long-term breast cancer prognosis and fasting glucose levels by retrospectively examining two large consecutive series of breast cancer patients. Furthermore, since high blood glucose is often associated with overweight, we also investigated whether glucose and body mass index (BMI) had independent or related effects on prognosis, and whether blood glucose mediates the association between BMI and prognosis. We evaluated the effects of these variables on any breast cancer recurrence, distant metastasis, and death for any cause.
Methods
Patients
We retrospectively examined two consecutive series of breast cancer (BC) patients treated at the National Cancer Institute of Milan (NCIM) in 1996 and 1999-2000, adhering to the following criteria: resident in the Region of Lombardy at moment of diagnosis, no previous cancer diagnosis (except non-melanoma skin cancer), fasting blood glucose determined peridiagnostically (and available), and stage I-III breast cancer (T1-T3, N0-N1, M0). We conducted a pilot investigation of cases from 1999 to 2000 and subsequently added 1996 cases to increase cohort size and lengthen median follow-up.
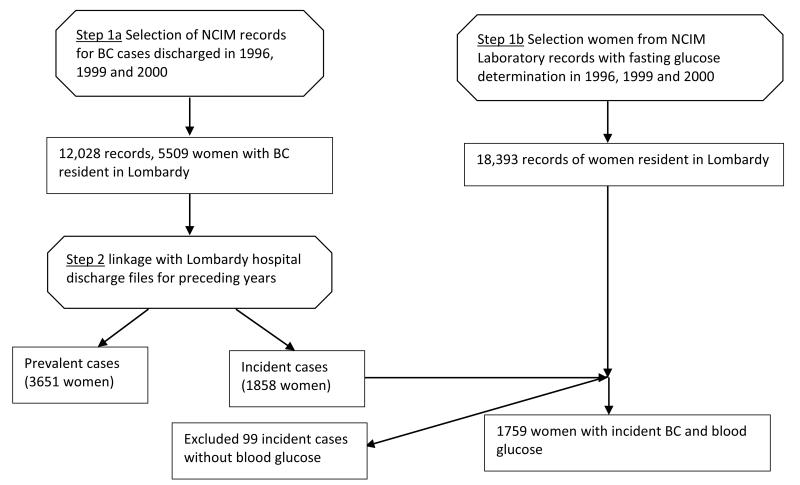
Cases were initially identified automatically from the prospectively maintained electronic database of NCIM case records (Step 1a, Figure 1). We next used the Epilink software to perform statistically-based linkage of NCIM cases to hospital discharge files for the entire Region of Lombardy [19]. This process also involved linkage to Region of Lombardy Social Security files in order to verify Region of Lombardy residency. The outcome of this procedure was that incident cases (Step 2, Figure 1) were separated from prevalent cases which were eliminated.
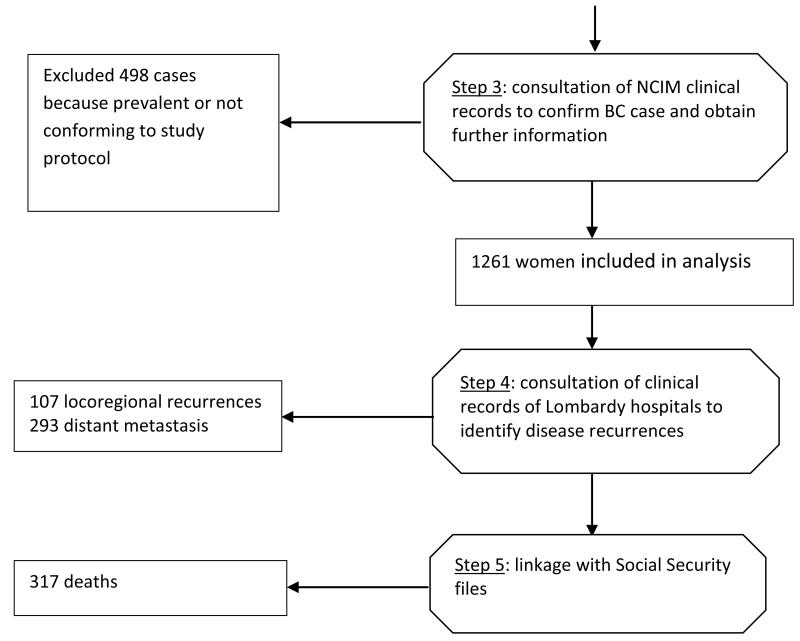
Fig. 1.
Flow-chart illustrating how incident breast cancer cases with fasting glucose determined peridiagnostically were identified and follow-up information obtained. Octagons show processes; rectangles show outputs.
In a separate procedure (Step 1b, Figure 1) we applied Epilink to cases archived in the NCIM analytical laboratory database, selecting female patients with fasting glucose determinations.
To identify BC cases (incident at NCIM in the study years) that also had peridiagnostic fasting glucose we manually checked the few records (1%) flagged by Epilink in Steps 1a and 1b as having uncertain linkage, and linked cases from Step 1b with those from Step 2. The next step (Step 3, Figure 1) was hand-searching the clinical records of identified cases. This served as an additional check that case details were correct, and provided the following additional information: BMI (kg/m2), disease stage (TNM), menopausal status at diagnosis, and tumour estrogen and progesterone receptor data. A series of algorithms included in the Open Registry software [20] was then applied to ascertain whether any adverse breast cancer events for these cases had been registered in the hospital discharge database of the Region of Lombardy from 1996 to 2009. Cases with an adverse event were then hand-searched in clinical records (Step 4, Figure 1) to confirm the event and obtain details (locoregional recurrence, distant metastasis, death). Finally (Step 5, Figure 1), the vital status of all cases was checked on Social Security files up to 31/12/2009 (end of follow-up). Thirty-one (2.5%) women were identified as having moved out of the Region of Lombardy before the close of follow-up and were considered lost to follow-up at the date of moving.
Statistical methods
Associations between glucose and other patient variables were analysed by chi-square test, with glucose categorized into quintiles, and age, menopausal status, tumour stage, BMI, and estrogen and progesterone receptor status, categorized as in Table 1. Spearman rank correlation coefficients were determined to examine correlations between selected variables.
Table 1.
Distribution of characteristics in 1261 breast cancer cases, according to quintiles peridiagnostic fasting blood glucose
| Quintiles of peridiagnostic fasting blood glucose (mg/dL) | N | |||||
|---|---|---|---|---|---|---|
| <87 | ≥87<93 | ≥93<99 | ≥99<108 | ≥108 | patients | |
| N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Age at diagnosis (years) P<0.001 | ||||||
| <48 | 107 (43) | 85 (36) | 64 (26) | 51 (18) | 21 ( 9) | 328 |
| ≥48<57 | 64 (26) | 61 (26) | 73 (30) | 78 (28) | 45(18) | 321 |
| ≥57<67 | 42(17) | 52 (22) | 53 (22) | 75 (26) | 79(32) | 301 |
| ≥67 | 37(14) | 39 (16) | 54 (22) | 79 (28) | 102(41) | 311 |
| Menopausal status P<0.001 | ||||||
| Pre- | 117 (47) | 100 (42) | 83 (34) | 78 (28) | 32 (13) | 410 |
| Peri- | 11 (4) | 10 (4) | 10 (4) | 8 (3) | 5 (2) | 44 |
| Post- | 120 (48) | 124 (53) | 149 (61) | 196 (69) | 204 (83) | 793 |
| Unknown | 2 (1) | 3 (1) | 2 (1) | 1 (0) | 6 (2) | 14 |
| Disease stage P<0.05 | ||||||
| I | 102 (41) | 95 (40) | 99 (41) | 105 (37) | 80 (33) | 481 |
| II | 114 (45) | 115 (49) | 105 (43) | 123 (44) | 107 (43) | 564 |
| III | 34 (14) | 27 (11) | 40 (16) | 55 (19) | 60 (24) | 216 |
| BMI P<0.001 | ||||||
| <18.5 | 19 (8) | 10 (4) | 6 (3) | 9 (3) | 2 (1) | 46 |
| ≥18.5<25 | 122 (49) | 107 (45) | 108 (44) | 104 (37) | 44 (18) | 485 |
| ≥25<30 | 45 (18) | 53 (22) | 64 (26) | 86 (30) | 88 (35) | 336 |
| ≥30 | 13 (5) | 18 (8) | 28 (11) | 36 (13) | 51 (21) | 146 |
| Unknown | 51 (20) | 49 (21) | 38 (16) | 48 (17) | 62 (25) | 248 |
| Estrogen receptor P=0.42 | ||||||
| Positive | 188 (75) | 194 (82) | 179 (73) | 217 (77) | 187 (76) | 965 |
| Negative | 50 (20) | 41 (17) | 54 (22) | 51 (18) | 57 (23) | 253 |
| Unknown | 12 (5) | 2 (1) | 11 (5) | 15 (5) | 3 (1) | 43 |
| Progesterone receptor P=0.73 | ||||||
| Positive | 166 (66) | 166 (70) | 154 (63) | 186 (66) | 164 (66) | 836 |
| Negative | 72 (29) | 69 (29) | 81 (33) | 81 (28) | 80 (33) | 383 |
| Unknown | 12 (5) | 2 (1) | 9 (4) | 16 (6) | 3 (1) | 42 |
The study had three end-points: any breast cancer recurrence, distant metastasis, and all-cause mortality. Some women contributed to all three endpoints. The Kaplan-Meier method was used to visualize the effects of variables on endpoints. Cox proportional hazard modelling was used to estimate hazard ratios (HRs) with 95% confidence intervals (CI) for any recurrence, distant metastasis, and all-cause death by quintiles of fasting blood glucose (lowest as reference), also by dichotomized BMI (<25 kg/m2 vs. ≥25 kg/m2, lower as reference), adjusting by age (five-year classes), menopausal status (according to Table 1 categories), estrogen and progesterone receptor status (positive or negative) and tumour stage. Postmenopausal status was defined as absence of menstruation for at least 12 months. Time to an event or end of follow-up was calculated from date of diagnosis. The proportional hazards assumption was tested by analysis of scaled Schoenfeld residuals, with p values for each variable estimated. When the hazard for a variable was suspected to be non-proportional over time, we performed additional analyses, substituting the conventional Cox model β coefficient (for a given variable) with a time-dependent function β(t) obtained by adding the smoothed scaled Schoenfeld residuals to the conventional β coefficient [21]. The results were evaluated as suggested by Grambsch et al. [19] and Bellera et al. [22]. When non-proportional hazards were found for covariates other than glucose and BMI, we stratified the Cox models by these covariates.
We ran semi-adjusted models that included all covariates except glucose (when assessing BMI) and BMI (when assessing glucose), and fully-adjusted models that included all covariates (including glucose and BMI). The methodology of MacKinnon et al. [23] was employed to test whether blood glucose mediates the causal relationship between BMI and breast cancer prognosis. To be considered a mediator of this relationship, blood glucose: (a) must be correlate with BMI; (b) must remain associated with prognosis after adjusting for BMI; (c) must partially or totally explain the effect of BMI on prognosis.
(a) was assessed by correlation analysis and (b) by Cox proportional hazards analysis. To ascertain (c) we calculated β for BMI in the semi-adjusted model without blood glucose, then ran the fully-adjusted model adding blood glucose into the analysis and obtaining a new coefficient (β’) for BMI. We then tested the β - β’ difference by the Freedman and Scatzkin test [24]. The percentage attenuation of β following the introduction of blood glucose introduction was calculated as ((β - β’) / β) * 100. The analyses were performed using the R statistical package [25].
Results
Cohort
A total of 12,028 women in the NCIM hospital discharge database, years 1996, 1999 and 2000, had a diagnosis of breast cancer. By linkage with Social Security files 5509 of these had a Region of Lombardy residence code. By linkage of these cases with Lombardy hospital discharge files for years prior to the study period, 3651 women were excluded as prevalent cases or as having a diagnosis of another cancer, and 1858 cases were identified as incident breast cancers. In a separate process, records of blood glucose tests performed by the NCIM analytical laboratory, in the years of the study, were linked with Social Security files, from which 18,393 records were identified as belonging to female residents in the Region of Lombardy. By linkage of these cases with the 1858 incident breast cancer cases, 1759 breast cancers cases with peridiagnostic glucose levels were identified, and 99 were excluded. Hand-searching of the clinical records of these cases led to the exclusion of a further 498 cases of prevalent (N=195), stage unknown (N=142), non-invasive (N=95), or metastatic (N=66) breast cancer at diagnosis. We conducted the analysis on 1261 cases, fully respecting the predefined study criteria.
The clinical characteristics of these cases are shown in Table 1. During a median follow-up of 9.5 years (maximum 13.5 years), 107 locoregional recurrences, 293 distant metastases and 317 deaths were identified (more than one event possible in any woman). Two thirds (65.2%) of the women were postmenopausal. Mean age was 57 years. Mean fasting glucose was 100.6 mg/dL (range 68-401). Women with high glucose were more likely to be older, more often menopausal, have higher BMI, and more advanced disease stage at diagnosis.
Disease progression and fasting glucose
Preliminary analyses identified non-proportional hazards in relation to estrogen receptor status, progesterone receptor status, disease stage, and age, so the final models were stratified for these variables.
In the semi-adjusted model (without BMI), HRs for distant metastasis were significantly above reference (lowest quintile) for women in the other four quintiles of the glucose distribution (Table 2). In the fully-adjusted model (with BMI), HRs were slightly lower but always significantly greater than reference.
Table 2.
Hazard ratios (HR) with 95%CIs, for disease recurrence, distant metastasis and death, according to quintiles of peridiagnostic fasting blood glucose
| Glucose (mg/dL) |
N patients |
Any recurrence * | Distant metastasis * | Death (any cause) * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N events |
Semi-adjusted HR (95% CI) |
Fully-adjusted HR (95% CI) |
N events |
Semi-adjusted HR (95% CI) |
Fully-adjusted HR (95% CI) |
N events |
Semi-adjusted HR (95% CI) |
Fully-adjusted HR (95% CI) |
||
| <87 | 250 | 48 | 1 | 1 | 39 | 1 | 1 | 48 | 1 | 1 |
| ≥87 <93 | 237 | 48 | 1.62 (1.02- 2.55) | 1.59 (1.01-2.52) | 47 | 2.03 (1.25-3.3) | 1.99 (1.23-3.24) | 55 | 1.59 (1.02-2.48) | 1.58 (1.01-2.46) |
| ≥93 <99 | 244 | 53 | 1.59 (1.01-2.5) | 1.53 (0.97-2.41) | 51 | 1.96 (1.21-3.18) | 1.85 (1.14-3.0) | 63 | 1.41 (0.90-2.2) | 1.37(0.88-2.15) |
| ≥99<108 | 283 | 60 | 1.64 (1.05-2.56) | 1.54 (0.98-2.42) | 55 | 1.87 (1.16-3.01) | 1.73 (1.07-2.8) | 73 | 1.57 (1.02-2.41) | 1.54 (1.00-2.36) |
| ≥108 | 247 | 57 | 1.81 (1.14-2.9) | 1.66 (1.03-2.68) | 55 | 2.13 (1.29-3.5) | 1.91 (1.15-3.17) | 78 | 1.79 (1.16-2.76) | 1.72 (1.11-2.67) |
Models adjusted for menopausal status and stratified by estrogen receptor status, progesterone receptor status, disease stage, and age. Semi-adjusted models exclude BMI, fully-adjusted models include BMI.
Risk of any breast cancer recurrence was also significantly above reference for women in the other four glucose quintiles in the semi-adjusted model, but were attenuated in the fully-adjusted model, when they were no longer significant for the third and fourth quintiles. The risk of all-cause death was significantly above reference for women in the second, fourth and fifth glucose quintiles in the fully-adjusted model (Table 2).
High glucose was associated with increased risk of distant metastasis in both categories of BMI. For <25 kg/m2 BMI women, HRs by increasing quintiles were 2.32 (95%CI 0.99-4.12), 2.33 (95%CI 0.89-3.97), 2.23 (95%CI 1.07-4.64), and 1.73 (95%CI 0.47-3.39). For those with BMI ≥25 kg/m2, the corresponding HRs were 1.22 (95%CI 0.49-3.01), 1.56 (95%CI 0.69-3.55), 1.13 (95%CI 0.52-2.47) and 1.74 (0.78-3.86) (fully adjusted models, data not shown in Tables). Analysis (fully adjusted model) indicated no interaction between fasting glucose and BMI.
High glucose was associated with increased risk of distant metastasis in both categories of menopausal status. For premenopausal women, HRs for increasing glucose quintiles were 2.49 (95%CI 1.27-4.90), 2.05 (95%CI 1.0-4.20), 2.30 (95%CI 1.07-4.97), and 0.64 (95%CI 0.14-2.93); for postmenopausal women the corresponding HRs were 1.43 (95%CI 0.68-2.99), 1.47 (95%CI 0.73-2.91); 1.37 (95%CI 0.70-2.62), and 1.89 (95%CI 0.99-3.62) (fully adjusted models, data not shown in Tables). The low HR and wide confidence limits for the upper quintile of premenopausal women is probably due to the fact that it contained few women (N=32). Analysis (fully adjusted model) indicated no interaction between fasting glucose and menopausal status.
To further probe the relationship between fasting glucose and prognosis we repeated the analyses with the covariate BMI categorized as recommended by the WHO (<18.5; ≥18.5<25; ≥25<30; ≥30 kg/m2) and also considering BMI as a continuous variable. The results (not shown in Tables) were closely similar to those shown in Tables 2 and 3. For example the HR for distant metastasis in the highest glucose quintile was 1.72 compared to reference, while with BMI as continuous variable the corresponding HR was 1.74. (c.f. HR 1.91 in Table 2).
Table 3.
Hazard ratios (HR) and 95% CIs for any recurrence, distant metastasis and death according to peridiagnostic BMI
| BMI (kg/m2) |
N cases | Any recurrence * | Distant metastasis * | Death (any cause) * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N events |
Semi-adjusted HR (95% CI) |
Fully-adjusted HR (95% CI) |
N events |
Semi-adjusted HR (95% CI) |
Fully-adjusted HR (95% CI) |
N events |
Semi-adjusted HR (95% CI) |
Fully-adjusted HR (95% CI) |
||
| <25 | 531 | 109 | 1 | 1 | 97 | 1 | 1 | 117 | 1 | 1 |
| ≥25 | 482 | 127 | 1.44 (1.09-1.91) | 1.38 (1.03-1.84) | 122 | 1.57 (1.17-2.10) | 1.49 (1.10-2.0) | 136 | 1.22 (0.93-1.60) | 1.16 (0.88-1.53) |
Models adjusted for menopausal status and stratified by estrogen receptor status, progesterone receptor status, disease stage, and age. Semi-adjusted models exclude fasting glusose, fully-adjusted models include fasting glucose.
Disease progression and BMI
Compared to reference (<25 kg/m2) women with BMI ≥25 kg/m2 had significantly greater risks of any recurrence and distant metastasis, but not death (Table 3). In the semi-adjusted model (excluding glucose) the HR for distant metastasis was 1.57 (95%CI 1.17-2.10) in the higher BMI category, and remained significant in the fully adjusted model. Risk of any recurrence was also significantly influenced by BMI both in the semi- and fully-adjusted models, but BMI had only a minor effect on all-cause mortality (Table 3).
Prognostic effects of high glucose and high BMI over time
Analyses of scaled Schoenfeld residuals showed that the proportional hazard assumption held for glucose over time. Specifically, for distant metastasis as outcome, p values for increasing glucose quintiles compared to the lowest were 0.11, 0.47, 0.42 and 0.06, indicating that the null hypothesis of no variation of hazard with time could not be rejected, and suggesting that the prognostic effect of glucose remained constant over the entire follow-up period. A similar result (P=0.48) was obtained for the upper BMI category compared to reference. Hazard proportionality was also investigated for all recurrences and mortality, and found to hold for glucose and BMI (data not shown), implying that the prognostic effect of glucose and BMI on these endpoints remained constant over the entire follow-up.
Mediation analysis of relationship between fasting glucose and BMI
Fasting glucose and BMI correlated with each other (Spearman’s r=0.28, p=0.002) hence satisfying the first MacKinnon et al. [23] criterion. Fasting glucose also remained associated with prognosis after the introduction of the BMI into the statistical model as shown above (criterion b). We next applied MacKinnon [23] criterion (c) to determine whether blood glucose partially or totally explained the effect of BMI on prognosis. With distant metastasis as outcome the attenuation percentage was 13%, and Freedman-Schatzkin test P value was 0.46, so the null hypothesis that blood glucose did not mediate the effect of BMI on distant metastasis could not be rejected. With any recurrence as outcome, attenuation percentage was 13.8%, Freedman-Schatzkin test P value 0.53, so again the null hypothesis that that blood glucose did not mediate the effect of BMI any recurrence could not be rejected. Finally BMI was not significantly associated with death either before or after the introduction blood glucose into the statistical model, so again the null hypothesis (that blood glucose did not mediate the effect of BMI on death recurrence) was not rejected. We also did mediation analysis with BMI categorized into four levels, and as a continuous variable: in all cases results indicated that the null hypothesis of no mediation.
Discussion
The principal finding of this retrospective study on 1261 women with invasive breast cancer is that risks of any recurrence and of distant metastasis were significantly higher for those with fasting blood glucose in the four high quintiles of the distribution, compared to those in the lowest quintile (<87 mg/dL). Risk of death for any cause showed a similarly significant pattern. These risks were attenuated, but still usually significant, in the fully-adjusted model that included BMI (Table 2); while models run for high and low BMI separately estimated similarly increased risks for all quintiles of glucose above reference, although these were not significant in most cases, probably due to the considerably lower numbers of cases in each category.
We also found that BMI was related to breast cancer prognosis: the risks of breast cancer recurrence and distant metastasis were significantly higher in overweight plus obese women (BMI ≥25 kg/m2) than normal weight plus underweight women (BMI <25 kg/m2). These risks were slightly attenuated in the fully adjusted model (with glucose as covariate). BMI was not significantly associated with all-cause death.
Importantly, our analysis also indicated that the worsened prognosis of women with high glucose and high BMI remained constant over the entire follow-up.
The recent study of Goodwin et al. investigated insulin-related (including fasting glucose) and obesity-related variables in 535 non-diabetic women treated for T1-3 N0-1 M0 breast cancer [18]. They found that insulin-related variables (including glucose) were significantly associated with distant metastasis only for the first five years of follow-up; by contrast our study suggests that the increased risk persists over the long term. In both studies the risks associated with BMI remained constant over time. Unlike the present study, Goodwin et al. found no significant association of fasting glucose with overall mortality.
The Diana-2 study investigated the relation of metabolic syndrome and its components to breast cancer prognosis [15]. Metabolic syndrome at baseline was associated with an adjusted HR for recurrence of 3.0 (95%CI 1.2-7.1) relative to no metabolic syndrome. High blood glucose (component of metabolic syndrome) was also associated with increased HRs for recurrence, but the association was not significant, probably in relation to the small numbers of both patients (N=110) and adverse events (N=31).
The HEAL study investigated relations of dietary fibre, glycaemic index and glycaemic load to adverse events in 688 breast cancer patients followed for 6.7 years [16]. There was some indication that high glycaemic index was related to increased breast cancer mortality and recurrences, but risk increases were not significant. Dietary glycaemic index and dietary glycaemic load may not correlate closely with fasting blood glucose.
With regard to the influence of obesity on breast cancer prognosis, this has been investigated by several studies [26-28]. Ewertz et al. [26] analysed 18,967 Danish women treated for early breast cancer between 1977 and 2006. For the first five years after diagnosis there was no association between BMI and distant metastasis, from five years on (at least 10 years of follow-up) obesity (BMI ≥30 kg/m2) and overweight (BMI 25-29 kg/m2) were associated with significantly greater risk of distant metastases after adjusting the models for disease characteristics.
A retrospective analysis of 2,887 lymph node-positive breast cancer patients enrolled in the BIG 02-98 study [27] also found that baseline obesity was associated with significantly poorer outcomes. A meta-analysis of 43 studies, published in 2008, found an HR for breast cancer-specific mortality of 1.33 (95%CI 1.19-1.50) in obese patients compared to non-obese patients [28].
The findings of the present study on the prognostic effect of fasting glucose are, therefore, consistent with the results of previous studies and are reinforced by plausible biological mechanisms for effects of glucose on breast cancer [1-5, 8-9, 12-13].
Our study is the first to use statistical methods investigate whether fasting glucose levels mediated the association between BMI and breast cancer prognosis. Our extensive analyses indicated that fasting glucose did not mediate this relationship, indicating that glucose and BMI have independent effects on BC prognosis.
A limitation of our study is that fasting glucose was only measured once. It would be useful to assess blood glucose at several time points during follow-up in the expectation that women with chronically high glucose would be at even greater risk of adverse events, and that those who correct hyperglycaemia and overweight have lowered risks.
Other limitation are the lack of information on comorbidities and biological factors like tumour proliferation rate and human epidermal growth factor receptor 2 overexpression, both of which are known to influence breast cancer prognosis, and lack of information about the use of diabetic medications to lower blood sugar levels medications.
It is noteworthy that 85% of our cohort had fasting serum glucose in the four highest quintiles, or BMI ≥25 kg/m2, or both. Thus a large vast majority of the patients in our cohort were at high risk of recurrence.
Conclusions
At the very least our data indicate that studies are necessary to determine whether long-term control of hyperglycaemia and reduction of obesity improves breast cancer outcomes.
Rapid and profound changes of in eating habits are occurring in many countries, particularly those in South Asia, Africa, Latin America and China, that have been linked to increased incidence of obesity and chronic diseases [29-32]. Thus in Brazil more sugar is consumed than recommended by the WHO, and added sugars from processed foods and manufactured beverages form an increasing proportion of sugar intake [33]. The World Health Organization estimates that by 2015 over 2.3 billion people will be overweight, and that deaths due to diabetes will have increased by more than 50% [34]. These projections suggest we are likely to see in increase in the proportion of breast cancer patients with high blood sugar levels, who will have, according to our findings, worsened prognosis for their disease compared to earlier periods. These considerations provide additional reasons why Governments should place food behaviour policy very high on their health agenda.
Acknowledgements
The authors thank Don Ward for help with the English and for critically reviewing the manuscript. This work was supported by the National Cancer Institute at the National Institutes of Health grant “Fasting Glucose in Long Term Breast Cancer Survival” (1R21 CA 106905 01).
Abbreviations
- BMI
body mass index
- HR
hazard ratio
- CI
confidence interval
- BC
breast cancer
- (NCIM)
National Cancer Institute of Milan
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PM and FB designed the study and coordinated data interpretation; PC and GT structured and tested automatic algorithms on electronic files to find and confirm cancer events; PC did the statistical analyses; MGD retrieved the clinical information; AM was responsible for laboratory examinations and their quality control; SF and MA managed the information system. All the authors contributed to data interpretation and critical review of the manuscript.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Koroljow S. Two cases of malignant tumors with metastases apparently treated successfully with hypoglycemic coma. Psychiatr Q. 1962;36:261–70. doi: 10.1007/BF01586115. [DOI] [PubMed] [Google Scholar]
- 3.Carroll KK. Dietary factors in hormone-dependent cancers. Curr Concepts Nutr. 1977;6:25–40. [PubMed] [Google Scholar]
- 4.Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schünemann HJ, Ram M, Freudenheim JL, Sieri S, Trevisan M, Berrino F. Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1361–8. [PubMed] [Google Scholar]
- 5.Sieri S, Muti P, Claudia A, Berrino F, Pala V, Grioni S, Abagnato CA, Blandino G, Contiero P, Schunemann HJ, Krogh V. Prospective study on the role of glucose metabolism in breast cancer occurrence. Int J Cancer. 2012;130(4):921–9. doi: 10.1002/ijc.26071. [DOI] [PubMed] [Google Scholar]
- 6.Holmes MD, Liu S, Hankinson SE, Colditz GA, Hunter DJ, Willett WC. Dietary carbohydrates, fiber, and breast cancer risk. Am J Epidemiol. 2004;15(8):732–9. doi: 10.1093/aje/kwh112. 159. [DOI] [PubMed] [Google Scholar]
- 7.Miao Jonasson J, Cederholm J, Eliasson B, Zethelius B, Eeg-Olofsson K, Gudbjörnsdottir S. HbA1C and cancer risk in patients with type 2 diabetes--a nationwide population-based prospective cohort study in Sweden. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garber K. Energy boost: the Warburg effect returns in a new theory of cancer. J Natl Cancer Inst. 2004;96(24):1805–6. doi: 10.1093/jnci/96.24.1805. [DOI] [PubMed] [Google Scholar]
- 9.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24(2):68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8(6):583–99. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- 11.Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444–5. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 12.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202(3):654–62. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 13.Krzeslak A, Wojcik-Krowiranda K, Forma E, Jozwiak P, Romanowicz H, Bienkiewicz A, Brys M. Expression of GLUT1 and GLUT3 Glucose Transporters in Endometrial and Breast Cancers. Pathol Oncol Res. 2012;18(3):721–8. doi: 10.1007/s12253-012-9500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng L, Biernacka KM, Holly JM, Jarrett C, Morrison AA, Morgan A, Winters ZE, Foulstone EJ, Shield JP, Perks CM. Hyperglycaemia confers resistance to chemotherapy on breast cancer cells: the role of fatty acid synthase. Endocr Relat Cancer. 2010;17(2):539–51. doi: 10.1677/ERC-09-0221. [DOI] [PubMed] [Google Scholar]
- 15.Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119(1):236–8. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 16.Belle FN, Kampman E, McTiernan A, Bernstein L, Baumgartner K, Baumgartner R, Ambs A, Ballard-Barbash R, Neuhouser ML. Dietary fiber, carbohydrates, glycemic index, and glycemic load in relation to breast cancer prognosis in the HEAL cohort. Cancer Epidemiol Biomarkers Prev. 2011;20(5):890–899. doi: 10.1158/1055-9965.EPI-10-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krone CA, Ely JT. Controlling hyperglycemia as an adjunct to cancer therapy. Integr Cancer Ther. 2005;4(1):25–31. doi: 10.1177/1534735404274167. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Taylor SK, Hood N. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30(2):164–171. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- 19.Contiero P, Tittarelli A, Tagliabue G, Maghini A, Fabiano S, Crosignani P, Tessandori R. The EpiLink record linkage software: presentation and results of linkage test on cancer registry files. Methods Inf Med. 2005;44(1):66–71. [PubMed] [Google Scholar]
- 20.Tagliabue G, Maghini A, Fabiano S, Tittarelli A, Frassoldi E, Costa E, Nobile S, Codazzi T, Crosignani P, Tessandori R, Contiero P. Consistency and accuracy of diagnostic cancer codes generated by automated registration: comparison with manual registration. Popul Health Metr. 2006;4:10. doi: 10.1186/1478-7954-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Therneau TM, Grambsch PM. Testing proportional hazards. In Modeling Survival Data: Extending The Cox Model. Springer-Verlag; New York: 2000. [Google Scholar]
- 22.Bellera CA, MacGrogan G, Debled M, de Lara CT, Brouste V, Mathoulin-Pélissier S. Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol. 2010;10:20. doi: 10.1186/1471-2288-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedman LS, Schatzkin A. Sample size for studying intermediate endpoints within intervention trails or observational studies. Am J Epidemiol. 1992;136(9):1148–59. doi: 10.1093/oxfordjournals.aje.a116581. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team R: a language and environment for statistical computing. 2007 http://www.r-project.org.
- 26.Ewertz M, Jensen MB, Gunnarsdóttir KÁ, Højris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB, Cold S. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 27.de Azambuja E, McCaskill-Stevens W, Francis P, Quinaux E, Crown JP, Vicente M, Giuliani R, Nordenskjöld B, Gutiérez J, Andersson M, Vila MM, Jakesz R, Demol J, Dewar J, Santoro A, Lluch A, Olsen S, Gelber RD, Di Leo A, Piccart-Gebhart M. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat. 2010;119(1):145–53. doi: 10.1007/s10549-009-0512-0. [DOI] [PubMed] [Google Scholar]
- 28.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123(3):627–3. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 29.Monteiro CA, Cannon G. The impact of transnational “big food” companies on the South: a view from Brazil. PLoS Med. 2012;9(7) doi: 10.1371/journal.pmed.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Igumbor EU, Sanders D, Puoane TR, Tsolekile L, Schwarz C, Purdy C, Swart R, Durão S, Hawkes C. “Big food,” the consumer food environment, health, and the policy response in South Africa. PLoS Med. 2012;9(7) doi: 10.1371/journal.pmed.1001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuckler D, McKee M, Ebrahim S, Basu S. Manufacturing epidemics: the role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med. 2012;9(6) doi: 10.1371/journal.pmed.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Wang J, Liu MM, Wang D, Liu YQ, Zhao Y, Huang MM, Liu Y, Sun J, Dong GH. Epidemiology of general obesity, abdominal obesity and related risk factors in urban adults from 33 communities of northeast china: the CHPSNE study. BMC Public Health. 2012;12:967. doi: 10.1186/1471-2458-12-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy RB, Claro RM, Bandoni DH, Mondini L, Monteiro CA. Availability of added sugars in Brazil: distribution, food sources and time trends. Rev Bras Epidemiol. 2012;15(1):3–12. doi: 10.1590/s1415-790x2012000100001. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization . Obesity and overweight. World Health Organization; Geneva: 2006. Fact sheet number 311. [Google Scholar]