Abstract
Prolactin (PRL) is a well-known regulator of ion and water transport within osmoregulatory tissues across vertebrate species, yet how PRL acts on some of its target tissues remains poorly understood. Using zebrafish as a model, we show that ionocytes in the gill directly respond to systemic PRL to regulate mechanisms of ion uptake. Ion-poor conditions led to increases in the expression of PRL receptor (prlra), Na+/Cl− cotransporter (ncc; slc12a10.2), Na+/H+ exchanger (nhe3b; slc9a3.2), and epithelial Ca2+ channel (ecac; trpv6) transcripts within the gill. Intraperitoneal injection of ovine PRL (oPRL) increased ncc and prlra transcripts, but did not affect nhe3b or ecac. Consistent with direct PRL action in the gill, addition of oPRL to cultured gill filaments stimulated ncc in a concentration-dependent manner, an effect blocked by a pure human PRL receptor antagonist (Δ1-9-G129R-hPRL). These results suggest that PRL signaling through PRL receptors in the gill regulates the expression of ncc, thereby linking this pituitary hormone with an effector of Cl− uptake in zebrafish for the first time.
Keywords: prolactin, zebrafish, gill, Na+/Cl− cotransporter, SLC12A10.2, Δ1-9-G129R-hPRL, receptor
1. Introduction
It has long been recognized that the endocrine system plays a central role in the homeostatic regulation of salt and water balance in vertebrates (McCormick and Bradshaw, 2006). Among the pituitary hormones, prolactin (PRL) has received considerable attention as an osmoregulatory hormone with conserved actions across vertebrate groups. In mammals, PRL influences solute and water transport across renal, intestinal, mammary and amniotic epithelial membranes (Bole-Feysot et al., 1998; Freeman et al., 2000). In teleost fishes, PRL is recognized as an important “freshwater-adapting hormone” regulating osmoregulatory functions within the gill, kidney and gastrointestinal tract by promoting ion conserving and water secreting processes (Hirano, 1986; Sakamoto and McCormick, 2006). The gill possesses a rich population of ionocytes that are capable of active Na+, Cl−, and Ca2+ uptake needed to support hydromineral balance in freshwater environments that are hyposmotic to body fluids (Kaneko et al., 2008). Varying and contending models have been proposed for the cellular mechanisms that regulate branchial ion-uptake in fresh water (Evans, 2011), and this incomplete understanding of the molecular mechanisms driving ion uptake in freshwater ionocytes has impeded progress in understanding how PRL promotes acclimation to freshwater environments.
The zebrafish (Danio rerio) has emerged as a new model organism for studying vertebrate physiology and is particularly well suited to detailed mechanistic and comparative studies of developmental endocrinology (McGonnell and Fowkes, 2006; Löhr and Hammerschmidt, 2011) and osmoregulation (Hwang, 2009). Zebrafish are regarded as being stenohaline and are naturally distributed in soft-water rivers and streams of the Indian subcontinent. Adult zebrafish can rapidly adapt to ion-poor conditions and can survive in deionized water for extended periods (Craig et al., 2007; Boisen et al., 2003). To persist in ion-poor waters, zebrafish have high capacity for Na+ and Cl− uptake (Boisen et al., 2003), despite strong opposing electrochemical gradients across gill epithelium. This capacity for ion-uptake, along with genetic and experimental accessibility, makes zebrafish particularly useful for studies aimed at elucidating how the endocrine system governs effectors of ion transport in vertebrates.
Zebrafish possess at least three distinct ionocyte sub-types characterized by the expression of specific integral membrane ion transporters/exchangers. Cells expressing the Na+/Cl− cotransporter (SLC12A10.2; NCC-cells) play a key role in Cl− ion uptake, while H+-ATPase-rich (HR-cells) and Na+-K+-ATPase-rich (NaR-cells) cells function in the uptake of Na+ and Ca2+, respectively (Pan et al., 2005; Esaki et al., 2007; Wang et al., 2009). NCC expression in the apical membrane of teleost ionocytes was first reported in Mozambique tilapia (Oreochromis mossambicus) (Hiroi et al., 2008); Horng et al. (2009) subsequently demonstrated that NCC-expressing cells actively absorb Cl−. As in tilapia, NCC is also expressed in a subset of ionocytes in the zebrafish gill and is essential for Cl− balance (Wang et al., 2009). In HR cells, a Na+/H+ exchanger (NHE3b; SLC9A3.2) provides the apical pathway for Na+ uptake from the external environment to the ionocyte interior where it is then transported into circulation (Yan et al., 2007). NaR cells specifically express an epithelial Ca2+ channel (ECaC; TRPV6) that facilitates the active uptake of Ca2+ from the external environment (Pan et al., 2005; Lin et al., 2011). The ion-absorptive functions of these three distinct zebrafish ionocytes have been demonstrated in zebrafish yolk integument, and all three genes are expressed in the gill (Liao et al., 2009). This characterization of ion transporters and cell types helps establish zebrafish as a new teleost model to assess the environmental and hormonal control of ion uptake capacities and mechanisms (Tseng et al., 2009; Chou et al., 2011; Lin et al., 2011).
While considerable progress has been made in establishing the cellular machinery supporting the functions of distinct ionocyte sub-types, the hormonal mechanisms that directly regulate ionocyte function, and thus ionoregulation by the gill, remain a mystery. PRL is a likely regulator of ionocytes based on the expression of teleost PRL receptors in gill tissue (Edery et al., 1984; Sandra et al., 1995; Weng et al., 1997; Santos et al., 2001; Lee et al., 2006), and the important role PRL plays in the osmoregulation of teleosts inhabiting freshwater environments. PRL can directly regulate gene expression in responding cells by binding to transmembrane receptors that activate the JAK/STAT signaling pathway (Bole-Feysot et al., 1998). There is evidence that the two zebrafish PRL receptors (denoted PRLRa and PRLRb) can regulate the transcription of distinct target genes upon PRL binding, at least in vitro (Chen et al., 2011). In Mozambique tilapia and black porgy (Acanthopagrus schlegeli), the expression of prlr1 and prlr2 transcripts (orthologous to zebrafish prlra and prlrb, respectively) in the gill is highly plastic, and the two prlrs are differentially influenced by osmotic and endocrine stimuli (Huang et al., 2007; Fiol et al., 2009; Breves et al., 2011). Modulation of prlr expression may provide a mechanism to regulate the sensitivity of target tissues to endocrine signaling. In fact, dynamic prlr expression in the gill appears to be an important aspect of adaptive responses to osmoregulatory challenges in euryhaline teleosts (Fiol et al., 2009; Breves et al., 2011; Flores and Shrimpton, 2012).
Here we show that PRL acts on ionocytes in the zebrafish gill by regulating the transcription of the ion cotransporter ncc, as well as the expression of prlra. The coordinated up-regulation of prlra and ncc in the gill upon transfer to ion-poor water, as well as following acute PRL treatment both in vivo and in vitro, suggests that PRL may be the key hormonal regulator of Cl− uptake mechanisms in zebrafish gill.
2. Materials and methods
2.1 Animals and rearing conditions
Sexually mature zebrafish (Danio rerio) were selected from stocks maintained at the University of Massachusetts, Amherst Zebrafish Facility. Fish were maintained in a recirculating system of dechlorinated reverse-osmosis municipal water (6.9 mM Na+, 6.6 mM Cl−, 0.12 mM Ca2+; pH 6.2–6.6) maintained at 26–27 °C. Fish were fed a flake diet supplemented with brine shrimp and maintained under a photoperiod of 14 h light: 10 h dark. The Institutional Animal Care and Use Committee of the University of Massachusetts approved the housing and maintenance of animals, and all experimental protocols.
2.2 Tissue distribution of PRL receptors
Tissues were collected from 10 adult zebrafish (5 males, 5 females, 1–2 g) maintained in standard rearing conditions for >1 year. Fish were lethally anesthetized with buffered tricaine methanesulfonate (MS-222; 250 mg/l), and the following tissues were collected: whole brain (olfactory bulb, telencephalon, optic tectum, cerebellum, diencephalon, and medulla), pituitary, gill, liver, body kidney, esophagus, anterior intestine and posterior intestine. Tissues were homogenized immediately in Trizol Reagent (Invitrogen, Carlsbad, CA) and stored at −80 °C until RNA isolation.
2.3 Transfer to ion-poor (ddH2O) water
Seven days prior to experimentation, adult zebrafish (1–2 g) maintained in standard rearing conditions for >1 year were distributed into 6 static aquaria (9 L; 8–10 fish/tank) maintained with filtration and aeration. At the time of transfer (0 h), fish from 2 aquaria were quickly netted and transferred directly to 2 additional aquaria containing ion-poor water (Millipore ddH2O; 0.2 mM Na+, 0.1 mM Cl−, 0.04 mM Ca2+; pH 7.0–7.2) with filtration and aeration. Control fish were netted and then returned to the same aquaria to control for potential handling effects. Fish were fed twice daily during the initial 7-day acclimation and then fasted for the duration of the transfer experiment. Water temperature was maintained at 26–28 °C. At the time of sampling, fish (n=8–10) from one system water- and one ion-poor water-containing aquaria were netted and anesthetized with a lethal dose of MS-222. Fish were sampled at 0, 2 and 7 days after transfer. Fish were rapidly decapitated and filaments from all branchial arches were homogenized immediately in Trizol Reagent and stored at −80 °C until RNA isolation. White muscle was sampled from the caudal musculature and the water content was measured gravimetrically after drying overnight at 90 °C.
2.4 In vivo effects of oPRL
Purified ovine PRL (oPRL; NIDDK-oPRL-21) was obtained from the National Hormone and Peptide Program and delivered in saline vehicle (0.9% NaCl; 20 µl/g body weight injection volume). Adult zebrafish (1–2 g) were administered oPRL (5 or 50 µg/g body weight) by two intraperitoneal (IP) injections. Fish were lightly anaesthetized with MS-222 and given the first injection. Twenty-four hours later, fish were netted, anaesthetized, and given a second injection. Fish were then returned to aquaria and left undisturbed for 24 h, after which time gill tissue was sampled as described above. The doses of oPRL were selected based on previous studies employing IP-injection in teleosts (Herndon et al., 1991; Eckert et al., 2001; Jackson et al., 2005; Breves et al., 2010).
2.5 Gill culture conditions and in vitro effects of oPRL and Δ1-9-G129R-hPRL
Gill filaments were isolated from adult zebrafish (1–2 g) and cultured according to McCormick and Bern (1989) with modifications. Fish were lethally anesthetized and branchial arches were removed and rinsed in pre-incubation Dulbecco's Modified Eagle Medium (DMEM; high glucose, HEPES, no phenol red; 311 mOsm; Invitrogen) containing 50 U/ml penicillin and 50 µg/ml streptomycin (Invitrogen). Gill filaments from a single fish were severed from the arches at the septum and placed in a single well (24-well cell culture plate; Corning Inc., Corning, NY) containing pre-incubation medium for 3 h. Each sample/well was designated as an individual fish. After the pre-incubation period, medium was replaced with freshly prepared control medium (DMEM + vehicle control: phosphate-buffered saline; PBS) or DMEM supplemented with oPRL (1 µg/ml) dissolved in PBS. For the initial time-course experiment, filaments (n=6) were incubated at 29 °C in a humidified chamber under atmospheric air for 4, 8, 12 and 24 h in either vehicle- or oPRL-supplemented DMEM. Gene expression was compared to pre-incubated filaments (0 h). For the subsequent concentration-response experiment, the effects of oPRL (0.01–10 µg/ml) were tested after 8 h of incubation. The recombinant human PRL receptor antagonist Δ1-9-G129R-hPRL was produced as described by Bernichtein et al. (2003). In an 8 h culture, Δ1-9-G129R-hPRL was used at concentrations ranging from 5–45 µg/ml in combination with 0.5 µg/ml oPRL. Gill cultures were terminated by removing the filaments from the culture wells and placing them in Trizol Reagent; filaments were homogenized immediately and stored at −80 °C until RNA isolation.
2.6 RNA extraction, cDNA synthesis and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from individual tissues using Trizol Reagent according to the manufacturer’s instructions. First strand cDNA was synthesized with a High-Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Relative amounts of mRNA were determined by qRT-PCR using the MxPro3000P system (Stratagene, La Jolla, CA). Primers for all genes are provided in Table 1, and when not previously reported were designed using Primer3. All primers were tested for non-specific product amplification and primer-dimer formation by melting curve analyses and gel electrophoresis. Assays were performed in a 10 µl reaction containing 1 µl cDNA template, 5 µl FastStart Universal SYBR Green Master (ROX) kit (Roche Diagnostics Corp., Indianapolis, IN), forward and reverse primers at 400 nM and nuclease-free water. The following cycling conditions were employed for all assays: 1 cycle of 95 °C for 15 min, 40 cycles of 95 °C for 15 sec, 60 °C for 30 sec, 72 °C for 30 sec and 1 cycle of 95 °C for 1 min, 55 °C for 30 sec and 95 °C for 30 sec. Three transcripts (β-actin, ef1α and 18s rRNA) were assessed for use as normalization genes in every experiment, a single gene was selected based upon its stable expression across treatments and time. qRT-PCR data were analyzed using the ΔΔCT method (Livak and Schmittgen, 2001). Standard curves were prepared from serial dilutions of untreated gill cDNA and included on each plate to calculate the PCR efficiencies for target and normalization genes. Relative gene expression is reported as a fold-change from controls. Intra-assay coefficients of variation ranged from 0.04 to 0.12.
Table 1.
Specific primer sequences for qRT-PCR.
| Name | Primer Sequence (5′-3′) | GenBank ID/Reference |
|---|---|---|
| β-actin | F: CACCTTCCAGCAGATGTGGA | Liao et al., 2007 |
| R: AAAAGCCATGCCAATGTTGTC | ||
| ef1α | F: CTGGTGTCCTCAAGCCTGGTA | Walpita et al., 2007 |
| R: ACTTGACCTCAGTGGTTACATTGG | ||
| 18s | F: TCGCTAGTTGGCATCGTTTATG | McCurley and Callard, 2008 |
| R: CGGAGGTTCGAAGACGATCA | ||
| prlra | F: AGGCAGTTCAATGCAGCACGA | EU517718.1 |
| R: GCACAGCGGCGGAAATCCTCAT | ||
| prlrb | F: GGATATCGTGCAGCCTCATCCTCCA | NM_001113500.1 |
| R: GGTTACCCATCCGGAGCGCG | ||
| ncc | F: GCCCCCAAAGTTTTCCAGTT | Wang et al., 2009 |
| R: TAAGCACGAAGAGGCTCCTTG | ||
| nhe3b | F: GTTTTCTGCAGACAGCGCCTCT | NM_001113479.1 |
| R: ATCCACACCAGCTCCAGTCGTGT | ||
| ecac | F: TTTGCAAGTCTTGTTGGTCTCGGT | NM_001001849.1 |
| R: TGCTGAAGGCGGAACCGCATT | ||
| b2m | F: GCCTTCACCCCAGAGAAAGG | McCurley and Callard, 2008 |
| R: GCGGTTGGGATTTACATGTTG | ||
| gapdh | F: CGCTGGCATCTCCCTCAA | Tang et al., 2007 |
| R: TCAGCAACACGATGGCTGTAG |
2.7 In situ hybridization
An antisense digoxigenin probe was generated from PCR product against ncc for the region spanning nucleotides 639–2162 as in Liao et al. (2009). Whole mount in situ hybridization was performed as previously described (Karlstrom et al., 1999) using NBT/BCIP as the chromogenic substrate (Roche Ltd., Basel, Switzerland). Colorimetric reaction times were identical for all samples. Following completion of the labeling reaction, filaments were cleared in 75% glycerol and examined using a dissecting microscope.
2.8 Statistical analyses
The tissue expression experiment (Fig. 1) was analyzed by two-way ANOVA (analysis of variance) with tissue and sex as main effects. A significant effect of tissue was followed up with Tukey’s honestly significant difference (HSD) test. The transfer experiment (Fig. 2) was analyzed by two-way ANOVA with treatment and time as main effects. Significant main effects of treatment or time were followed up by Student’s t-test or Dunnett’s test, respectively. Group comparisons for the in vivo injection (Fig. 3) and in vitro concentration-response (Fig. 5) experiments were conducted with Tukey’s HSD. When data were not normally distributed, a nonparametric ANOVA was performed on ranked data, followed by Tukey’s HSD to determine differences between groups. For the in vitro time-course (Fig. 4) and PRL receptor antagonist experiments (Fig. 6), two-way ANOVA was followed by a Student’s t-test and Tukey’s HSD test. All analyses were conducted using GraphPad Prism 5.0 (San Diego, CA, USA). Significance for all tests was set at P<0.05.
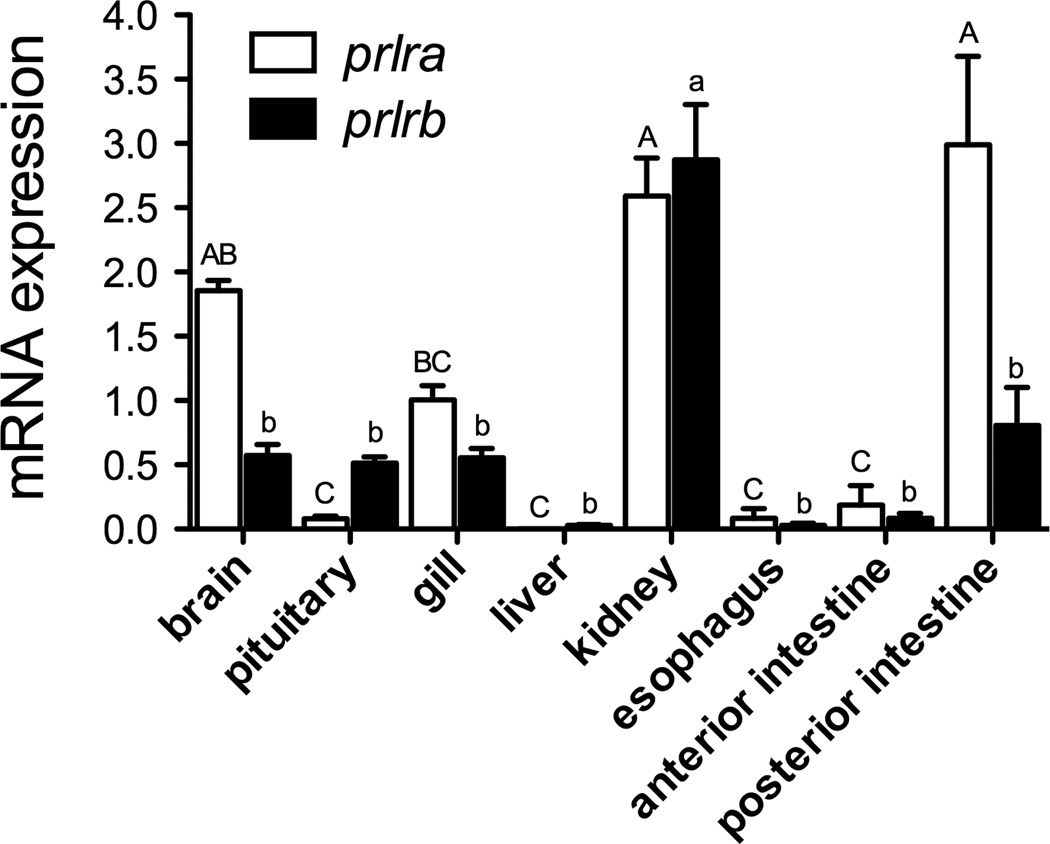
Figure 1.
Gene expression of prlra (open bars) and prlrb (solid bars) in the tissues of adult zebrafish maintained in normal fresh water. Data were normalized using ef1α as a reference gene and are presented relative to the amount of prlra mRNA in gill. Means ± SEM (n=10; 5 males, 5 females). For a given transcript, denoted by upper- or lower-case letters, groups not sharing the same letter are significantly different (ANOVA, Tukey’s HSD, P<0.05).
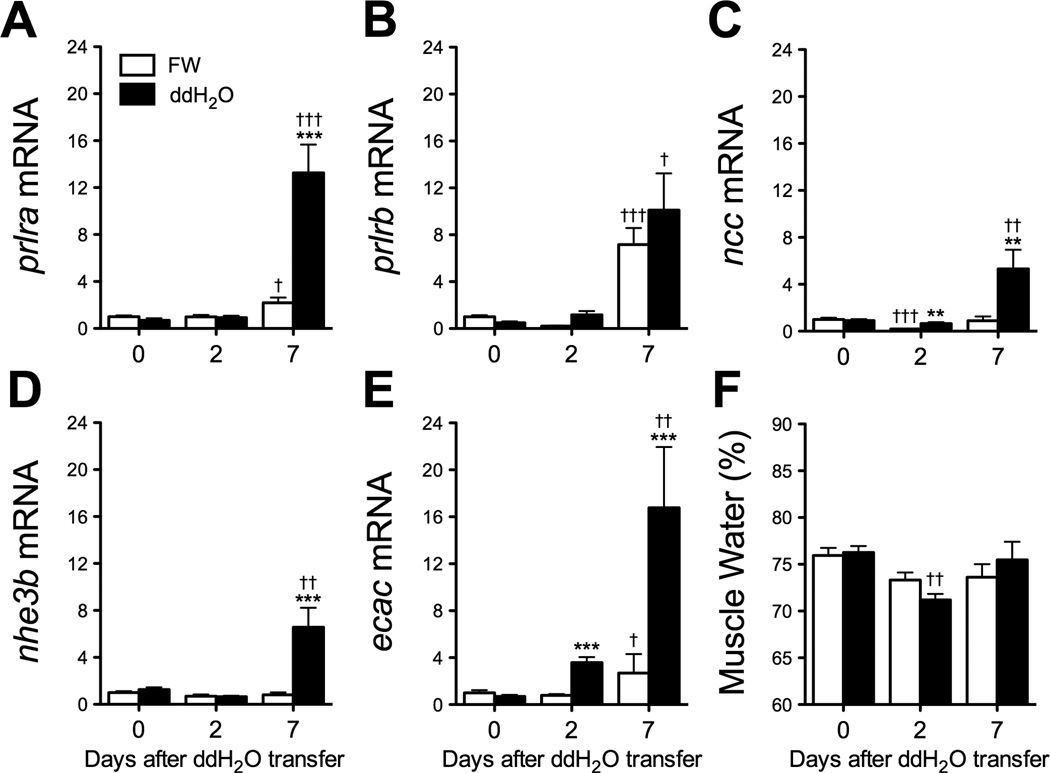
Figure 2.
Changes in branchial prlra (A), prlrb (B), ncc (C), nhe3b (D) and ecac (E) gene expression and muscle water content (F) at 0, 2 and 7 days after transfer of zebrafish adults from fresh water (FW) to ion-poor (ddH2O) water (solid bars). Means ± SEM (n=8–10). Time-matched control fish were maintained in FW (open bars). Gene expression is presented as fold-change from FW controls at time 0. Differences among groups were evaluated by two-way ANOVA. **, *** Significantly different from time-matched FW controls at P<0.01 and 0.001, respectively, by Student’s t-test. †, ††, ††† Significantly different from time 0 at P<0.05, 0.01 and 0.001, respectively, by Dunnett’s test.
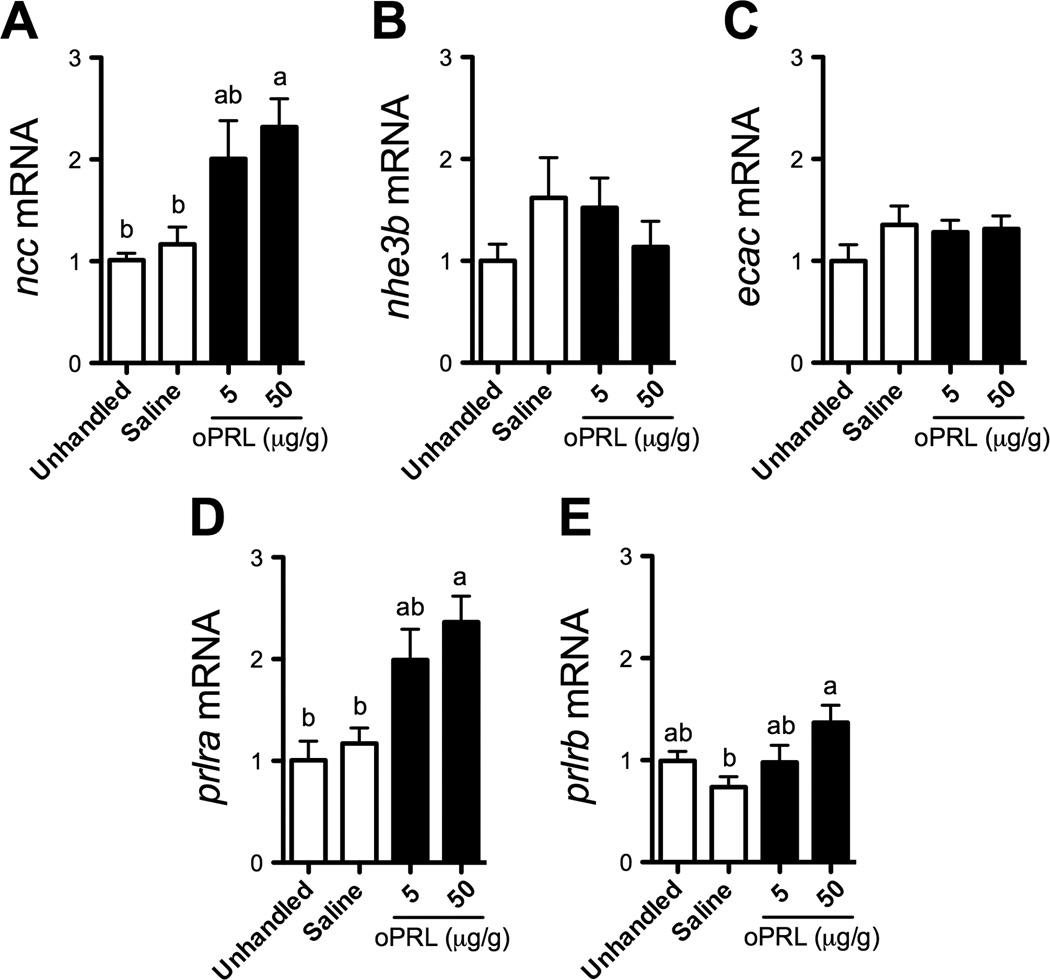
Figure 3.
Branchial gene expression of ncc (A), nhe3b (B), ecac (C), prlra (D) and prlrb (E) following intraperitoneal (IP) injection of oPRL. Means ± SEM (n=6–10). Fish were maintained in system water and administered two IP injections (20 µl/g body weight) of saline or oPRL (5 and 50 µg/g) (solid bars) separated by 24 hours. Fish were sampled 48 h after the first injection. Unhandled and saline-injected fish were included as controls (open bars). Gene expression is presented as fold-change from unhandled fish. Means not sharing the same letter are significantly different (ANOVA, Tukey’s HSD, P<0.05).
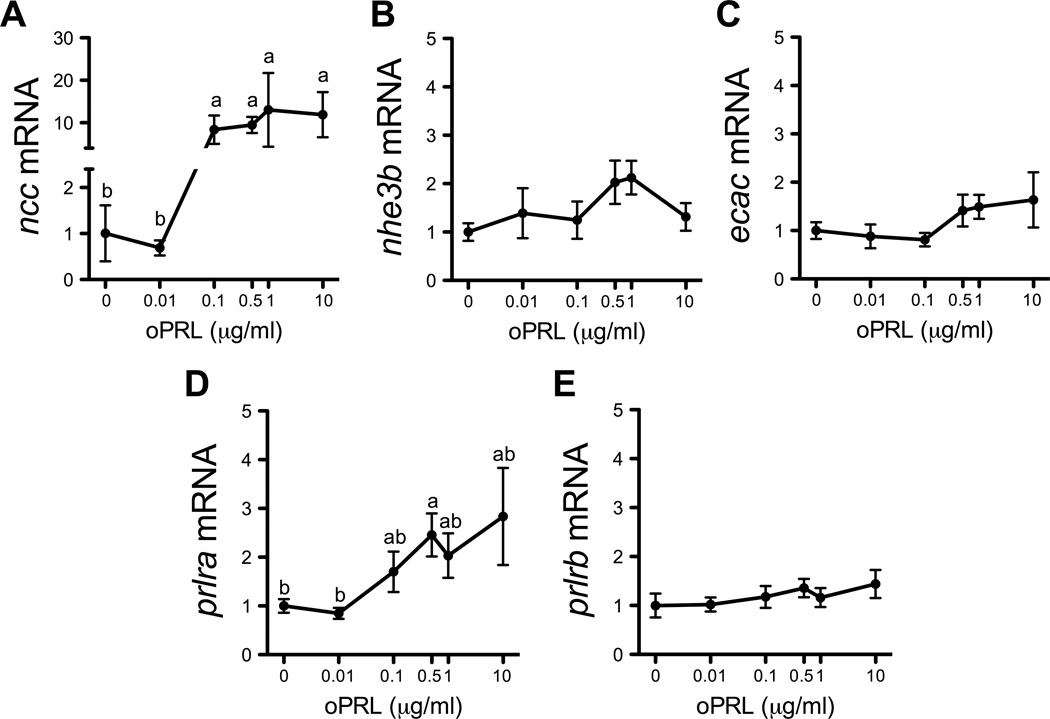
Figure 5.
Effects of oPRL concentration on ncc (A), nhe3b (B), ecac (C), prlra (D) and prlrb (E) gene expression in cultured gill filaments. Means ± SEM (n=6). Filaments were pre-incubated for 3 h, and then incubated with oPRL-supplemented (0.01–10 µg/ml) medium for 8 h. Gene expression is presented as fold-change from the 0 oPRL group. Groups not sharing the same letter are significantly different (ANOVA, Tukey’s HSD, P<0.05).
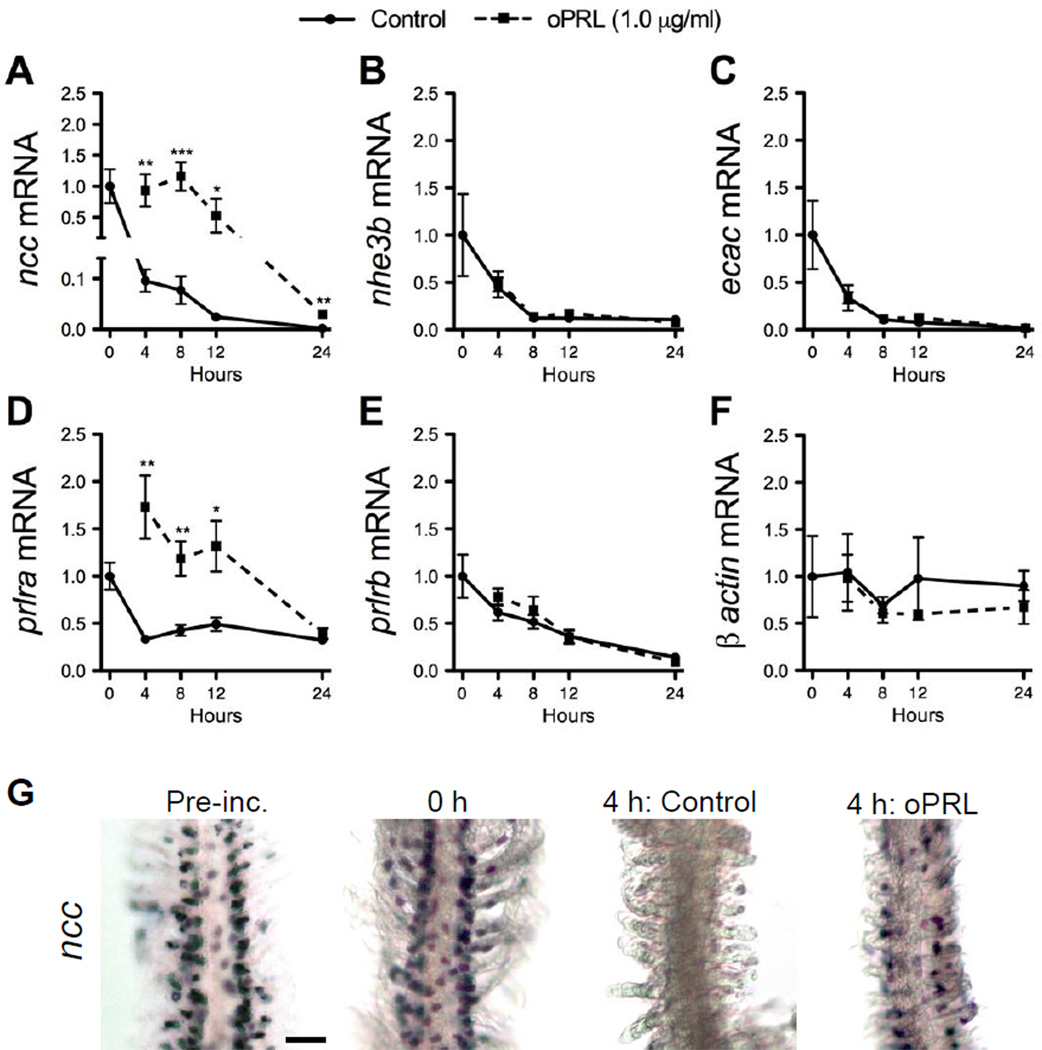
Figure 4.
Effects of incubation time and oPRL on ncc (A), nhe3b (B), ecac (C), prlra (D), prlrb (E) and β -actin (F) gene expression in cultured gill filaments. Solid line, control; dashed line, oPRL (1.0 µg/ml). Gene expression is presented as fold-change from 0 h. Means ± SEM (n=6). Filaments were dissected from anesthetized fish and pre-incubated for 3 h prior to treatment with oPRL-supplemented medium. Effects of hormone treatment and time were evaluated by two-way ANOVA. For clarity, only the results of follow-up analyses for a significant main effect of hormone treatment are presented. *, **, *** Significantly different from the time-matched controls at P<0.05, 0.01 and 0.001, respectively, by Student’s t-test. Whole-mount in situ hybridization showing ncc expression in gill filaments cultured for 4 h in the presence or absence of oPRL as compared with pre-incubated and 0 h control filaments (G). Scale bar = 50 µm.
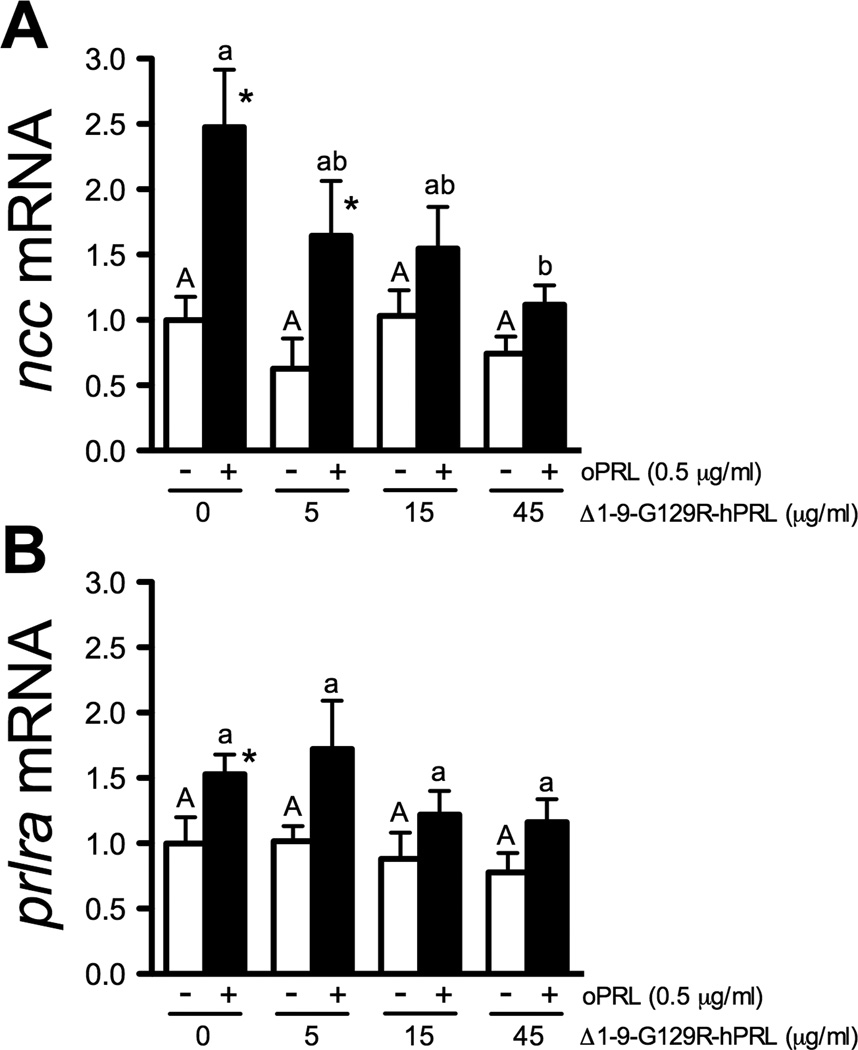
Figure 6.
Effects of Δ1-9-G129R-hPRL concentration on basal (open bars) and oPRL-stimulated (0.5 µg/ml; solid bars) ncc (A) and prlra (B) gene expression in gill filaments cultured for 8 h. Means ± SEM (n=6). Gene expression is presented as fold-change from the 0 oPRL and 0 Δ1-9-G129R-hPRL group. Main effects of oPRL treatment and antagonist concentration were evaluated by two-way ANOVA. * Significantly different from dose-matched 0 oPRL controls at P<0.05 by Student’s t-test. Within a given series, denoted by uppercase or lowercase letters, means not sharing the same letter are significantly different (Tukey’s HSD, P<0.05).
3. Results
3.1 PRL receptor gene expression in osmoregulatory tissues
We first verified that the two known zebrafish PRL receptor transcripts (prlra and prlrb) are expressed in the gill and established the relative amounts of expression across zebrafish tissues relative to gill prlra expression (Fig. 1). prlra mRNA was highly expressed in brain, gill, kidney and posterior intestine, with lower expression in other tissues. prlrb was also highly expressed in the kidney with lower expression in other tissues. In the gill, the relative expression of prlra versus prlrb was comparable, while in other tissues such as brain, pituitary, and posterior intestine, prlra and prlrb were expressed at distinct amounts. The expression of prlra and prlrb was not significantly different in males vs. females (data not shown), thus data from both sexes were pooled (Fig. 1).
3.2 Transfer to ion-poor water led to an increase in PRL receptor and ion transporter/exchanger gene expression
To determine whether transfer to ion-poor conditions affects expression of PRL receptor genes, as well as genes known to be involved in ion transport, we performed qRT-PCR on gill tissues 0, 2 and 7 days following transfer of adult fish to ddH2O (Fig. 2A-E). Transfer to ddH2O did not overtly affect adult zebrafish; there were no mortalities and no detectable changes in muscle water content (Fig. 2F). prlra gene expression remained unchanged after 2 days in ddH2O, but was increased 6-fold from FW-controls following 7 days in ddH2O (Fig. 2A). In contrast, prlrb was unchanged relative to FW controls after 2 and 7 days in ddH2O (Fig. 2B). prlrb expression increased variably at 7 days, but this increase was seen irrespective of treatment. Expression of ncc mRNA increased approximately 3-fold from time-matched FW controls after 2 days in ddH2O, and nearly 6-fold after 7 days (Fig. 2C). Expression of nhe3b mRNA was relatively unchanged after 2 days in ddH2O, but was increased over 8-fold from time-matched controls after 7 days (Fig. 2D). Similar to ncc, ecac was increased 4.6- and 6.2-fold from time-matched controls after 2 and 7 days in ddH2O, respectively (Fig. 2E).
3.3 Increased PRL levels induced gene expression of ncc and prlra in vivo
We next examined whether increased systemic PRL levels could affect the expression of the same genes that were induced by ion-poor water conditions. Adult zebrafish were IP-injected two times at t=0 and t=24 h with 5 or 50 µg/g of purified oPRL. At t=48 h, 50 µg/g of oPRL led to a ~2-fold increase in branchial expression of ncc mRNA (Fig. 3A). oPRL injections did not affect nhe3b or ecac expression (Fig. 3B,C), suggesting PRL effects on gill gene expression are specific. Similar to ncc, a 2-fold increase in prlra expression occurred following injection of 50 µg/g oPRL (Fig 3D). There was no clear effect of oPRL on the expression of prlrb. While prlrb expression was significantly different between saline- and oPRL-injected fish (50 µg/g), there were no significant differences between unhandled and oPRL-injected fish (Fig. 3E). Since high oPRL levels may bind teleost growth hormone (GH) receptors (Prunet and Auperin, 1994), we tested whether oGH influenced the expression of these genes in the gill. IP injection of oGH did not alter branchial prlra or ncc gene expression at doses up to 50 µg/g (data not shown).
3.4 PRL directly induced ncc and prlra gene expression in cultured gill
To determine whether oPRL acts directly on gill tissue to regulate expression of ionoregulatory genes, we cultured zebrafish gills in the presence or absence of 1 µg/ml oPRL and assayed gene expression by qRT-PCR and in situ hybridization. As expected, ncc, nhe3b, and ecac declined during the culture period (Fig. 4A-C) consistent with the need for systemic signals to maintain expression of these ionoregulatory genes. Expression of prlra and prlrb also gradually declined under these culture conditions, but with distinct kinetics (Fig. 4D,E). Importantly, the amount of β -actin (Fig. 4F), β -2-microglobulin (b2m) and glyceraldehyde 3-phosphate dehydrogenase (gapdh) mRNA was unchanged through the culture period (data not shown), revealing the overall stability of gene expression within the cultured tissue. The supplementation of medium with oPRL resulted in markedly higher (~10-fold) expression of ncc mRNA at 4, 8, 12 and 24 h versus time-matched controls (Fig. 4A). In contrast, nhe3b and ecac expression was unaffected by oPRL (Fig. 4B,C). prlra mRNA was elevated from time-matched controls at 4, 8 and 12 h by oPRL treatment (Fig. 4D) while prlrb mRNA was unaffected by oPRL (Fig. 4E). In the absence of PRL in culture, ncc-positive cells disappeared by 4 h (Fig. 4G). oPRL treatment led to the maintenance of ncc expression in discrete cells located along the length of the gill filaments.
This effect of oPRL on the maintenance of ncc expression in culture was concentration-dependent, further supporting the specific nature of this effect. Treatment with 0.1, 0.5, 1 and 10 µg/ml of oPRL for 8 h induced 8.4-fold, 9.5-fold, 13.0-fold and 11.9-fold higher ncc expression from controls, respectively (Fig. 5A). Again, there were no effects of oPRL on either nhe3b or ecac (Fig. 5B,C). There was a modest effect of oPRL at 0.5 µg/ml on prlra mRNA with a 2.5-fold increase from controls (Fig. 5D). prlrb was unaffected by oPRL treatment at all doses (Fig. 5E). We confirmed that addition of oGH had no effect on ncc expression in vitro (data not shown).
3.5 The PRL receptor antagonist Δ1-9-G129R-hPRL blocked the effects of oPRL on gill gene expression
To further test whether oPRL specifically affects ncc and prlra expression via PRL receptor mediated signaling, we took advantage of a modified human PRL peptide that antagonizes signaling by blocking PRL binding and subsequent PRL receptor activation (Bernichtein et al., 2003). As in the previous experiment (Fig. 5A), ncc expression was maintained in the presence of 0.5 µg/ml oPRL after 8 h in culture. Co-incubation with Δ1-9-G129R-hPRL blocked this effect on ncc in a concentration-dependent manner (Fig. 6A). Similarly, addition of Δ1-9-G129R-hPRL blocked the effect of oPRL on prlra expression (Fig. 6B). Importantly, incubation with Δ1-9-G129R-hPRL alone had no significant effect on ncc and prlra expression (Fig. 6A,B).
4. Discussion
4.1 PRL as an evolutionarily conserved osmoregulatory hormone
PRL has been identified as a freshwater-adapting hormone in fish through its actions on water permeability and ion retention in the gill, gut, kidney and integument (Hirano, 1986; Sakamoto and McCormick, 2006). However, there is little direct evidence of PRL stimulating ion uptake across branchial epithelia, and limited information on the actual ion-transport pathways regulated by PRL (Zhou et al., 2003). Here we present the first in vivo and in vitro evidence in a stenohaline freshwater teleost that PRL directly regulates branchial expression of ncc, a gene encoding the Na+/Cl− cotransporter that is central to the maintenance of Cl− balance (Hiroi et al., 2008; Horng et al., 2009; Wang et al., 2009). Combined with our recent work showing that pituitary-derived PRL regulates ionocyte ncc expression in a euryhaline cichlid, the Mozambique tilapia (Breves et al., 2010), our data suggest that ncc may represent a conserved transcriptional target of PRL in fishes that employ NCC-dependent Cl− uptake pathways.
We show that the two zebrafish prlr genes (prlra and prlrb) are robustly expressed in the gill (Fig. 1). This expression is in agreement with reports of radiolabeled-PRL binding and prlr1/prlr2 expression in branchial tissue of tilapia, sea bream (Sparus aurata) and goldfish (Dauder et al., 1990; Prunet and Auperin, 1994; Tse et al., 2000; Santos et al., 2001; Pierce et al., 2007; Fiol et al., 2009) and strongly suggests gill tissue is competent to respond to PRL signaling. In teleosts, multiple GH and PRL receptor family genes have been retained following genome duplication events (Fukamachi and Meyer, 2007). These multiple forms have distinct expression patterns (Huang et al., 2007; Fiol et al., 2009; Breves et al., 2011) and capacities to activate intracellular signaling pathways (Huang et al., 2007; Fiol et al., 2009; Chen et al., 2011). The idea that PRL receptor proteins are functional in the gill is further supported by our finding that prlra expression is positively regulated by oPRL (Fig. 3D) and by the fact that plasma PRL levels seemingly regulate transcription of clade 1 PRL receptors (prlr1) in other systems (Pierce et al., 2007; Breves et al., 2010).
Our finding that prlra expression increases in the zebrafish gill following transfer to ddH2O (Fig. 2A) is the first evidence for this response in a stenohaline teleost. The ability of zebrafish to rapidly acclimate to dramatic reductions in environmental ion concentrations may allow them to adapt to natural variations in their native habitat, freshwater streams in the Indian subcontinent with significant seasonal fluctuations (Boisen et al., 2003). In euryhaline species including tilapia and rainbow trout (Oncorhynchus mykiss), branchial prlr1 gene expression is similarly enhanced in parallel with freshwater acclimation responses (Pierce et al., 2007; Fiol et al., 2009; Breves et al., 2011; Flores and Shrimpton, 2012). Combined, these data suggest an evolutionarily conserved, PRL-mediated low salinity (freshwater) acclimation response.
Zebrafish can tolerate transfer to ion-poor water without apparent distress or severe perturbations of plasma ion levels (Fig. 2F; Craig et al., 2007; Boisen et al., 2003; Liao et al., 2009). To maintain hydromineral balance in such dynamic conditions, gill tissue rapidly modulates the transcription of genes encoding effectors of ion transport (Fiol and Kültz, 2007). NCC was previously shown to be a key effector of Cl− uptake in at least a subset of teleost species (Hiroi et al., 2008), including zebrafish (Wang et al., 2009), with inward Cl− currents detectable in the immediate vicinity of NCC-expressing tilapia ionocytes (Horng et al., 2009). Reduced ncc function in larval zebrafish significantly impacted Cl− influx and tissue Cl− content (Wang et al., 2009), confirming that NCC is a key component of physiological responses underlying Cl− balance.
Our findings that PRL is 1) sufficient to upregulate gill ncc expression in vivo (Fig. 3A) and 2) required for maintenance of ncc expression in cultured gill tissue (Figs. 4–6) strongly suggest that PRL signaling is central to appropriate Cl− regulation. The observation that increased ncc expression appears to precede an increase in prlra expression by several days following low ion exposure in vivo (Fig. 2A,C) suggests that basal prlra expression is sufficient to mediate the ncc response to PRL, with increased receptor expression helping maintain or induce additional osmoregulatory responses. It is also certainly the case that other hormones help trigger and modify adaptive responses to hyposmotic conditions in vivo. To confirm our findings and begin to uncover the kinetics of individual hormonal responses to ionoregulatory challenges, it will thus be important to develop sensitive radioimmunoassays for PRL, GH and other putative osmoregulatory hormones to determine plasma levels in zebrafish.
4.2 Cellular mechanisms of PRL action in the gill
The filament culture system is particularly useful in uncovering cellular responses in the gill that do not require input from the whole animal, with gill-autonomous cellular functions being maintained for up to 4 days (McCormick et al., 1989; Kiilerich et al., 2007). We found that expression of prlra, as well as several ionoregulatory genes (ncc, nhe3b, and ecac) declined within 4 h of culture, as would be expected for endocrine regulated genes (Fig. 4). Addition of oPRL maintained the expression of prlra and ncc for up to 12 h in a concentration-dependent fashion, but did not affect nhe3b and ecac expression (Fig. 4A-C). Consistent with highly specific hormonal action on ionocytes, recent work by others showed that the nhe3b and ecac genes are regulated by distinct hormonal signals including stanniocalcin, isotocin, and/or cortisol (Tseng et al., 2009; Chou et al., 2011; Lin et al., 2011; Kumai et al., 2012).
In situ hybridization confirmed that ncc is expressed in discrete cells of the gill filaments, both at the beginning of the culture period as well as in cultures exposed to oPRL (Fig. 4G). NCC ionocyte number and distribution appeared normal in oPRL treated cultures, strongly suggesting that under these conditions PRL acts to maintain ncc expression in existing ionocytes rather than to induce expression in other cells of the gill. We are now developing the tools needed to determine PRL receptor expression in relation to differentiated ionocytes, and ionocyte precursors in vivo (reviewed by Chang and Hwang, 2011), in order to gain further insight on which cells directly respond to PRL during salinity acclimation.
Direct action of PRL through transmembrane PRL receptors in the gill is further supported by our experiments employing a specific PRL receptor antagonist (Δ1-9-G129R-hPRL) that binds PRL receptors with high affinity and prevents the receptor dimerization required for the activation of JAK/STAT signaling (Bernichtein et al., 2003). Δ1-9-G129R-hPRL is a variant of the human PRL sequence with a glycine to arginine substitution at position 129 and the deletion of nine residues at the N-terminus (Bernichtein et al., 2003). Glycine 129, which lies within the second receptor-binding site, is conserved from zebrafish to humans (Huang et al., 2009), which suggested Δ1-9-G129R-hPRL could act as an antagonist in zebrafish. Addition of Δ1-9-G129R-hPRL eliminated the ability of PRL to maintain ncc expression in a dose dependent manner, with 45 µg/ml Δ1-9-G129R-hPRL eliminating ncc gene expression seen when gills were maintained in 0.5 µg/ml oPRL (Fig. 6). This dose relationship is r emarkably similar to that reported for the murine PRL receptor (Bernichtein et al., 2003). The lack of an effect of Δ1-9-G129R-hPRL alone on ncc expression confirmed findings from mammalian models that Δ1-9-G129R-hPRL does not operate as an agonist at high doses.
Our in vivo studies revealed that prlra, ncc, nhe3b and ecac gene expression increased following transfer to ion-poor conditions or PRL injections, and that these changes occurred on a multi-day time scale (Figs. 2,3). This slower response may point to more complex regulation within the whole organism, and/or may suggest that cell number regulation may also provide a mechanism for maintaining ion balance. Recent studies provide evidence that osmoregulatory hormones can act upon ionocyte progenitor populations to induce cell differentiation events that contribute to detectable changes in the expression of the ion transporters/exchangers that define mature ionocytes (Chou et al., 2011; Cruz et al., 2013). PRL signaling may thus work at both the transcriptional level and the level of cell differentiation to regulate immediate and long-term responses to osmotic challenges, respectively.
4.3 Conclusions
Taken together our data indicate that PRL is an endocrine signal that is necessary and sufficient to regulate expression of ncc in the zebrafish gill, and may thus be key to adaptive ionoregulatory physiology. This work helps establish zebrafish as a model for investigating the molecular and cellular mechanisms underlying this physiological response, including the regulatory networks in the hypothalamus and pituitary that confer osmoresponsive PRL expression and release (Liu et al., 2006; Hoshijima and Hirose, 2007). PRL binding has been documented in other key osmoregulatory tissues in both fish and mammals, including the kidney and intestine (Bole-Feysot et al., 1998). Given its power as an embryological model, the zebrafish promises to greatly facilitate the study of the interplay between endocrine system ontogeny and the onset of extra-branchial (renal and gastrointestinal) processes that underlie hydromineral balance. Finally, given the remarkable level of conservation in these regulatory mechanisms, this work in zebrafish promises to help uncover how defects in PRL-regulated ion and water metabolism might underlie hydromineral imbalances associated with a wide variety of human diseases.
Highlights.
► We investigated gene targets of PRL that underlie zebrafish ionoregulation ► Branchial ncc and prlra are activated in parallel by ion-poor water. ► In vivo and in vitro treatment with oPRL stimulated ncc and prlra. ► Human PRL receptor antagonist, Δ1-9-G129R-hPRL, blocked PRL-stimulated ncc expression.
Acknowledgments
This work was supported by training grants from the National Institute of Mental Health (T32-MH020051-07) and the National Institute of Diabetes and Digestive and Kidney Diseases (F32-DK095575) to J.P.B, a Northeast Alliance for Graduate Education and the Professoriate fellowship (NSF HRD 0450339 and PREP S1111000000063) to S.B.S., and NIH NS039994 (R.O.K.). We are grateful to Professor Geert de Vries for encouragement during the course of this study. We also appreciate the invaluable laboratory assistance provided by Ms. Meng-Chieh Shen, Ms. Judy Bennett and Ms. Theresa Ortiz.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bayaa M, Vulesevic B, Esbaugh A, Braun M, Ekker ME, Grosell M, Perry SF. The involvement of SLC26 anion transporters in chloride uptake in zebrafish (Danio rerio) larvae. J. Exp. Biol. 2009;212:3283–3295. doi: 10.1242/jeb.033910. [DOI] [PubMed] [Google Scholar]
- Bernichtein S, Kayser C, Dillner K, Moulin S, Kopchick JJ, Martial JA, Norstedt G, Isaksson O, Kelly PA, Goffin V. Development of pure prolactin receptor antagonists. J. Biol. Chem. 2003;278:35988–35999. doi: 10.1074/jbc.M305687200. [DOI] [PubMed] [Google Scholar]
- Boisen AM, Amstrup J, Novak I, Grosell M. Sodium and chloride transport in soft water and hard water acclimated zebrafish (Danio rerio) Biochim. Biophys. Acta. 2003;1618:207–218. doi: 10.1016/j.bbamem.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- Breves JP, Seale AP, Helms RE, Tipsmark CK, Hirano T, Grau EG. Dynamic gene expression of GH/PRL-family hormone receptors in gill and kidney during freshwater-acclimation of Mozambique tilapia. Comp. Biochem. Physiol. A. 2011;158:194–200. doi: 10.1016/j.cbpa.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Breves JP, Watanabe S, Kaneko T, Hirano T, Grau EG. Prolactin restores branchial mitochondrion-rich cells expressing Na+/Cl− cotransporter in hypophysectomized Mozambique tilapia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;29958:R702–R710. doi: 10.1152/ajpregu.00213.2010. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Hwang PP. Development of zebrafish epidermis. Birth Defects Res. C. 2011;93:205–214. doi: 10.1002/bdrc.20215. [DOI] [PubMed] [Google Scholar]
- Chen M, Huang X, Yuen DSH, Cheng CHK. A study on the functional interaction between the GH/PRL family of polypeptides with their receptors in zebrafish: Evidence against GHR1 being the receptor for somatolactin. Mol. Cell. Endocrinol. 2011;337:114–121. doi: 10.1016/j.mce.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Chou MY, Hung JC, Wu LC, Hwang SPL, Hwang PP. Isotocin controls ion regulation through regulating ionocyte progenitor differentiation and proliferation. Cell. Mol. Life Sci. 2011;68:2797–2809. doi: 10.1007/s00018-010-0593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig PM, Wood CM, McClelland GB. Gill membrane remodeling with soft-water acclimation in zebrafish (Danio rerio) Physiol. Genomics. 2007;30:53–60. doi: 10.1152/physiolgenomics.00195.2006. [DOI] [PubMed] [Google Scholar]
- Cruz AA, Chao PL, Hwang PP. Cortisol promotes differentiation of epidermal ionocytes through Foxi3 transcription factors in zebrafish (Danio rerio) Comp. Biochem. Physiol. A. 2013;164:249–257. doi: 10.1016/j.cbpa.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Dauder S, Young G, Hass L, Bern HA. Prolactin receptors in liver, kidney, and gill of the tilapia (Oreochromis mossambicus): characterization and effect of salinity on specific binding of iodinated ovine prolactin. Gen. Comp. Endocrinol. 1990;77:368–377. doi: 10.1016/0016-6480(90)90226-c. [DOI] [PubMed] [Google Scholar]
- Eckert SM, Yada T, Shepherd BS, Stetson MH, Hirano T, Grau EG. Hormonal control of osmoregulation in the channel catfish Ictalurus punctatus. Gen. Comp. Endocrinol. 2001;122:270–286. doi: 10.1006/gcen.2001.7633. [DOI] [PubMed] [Google Scholar]
- Edery M, Young G, Bern HA, Steiny S. Prolactin receptors in tilapia (Sarotherodon mossambicus) tissues: binding studies using I-125 labeled ovine prolactin. Gen. Comp. Endocrinol. 1984;56:19–23. doi: 10.1016/0016-6480(84)90056-x. [DOI] [PubMed] [Google Scholar]
- Esaki M, Hoshijima K, Kobayashi S, Fukuda H, Kawakami K, Hirose S. Visualization in zebrafish larvae of Na+ uptake in mitochondria-rich cells whose differentiation is dependent on foxi3a. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R470–R480. doi: 10.1152/ajpregu.00200.2006. [DOI] [PubMed] [Google Scholar]
- Evans DH. Freshwater fish gill ion transport: August Krogh to morpholinos and microprobes. Acta Physiol. 2011;202:349–359. doi: 10.1111/j.1748-1716.2010.02186.x. [DOI] [PubMed] [Google Scholar]
- Fiol DF, Kültz D. Osmotic stress sensing and signaling in fishes. FEBS J. 2007;274:5790–5798. doi: 10.1111/j.1742-4658.2007.06099.x. [DOI] [PubMed] [Google Scholar]
- Fiol DF, Sanmarti E, Sacchi R, Kültz D. A novel tilapia prolactin receptor is functionally distinct from its paralog. J. Exp. Biol. 2009;212:2007–2015. doi: 10.1242/jeb.025601. [DOI] [PubMed] [Google Scholar]
- Flores AM, Shrimpton MJ. Differential physiological and endocrine responses of rainbow trout, Oncorhynchus mykiss transferred from fresh water to ion-poor or salt water. Gen. Comp. Endocrinol. 2012;175:244–250. doi: 10.1016/j.ygcen.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: Structure, Function and Regulation of Secretion. Physiol. Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Fukamachi S, Meyer A. Evolution of Receptors for Growth Hormone and Somatolactin in Fish and Land Vertebrates: Lessons from the Lungfish and Sturgeon Orthologues. J. Mol. Evol. 2007;65:359–372. doi: 10.1007/s00239-007-9035-7. [DOI] [PubMed] [Google Scholar]
- Herndon TM, McCormick SD, Bern HA. Effects of prolactin on chloride cells in opercular membrane of seawater-adapted tilapia. Gen. Comp. Endocrinol. 1991;83:283–289. doi: 10.1016/0016-6480(91)90032-2. [DOI] [PubMed] [Google Scholar]
- Hirano T. The spectrum of prolactin action in teleosts. Prog. Clin. Biol. Res. 1986;205:53–74. [PubMed] [Google Scholar]
- Hiroi J, Yasumasu S, McCormick SD, Hwang PP, Kaneko T. Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish. J. Exp. Biol. 2008;211:2584–2599. doi: 10.1242/jeb.018663. [DOI] [PubMed] [Google Scholar]
- Horng JL, Hwang PP, Shih TH, Wen ZH, Lin CS, Lin LY. Chloride transport in mitochondrion-rich cells of euryhaline tilapia (Oreochromis mossambicus) larvae. Am. J. Physiol. Cell Physiol. 2009;297:C845–C854. doi: 10.1152/ajpcell.00218.2009. [DOI] [PubMed] [Google Scholar]
- Hoshijima K, Hirose S. Expression of endocrine genes in zebrafish larvae in response to environmental salinity. J. Endocrinol. 2007;193:481–491. doi: 10.1677/JOE-07-0003. [DOI] [PubMed] [Google Scholar]
- Huang X, Hui MNY, Liu Y, Yuen DSH, Zhang Y, Chan WY, Lin HR, Cheng SH, Cheng CHK. Discovery of a novel prolactin in non-mammalian vertebrates: evolutionary perspectives and its involvement in teleost retina development. PLoS One. 2009;4:e6163. doi: 10.1371/journal.pone.0006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Jiao B, Fung CK, Zhang Y, Ho WK, Chan CB, Lin H, Wang D, Cheng CHK. The presence of two distinct prolactin receptors in seabream with different tissue distribution patterns, signal transduction pathways and regulation of gene expression by steroid hormones. J. Endocrinol. 2007;194:373–392. doi: 10.1677/JOE-07-0076. [DOI] [PubMed] [Google Scholar]
- Hwang PP. Ion uptake and acid secretion in zebrafish (Danio rerio) J. Exp. Biol. 2009;212:1745–1752. doi: 10.1242/jeb.026054. [DOI] [PubMed] [Google Scholar]
- Jackson LF, McCormick SD, Madsen SS, Swanson P, Sullivan CV. Osmoregulatory effects of hypophysectomy and homologous prolactin replacement in hybrid striped bass. Comp. Biochem. Physiol. B. 2005;140:211–218. doi: 10.1016/j.cbpc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Watanabe S, Lee KM. Functional morphology of mitochondrion-rich cells in euryhaline and stenohaline teleosts. Aqua-BioSci. Monogr. 2008;1:1–62. [Google Scholar]
- Karlstrom RO, Talbot WS, Schier AF. Comparative synteny cloning of zebrafish you-too: mutations in the Hedgehog target gli2 affect ventral forebrain patterning. Genes Dev. 1999;13:388–393. doi: 10.1101/gad.13.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiilerich P, Kristiansen K, Madsen SS. Cortisol regulation of ion transporter mRNA in Atlantic salmon gill and the effect of salinity on the signaling pathway. J. Endocrinol. 2007;194:417–427. doi: 10.1677/JOE-07-0185. [DOI] [PubMed] [Google Scholar]
- Kumai Y, Nesan D, Vijayan MM, Perry SF. Cortisol regulates Na+ uptake in zebrafish, Danio rerio, larvae via the glucocorticoid receptor. Mol. Cell. Endocrinol. 2012;364:113–125. doi: 10.1016/j.mce.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Lee KM, Kaneko T, Aida K. Prolactin and prolactin receptor expression in a marine teleost, pufferfish Takifugu rubripes. Gen. Comp. Endocrinol. 2006;146:318–328. doi: 10.1016/j.ygcen.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Liao BK, Deng AN, Chen SC, Chou MY, Hwang PP. Expression and water calcium dependence on calcium transporter isoforms in zebrafish gill mitochondrion-rich cells. BMC Genomics. 2007;8:354. doi: 10.1186/1471-2164-8-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao BK, Chen RD, Hwang PP. Expression regulation of Na+-K+-ATPase alpha1-subunit subtypes in zebrafish gill ionocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1897–R1906. doi: 10.1152/ajpregu.00029.2009. [DOI] [PubMed] [Google Scholar]
- Lin CH, Tsai IL, Su CH, Tseng DY, Hwang PP. Reverse effect of mammalian hypocalcemic cortisol in fish: cortisol stimulates Ca2+ uptake via glucocorticoid receptor-mediated vitamin D3 metabolism. PLoS One. 2011;6(8):e23689. doi: 10.1371/journal.pone.0023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NA, Liu Q, Wawrowsky K, Yang Z, Lin S, Melmed S. Prolactin receptor signaling mediates the osmotic response of embryonic zebrafish lactotrophs. Mol. Endocrinol. 2006;20:871–80. doi: 10.1210/me.2005-0403. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Löhr H, Hammerschmidt M. Zebrafish in endocrine systems: recent advances and implications for human disease. Annu. Rev. Physiol. 2011;73:183–211. doi: 10.1146/annurev-physiol-012110-142320. [DOI] [PubMed] [Google Scholar]
- McCurley AT, Callard GV. Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008;9:102. doi: 10.1186/1471-2199-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonnell IM, Fowkes RC. Fishing for gene function - endocrine modeling in the zebrafish. J. Endocrinol. 2006;189:425–439. doi: 10.1677/joe.1.06683. [DOI] [PubMed] [Google Scholar]
- McCormick SD, Bern HA. In vitro stimulation of Na+-K+-ATPase activity and ouabain binding by cortisol in coho salmon gill. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1989;256:707–715. doi: 10.1152/ajpregu.1989.256.3.R707. [DOI] [PubMed] [Google Scholar]
- McCormick SD, Bradshaw D. Hormonal control of salt and water balance in vertebrates. Gen. Comp. Endocrinol. 2006;147:3–8. doi: 10.1016/j.ygcen.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Pan TC, Liao BK, Huang CJ, Lin LY, Hwang PP. Epithelial Ca2+ channel expression and Ca2+ uptake in developing zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1202–R1211. doi: 10.1152/ajpregu.00816.2004. [DOI] [PubMed] [Google Scholar]
- Pierce AL, Fox BK, Davis LK, Visitacion N, Kitahashi T, Hirano T, Grau EG. Prolactin receptor, growth hormone receptor, and putative somatolactin receptor in Mozambique tilapia: tissue specific expression and differential regulation by salinity and fasting. Gen. Comp. Endocrinol. 2007;154:31–40. doi: 10.1016/j.ygcen.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Prunet P, Auperin B. Prolactin receptors. In: Sherwood NM, Hew CL, editors. Fish Physiology, Vol. 13: Molecular endocrinology of fish. New York, NY, USA: Academic Press; 1994. pp. 367–391. [Google Scholar]
- Sakamoto T, McCormick SD. Prolactin and growth hormone in fish osmoregulation. Gen. Comp. Endocrinol. 2006;147:24–30. doi: 10.1016/j.ygcen.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Sandra O, Sohm F, de Luze A, Prunet P, Edery M, Kelly PA. Expression cloning of a cDNA encoding a fish prolactin receptor. Proc. Natl. Acad. Sci. USA. 1995;92:6037–6041. doi: 10.1073/pnas.92.13.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CRA, Ingleton PM, Cavaco JEB, Kelly PA, Edery M, Power DM. Cloning, characterization, and tissue distribution of prolactin receptor in the sea bream (Sparus aurata) Gen. Comp. Endocrinol. 2001;121:32–47. doi: 10.1006/gcen.2000.7553. [DOI] [PubMed] [Google Scholar]
- Sbrogna JL, Barresi MJ, Karlstrom RO. Multiple roles for Hedgehog signaling in zebrafish pituitary development. Dev. Biol. 2003;254:19–35. doi: 10.1016/s0012-1606(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Tang R, Dodd A, Lai D, McNabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin. 2007;39:384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse DLY, Chow BKC, Chan CB, Lee LTO, Cheng CHK. Molecular cloning and expression studies of a prolactin receptor in goldfish (Carassius auratus) Life Sci. 2000;66:593–605. doi: 10.1016/s0024-3205(99)00632-3. [DOI] [PubMed] [Google Scholar]
- Tseng DY, Chou MY, Tseng YC, Hsiao CJ, Kaneko T, Hwang PP. Effects of stanniocalcin 1 on calcium uptake in zebrafish (Danio rerio) embryo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R549–R557. doi: 10.1152/ajpregu.90742.2008. [DOI] [PubMed] [Google Scholar]
- Walpita CN, Van der Geyten S, Rurangwa E, Darras VM. The effect of 3,5,3′-triiodothyronine supplementation on zebrafish (Danio rerio) embryonic development and expression of iodothyronine deiodinases and thyroid hormone receptors. Gen. Comp. Endocrinol. 2007;152:206–214. doi: 10.1016/j.ygcen.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Wang YF, Tseng YC, Yan JJ, Hiroi J, Hwang PP. Role of SLC12A10.2, a Na-Cl cotransporter-like protein, in a Cl uptake mechanism in zebrafish (Danio rerio) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1650–R1660. doi: 10.1152/ajpregu.00119.2009. [DOI] [PubMed] [Google Scholar]
- Weng CF, Lee TH, Hwang PP. Immune localization of prolactin receptor in the mitochondria-rich cells of the euryhaline teleost (Oreochromis mossambicus) gill. FEBS Lett. 1997;405:91–94. doi: 10.1016/s0014-5793(97)00162-2. [DOI] [PubMed] [Google Scholar]
- Yan JJ, Chou MY, Kaneko T, Hwang PP. Gene expression of Na+/H+ exchanger in zebrafish H+-ATPase-rich cells during acclimation to low-Na+ and acidic environments. Am. J. Physiol. Cell Physiol. 2007;293:C1814–C1823. doi: 10.1152/ajpcell.00358.2007. [DOI] [PubMed] [Google Scholar]
- Zhou B, Kelly SP, Ianowski JP, Wood CM. Effects of cortisol and prolactin on Na+ and Cl− transport in cultured branchial epithelia from FW rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1305–R1316. doi: 10.1152/ajpregu.00704.2002. [DOI] [PubMed] [Google Scholar]