Abstract
End-stage renal disease (ESRD) is associated with a significant propensity for development of atherosclerosis and cardiovascular mortality. The atherogenic diathesis associated with ESRD is driven by inflammation, oxidative stress and dyslipidemia. Reduced high density lipoprotein cholesterol (HDL-C) level and HDL dysfunction are the hallmarks of ESRD-related dyslipidemia. Clinical and laboratory studies have revealed that ESRD is associated with significantly reduced serum apolipoprotein A-I (ApoA-I) and HDL-C level as well as altered HDL-C composition. Furthermore, while ESRD is associated with impaired HDL antioxidant and anti-inflammatory properties in the majority of patients, in a small subset, HDL may in fact have a pro-oxidant and pro-inflammatory effect. Therefore, it is no surprise that serum HDL-C level is not a dependable indicator of cardiovascular disease burden in ESRD, and markers such as HDL function are critical to accurately identifying patients at risk for cardiovascular disease and mortality in ESRD.
Keywords: cardiovascular diseases, end-stage renal disease, high density lipoprotein
Introduction
The number of patients with end-stage renal disease (ESRD) requiring maintenance dialysis in the United States currently stands at approximately 600,000 (1). Despite many recent improvements in dialysis treatment and the strict adherence of patients and physicians to the quality measures set forth by guidelines, ESRD patients continue to experience an annual mortality rate of approximately 20% in the United States. While the causes of death in these patients are diverse, approximately half of all death of dialysis patients is directly attributed to cardiovascular disease (1). Several factors are involved in the pathogenesis of atherosclerosis and cardiovascular disease in the entire spectrum of chronic kidney disease (CKD) including ESRD. These include oxidative stress, inflammation and wasting syndrome, hypertension, endothelial dysfunction, vascular calcification and dyslipidemia. While dyslipidemia in the general population is characterized by elevate low density lipoprotein cholesterol (LDL-C), in ESRD patients it is marked by elevated triglyceride-rich lipoproteins and decreased HDL-C (2–4) Therefore, while in the general population therapeutic strategies have focused on lowering LDL-C, primarily by the use of statins, this strategy has not been fruitful in patients with ESRD as indicated by the results of 4D, AURORA and SHARP trials. Hence, understanding the mechanisms responsible for HDL deficiency and dysfunction are critical steps in devising effective therapies aimed at improving HDL-C level and function in ESRD.
HDL As an Atheroprotective Molecule
Generation and life cycle of HDL begins with the secretion of its major protein component, (ApoA-I), from the liver. ApoA-I binds circulating phospholipids and cholesterol forming nascent discoid lipid-poor HDL particles (2,5). Furthermore, ApoA-I triggers free cholesterol efflux from subendothelial macrophages and fibroblasts via it’s interactions with ATP-binding cassette transporter A1 (ABCA1). Free cholesterol is then esterified via lecithin cholesterol acyltransferase (LCAT) and stored in the core of the HDL molecule as a hydrophobic cholesterol ester (2,5). This step is critical to HDL maturation and results in HDL particles obtaining a spherical shape, with the two main mature particles being called HDL2 and HDL3. Subsequently, HDL particles deliver their cholesterol cargo either directly to the liver via scavenger receptor B-I (SR-BI) or indirectly by transferring cholesterol to very low density lipoprotein (VLDL) or LDL particles, which in turn are taken up by the liver via the LDL-receptor (2,4). This transfer is mediated via cholesterol ester transfer protein (CETP), a protein associated with HDL. This entire process is called reverse cholesterol transport (RCT) and is mediated by HDL and serves as a major component of its atheroprotective properties.
Another anti-atherosclerotic property of HDL, which is thought to be just as important if not more critical than RCT in prevention of cardiovascular disease, is its anti-inflammatory and antioxidant effects (Figure 1-A) (2,5). Atherosclerosis is an inflammatory disease. Monocyte adhesion, infiltration and differentiation into macrophages and their ultimate conversion to foam cells are the primary steps in plaque formation. Foam cell formation is the result of increased uptake of oxidized or otherwise modified LDL and remnant lipoproteins by macrophages in the artery wall. HDL and ApoA-I inhibit the expression of endothelial adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and prevent production of monocyte chemoattractant protein-1(MCP-1), steps critical to leukocyte infiltration and reactive oxygen species (ROS) production in the artery wall. Furthermore, in addition to ApoA-I’s potent anti-oxidant properties, HDL is associated with several anti-oxidant enzymes, such as paraoxonase (PON) and glutathione peroxidase (GPX) as well as LCAT and platelet-activating factor acetylhydrolase (2,5). Also, oxidized lipids can be transferred from oxLDL to HDL by CETP and subsequently cleared via the liver. Thus, HDL plays a key role in prevention and reversal of lipid oxidation, a key step in generation of oxidative stress and inflammation which lead to formation and progression of atherosclerosis.
Figure 1.
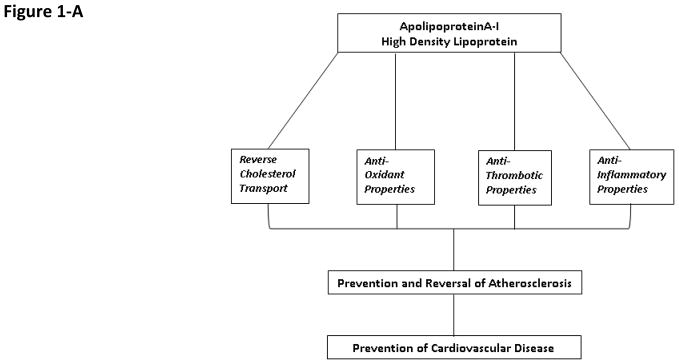
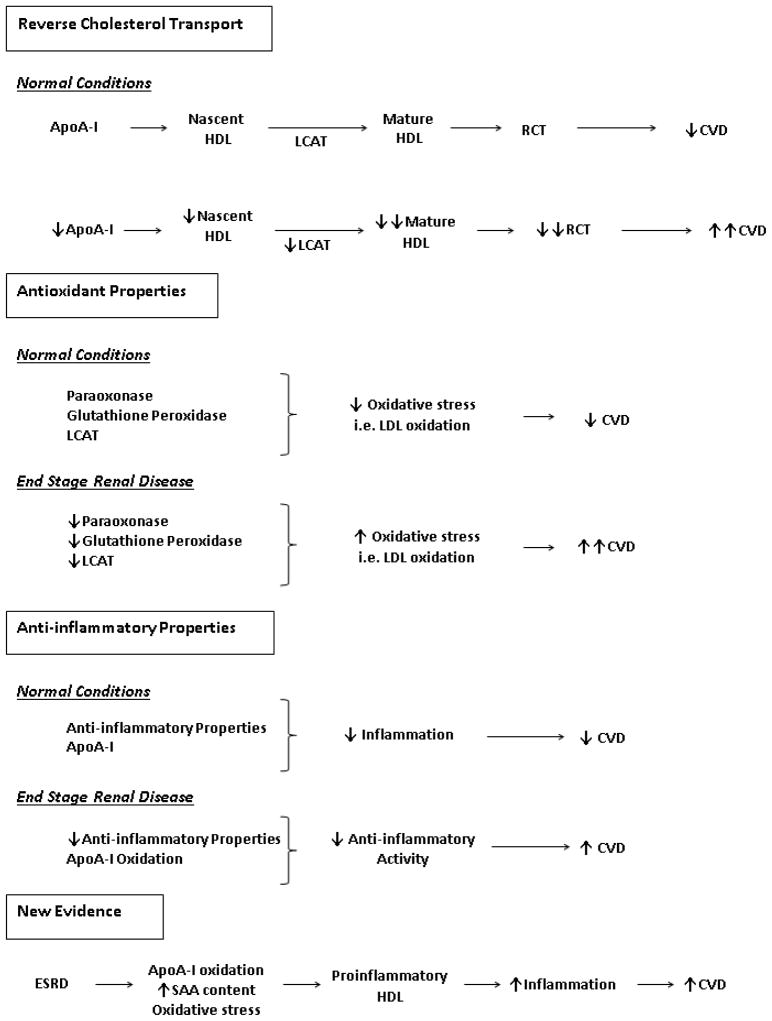
A- Mechanisms responsible for HDL’S atheroprotective properties.
B-HDL abnormalities associated with ESRD. Reverse cholesterol transport (RCT), Cardiovascular disease (CVD), Serum amyloid A (SAA), lecithin cholesterol acyltransferase (LCAT), Apolipoprotein A-I (ApoA-I).
Impact of ESRD on HDL-C Level and Composition
In 1977, the Framingham study was the first large-scale study which showed that low levels of HDL-C are a major risk factor for coronary artery disease (CAD) (6). In addition, it has been shown that with each increase by 1 mg/dl in HDL-C, there is a 2–3% decrease in CAD incidence (7). Numerous studies have shown that ESRD is associated with a significant decrease in plasma ApoA-I and HDL-C level (2,5). There are two mechanisms by which HDL-C deficiency in ESRD is mediated, increased catabolism and reduced production of this molecule. While one study showed ESRD was associated with increased catabolism of HDL particles, another study showed decreased HDL production as the main culprit responsible for reduced HDL-C levels (8–9). Indeed, uremia results in a marked reduction of hepatic production and plasma concentration of ApoA-I and HDL in animals with chronic kidney disease (CKD) (5). The inhibitory influence of uremic milieu on ApoA-I biosynthesis has also been confirmed by in-vitro studies which revealed inhibition of ApoA-I production by uremic plasma in cultured hepatocytes (5). We recently showed that this phenomenon is mediated via a post-transcriptional mechanism at the level of RNA stability (10). Clearly, the ESRD-induced deficiency of ApoA-I contributes to the reduction of plasma HDL in advanced CKD. In addition to HDL and ApoA-I deficiency, detailed analysis of HDL particles in patients with ESRD has revealed that the proportion of lipid-poor premature HDL is increased and maturation of cholesterol ester-poor HDL to cholesterol ester-rich HDL-2 is impaired in the ESRD population. These findings are clinically relevant as small-sized HDL particles alone and combined with elevated high sensitivity C-reactive protein (hsCRP) concentrations have been found to be independent predictors of reduced survival in patients with ESRD (11). The mechanisms responsible for the ESRD-associated alterations in HDL composition are twofold. Oxidative modification of ApoA-I and HDL impairs its ability to bind to ATP binding cassette transporter A-1 (ABCA-1), which is an essential step in efflux of free cholesterol from cells to HDL (5). In this context, several recent studies have revealed oxidative modification of HDL and ApoA-I in patients with end-stage renal disease.
Furthermore, hepatic production, plasma concentration and activity of LCAT are consistently reduced in patients with ESRD (5). Given the critical role that LCAT plays in HDL maturation, ESRD-induced LCAT deficiency further contributes to the reduction of plasma HDL concentration and impaired HDL maturation. These mechanisms lead to altered HDL particle composition in patients with ESRD which may lead to atherosclerosis and cardiovascular disease. This was recently shown by Holzer et al. using mass spectrometry and biochemical analyses to assess the composition of HDL particles in patients with ESRD. They found a significant increase in quantity of acute phase protein serum amyloid A1, albumin, lipoprotein-associated phospholipase A2, and apoC-III in HDL from patients with ESRD when compared with normal individuals. Furthermore, these HDL particles contained reduced phospholipid and increased triglyceride content and had an impaired ability to promote cholesterol efflux from macrophages (12).
Impact of ESRD on HDL Function
In a series of earlier studies we found that compared to the HDL from the healthy controls, the HDL from patients with ESRD exhibits markedly reduced antioxidant and anti-inflammatory activity (5,13). In these experiments, we found that the ability of HDL to inhibit LDL-induced monocyte chemotactic activity was dramatically reduced in patients with ESRD when compared with normal control individuals (13). These findings were associated with and, in part, due to a significant reduction in plasma PON and GPX activity in addition to decreased LCAT concentrations. These are key HDL-associated antioxidant enzymes which are crucial for its antioxidant-anti-inflammatory functions (14). The clinical significance of these findings were underlined by in a study which showed that depressed antioxidant capacity of HDL is associated with increased risk of cardiovascular and overall mortality in a cohort of dialysis-dependent CKD patients (15).
Another clinically intriguing observation was reported by Kilpatrick et al who on examination of a large cohort of prevalent hemodialysis patients did not find a clear association between serum HDL cholesterol level and survival (16). This can be explained by the growing body of evidence which indicate that in the presence of oxidative stress and inflammation, HDL is transformed from an antioxidant and anti-inflammatory to a pro-oxidant, pro-inflammatory particle known as acute-phase HDL (17). In fact, Honda et al. have recently shown that in a cohort of Japanese maintenance hemodialysis (MHD) patients, higher HDL cholesterol concentrations were associated with higher levels of oxidized-HDL. Furthermore, they found that elevated oxidized-HDL concentrations were associated with increased cardiovascular mortality. Hence, they concluded that excess oxidative stress may have yielded dysfunctional HDL in patients on with ESRD, and patients with high HDL-cholesterol under these conditions may have enriched oxidized HDL which can then result in increased cardiovascular disease burden and mortality (18). In addition, while evaluating the HDL from patients with ESRD, Weichhart et al. found that HDL from ESRD patients not only was deficient in preventing inflammation but it in fact promoted inflammatory cytokine production. Using shotgun proteomics they identified 49 HDL-associated proteins, including the serum amyloid A protein (SAA) which they postulate may be responsible for the pro-inflammatory properties of HDL described in these patients (19). Yamamoto et al also recently confirmed that HDL from patients with ESRD not only had reduced anti-chemotactic ability but it caused increased macrophage cytokine response (tumor necrosis factor alpha, interleukin 6, and interleukin 1 beta), when compared to HDL from healthy controls (20). Given the mentioned results, one can predict that in particular circumstances, HDL from patients with ESRD may in fact have a deleterious effect on formation and progression of cardiovascular disease.
Future Directions
ESRD is associated with decreased HDL-C and ApoA-I level and altered HDL composition. While these abnormalities can contribute to the atherogenic diathesis in ESRD, there is also significant HDL dysfunction associated with this disease which can further exacerbate the cardiovascular disease burden in this patient population. In fact, the latter findings can be responsible for the paradox that contrary to the general population, in patients with ESRD, there is no clear association between serum HDL cholesterol level and survival. Indeed, based on some of the evidence noted in this review there may be some circumstances in which elevated serum levels of HDL can be associated with an increased risk of CV mortality in patients with ESRD (Figure 1-B). Therefore, solely relying on HDL-C level to predict cardiovascular disease and mortality in ESRD may be misleading. Hence, it is essential that future studies focus on deciphering the role of HDL function rather than level in CV mortality. Furthermore, novel actionable biomarkers are needed which can predict HDL level as well as function thereby providing prognostic tools as well as potential new targets for therapy.
Acknowledgments
FUNDING SOURCE
KK-Z is supported in part by NIH grants K24-DK091419 and R01-DK078106. HM is supported by NIH grant F32-DK082130.
Footnotes
RELEVANT POTENTIAL CONFLICT OF INTEREST
Authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Renal Data System: Excerpts from the USRDS. Annual Data Report: Atlas of End-Stage Renal Disease in the United States 2012 [Google Scholar]
- 2.Vaziri ND, Moradi H. Mechanisms of dyslipidemia of chronic renal failure. Hemodial Int. 2006;10:1–7. doi: 10.1111/j.1542-4758.2006.01168.x. [DOI] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Inverse Association between Lipid Levels and Mortality in Men with Chronic Kidney Disease Who Are Not Yet on Dialysis: Effects of Case Mix and the Malnutrition-Inflammation-Cachexia Syndrome. J Am Soc Nephrol. 2007;18:304–311. doi: 10.1681/ASN.2006060674. [DOI] [PubMed] [Google Scholar]
- 4.Noori N, Caulfield MP, Salameh WA, Reitz RE, Nicholas SB, Molnar MZ, Nissenson AR, Kovesdy CP, Kalantar-Zadeh K. Novel lipoprotein subfraction and size measurements in prediction of mortality in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2861–2870. doi: 10.2215/CJN.03650411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaziri ND. Lipotoxicity and impaired high density lipoprotein-mediated reverse cholesterol transport in chronic kidney disease. J Ren Nutr. 2010;20:S35–43. doi: 10.1053/j.jrn.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 7.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Okubo K, Ikewaki K, Sakai S, Tada N, Kawaguchi Y, Mochizuki S. Abnormal HDL apolipoprotein A-I and A-II kinetics in hemodialysis patients: a stable isotope study. J Am Soc Nephrol. 2004;15:1008–15. doi: 10.1097/01.asn.0000117286.85443.7d. [DOI] [PubMed] [Google Scholar]
- 9.Effect of chronic renal failure on high-density lipoprotein kinetics. Fuh MM, Lee CM, Jeng CY, Shen DC, Shieh SM, Reaven GM, Chen YD. Kidney Int. 1990;37:1295–300. doi: 10.1038/ki.1990.114. [DOI] [PubMed] [Google Scholar]
- 10.Moradi H, Said HM, Vaziri ND. Post-transcriptional nature of uremia-induced downregulation of hepatic apolipoprotein A-I production. Transl Res. 2012 Dec 3; doi: 10.1016/j.trsl.2012.11.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vekic J, Zeljkovic A, Bogavac-Stanojevic N, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V, Simic-Ogrizovic S, Dopsaj V, Spasic S. Cox proportional hazard model analysis of survival in end-stage renal disease patients with small-sized high-density lipoprotein particles. Clin Biochem. 2011;44:635–41. doi: 10.1016/j.clinbiochem.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G. Uremia alters HDL composition and function. J Am Soc Nephrol. 2011;22:1631–41. doi: 10.1681/ASN.2010111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 2009;76:437–44. doi: 10.1038/ki.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moradi H, Pahl MV, Elahimehr R, Vaziri ND. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl Res. 2009;153:77–85. doi: 10.1016/j.trsl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Kopple JD, Kamranpour N, Fogelman AM, Navab M. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 2007;72:1149–56. doi: 10.1038/sj.ki.5002491. [DOI] [PubMed] [Google Scholar]
- 16.Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar-Zadeh K. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol. 2007;18:293–303. doi: 10.1681/ASN.2006070795. [DOI] [PubMed] [Google Scholar]
- 17.Navab M, Reddy S, Van Lenten BJ, et al. Role of dysfunctional HDL in atherosclerosis. J Lipid Res. 2008;50:S145–9. doi: 10.1194/jlr.R800036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda H, Ueda M, Kojima S, Mashiba S, Michihata T, Takahashi K, Shishido K, Akizawa T. Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis. 2012;220:493–501. doi: 10.1016/j.atherosclerosis.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 19.Weichhart T, Kopecky C, Kubicek M, Haidinger M, Döller D, Katholnig K, Suarna C, Eller P, Tölle M, Gerner C, Zlabinger GJ, van der Giet M, Hörl WH, Stocker R, Säemann MD. Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol. 2012;23:934–47. doi: 10.1681/ASN.2011070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, Fazio S, Kon V. Dysfunctional High-Density Lipoprotein in Patients On Chronic Hemodialysis. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.09.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]