Abstract
BACKGROUND
The cardiometabolic syndrome comprised of multiple correlated traits, but its origin is incompletely understood. Chromogranin A (CHGA) is required for formation of the catecholamine secretory pathway in sympathochromaffin cells. In twin pair studies, we found that CHGA traits aggregated with body mass index (BMI), as well as its biochemical determinant leptin.
METHODS
Here we used the twin method to probe the role of heredity in generating such risk traits, and then investigated the role of risk-trait-associated CHGA promoter genetic variation in transfected chromaffin cells. Trait heritability (h2) and shared genetic determination among traits (pleiotropy, genetic covariance, ρG) were estimated by variance components in twin pairs.
RESULTS
CHGA, BMI, and leptin each displayed substantial h2, and the traits also aggregated with several features of the metabolic syndrome (e.g., insulin resistance, blood pressure (BP), hypertension, catecholamines, and C-reactive protein (CRP)). Twin studies demonstrated genetic covariance (pleiotropy, ρG) for CHGA, BMI, and leptin with other metabolic traits (insulin resistance, BP, and CRP). We therefore investigated the CHGA locus for mechanisms of codetermination with such metabolic traits. A common functional variant in the human CHGA promoter (G-462A, rs9658634, minor allele frequency ~21%) was associated with leptin and CRP secretion, as well as BMI, especially in women; marker-on-trait effects on BMI were replicated across twin populations on two continents. In CHGA promoter/luciferase reporter plasmids transfected into chromaffin cells, G-462A alleles differed markedly in reporter expression. The G-462A variant disrupted predicted transcriptional control by a PPARγ/RXRα motif and costimulation by PPARγ/RXRα and their cognate ligands, differentially activated the two alleles. During chromatin immunoprecipitation, endogenous PPARγ bound the motif.
CONCLUSIONS
Multiple features of the metabolic syndrome are thus under joint (pleiotropic) genetic determination, with CHGA as one such contributory locus: a common polymorphism in the promoter (G-462A) of CHGA predicts such heritable metabolic traits as BMI and leptin. CHGA promoter variant G-462A was not only associated with such metabolic traits but also disrupted a PPARγ/RXRα motif and responded differentially to characteristic trans-activators of that motif. The results suggest novel links between the catecholaminergic system and risk for the metabolic syndrome as well as systemic hypertension.
Keywords: adrenal, blood pressure, BMI, C-reactive protein, catecholamine, chromaffin, chromogranin, hypertension, leptin, metabolic syndrome, twin study
Systemic hypertension clusters with multiple other cardiovascular risk traits, including several features of the metabolic syndrome. The adipocyte-secreted, appetite-suppressing hormone leptin, encoded by the LEP locus at human chromosome 7q31,1 functions as a regulator of not only food intake but also neuroendocrine outflow, metabolism, and fat accumulation.2 In addition to its endocrine effects, leptin influences autonomic and cardiovascular traits. Central nervous system leptin infusion increases sympathetic nervous system activity,3 and chronic hyperleptinemia increases heart rate and arterial blood pressure; such effects are abolished by α plus β adrenergic blockade, indicating mediation by adrenergic activity.4,5 Haynes et al. reported that leptin activates sympathetic responses in vascular and renal districts,6 while Prior et al. found that visceral fat accumulation leads to leptin-dependent sympathetic activation.7 Thus, leptin is associated with blood pressure and contributes to the occurrence of hypertension through sympathetic activation in resistance vessels or kidney.8,9 Indeed, leptin is elevated in established hypertension,10 and early increases in leptin secretion predict later incident hypertension.11,12
Chromogranin A (CHGA), a 48-kDa acidic polypeptide,13,14 is the major protein costored and coreleased with catecholamines from secretory vesicles in adrenal medulla and postganglionic sympathetic axons.15 Catecholamine storage vesicles (or chromaffin granules) of the adrenal medulla contain remarkably high concentrations of CHGA, catecholamines, ATP, and Ca2+, wherein CHGA seems to bind and store both catecholamines and Ca2+.16 CHGA is required for the formation of catecholamine secretory vesicles in chromaffin cells,17 and its expression may be sufficient to induce a regulated secretory system even in nonsecretory cells.18 We systematically discovered genetic variation across the CHGA locus in several human populations19 and found that genetic variants in the CHGA promoter may regulate environmental stress-induced changes in blood pressure.20 During targeted ablation of the Chga locus in the mouse, we found substantial changes in leptin expression, in both adipocytes and plasma.21 Here, we explore whether CHGA promoter polymorphism contributes to heritable human metabolic trait variation and whether leptin levels influence blood pressure and hypertension differentially by gender. Our results suggest novel functional links in humans between the adrenergic pathway, leptin, the metabolic syndrome, and hypertension.
METHODS
University of California San Diego twin pairs
Twin recruitment included access to a population birth record-based twin registry,22 as well as by newspaper advertisement, as described.23 The 362 subjects in the twin heritability and allelic association studies were all white (European ancestry) with 121 monozygotic pairs (25 male pairs and 96 female pairs) and 60 dizygotic pairs (13 male pairs, 43 female pairs, and 4 male–female pairs). Zygosity was confirmed by use of either >100 microsatellites (chromosomes 1 and 2) for self-identified dizygotic twins or single nucleotide polymorphism data (11–177 single-nucleotide polymorphisms) as well as the TH (TCAT)n microsatellite 23 for self-reported monozygotic and dizygotic pairs. Ethnic status was ascertained by self-identification of participants, and also for both parents and all four grandparents. Twins were between 18 and 81 years of age (with a mean of ~41 years). Definitions of twin subject characteristics have been published.24 Study participants were twin volunteers from southern California. Written informed consent was obtained from each participant, and the research protocol was approved by the institutional review board of University of California San Diego (UCSD). Blood pressure status (high vs. normal) was defined by history (medical record or self-report), presence or absence of antihypertensive medications, and measurement of seated blood pressure by arm cuff (hypertension: either/or ≥140/≥90 mm Hg systolic blood pressure (SBP)/diastolic blood pressure (DBP), or both). None of the subjects had a history of renal failure, and serum creatinine concentrations were ≤1.5 mg/dl. The prevalence of obesity (defined as body mass index (BMI) ≥30 kg/m2) was 13.0%. Supplementary Table S1 online provides detailed descriptive statistics for UCSD twins, divided by gender.
Australia twin pairs
These subjects enabled replication of the BMI associations only (leptin was not measured). Characteristics of twin pairs from Queensland Institute of Medical Research in Brisbane (Queensland, Australia) have been reported.25 They completed a questionnaire in 1989, a telephone interview in 1993–1994, and provided a blood sample in 1993–1996. Zygosity was determined from responses to questions about physical similarity and the inability of others to tell them apart, supplemented by blood group information and extensive microsatellite or single-nucleotide polymorphism genotyping. Participants gave informed consent to the questionnaire, interview, and blood collection, and the studies were approved by the appropriate ethics review committees. Blood pressure was measured on the occasion when blood was collected, with the subjects sitting, using an automated blood pressure recorder (Dinamap 845 Vital Signs Monitor; Critikon, Tampa, FL). The mean of two results taken at 1-min intervals was calculated. Supplementary Table S1 online provides the descriptive statistics of Queensland Institute of Medical Research twins by gender; the mean age was ~44 (men)–~46 (women) years.
Physiological phenotyping in vivo
Subjects were studied before genotyping. Brachial arterial cuff blood pressure (in mm Hg) and heart rate (beats/min) were obtained in seated subjects with a DynaPulse device (PulseMetric; Vista, CA), as previously described and validated.26 Triplicate determinations of blood pressure and heart rate were made, until each value was within ± 10% of the mean value in that individual.
Biochemical phenotyping
Leptin
Leptin was measured by radioimmunoassay with [125I]-human leptin (~135 μCi/μg; Linco Research; St. Charles, MO; Cat. #HL-81K), in 100 μl samples of EDTA-plasma, as described.27 Blood samples in subjects who had not consumed food for at least 3 h were drawn into EDTA anticoagulant tubes, and the plasma was frozen at −70 °C prior to assay in batch. The lower limit of leptin detection was 0.5 ng/ml (in 100 μl plasma), and the assay did not recognize insulin, proinsulin, C-peptide, glucagon, or IGF-1. Assay coefficients of variation were as follows: within-assay 3.4–8.3% and between-assay 3.6–6.2%. Recovery of exogenous human leptin added to serum was 103–105%.
Catecholamines
Spot/untimed urine samples were collected and similarly stored at −70°C. Batched, previously unthawed samples were subjected to a sensitive radioenzymatic assay based on catechol-O-methylation.28 Intra-assay coefficients of variation were as follows: norepinephrine 4% and epinephrine 13%. Inter-assay coefficients of variation were as follows: norepinephrine 10% and epinephrine 16%. Urine catecholamine values were normalized to creatinine excretion in the same sample.
Other assays
Plasma insulin was measured by immunoassay, while high sensitivity C-reactive protein (CRP) was measured by ELISA,24 in a high-sensitivity sandwich immunoassay for quantitative determination of CRP. Plasma glucose, and plasma and urine electrolytes were measured by autoanalyzer (Beckman-Coulter; Brea, CA).
Calculations
BMI, a measure of body fat based on height and weight that applies to both adult men and women, was estimated as (weight in kg)/(height in m)2. Endogenous insulin sensitivity (or resistance) was estimated from plasma glucose and insulin values by HOMeostatic Assessment model (HOMA); an index of insulin resistance29 and QUantitative Insulin sensitivity ChecK Index (QUICKI); an index of insulin sensitivity.24
Molecular methods: Function of CHGA promoter variant G-462A
Genomics, cell culture, CHGA promoter/luciferase reporter plasmids, transfection/transcription/reporter assay, and chromatin immunoprecipitation are detailed in Supplementary Methods online.
Statistical analyses
Data were stored in Microsoft Access, and analyses were conducted in SAS (Statistical Analysis System; Cary, NC), or Sequential Oligogenic Linkage Analysis Routines (SOLAR). Descriptive statistics (mean ± s.e.m.) were computed across all of the twins, using generalized estimating equations (GEE; PROC GENMOD), in SAS (Statistical Analysis System), to take into account intra-twin-pair correlations.30 Raw CHGA116–439, CHGA361–372, and CRP values were log10-transformed, resulting in improved normality of the distribution for parametric statistical analyses. Analyses were routinely adjusted for the covariates of age and sex. Trait-on-trait nonparametric Spearman correlations were performed with one individual per twin pair, to avoid false-positive conclusions from nonindependent observations.
Estimates of heritability (h2 = VG/VP, where VG is additive genetic variance and VP is total phenotypic variance) were obtained using the variance-component methodology implemented in the SOLAR package31 available at http://www.sfbr.org.solar/. This method maximizes the likelihood assuming a multivariate normal distribution of phenotypes in twin pairs (monozygotic vs. dizygotic) with a mean dependent on a particular set of explanatory covariates. The null hypothesis (H0) of no heritability is tested by comparing the full model, which assumes genetic variation (VG), and a reduced model, which assumes no genetic variation, using a likelihood ratio test. Heritability estimates were adjusted for age and sex, because of the effects of these covariates on several traits (Table 1). Pleiotropy (genetic covariance for two correlated, heritable traits, i.e., the cross-product of trait heritabilities)32 was estimated as the parameter ρG in SOLAR.32 SOLAR also estimated the environmental covariance, as parameter ρE.
Table 1.
Plasma leptin stratification into upper and lower quantiles: effects on multiple physiological and biochemical traits in twins.
| Phenotype | Plasma leptin strata | ||||
|---|---|---|---|---|---|
| Leptin ≤9.87 ng/ml | Leptin >9.87 ng/ml | P value | |||
| N | Mean ± s.e.m. | N | Mean ± s.e.m. | Sig. (2-tailed) | |
| Age (years) | 181 | 37.5 ± 1.2 | 181 | 43.9 ± 1.3 | 2.17E-04* |
| Sex (M/F) | 181 | 64 (35.4)/117 (64.6) | 181 | 16 (8.8)/165 (91.2) | 1.26E-9* |
| BP status, NT/HTN (%) | 181 | 172 (95.0)/9 (5.0) | 181 | 153 (84.5)/28 (15.5) | 0.001* |
| Physical | |||||
| Body mass index, kg/m2 | 181 | 22.6 ± 0.2 | 181 | 27.3 ± 0.5 | 4.82E-19* |
| Physiological | |||||
| SBP (mm Hg) | 179 | 126.7 ± 1.1 | 175 | 134.4 ± 1.2 | 5.57E-06* |
| DBP (mm Hg) | 179 | 68.8 ± 0.7 | 175 | 73.5 ± 0.8 | 1.07E-05* |
| Metabolic | |||||
| Plasma glucose (mg/dl) | 181 | 79.4 ± 1.0 | 181 | 84.5 ± 1.8 | 0.012* |
| Plasma insulin (μUnit/ml) | 179 | 10.14 ± 0.54 | 181 | 16.95 ± 1.14 | 1.65E-07* |
| QUICKI (insulin sensitivity) | 179 | 0.36 ± 0.003 | 181 | 0.34 ± 0.005 | 7.44E-05* |
| HOMA (insulin resistance) | 176 | 0.01 ± 0.01 | 179 | 0.07 ± 0.02 | 0.004* |
| Plasma leptin (ng/ml) | 181 | 5.85 ± 0.18 | 181 | 17.78 ± 0.56 | 1.75E-51* |
| Biochemical | |||||
| Epinephrine in urine (ng/gm) | 167 | 13,641 ± 542 | 168 | 13,014 ± 435 | 0.367 |
| Norepinephrine in urine (ng/gm) | 167 | 26,890 ± 980 | 168 | 31,864 ± 1,167 | 0.001* |
| CHGA116–439 (nmol/l) | 174 | 3.9 ± 0.13 | 177 | 4.06 ± 0.3 | 0.631 |
| CHGA361–372 (nmol/l) | 174 | 1.21 ± 0.04 | 179 | 1.39 ± 0.05 | 0.01* |
| C-reactive protein (ng/ml) | 176 | 1,308 ± 146 | 179 | 3,496 ± 346 | 8.73E-14* |
Descriptive and inferential statistics for twin study population. Values are mean ± s.d. of the mean (or n and %) derived from GEE. Leptin is dichotomized around the median concentration of 9.87 ng/ml. Numbers (‘n’) describe the number of analyzed individuals available for that particular trait. The mean or N (%) gives the mean for continuous variables, or percentage of the trait observed for dichotomous variables (listed in parenthesis). Values in urine are normalized to creatinine concentration.
BP, blood pressure; CHGA, Chromogranin A; DBP, diastolic blood pressure; GEE, generalized estimating equations; HOMA, HOmeostatic Model Assessment (insulin resistance); HTN, hypertension; NT, normotension; QUICKI, QUantitative Insulin sensitivity ChecK Index (insulin sensitivity); SBP, systolic blood pressure.
P < 0.05.
Bioinformatics
Promoter motif matches used the TRANSFAC-7.0-Public-200533 position weight matrix database http://www.gene-regulation.com, accessed by the graphical user interfaces at Chip Mapper 34 http://mapper.chip.org/mapper or JASPAR35 at http://jaspar.genereg.net/. Interspecies multiple sequence alignments were done at Clustal-W version-2.0.10 at http://www.ebi.ac.uk/Tools/clustalw2/index.html.
RESULTS
Twin phenotypes: Descriptive statistics of twin study populations, stratified by sex
Supplementary Table S1 online describes the UCSD twin subject population (n = 362 individuals). Women (n = 282) had higher plasma leptin (P = 7.39E-12), urinary norepinephrine (P = 3.10E-03), CHGA361–372 (P = 3.00E-02), and CRP (P = 5.55E-04), though lower SBP (P = 0.005) than men (n= 80).
Leptin, BMI, and hypertension
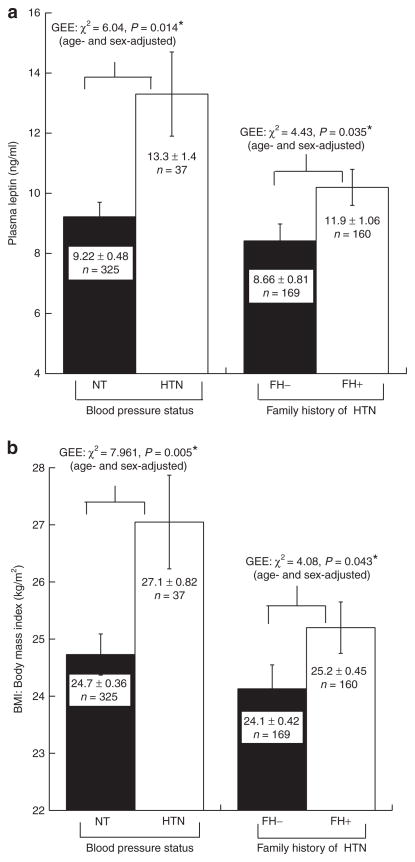
Figure 1 stratifies subjects by blood pressure (BP) status (hypertensive (n = 37/362, or ~10%) vs. normotensive (n = 325)) and illustrates the effects of BP and family history stratification on the quantitative traits leptin (Figure 1a) and BMI (Figure 1b). Despite the relatively small number of subjects with hypertension in the twin cohort, we observed that subjects with hypertension also displayed elevated leptin (P = 0.014, Figure 1a) and BMI (P = 0.005, Figure 1b). We also found that positive family history for hypertension was associated with elevated leptin (P = 0.035, Figure 1a) and BMI (P = 0.043, Figure 1b). In n = 1465 Australia/Queensland Institute of Medical Research twins, BMI was also higher in those with hypertension (n= 155, P= 7.97E-4).
Figure 1.
Blood pressure (BP): Aggregation with leptin and BMI traits in twin pairs. Traits are age- and sex-adjusted. Statistical analyses were by generalized estimating equations (GEE). Family history (FH) was obtained by self-report of the individual; positive family history was defined as onset of hypertension in one or both parents, before the age of 60 years. *P < 0.05. (a) Leptin, hypertension, and family history. BP status (normotensive (NT) vs. hypertensive (HT)) status (χ2 = 6.04, P = 0.014*), or family history of hypertension (χ2 = 4.43, P = 0.035*): effects on the leptin quantitative trait; (b) BMI, hypertension, and family history. BP status (χ2 = 7.961, P = 0.005*), or family history of hypertension (χ2 = 4.08, P = 0.043*): effects on the BMI quantitative trait. BMI, body mass index.
Plasma leptin quantiles: Aggregated traits
Table 1 shows multiple traits in twin stratified by the leptin median value of ~9.87 ng/ml. Individuals with higher plasma leptin displayed a number of significant trait differences, biochemical (chromogranin, catecholamine, and inflammatory), metabolic (plasma insulin, QUICKI, and HOMA), and physiological (SBP/DBP/hypertension). Once again, several of the traits are components of the cardiometabolic syndrome.
Pleoitropy (shared h2): Genetic covariance with leptin
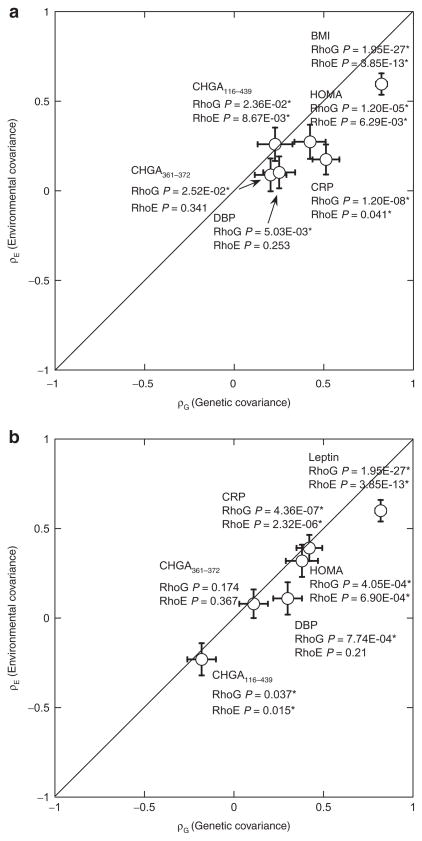
We tested several heritable leptin-correlated traits for shared genetic determination (pleiotropy, ρG) with leptin (Table 2). Leptin shared significant genetic determination with BMI, SBP/DBP, glucose, insulin, HOMA, QUICKI, CRP, CHGA116–439, and CHGA361–372. By contrast, several correlated traits also shared significant environmental determination (environmental covariance, ρE) with leptin: BMI, insulin, HOMA, CHGA116–439, CHGA361–372, and C-reactive protein; these six traits displayed both ρG and ρE with leptin. Figure 2a illustrates two examples of traits displaying different patterns of codetermination with leptin: BMI, CRP, and CHGA116–439 with significance for both ρG and ρE, and CHGA361–372, with significant ρG but not ρE.
Table 2.
Leptin: Shared genetic determination (genetic covariance, RHoG (ρG), pleiotropy) and environmental determination (environmental covariance, RHoE (ρE)) for metabolic syndrome traits correlated with plasma leptin in twins
| Trait | Heritability | Nonparametric overall correlation | Shared environmental determination | Shared genetic determination (pleiotropy) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| h2 ± s.e.m. | P value | Spearman rho | P value | ρE | s.e.m. | P value | ρG | s.e.m. | P value | |
| Physical | ||||||||||
| Body mass index (kg/m2) | 0.86 ± 0.02 | 1.63E-42* | 0.535 | 3.85E-28* | 0.596 | 0.06 | 3.58E-13* | 0.822 | 0.031 | 1.95E-27* |
| Physiological | ||||||||||
| SBP (mm Hg) | 0.46 ± 0.06 | 1.17E-09* | 0.383 | 8.40E-14* | −0.003 | 0.09 | 0.977 | 0.351 | 0.089 | 2.07E-04* |
| DBP (mm Hg) | 0.52 ± 0.06 | 4.58E-12* | 0.363 | 1.83E-12* | 0.103 | 0.089 | 0.253 | 0.259 | 0.089 | 5.03E-03* |
| Metabolic | ||||||||||
| Plasma glucose (mg/dl) | 0.34 ± 0.07 | 2.00E-06* | 0.349 | 8.83E-12* | −0.04 | 0.082 | 0.672 | 0.55 | 0.101 | 4.90E-07* |
| Plasma insulin (μUnit/ml) | 0.50 ± 0.09 | 1.60E-06* | 0.274 | 1.22E-07* | 0.294 | 0.095 | 3.83E-03* | 0.381 | 0.093 | 2.14E-04* |
| QUICKI (insulin sensitivity) | 0.42 ± 0.06 | 2.79E-09* | −0.305 | 3.45E-09* | 0.04 | 0.08 | 0.66 | −0.49 | 0.09 | 1.36E-06* |
| HOMA (insulin resistance) | 0.55 ± 0.08 | 1.18E-08* | 0.305 | 3.45E-09* | 0.274 | 0.095 | 6.29E-03* | 0.423 | 0.087 | 1.20E-05* |
| Biochemical | ||||||||||
| Epinephrine in urine (ng/gm) | 0.72 ± 0.04 | 1.55E-20* | −0.052 | 0.343 | 0.106 | 0.099 | 0.2879 | 0.016 | 0.099 | 0.8745 |
| Norepinephrine in urine (ng/gm) | 0.47 ± 0.06 | 4.75E-10* | 0.013 | 0.813 | 0.003 | 0.095 | 0.9718 | 0.019 | 0.115 | 0.8673 |
| CHGA116–439 (nmol/l) (log10) | 0.33 ± 0.09 | 3.6E-04* | −0.153 | 4.00E-03* | −0.26 | 0.093 | 8.67E-03* | −0.228 | 0.097 | 2.36E-02* |
| CHGA361–372 (nmol/l) (log10) | 0.65 ± 0.05 | 6.72E-21* | 0.14 | 3.85E-28* | 0.089 | 0.092 | 0.341 | 0.203 | 0.088 | 2.52E-02* |
| C-reactive protein (ng/ml) (log10) | 0.60 ± 0.05 | 3.79E-15* | 0.477 | 2.27E-11* | 0.175 | 0.084 | 0.041* | 0.512 | 0.075 | 1.20E-08* |
RhoG and RhoE were determined from variance components in SOLAR. Values in urine are normalized to creatinine concentration. (−) RhoG or RhoE typically indicates that the primary trait-on-trait correlation is negative. The heritability for leptin is 72.9 ± 3.88% (P = 1.67E-24).
CHGA, Chromogranin A; DBP, diastolic blood pressure; HOMA, HOmeostatic Model Assessment, insulin resistance; QUICKI, QUantitative Insulin sensitivity ChecK Index; SBP, systolic blood pressure; SOLAR, Sequential Oligogenic Linkage Analysis Routines.
P < 0.05.
Figure 2.
Genetic pleiotropy: Codetermination of traits in twin pairs. The diagonal lines are the theoretical lines of identity (Y= X). Results are shown as the mean ± s.e.m. for the covariance estimates, plotting RhoG (genetic covariance) as a function of RhoE (environmental covariance). Significance of RhoG or RhoE estimates is shown by P values. *P < 0.05. (a) Genetic pleiotropy for leptin with correlated traits; (b) genetic pleiotropy for BMI with correlated traits. BMI, body mass index; CHGA, chromogranin A; CRP, C-reactive protein; DBP, diastolic blood pressure; HOMA, HOMeostatic Assessment model.
BMI stratification in twin pairs: Aggregated traits
Multiple physiological and biochemical traits differed in UCSD twins upon BMI stratification about the median (23.8 kg/m2) into upper vs. lower quantiles (Table 3). The higher BMI group displayed greater SBP/DBP (and frequency of hypertension), insulin, HOMA (insulin resistance), leptin, CHGA361–372, and C-reactive protein, with lower QUICKI (insulin sensitivity) than the lower BMI group. Since higher and lower BMI groups differed in age and sex, genetic marker-on-trait analyses were adjusted for these two demographic traits.
Table 3.
BMI (body mass index) stratification into upper and lower quantiles: Effects on multiple physiological and biochemical traits in twins (by GEE, adjusted for age, sex)
| Phenotype | BMI strata | P value | |||
|---|---|---|---|---|---|
| Lower BMI (<23.8 kg/m2) | Higher BMI (≥23.8 kg/m2) | ||||
| N | Mean ± s.e.m. | N | Mean ± s.e.m. | ||
| Age (years) | 181 | 37.0 ± 1.2 | 181 | 44.4 ± 1.3 | 1.79E-05* |
| Sex (M/F) | 181 | 30 (16.6)/151 (83.4) | 181 | 50 (27.6)/131 (72.4) | 0.016* |
| BP status, NT/HTN (%) | 181 | 174 (96.1)/7 (3.9) | 181 | 151 (83.4)/30 (16.6) | 8.64E-05* |
| Physical | |||||
| Body mass index (kg/m2) | 181 | 22.0 ± 0.24 | 181 | 27.8 ± 0.42 | 6.36E-43* |
| Physiological | |||||
| SBP (mm Hg) | 179 | 127.0 ± 1.2 | 175 | 134.0 ± 1.2 | 1.67E-05* |
| DBP (mm Hg) | 179 | 68.4 ± 0.7 | 175 | 73.9 ± 0.8 | 6.73E-08* |
| Metabolic | |||||
| Plasma glucose (mg/dl) | 181 | 80.2 ± 1.1 | 181 | 83.6 ± 1.9 | 0.112 |
| Plasma insulin (μUnit/ml) | 179 | 11.1 ± 0.64 | 181 | 16.0 ± 1.23 | 3.38E-04* |
| QUICKI (insulin sensitivity) | 179 | 0.35 ± 0.003 | 181 | 0.34 ± 0.005 | 0.015* |
| HOMA (insulin resistance) | 188 | 2.33 ± 0.16 | 172 | 3.51 ± 0.33 | 0.001* |
| Plasma leptin (ng/ml) | 190 | 8.53 ± 0.44 | 172 | 15.1 ± 0.70 | 2.22E-16* |
| Biochemical | |||||
| Epinephrine in urine (ng/gm) | 166 | 13,537 ± 590 | 169 | 13,141 ± 583 | 0.622 |
| Norepinephrine in urine (ng/gm) | 174 | 29,744 ± 1,175 | 161 | 29,053 ± 1,052 | 0.649 |
| CHGA116–439 (nmol/l) | 176 | 4.11 ± 0.17 | 177 | 3.86 ± 0.27 | 0.376 |
| CHGA361–372 (nmol/l) | 176 | 1.23 ± 0.05 | 177 | 1.36 ± 0.05 | 0.017* |
| C-reactive protein (ng/ml) | 173 | 1,457 ± 178 | 177 | 3,386 ± 355 | 4.41E-07* |
Descriptive and inferential statistics for twin study population. Values are mean ± s.e.m. (or n and %) derived from GEE. BMI (dichotomized around the median concentration of 23.8 kg/m2) is listed for all individuals. Numbers ‘N’ describe the number of analyzed individuals available for that particular trait. The mean or N (%) gives the mean for continuous variables or percentage of the trait observed for dichotomous variables (listed in parenthesis). Values in urine are normalized to creatinine concentration.
BP, blood pressure; CHGA, Chromogranin A; DBP, diastolic blood pressure; GEE, generalized estimating equations; HOMA, HOmeostatic Model Assessment; insulin resistance; HTN, hypertension; NT, normotension; QUICKI, QUantitative Insulin sensitivity ChecK Index; SBP, systolic blood pressure.
P < 0.05.
Pleiotropy (shared h2): Genetic covariance with BMI
We tested several heritable BMI-correlated metabolic traits for shared genetic or environmental determination (Table 4). The results indicate that BMI shares genetic determination (pleiotropy, ρG) with SBP/DBP, glucose, insulin, HOMA, QUICKI, leptin, CHGA116–439, and C-reactive protein. By contrast, five correlated traits also shared significant environmental determination (environmental covariance, ρE) with BMI: insulin, HOMA, leptin, CHGA116–439, and C-reactive protein; these five traits displayed both ρG and ρE with BMI. Figure 2b illustrates traits displaying different patterns of codetermination with BMI: leptin, CRP, and CHGA116–439 with significance for both ρG and ρE, but CHGA361–372 with significance for neither ρG nor ρE.
Table 4.
BMI: Shared genetic determination (genetic covariance, RHoG (ρG), pleiotropy) and environmental determination (environmental covariance, RHoE (ρE)) for metabolic syndrome traits correlated with BMI in twins
| Trait | Heritability | Nonparametric overall correlation | Environmental covariance | Genetic covariance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| h2 ± s.e.m. | P value | Spearman rho | P value | ρE | s.e.m. | P value | ρG | s.e.m. | P value | |
| Physiological | ||||||||||
| SBP (mm Hg) | 0.46 ± 0.06 | 1.17E-09* | 0.248 | 2.41E-06* | 0.03 | 0.09 | 0.76 | 0.36 | 0.09 | 9.31E-05* |
| DBP (mm Hg) | 0.52 ± 0.06 | 4.58E-12* | 0.294 | 1.75E-08* | 0.11 | 0.09 | 0.21 | 0.3 | 0.08 | 7.74E-04* |
| Metabolic | ||||||||||
| Plasma glucose (mg/dl) | 0.34 ± 0.07 | 2.00E-06* | 0.228 | 1.20E-05* | −0.06 | 0.08 | 0.48 | 0.46 | 0.11 | 9.79E-05* |
| Plasma insulin (μUnit/ml) | 0.50 ± 0.09 | 1.60E-06* | 0.364 | 1.05E-12* | 0.36 | 0.09 | 2.00E-04* | 0.39 | 0.10 | 7.95E-04* |
| QUICKI (insulin sensitivity) | 0.42 ± 0.06 | 2.79E-09* | −0.386 | 3.17E-14* | −0.13 | 0.08 | 0.13 | −0.31 | 0.09 | 0.0014* |
| HOMA (insulin resistance) | 0.55 ± 0.08 | 1.18E-08* | 0.157 | 3.00E-03* | 0.32 | 0.09 | 6.90E-04* | 0.38 | 0.09 | 4.05E-04* |
| Plasma leptin (ng/ml) | 0.73 ± 0.04 | 1.67E-24* | 0.535 | 9.82E-53* | 0.6 | 0.06 | 3.58E-13* | 0.82 | 0.03 | 1.95E-27* |
| Biochemical | ||||||||||
| Epinephrine in urine (ng/gm) | 0.72 ± 0.04 | 1.55E-20* | −0.071 | 0.36 | −0.1 | 0.09 | 0.264 | −0.06 | 0.09 | 0.465 |
| Norepinephrine in urine (ng/gm) | 0.47 ± 0.06 | 4.75E-10* | 0.064 | 0.413 | 0.02 | 0.09 | 0.782 | −0.01 | 0.10 | 0.908 |
| CHGA116–439 (nmol/l) (log10) | 0.72 ± 0.05 | 3.57E-15* | −0.153 | 4.00E-03* | −0.23 | 0.09 | 0.015* | −0.18 | 0.08 | 0.037* |
| CHGA361–372 (nmol/l) (log10) | 0.65 ± 0.05 | 6.72E-21* | 0.140 | 8.00E-03* | 0.08 | 0.08 | 0.367 | 0.11 | 0.08 | 0.174 |
| C-reactive protein (ng/ml) (log10) | 0.60 ± 0.05 | 3.79E-15* | 0.406 | 2.29E-08* | 0.39 | 0.07 | 2.32E-06* | 0.42 | 0.07 | 4.36E-07* |
RhoG and RhoE were determined from variance components in SOLAR. Values in urine are normalized to creatinine concentration. (−) RhoG or RhoE typically indicates that the primary trait-on-trait correlation is negative. Heritability for BMI is: 85.8 ± 2.12% (P = 1.63e-42*).
BMI, body mass index; CHGA, Chromogranin A; DBP, diastolic blood pressure; HOMA, HOmeostatic Model Assessment; insulin resistance; QUICKI, QUantitative Insulin sensitivity ChecK Index; SBP, systolic blood pressure.
P < 0.05.
CHGA promoter polymorphism: Pleiotropic effects of G-462A on leptin and metabolic traits
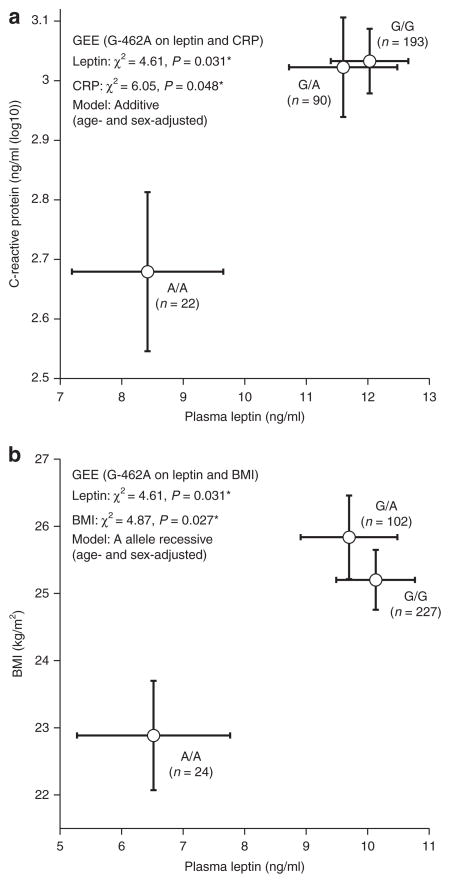
Because the leptin and CHGA traits displayed genetic covariance (Table 2, Figure 2a), we tested whether CHGA promoter variant G-462A influenced the leptin trait; we focused on G-462A because this variant plays the major functional role within the CHGA promoter block.19 The G-462A minor allele (in bold) was associated with lower leptin (P = 0.031, Figure 3a), BMI (P = 0.027, Figure 3b), and C-reactive protein (P = 0.048, Figure 3a) in UCSD twins.
Figure 3.
Pleiotropic effects of CHGA promoter variant G-462A on multiple metabolic syndrome traits in UCSD twins. *P < 0.05. (a) Joint effects of CHGA promoter variant G-462A on leptin (P = 0.031*) and CRP (P = 0.048*) are illustrated; (b) joint effects of CHGA promoter variant G-462A on leptin (P = 0.031*) and BMI (P = 0.027*) are illustrated. BMI, body mass index; CHGA, Chromogranin A; CRP, C-reactive protein; GEE, generalized estimating equations; UCSD, University of California San Diego.
Interactive effects of CHGA promoter polymorphism and sex: Replication across populations
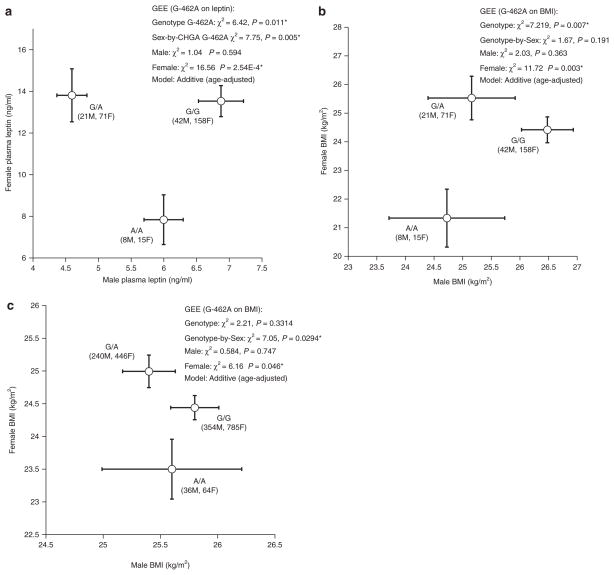
In UCSD twins (Supplementary Table S1 online), women had ~2-fold higher leptin than men, while BMI did not differ between the sexes. We then tested whether the CHGA G-462A effect varied by sex. In UCSD twins, there was a significant (P = 0.005) genotype-by-sex interaction on leptin (Figure 4a), though not BMI (Figure 4b). In women, leptin differed substantially between homozygote (G/G, A/A) classes (P = 2.54E-4, Figure 4a). The allele -462A was associated with both lower leptin (P = 0.029, Figure 4a) and BMI (P = 0.003, Figure 4b) in UCSD female twins. In men, G-462A was not associated with either leptin or BMI.
Figure 4.
CHGA promoter variant G-462A: Sex-dependent effects on traits in twins. The effect of CHGA promoter variant G-462A on traits leptin is illustrated separately for men and women. *P < 0.05. (a) Leptin in UCSD twins. There is a significant overall effect for genotype (P = 0.011*), as well as an effect in women alone (P = 2.54E-4*); (b) BMI in UCSD twins. There is a significant overall effect for genotype (P = 0.007), as well as an effect in women alone (P = 0.002); (c) BMI in Australia (QIMR) twins. There is a significant effect in women alone (P = 0.046), as well as a gene-by-sex interaction (P = 0.029). BMI, body mass index; CHGA, Chromogranin A; GEE, generalized estimating equations; QIMR, Queensland Institute of Medical Research; UCSD, University of California San Diego.
Replication
The gene-by-sex effects on BMI were replicated in an independent (Australia/Queensland Institute of Medical Research) twin sample. We found that there was significant genotype-by-sex interaction on BMI (P = 0.0294); the minor allele (in bold) at position G-462A predicted lower BMI in Australian female twins (P= 0.046), though not in male twins (Figure 4c).
Leptin and BMI: Correlated traits or covariates
Since leptin and BMI are highly correlated (Spearman rho = 0.535, P = 3.85E-28; Table 2, UCSD twins), we evaluated whether entry of BMI as a covariate affected statistical predictions of leptin. Inclusion of BMI as a covariate abrogated the significant predictions of leptin by BP (Figure 1a) and CHGA genotype in the entire UCSD cohort (Figure 3a, b); however, significant prediction of leptin by CHGA genotype persisted in women (Figure 4a). Since the leptin and BMI traits are correlated (Table 2), it is difficult to discern the sequential causal pathway of the CHGA gene effect upon the clinical traits; however, genetic covariance estimates (Figure 2a, b) document joint heritability between leptin, CHGA, and BMI, indicating that these traits experience partial determination by a shared set of genes.
CHGA polymorphism: Role of promoter variant G-462a and trans-activation
Sequence conservation and alignment
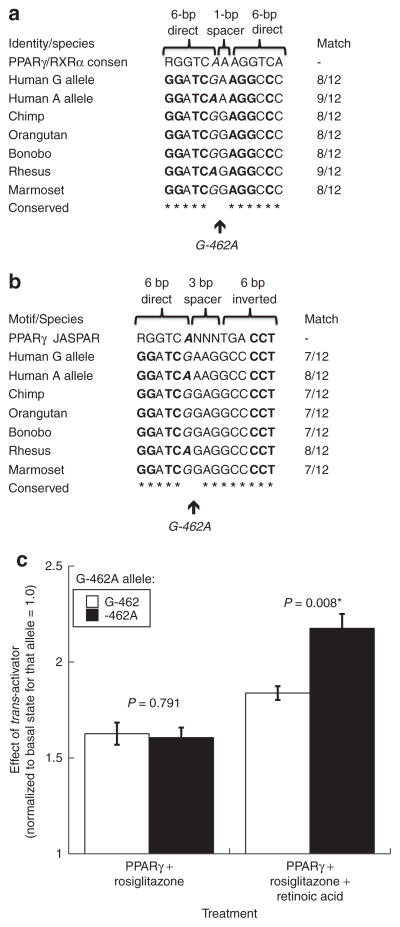
G-462A is located in a region highly conserved across sequenced primates (Figure 5a, b), with the G allele likely ancestral in the primate lineage (based on chimpanzee G). Since G-462A exhibited pleiotropic effects on multiple metabolic syndrome traits BMI, leptin, C-reactive protein, and BP,20 we focused on this variant. G-462A displayed a partial consensus match for two transcription factor binding motifs, of the nuclear hormone receptor variety: a PPARγ/RXRα heterodimer (DR1, direct repeat) motif and a PPARγ homodimer (Pal3, palindrome) motif. At the PPARγ/RXRα heterodimer motif, the A allele provided a better match than G (9/12 vs. 8/12 bp; Figure 5a), while at the PPARγ homodimer motif, the A allele also displayed a better match (8/12 vs. 7/12 bp; Figure 5a). Among sequenced mammals, all had either G or A at the equivalent position of human G-462A (Figure 5a, b).
Figure 5.
Human CHGA promoter common variant G-462A: transcriptional motif disruption. (a) PPARγ/RXRα heterodimer recognition motif is shown as two direct repeats (RGGTCA) separated by a one nucleotide spacer. Italics: Position of G-462A (rs9658634). Bold: Match to PPARγ/RXRα heterodimer DR1 (direct repeat) motif. PPARγ/RXRα heterodimer DR1 motif: MA0065 at JASPAR <http://jaspar.genereg.net>. Computed at ref. 54; (b) PPARγ homodimer recognition motif is shown as one direct repeat (RGGTCA) and one inverted repeat (TGACCT) separated by a three nucleotide spacer. Italics: Position of G-462A (rs9658634). Bold: Match to PPARγ homodimer Pal3 (palindrome) motif. PPARγ homodimer Pal3 motif: MA0066.1 at JASPAR <http://jaspar.genereg.net>. Computed at ref. 54; (c) human CHGA promoter G-462A and nuclear receptor activation: effects on transcriptional activity in chromaffin cells. Allele G-462 occurs naturally as part of Hap-A (TTgTC); on the Hap-A background, G-462 was point mutated to -462A, creating nonnatural/artificial haplotype TTaTC. Results for TTgTC vs. TTaTC are compared with t-test. Chromaffin (PC12) cell transfection results are shown. A PPARγ expression plasmid (pCMV promoter) was cotransfected with CHGA promoter haplotypes into PC12 cells, wherein stimulation was 10 μM rosiglitazone alone (left), vs. 10 μM rosiglitazone plus 1 μM retinoic acid (right). *P < 0.05. CHGA, chromogranin A.
PPARγ and retinoic acid stimulation of CHGA G-462A
To probe the significance of the putative PPARγ/RXRα motifs, we tested the effect of PPARγ and its thiazolidinedione ligand rosiglitazone, by cotransfection with CHGA promoter/reporters bearing either G-462A allele (Figure 5c). Each promoter variant was stimulated ~1.6-fold by PPARγ/rosiglitazone, though the increment did not differ by G-462A allele (Figure 5c). Upon addition of retinoic acid (Figure 5c), both G-462A promoter variants were further activated, but the increment was greater for the -462A allele.
Chromatin immunoprecipitation
After immunoisolation of nucleosomes, PCR with a 152-bp amplicon spanning G-462A detected PPARγ binding to the CHGA promoter on both alleles (G and A; data not shown).
DISCUSSION
Overview: Leptin, BMI, and heredity
We first noted the association of CHGA genetic variation with leptin and then found that CHGA genetic variation or secretion predicted not only leptin but also BMI and CRP in a series of twin pairs. We demonstrate that both leptin and BMI are substantially heritable (Tables 2 and 4), and each aggregates with multiple traits: SBP/DBP, glucose, insulin, HOMA, QUICKI, leptin, CHGA116–439, CHGA361–372, and CRP (Tables 1 and 3). Individuals with higher plasma leptin and BMI displayed a number of significant trait differences, both biochemical (chromogranin, catecholamine, and inflammatory) and metabolic (insulin resistance; Tables 1 and 3), and both plasma leptin and BMI are associated with systemic hypertension (Figure 1a, b, Tables 1 and 3).
An unusual feature of this study was the use of the twin method, to dissect out complex features of the mode of inheritance of leptin and BMI, including heritability and pleiotropy (Tables 2 and 4). Since CHGA shared heritability with both leptin and BMI (Tables 2 and 4), we tested the CHGA locus for effects on the BMI and leptin traits; we found that common functional variation in the CHGA promoter exerted pleiotropic effects on both traits (Figure 3b); moreover, we found the minor (A) allele at G-462A was associated with lower levels of both leptin and BMI.
We found that plasma leptin is approximately twofold greater in female than male subjects (13.3 ± 0.45 vs. 6.67 ± 0.85, P = 2.59E-11; Supplementary Table S1 online). In a large cross-sectional study of children of both sexes, girls had higher leptin concentrations than boys,36 a finding evident even in the youngest age group studied. Tome et al. even found sex differences in leptin measured in umbilical cord serum at birth, suggesting differences in the regulation of leptin production by fetal adipose tissue.37 Sexual dimorphism in serum leptin persisted even after accounting for the effects of body fat.38 Likewise, we found that G-462A was associated with BMI in both UCSD and Australian female twins (Figure 4c).
In mice with targeted ablation of the Chga locus, we previously found substantial elevations in leptin expression in both adipose tissue and plasma;21 such reciprocal (opposite) changes between Chga and Lep gene expression are mirrored by the inverse overall correlation between CHGA116–439 and leptin protein expression in the UCSD twins (Spearman rho = −0.153, P = 4.00E-03; Table 2). Overall, the results suggest novel functional links between adrenergic pathways, the metabolic syndrome and hypertension.
Catecholamines and leptin in hypertension
Thomopoulos et al. observed that free leptin predicts incident hypertension in a Danish cohort.39 Leptin may regulate blood pressure through sympathetic activation.40 In our study, we found elevated plasma leptin level was associated with systemic hypertension: mean leptin was significantly higher in hypertensive than normotensive individuals (P = 0.014) (Figure 1), as well as in subjects with positive family history of hypertension (P = 0.035) (Figure 1).
Such effects of leptin on BP might be mediated through several pathways: interaction between leptin and insulin,41 up-regulation of angiotensinogen,42 or actions of leptin on its receptor in the hypothalamus.43,44 Leptin activates sympathetic responses in vascular and renal districts.6,45 Indeed, we found that leptin aggregates with norepinephrine more significantly than does BMI (Tables 1 and 3), suggesting that leptin itself may participate in sympathetic activation.6
Previous studies demonstrate that CHGA plays a pivotal role in the sympathochromaffin system, both in formation of secretory vesicles and in regulation of transmitter release.13,17 Genetic variation at the CHGA locus alters transmitter storage and secretion in both humans and experimental animals.46 We have previously demonstrated that the G-462A variant in the CHGA promoter alters autonomic activity and blood pressure.20 Thus, our results suggest novel functional links between autonomic activity and leptin.
Complex inheritance of leptin: Genetic pleiotropy
The twin design allowed us to explore pleiotropy (shared genetic determination of two or more traits), documented as the genetic covariance (Table 2) between correlated traits. Of the many traits correlated or associated with leptin, genetic pleiotropy (ρG) occurred with BMI, SBP/DBP, CHGA, glucose, insulin, HOMA, and QUICKI. What specific genes might jointly contribute to such traits? We explored the role of genetic variation at the CHGA locus, since CHGA and leptin shared ρG (Table 2), yet polymorphisms at other genes not directly scored in this report could jointly contribute to multiple facets of the metabolic syndrome. Heritability for leptin and BMI traits has been reported previously, with evidence of shared genetic determination.47,48
Functional nature of CHGA promoter polymorphism
CHGA is required for the formation of catecholamine secretory vesicles in chromaffin cells, and its expression may be sufficient to induce a regulated secretory system even in nonsecretory cells.18
Previously, we found that the proximal promoter region of CHGA governs its transcription, under both basal circumstances and secretory stimulation, consistent with the notion of “stimulus–secretion–synthesis” or “stimulus–transcription” coupling.49–51 The cyclic AMP response element site in the very proximal CHGA promoter was initially identified to be crucial in neuroendocrine-specific expression of CHGA,52,53 and its response to secretory stimulation. Previously we reported that interindividual expression differences were maximally effected by promoter variant G-462A, which influenced not only transcriptional strength in transfected promoter/reporter constructs, but also exhibited an effect on basal and stress-augmented BPs in the population.20 Here we found that G-462A altered a consensus PPARγ/RXRα transcriptional motif;54 site-directed mutagenesis of the site altered the response to PPARγ/RXRα activated by the cognate ligands rosiglitazone/retinoic acid (Figure 5c), and the motif bound endogenous PPARγ.
Of note for the potential physiological significance of these results, transcripts for both PPARγ and RXRα are highly expressed in PC12 chromaffin cells55 (NCBI Gene Expression Omnibus/GEO http://www.ncbi.nlm.gov/geo, datasets GDS2555,55 GDS1234,56 and GDS103857). Indeed, when PPARγ and RXRα are coexpressed, the PPARγ/RXRα heterodimer (Figure 5c), rather than the PPARγ/PPARγ homodimer (Figure 5c), tends to be activated by PPARγ ligands.54 Previously we noted that CHGA G-462A may disrupt the recognition motif for another member of the nuclear hormone receptor family, COUP-TF (or “chicken ovalbumin upstream promoter” element-binding transcription factor);20 however, unlike PPARγ/RXRα, COUP-TF does not have a known activating ligand, and the G-462A region motif match for COUP-TF (at 7–8 of 12 bp) is slightly inferior to the match for PPARγ/RXRα (at 8–9 of 12 bp; Figure 5a).
Several findings in this study suggested that polymorphism in the promoter of CHGA might be functionally important for interindividual variation in autonomic as well as metabolic syndrome traits. First, the promoter variant G-462A was associated with BMI and leptin (Figure 3b). Second, the promoter variant G-462A influenced gene expression in cella.20 Third, the changes conferred by allelic variation at G-462A seemed to be directionally consistent: the -462A (minor) allele decreased gene expression both in cella 20 and in vivo (Figure 3a–c). Finally, the findings on BMI were consistent in vivo between two independent twin samples (Figure 4a, b).
Advantages and limitations of this study
Advantages
We used the classical twin design in the search for trait-associated polymorphisms.58 Multiple phenotypes were measured in twins, which permitted estimation of trait heritability and genetic covariance (shared heritability) as well as defining the effects of particular genetic variants at CHGA on the correlated traits. We probed the effects of CHGA variation upon early “intermediate” phenotypes for disease and confirmed the effects of CHGA G-462A variation upon BMI in an independent group (Australia twin pairs).
Limitations
To minimize artifacts in genetic association, we initially confined our analyses to only one ethnicity, and the majority of the study subjects were healthy. Thus we cannot readily extrapolate our conclusions to other population groups, or other cardiovascular diseases.
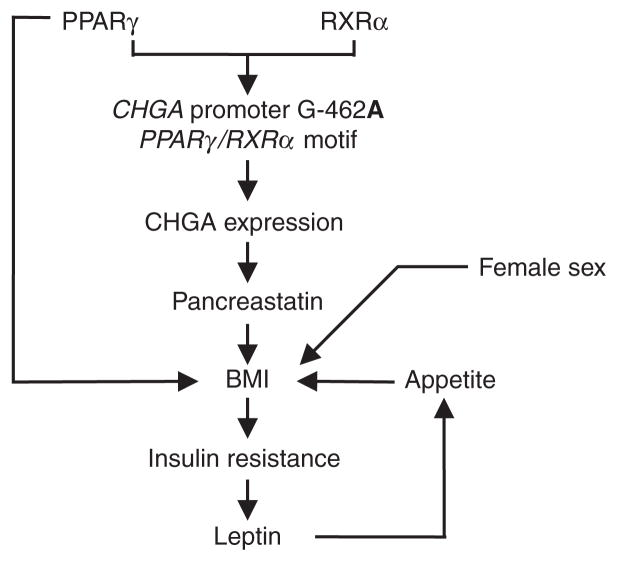
We conclude that CHGA shares joint genetic determination (or pleiotropy) with such phenotypes as BMI, SBP/DBP, glucose, insulin, HOMA, and QUICKI, as well as the emerging risk trait of leptin (Table 1). Based on pleiotropic genetic control of leptin and BMI with CHGA (Tables 2 and 4), we tested the role of CHGA genetic variation and found that promoter G-462A contributes to both BMI and leptin traits (Figure 3b). The findings are consistent with a cascade of events (Figure 3a–c), beginning with differential stimulation of G-462A by PPARγ/RXRα, and eventuating in altered leptin and BMI. Our results thus propose novel pathophysiological links between the CHGA gene, leptin, multiple features of the metabolic syndrome, and hypertension and suggest new strategies for probing the role and actions of PPARγ/RXRα, CHGA, and leptin within this setting (Figure 6).
Figure 6.
Human CHGA promoter variant G-462A: integrated hypothesis for metabolic traits. A framework hypothesis is shown for the actions of PPARγ/RXRα on CHGA promoter variant G-462A, to influence metabolic syndrome traits, and ultimately blood pressure. BMI, body mass index; CHGA, chromogranin A.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health and the Department of Veterans Affairs. We appreciate the assistance of the NIH/NCRR-supported General Clinical Research Center at UCSD (RR00827) and the NIH/NCMHD EXPORT minority health center (MD000220).
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/ajh
Disclosure: The authors declared no conflict of interest.
References
- 1.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 2.van Dijk G. The role of leptin in the regulation of energy balance and adiposity. J Neuroendocrinol. 2001;13:913–921. doi: 10.1046/j.1365-2826.2001.00707.x. [DOI] [PubMed] [Google Scholar]
- 3.Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, Ebihara K, Masuzaki H, Hosoda K, Yoshimasa Y, Nakao K. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48:1787–1793. doi: 10.2337/diabetes.48.9.1787. [DOI] [PubMed] [Google Scholar]
- 4.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 5.da Silva AA, Tallam LS, Liu J, Hall JE. Chronic antidiabetic and cardiovascular actions of leptin: role of CNS and increased adrenergic activity. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1275–R1282. doi: 10.1152/ajpregu.00187.2006. [DOI] [PubMed] [Google Scholar]
- 6.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30:619–623. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- 7.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 8.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 9.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14:103S–115S. doi: 10.1016/s0895-7061(01)02077-5. [DOI] [PubMed] [Google Scholar]
- 10.Shankar A, Xiao J. Positive relationship between plasma leptin level and hypertension. Hypertension. 2010;56:623–628. doi: 10.1161/HYPERTENSIONAHA.109.148213. [DOI] [PubMed] [Google Scholar]
- 11.Asferg C, Møgelvang R, Flyvbjerg A, Frystyk J, Jensen JS, Marott JL, Appleyard M, Jensen GB, Jeppesen J. Leptin, not adiponectin, predicts hypertension in the Copenhagen City Heart Study. Am J Hypertens. 2010;23:327–333. doi: 10.1038/ajh.2009.244. [DOI] [PubMed] [Google Scholar]
- 12.Kramer CK, von Mühlen D, Barrett-Connor E. Does leptin predict incident hypertension in older adults? Clin Endocrinol (Oxf) 2010;73:201–205. doi: 10.1111/j.1365-2265.2010.03781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 14.Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992;49:497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takiyyuddin MA, Cervenka JH, Hsiao RJ, Barbosa JA, Parmer RJ, O’Connor DT, Chromogranin A. Storage and release in hypertension. Hypertension. 1990;15:237–246. doi: 10.1161/01.hyp.15.3.237. [DOI] [PubMed] [Google Scholar]
- 16.Videen JS, Mezger MS, Chang YM, O’Connor DT. Calcium and catecholamine interactions with adrenal chromogranins. Comparison of driving forces in binding and aggregation. J Biol Chem. 1992;267:3066–3073. [PubMed] [Google Scholar]
- 17.Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 19.Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, Rao F, Stridsberg M, Smith DW, Mahboubi P, Schork NJ, O’Connor DT, Hamilton BA. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Rao F, Rodriguez-Flores JL, Mahapatra NR, Mahata M, Wen G, Salem RM, Shih PA, Das M, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, O’Connor DT. Common genetic variants in the chromogranin A promoter alter autonomic activity and blood pressure. Kidney Int. 2008;74:115–125. doi: 10.1038/ki.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gayen JR, Saberi M, Schenk S, Biswas N, Vaingankar SM, Cheung WW, Najjar SM, O’Connor DT, Bandyopadhyay G, Mahata SK. A novel pathway of insulin sensitivity in chromogranin A null mice: a crucial role for pancreastatin in glucose homeostasis. J Biol Chem. 2009;284:28498–28509. doi: 10.1074/jbc.M109.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cockburn M, Hamilton A, Zadnick J, Cozen W, Mack TM. The occurrence of chronic disease and other conditions in a large population-based cohort of native Californian twins. Twin Res. 2002;5:460–467. doi: 10.1375/136905202320906282. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Rao F, Wessel J, Kennedy BP, Rana BK, Taupenot L, Lillie EO, Cockburn M, Schork NJ, Ziegler MG, O’Connor DT. Functional allelic heterogeneity and pleiotropy of a repeat polymorphism in tyrosine hydroxylase: prediction of catecholamines and response to stress in twins. Physiol Genomics. 2004;19:277–291. doi: 10.1152/physiolgenomics.00151.2004. [DOI] [PubMed] [Google Scholar]
- 24.Wessel J, Moratorio G, Rao F, Mahata M, Zhang L, Greene W, Rana BK, Kennedy BP, Khandrika S, Huang P, Lillie EO, Shih PA, Smith DW, Wen G, Hamilton BA, Ziegler MG, Witztum JL, Schork NJ, Schmid-Schönbein GW, O’Connor DT. C-reactive protein, an ‘intermediate phenotype’ for inflammation: human twin studies reveal heritability, association with blood pressure and the metabolic syndrome, and the influence of common polymorphism at catecholaminergic/beta-adrenergic pathway loci. J Hypertens. 2007;25:329–343. doi: 10.1097/HJH.0b013e328011753e. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, Mahata SK, Mahata M, Wang L, Zhang K, Greenwood TA, Shih PA, Cockburn MG, Ziegler MG, Stridsberg M, Martin NG, Whitfield JB. Heritability and genome-wide linkage in US and australian twins identify novel genomic regions controlling chromogranin a: implications for secretion and blood pressure. Circulation. 2008;118:247–257. doi: 10.1161/CIRCULATIONAHA.107.709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinton TJ, Cotter B, Kailasam MT, Brown DL, Chio SS, O’Connor DT, DeMaria AN. Development and validation of a noninvasive method to determine arterial pressure and vascular compliance. Am J Cardiol. 1997;80:323–330. doi: 10.1016/s0002-9149(97)00353-6. [DOI] [PubMed] [Google Scholar]
- 27.Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH, Landt M. Radioimmunoassay of leptin in human plasma. Clin Chem. 1996;42:942–946. [PubMed] [Google Scholar]
- 28.Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–2153. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- 29.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 30.Do KA, Broom BM, Kuhnert P, Duffy DL, Todorov AA, Treloar SA, Martin NG. Genetic analysis of the age at menopause by using estimating equations and Bayesian random effects models. Stat Med. 2000;19:1217–1235. doi: 10.1002/(sici)1097-0258(20000515)19:9<1217::aid-sim421>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 31.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4. England: Longman; 1996. Essex ed. [Google Scholar]
- 33.Knüppel R, Dietze P, Lehnberg W, Frech K, Wingender E. TRANSFAC retrieval program: a network model database of eukaryotic transcription regulating sequences and proteins. J Comput Biol. 1994;1:191–198. doi: 10.1089/cmb.1994.1.191. [DOI] [PubMed] [Google Scholar]
- 34.Marinescu VD, Kohane IS, Riva A. MAPPER: a search engine for the computational identification of putative transcription factor binding sites in multiple genomes. BMC Bioinformatics. 2005;6:79. doi: 10.1186/1471-2105-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasserman WW, Sandelin A. Applied bioinformatics for the identification of regulatory elements. Nat Rev Genet. 2004;5:276–287. doi: 10.1038/nrg1315. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J Clin Endocrinol Metab. 1997;82:2849–2855. doi: 10.1210/jcem.82.9.4235. [DOI] [PubMed] [Google Scholar]
- 37.Tome MA, Lage M, Camiña JP, Garcia-Mayor RV, Dieguez C, Casanueva FF. Sex-based differences in serum leptin concentrations from umbilical cord blood at delivery. Eur J Endocrinol. 1997;137:655–658. doi: 10.1530/eje.0.1370655. [DOI] [PubMed] [Google Scholar]
- 38.Martin LJ, Mahaney MC, Almasy L, MacCluer JW, Blangero J, Jaquish CE, Comuzzie AG. Leptin’s sexual dimorphism results from genotype by sex interactions mediated by testosterone. Obes Res. 2002;10:14–21. doi: 10.1038/oby.2002.3. [DOI] [PubMed] [Google Scholar]
- 39.Thomopoulos C, Tsioufis C, Makris T, Stefanadis C. Free leptin predicts incident (clinic) hypertension in a Danish cohort. Am J Hypertens. 2010;23:814. doi: 10.1038/ajh.2010.107. author reply 815. [DOI] [PubMed] [Google Scholar]
- 40.Ma D, Feitosa MF, Wilk JB, Laramie JM, Yu K, Leiendecker-Foster C, Myers RH, Province MA, Borecki IB. Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension. 2009;53:473–479. doi: 10.1161/HYPERTENSIONAHA.108.118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities–the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 42.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 43.Campfield LA, Smith FJ, Pénicaud L, Burn P. OB protein and its receptor: signal transduction between adipose tissue and central nervous system. Journ Annu Diabetol Hotel Dieu. 1997:131–148. [PubMed] [Google Scholar]
- 44.Wolf G. Neuropeptides responding to leptin. Nutr Rev. 1997;55:85–88. doi: 10.1111/j.1753-4887.1997.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 45.Cao GY, Considine RV, Lynn RB. Leptin receptors in the adrenal medulla of the rat. Am J Physiol. 1997;273:E448–E452. doi: 10.1152/ajpendo.1997.273.2.E448. [DOI] [PubMed] [Google Scholar]
- 46.Jirout ML, Friese RS, Mahapatra NR, Mahata M, Taupenot L, Mahata SK, Kren V, Zídek V, Fischer J, Maatz H, Ziegler MG, Pravenec M, Hubner N, Aitman TJ, Schork NJ, O’Connor DT. Genetic regulation of catecholamine synthesis, storage and secretion in the spontaneously hypertensive rat. Hum Mol Genet. 2010;19:2567–2580. doi: 10.1093/hmg/ddq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaprio J, Eriksson J, Lehtovirta M, Koskenvuo M, Tuomilehto J. Heritability of leptin levels and the shared genetic effects on body mass index and leptin in adult Finnish twins. Int J Obes Relat Metab Disord. 2001;25:132–137. doi: 10.1038/sj.ijo.0801526. [DOI] [PubMed] [Google Scholar]
- 48.Jordan J, Brabant G, Brinsuk M, Tank J, Horn R, Luft FC, Busjahn A. Heritability of free and receptor-bound leptin in normal twins. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1411–R1416. doi: 10.1152/ajpregu.00446.2004. [DOI] [PubMed] [Google Scholar]
- 49.Tang K, Wu H, Mahata SK, Mahata M, Gill BM, Parmer RJ, O’Connor DT. Stimulus coupling to transcription versus secretion in pheochromocytoma cells. Convergent and divergent signal transduction pathways and the crucial roles for route of cytosolic calcium entry and protein kinase C. J Clin Invest. 1997;100:1180–1192. doi: 10.1172/JCI119630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taupenot L, Mahata M, Mahata SK, O’Connor DT. Time-dependent effects of the neuropeptide PACAP on catecholamine secretion: stimulation and desensitization. Hypertension. 1999;34:1152–1162. doi: 10.1161/01.hyp.34.5.1152. [DOI] [PubMed] [Google Scholar]
- 51.Taupenot L, Mahata SK, Wu H, O’Connor DT. Peptidergic activation of transcription and secretion in chromaffin cells. Cis and trans signaling determinants of pituitary adenylyl cyclase-activating polypeptide (PACAP) J Clin Invest. 1998;101:863–876. doi: 10.1172/JCI1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu H, Mahata SK, Mahata M, Webster NJ, Parmer RJ, O’Connor DT. A functional cyclic AMP response element plays a crucial role in neuroendocrine cell type-specific expression of the secretory granule protein chromogranin A. J Clin Invest. 1995;96:568–578. doi: 10.1172/JCI118069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu H, Rozansky DJ, Webster NJ, O’Connor DT. Cell type-specific gene expression in the neuroendocrine system. A neuroendocrine-specific regulatory element in the promoter of chromogranin A, a ubiquitous secretory granule core protein. J Clin Invest. 1994;94:118–129. doi: 10.1172/JCI117297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okuno M, Arimoto E, Ikenobu Y, Nishihara T, Imagawa M. Dual DNA-binding specificity of peroxisome-proliferator-activated receptor gamma controlled by heterodimer formation with retinoid X receptor alpha. Biochem J. 2001;353:193–198. doi: 10.1042/0264-6021:3530193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lattanzi W, Bernardini C, Gangitano C, Michetti F. Hypoxia-like transcriptional activation in TMT-induced degeneration: microarray expression analysis on PC12 cells. J Neurochem. 2007;100:1688–1702. doi: 10.1111/j.1471-4159.2006.04331.x. [DOI] [PubMed] [Google Scholar]
- 56.Nowroozi N, Raffioni S, Wang T, Apostol BL, Bradshaw RA, Thompson LM. Sustained ERK1/2 but not STAT1 or 3 activation is required for thanatophoric dysplasia phenotypes in PC12 cells. Hum Mol Genet. 2005;14:1529–1538. doi: 10.1093/hmg/ddi161. [DOI] [PubMed] [Google Scholar]
- 57.Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, McWeeney S, Dunn JJ, Mandel G, Goodman RH. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119:1041–1054. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 58.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.