SUMMARY
Dried blood spots (DBS) are collected uniformly from U.S. newborns to test for metabolic and other disorders. Because evidence exists for prenatal origins of some diseases, DBS may provide unique prenatal exposure records. Some states retain residual DBS and permit their use in etiologic studies. The primary study aim was to assess the feasibility of obtaining residual DBS from state newborn screening programs for pediatric and adolescent cancer patients nationwide with parental/subject consent/assent. Families of leukemia and lymphoma patients aged ≤21 years diagnosed from 1998–2007 at randomly selected Children’s Oncology Group institutions across the U.S. were queried (n=947). Parents/guardians and patients aged ≥18 years were asked to release DBS to investigators in spring 2009. DBS were then requested from states. Overall, 299 families (32%) released DBS. Consenting/assenting patients were born in 39 U.S. states and 46 DBS were obtained from 5 states; 124 DBS were unobtainable because patients were born prior to dates of state retention. State policies are rapidly evolving and there is ongoing discussion regarding DBS storage and secondary research uses. Currently, population-based DBS studies can be conducted in a limited number of states; fortunately, many have large populations to provide reasonably sized pediatric subject groups.
MeSH keywords: biological specimen banks, child, epidemiologic methods, informed consent, neonatal screening, neoplasms
INTRODUCTION
Dried blood spots (DBS) are collected from nearly all neonates (>95%)1 in the U.S. per a standard protocol2 to test for metabolic and other disorders.3 Newborn screening (NBS) programs are mandated by U.S. states, resulting in considerable variability in implementation nationally.4, 5 Each state selects the tests to be performed, establishes consent and refusal processes, and acts as a steward of residual DBS. Some states retain residual DBS for ≥5 years, potentially permitting their use in etiologic research.5 There is ongoing discussion among the public, NBS, and research communities, however, regarding individual and societal benefits and potential risks of DBS storage and secondary research uses.
Many childhood conditions, including cancers, are thought to have prenatal origins.6–10 One important line of evidence in support of prenatal origins is the detection of chromosomal translocations in DBS collected at birth from childhood leukemia cases.7, 8 These translocations may constitute a first genetic hit in a multistep pathway,8 with subsequent hits from endogenous or exogenous factors. It is therefore of keen interest to elucidate the role of in utero exposures in carcinogenesis.
Recall bias is a potentially serious limitation of retrospective case-control studies of rare pediatric/adolescent disorders. To our knowledge, DBS constitute the only consistent source of prediagnostic biospecimens for affected children, and for a representative population of control children, and may provide an unbiased record of in utero exposures, since they are collected at birth. Their potential research value is great since theoretically, any substrate measured in whole blood or blood products can also be analyzed in DBS.1 Accordingly, several analytes have been evaluated in DBS, such as amino acids, enzymes, human and viral DNA, antibodies, markers of inflammation, steroids, metals, protein adducts, pesticides and cotinine.1, 11–14
Prior studies assessing DBS retrieval have been limited to single states and did not include study-specific subject consent processes prior to specimen retrieval.15–17 Our primary aim was to assess the feasibility of obtaining 1) parental/subject consent/assent for DBS release from childhood/adolescent hematologic cancer cases on a nationwide basis and 2) DBS from state newborn screening programs.
METHODS
Patients were eligible for this Children’s Oncology Group (COG) study (AEPI08N1) if they were diagnosed with leukemia or lymphoma at 0–21 years of age in 1998–2007, were born in the U.S., and they or their parent/guardian participated in a prior COG protocol (AADM01P1),18 agreed to future contact, and spoke English or Spanish. Parents/guardians of deceased patients were eligible.
Briefly, the prior COG protocol (AADM01P1) from which eligible subjects were identified was conducted at a 10% random sample of COG institutions in North America (n=23). Between May 2001 and January 2007, 2,233 families of childhood cancer cases were approached and 2,136 (96%) agreed to future contact regarding COG-approved non-therapeutic studies. Since COG institutions treat the vast majority of leukemia and lymphoma cases ages 0–14 years in the U.S.19, 20 and since nearly all families agreed to future contact, the use of this dataset in subject ascertainment provided a reasonably representative sample of cases.
COG diagnosing institutions were provided with study summaries and were asked to either supply last known contact information for parents/guardians or subjects providing consent for future contact, or any reasons why families should not be contacted (e.g., child recently died). Of note, contact information provided by COG institutions was last verified several years prior for many families. Families were contacted in a series of mailings (introductory letter, study packet, reminder/thank you postcard) over a period of 4 weeks in the spring of 2009, followed by a phone call and/or a final mailing (second study packet). Spanish translations were sent to known Spanish speakers.
Parents/guardians of children <18 years and deceased children were provided with a description of the study and were asked to consider three levels of written consent, including release of their child’s DBS to study investigators for prenatal exposure assessment, long-term DBS storage by investigators, and future contact regarding this study. For children ≥18 years, contact information was requested (from parents/guardians) so they could be asked to provide consent regarding their own DBS. Children ages 8–17 years were asked to provide written assent, as children possessing the cognitive ability and maturity to comprehend a research study should be given the autonomy to decide about their participation. In addition, biological mothers, if available, were asked to complete self-administered questionnaires regarding prenatal exposures and birth characteristics.
Non-respondents were traced via telephone and reverse directories and on-line methods. Multiple attempts were made to reach non-respondents via telephone at different times of day/days of the week; voicemail/answering machine messages were left on third or subsequent failed attempts. Families who did not respond via mail, who were not reached via telephone, and whose outgoing message did not clearly identify them were classified as “contact unknown,” given that the U.S. Postal Service would not return study materials to sender for addresses that had expired several years prior.
State newborn screening programs were provided with signed consent/assent forms and asked to release DBS. States were also asked to provide DBS retention, storage, and release policies. We contacted states of birth for all participating cases (39 states) regardless of prior responses to surveys,4, 5 as state policies are evolving.
The protocol was approved by the Institutional Review Boards of the University of Minnesota and of states releasing DBS, as needed.
Statistical analysis
Primary outcomes are the proportions of families consenting to DBS release and proportions of DBS retrieved. Three “rates” (contact, cooperation, and response) were calculated as outlined by Slattery et al21 to facilitate discussion of results. Contact rates are the proportion of the total sample for whom contact was established, while cooperation rates are the proportion of those contacted that participated, and response rates are the proportion of the total that participated. Thus, response rates are equal to contact rates multiplied by cooperation rates.
Univariate unconditional logistic regression (SAS v9.2, SAS Institute, Cary, North Carolina) was also performed to assess whether baseline participant characteristics (shown in Table 1) were statistically significant predictors of consent, assent, provision of adult child contact information, questionnaire completion, or DBS retrieval; odds ratios (ORs) and 95% confidence intervals (CIs) were produced. In addition, multivariable logistic regression was performed to determine the significance of predictors after adjustment for all other factors.
Table 1.
Characteristics of 947 Eligible Pediatric and Adolescent Hematologic Cancer Cases, United States, 2009–2010.
| Characteristic | N (%) |
|---|---|
| Initial contact | |
| Mother | 887 (93.7) |
| Parent/Guardian | 57 (6.0) |
| Child ≥18 years at diagnosis | 3 (0.3) |
| Language | |
| English | 877 (92.6) |
| Spanish | 46 (4.9) |
| Unknown | 24 (2.5) |
| Age at diagnosis | |
| 0–4 years | 375 (39.6) |
| 5–9 years | 204 (21.5) |
| 10–14 years | 181 (19.1) |
| 15–20 years | 187 (19.8) |
| Year of birth | |
| 1982–1986 | 86 (9.1) |
| 1987–1991 | 208 (22.0) |
| 1992–1996 | 197 (20.8) |
| 1997–2001 | 326 (34.4) |
| 2002–2006 | 130 (13.7) |
| Years since diagnosis | |
| 2–4 years | 386 (40.8) |
| 5–10 years | 561 (59.2) |
| Vital status | |
| Alive | 844 (89.1) |
| Deceased | 83 (8.8) |
| Unknown | 20 (2.1) |
RESULTS
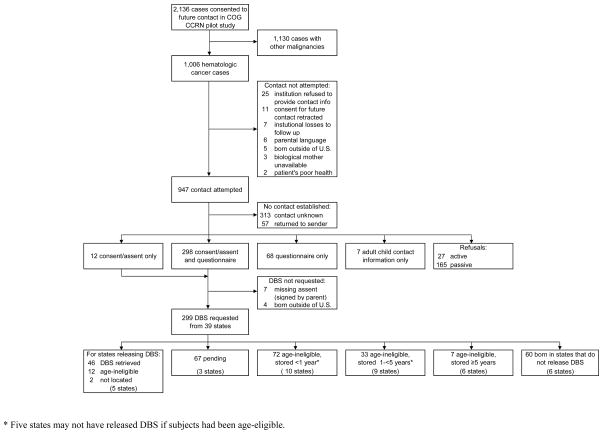
Nineteen U.S. COG institutions provided contact information and reasons not to contact subjects, while 2 refused for administrative reasons (1 institution was no longer participating in COG and 1 had closed the original AADM01P1 study with their IRB and could not prioritize reopening it) and 2 Canadian institutions were excluded (Canada has no provision to provide DBS). Figure 1 provides a flow diagram for subject eligibility and participation. Of 1,006 hematologic cancer cases in the original AADM01P1 dataset, contact was attempted for 947 in the current study.
Figure 1.
Flow Diagram of Subject Participation and Dried Blood Spot Retrieval for 947 Families of Pediatric and Adolescent Hematologic Cancer Cases and 39 State Newborn Screening Programs, United States, 2009–2010.
For most of the 947 families, the first points of contact were mothers (94%) and English speakers (93%) (Table 1). Forty percent of cases were diagnosed at <5 years and approximately 20% were diagnosed in the other age categories. Half were born in 1997 or thereafter (48%), while 59% were diagnosed ≥5 years prior to contact (median=5 years). Most cases were last known to be alive (89%).
Contact, response, and cooperation rates are summarized in Table 2. Of the 947 families, 385 participated in some manner, resulting in an overall response rate of 41%, while 27 actively refused (3%) and materials from 57 were returned to sender (6%). An additional 165 were passive refusals (17%), meaning contact was confirmed via telephone. It was unknown if contact was made with the remaining 313 (33%) (see Methods for further information).
Table 2.
Contact, Cooperation, and Response Rates for 947 Families of Pediatric and Adolescent Hematologic Cancer Cases and 39 State Newborn Screening Programs, United States, 2009–2010.
| Contact rate a | Cooperation ratea | Response rate a | ||||
|---|---|---|---|---|---|---|
| P+R / P+R+NC | % | P / P+R | % | P / P+R+NC | % | |
| Subjects | ||||||
| Any participation | 577 / 947 | 60.9 | 385 / 577 | 66.7 | 385 / 947 | 40.7 |
| Consent | 537 / 835 | 64.3 | 310 / 537 | 57.7 | 310 / 835 | 37.1 |
| Parents/guardians | 404 / 700 | 57.7 | 235 / 404 | 58.2 | 235 / 700 | 33.6 |
| Subjects ≥18 years | 133 / 135 | 98.5 | 75 / 133 | 56.4 | 75 / 135 | 55.6 |
| Assent (subjects 8–17 years) | 268 / 463 | 57.9 | 148 / 268 | 55.2 | 148 / 463 | 32.0 |
| Parent/guardian provision of contact information for subjects ≥18 years | 172 / 244 | 70.5 | 132 / 172 | 76.7 | 132 / 244 | 54.1 |
| Questionnaire (biological mothers only) | 549 / 887 | 61.9 | 366 / 549 | 66.7 | 366 / 887 | 41.3 |
| States | ||||||
| DBS retrieved b | 299 / 947 | 31.6 | 46 / 299 | 15.4 | 46 / 947 | 4.9 |
NC = no contact established; P = participant; R = refusal (includes active and passive refusals)
Rates are based on field rates defined by Slattery et al.21 Notably, response rate = contact rate X cooperation rate.
Although 310 consents were received, only 299 DBS were requested from state newborn screening programs because 7 assents were not signed by children and 4 subjects were foreign born.
Fifty-four percent of parents/guardians of children ≥18 years at the time of contact provided names and addresses. In addition, 3 subjects were adults (≥18 years) at diagnosis and had provided consent for future contact, for a total of 135 adult children queried.
Thirty-seven percent of families returned signed consent forms, including 228 mothers (34%), 7 parent/guardians (21%), and 75 adult children contacted upon receipt of information from parents/guardians (57%). (Only 1 of 3 adult subjects was successfully contacted and passively refused participation.) Of those providing consent, 304 agreed to DBS release, storage, and future contact, while 2 agreed to release and storage, 2 agreed to release and future contact, and 2 agreed to release only. One consent was signed by a minor and written consent could not be obtained from that parent/guardian. Families of cases diagnosed at 15–20 years and cases born in 1987–1991 were approximately 2 times more likely to consent compared to cases aged 0–4 years (OR=1.95, 95% CI:1.28, 2.97), and those born in 1997–2001 (OR=2.08, 95% CI:1.38, 3.15), respectively. Spanish speakers were significantly less likely to consent than English speakers (OR=0.33, 95% CI:0.14, 0.75). Upon adjustment for all factors, only Spanish language remained a significant predictor of response (OR=0.32, 95% CI:0.14, 0.74).
Thirty-two percent of children ages 8–17 years returned signed assent forms (n=148). In addition, 7 were signed by parents/guardians and signed assents were not obtained from these children.
Forty-one percent of mothers returned completed questionnaires. Notably, 82% of families providing completed questionnaires also returned signed consents for DBS release. Similar to consent response, diagnosis at ages 15–20 years (OR=2.05, 95% CI:1.41, 2.98), birth in the years 1987–1991 (OR=1.85, 95% CI:1.29, 2.66), and Spanish language (OR=0.39, 95% CI:0.19, 0.79) were significant predictors of questionnaire completion. Spanish language was the only significant predictor in the multivariable model (OR=0.38, 95% CI:0.19, 0.79).
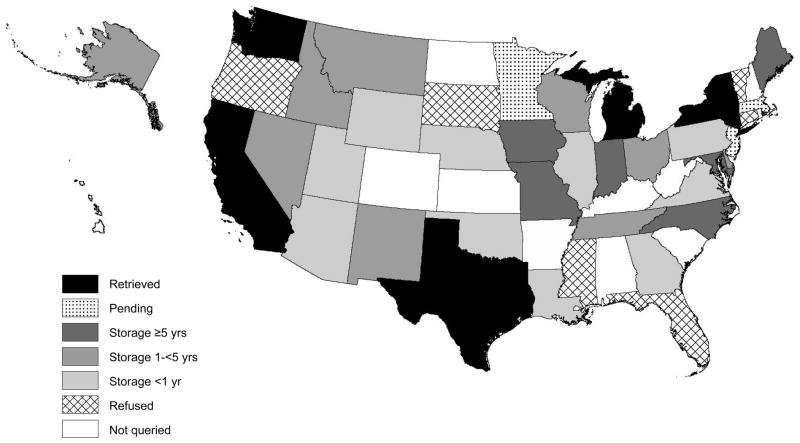
Respondents were born in 42 U.S. states and those providing consent for DBS release were born in 39 of these. DBS were requested for 299 cases (and were not requested for 7 missing signed assents and 4 not born in the U.S.). Of 39 states queried, 5 (CA, MI, NY, TX, WA) released 46 DBS (Figure 2). (The specimens await analysis for prenatal exposures.) These states did not have DBS for 12 “age-ineligible” cases (those born prior to oldest date of state retention) and could not locate spots for 2 age-eligible cases based on the information provided (child’s last name, date of birth, mother’s full name, hospital name and city). None of the factors examined were significant predictors of DBS retrieval, although this may be attributable to the small number of retrieved spots.
Figure 2.
State Newborn Screening Program Policies on Residual Dried Blood Spot Retention and Release for 39 Queried States, United States, 2009–2010. Black = DBS retrieved, dotted = request pending, dark gray = DBS stored ≥5 years, gray = DBS stored 1-<5 years, light gray = DBS stored <1 year, hatched = refused, white = not queried. (United States map courtesy of Minnesota Population Center. National Historical Geographic Information System: Pre-release Version 0.1. Minneapolis, MN: University of Minnesota 2004 (http://www.nhgis.org). Shading via ArcGIS mapping software version 9.3, Esri, Relands, California.)
DBS were unobtainable for 112 cases because states store spots for <1 year (n=72, AZ, GA, IL, LA, NE, OK, PA, UT, VA, WY), 1-<5 years (n=33, AK, DE, ID, MT, NM, NV, OH, TN, WI) or ≥5 years (n=7, IA, IN, MD, ME, MO, NC) and cases were age-ineligible. Of note, policies may not allow for DBS retrieval in 5 of these (GA, IL, NM, NV, WY). Policies in 6 states (CT, FL, MS, OR, SD, VT) do not allow for release of identifiable DBS for research (n=60). Requests are pending in 3 states (n=67), including one involved in ongoing litigation unrelated to the current study (MN), a second currently reviewing its storage/release policies (NJ), and a third performing a protocol review (MA). Supplementary Table 1 (available online) summarizes length of retention, willingness to release spots with written consent, and storage conditions for the 39 queried states.
DISCUSSION
In this national feasibility study, 37% of families of pediatric and adolescent cancer patients contributed written consent/assent authorizing DBS release to investigators and 39 state NBS programs were approached with signed consent/assent forms. This response rate may not accurately portray subject interest in DBS research, however, since we are unable to determine whether one-third of families were reached. When we calculated cooperation rates based on families with confirmed contact, the consent rate was considerably higher (58%, Table 2). Nevertheless, due to limited state retention and release practices, DBS were retrieved for only a small fraction of those requested (46/299, 15%). Notably, at least 25 of the 39 queried states were willing to release DBS with subject consent. However, in most of the states DBS were not stored long enough to permit their release.
Families of older cases and those diagnosed less recently were more likely to participate. This pattern may signal limitations on subjects’ time, as families with younger children anecdotally indicated in telephone conversations with interviewers that time constraints were preventing them from participating. Spanish-speaking subjects were also less likely to participate after adjustment for all other factors, although materials were provided in their native language. These results likely reflect the greater inability to reach these families (materials were returned to sender in 20% of families and no contact was established in 59% of families vs. 5% and 32%, respectively, in English-speaking families). It is also possible that most families were reached and elected not to respond, which might signify a lack of trust in this research.22, 23
This was also the first DBS study to assess participation of subjects ages ≥18 years, in whom higher levels of consent were observed compared with parents/guardians. Notably, adult children would have been aware of their cancers as they were diagnosed 2–9 years prior to the current study. They may not have had complete autonomy with regard to their participation, however, since parents/guardians were the initial points of contact for most. Presumably, if parents/guardians could not be reached, were opposed to DBS research, or did not wish for their adult children to be asked about this study, no contact information was provided, and adult children were not asked. Conversely, very interested parents/guardians may have placed expectations on their children regarding participation. Similar to our results, a prior survey of adult subjects’ attitudes indicated that a majority would be willing to provide consent for research on their pediatric specimens.24 Future studies should attempt to contact adult subjects directly to remove the parental element from the decision.
A complex array of ethical considerations is implicated with the storage and use of DBS for secondary research purposes. As such, this topic is currently the subject of active dialogue on a national level, with a focus on balancing societal/public health benefits and respect for individuals.4, 25, 26 Although limited, the available evidence indicates support for DBS storage and research among the general public,27–29 and among NBS professionals and researchers,12, 30–32 with some caveats. For example, a majority of participants in a nationally representative survey of parental attitudes27 and a Michigan citizen’s deliberative jury28 supported DBS storage and release if their permission was sought. Far fewer were amenable to research conducted without permission, however. Notably, no consent forms or DBS were collected in those studies.27, 28
There are important ethical considerations with respect to NBS itself (see 33). Ensuring universal screening is of primary importance to NBS programs, in order to protect children from rare debilitating and/or potentially fatal conditions by early detection and intervention.32 Thus, secondary research uses of DBS should not interfere with this core mission.32
Residual DBS are a fortunate NBS byproduct that could be used to facilitate valuable research on additional debilitating and/or fatal conditions, if the difficult outstanding ethical issues could be addressed in a manner acceptable to all stakeholders. A principal area of concern is ownership of the spots. DBS ownership is assigned to the state in 5 states, while 5 others classify genetic information as personal property.31 Regardless of formal policies/laws, however, parents or children may feel ownership regarding DBS28, 29 and/or genetic information generated from them. DNA can be extracted from DBS, potentially allowing individual and/or familial disease susceptibility to be uncovered. Thus, research on identifiable specimens could lead to loss of privacy, psychological harms, or discrimination by third parties (e.g., employers or insurance providers),25, 34 although there have been no documented abuses to date.31 Other important concerns involve failure to disclose and obtain active consent for long-term storage and secondary uses, timing and type of consent needed (e.g., blanket vs. study-specific), insufficient security measures, potential government and/or forensic uses, potential to reveal paternity, inequitable distribution of research benefits, and the potential for group-level harms4, 25, 26, 32. Many of these issues suggest a need for increased transparency, oversight, and education to improve public trust.32, 35 Notably, 12 states describe DBS storage and release policies in education materials, and a minority have explicit consent mechanisms for future use.28, 31
If the unresolved concerns are not adequately addressed, public unease may lead to lower NBS rates and/or reduced DBS availability. For example, genetic privacy advocacy groups sued the states of Texas and Minnesota in 2009, calling for destruction of specimens stored without explicit parental consent.36, 37 Although DBS were spared for our study, the Texas settlement resulted in the destruction of 5 million DBS.38 A second lawsuit has recently been filed by the same plaintiffs.39 The Minnesota lawsuit was dismissed40 but an appeal has recently been filed with the state supreme court. Other states are reviewing their policies (see Supplementary Table 1), presumably due to similar concerns. Importantly, recommendations have recently been issued to assist states as they develop comprehensive DBS policies.31
Other factors have been associated with DBS acquisition. In our study, there was no obvious explanation for the 2 unretrievable spots in states providing DBS; identical data were provided for these cases as for those with retrieved specimens. In a prior study conducted in Maryland, significantly lower retrieval rates were observed among cases with congenital heart defects (65%) than among population-based controls (84%).17 Among controls, a sizable number either had no laboratory number linking participants and DBS (10%) or could not be located (6%). Observed retrieval rates were also significantly lower among low birthweight and preterm infants.
This study features unique strengths. To our knowledge, it is the first U.S. study to attempt DBS retrieval with parental/subject consent/assent on a national level. Study-specific consent/assent allowed subjects to evaluate content of proposed research28 and measures to protect privacy/confidentiality. Additionally, the randomly selected study population should reasonably represent U.S. pediatric/adolescent cancer cases, as described above. Correspondingly, consenting cases were born in 39 states, providing an excellent survey of the national feasibility of this methodology. Further, it included children ≥18 years, although many were not approached because parents/guardians were not reached or chose not to provide contact information. Finally, these results are applicable to etiologic studies of other rare pediatric/adolescent conditions requiring retrospective designs.
The chief limitation is that state DBS policies are continually evolving; this report represents a snapshot in time. Some states have recently passed legislation allowing long-term storage of DBS (Supplementary Table 1). Conversely, 2 states are involved in ongoing legislation due to data privacy concerns, resulting in destruction of stored DBS in 1, and others are reviewing their policies. Additionally, the sizable proportion of families for whom contact was uncertain limits calculation of accurate response and cooperation rates and generalizability of results. Failure to reach these families is likely a function of the dataset used in case ascertainment, as subjects were diagnosed up to 10 years prior. We have anecdotally observed that a cancer diagnosis in a child can induce financial turmoil and familial instability, leading to transient housing situations and therefore, difficulty in locating families for research. We anticipate that the use of a dataset involving a more recently diagnosed patient population would greatly minimize this concern, as treating institutions would have current contact information for subjects in active treatment or follow-up. Notably, 82% of families returning questionnaires also released DBS, indicating high participation levels if families are reached and engaged. Lastly, etiologic studies of pediatric/adolescent cancers usually utilize retrospective case-control designs, however, we limited the scope of this initial feasibility study to families of cases who had agreed to future contact regarding research. Parents of healthy children may not be aware of the importance or utility of DBS research27 or may prioritize data privacy concerns over desire to participate and therefore may be less likely to participate. Consistent with this idea, Tarini et al found that parents with children with very good and excellent health reported they would be less likely to agree to DBS release or storage.27 An alternate control strategy may be the use of randomly selected, anonymized DBS from states of birth of cases. Future research should address the need for DBS from healthy controls.
From a research perspective, DBS from all U.S. infants would ideally be cataloged and properly stored such that they could be retrieved and released with appropriate scientific justification, ethics review board oversight, and subject consent. In practice, state retention policies are evolving. In our experience, DBS studies of pediatric conditions are currently limited to a few states. Fortunately, most have large populations to provide reasonable pediatric sample sizes. Depending on subject ages and future policies, research may be possible in additional states storing DBS ≥5 years. Studies of conditions in young children may have the greatest success and relevance to prenatal exposures. It will be of interest to observe state policies as they unfold.
Supplementary Material
Acknowledgments
Research supported by National Institutes of Health Grants T32 CA099936, U10 CA13539, and U10 CA98543, and the Children’s Cancer Research Fund, Minneapolis, MN.
Participating Children’s Oncology Group (COG) institutions included: Cancer Research Center of Hawaii, Honolulu, HI; Children’s Hospitals and Clinics of Minnesota, Minneapolis, MN; Children’s Medical Center, Dayton, OH; Connecticut Children’s Medical Center, Hartford, CT; Dartmouth-Hitchcock Medical Center, Lebanon, NH; Driscoll Children’s Hospital, Corpus Christi, TX; East Tennessee Children’s Hospital, Knoxville, TN; Hackensack University Medical Center, Hackensack, NJ; Lutheran General Children’s Medical Center, Park Ridge, IL; Michigan State University, Lansing, MI; Miller Children’s Hospital/Harbor-UCLA, Long Beach, CA; Mission Hospitals, Asheville, NC; New York University Medical Center, New York, NY; Primary Children’s Medical Center, Salt Lake City, UT; St. Christopher’s Hospital for Children, Philadelphia, PA; The Children’s Hospital of Southwest Florida, Lee Memorial Health System, Fort Myers, FL; University of Florida, Gainesville, FL; University of Texas Health Science Center at San Antonio, San Antonio, TX; and University of Vermont College of Medicine, Burlington, VT.
The authors wish to extend a sincere thanks to the state newborn screening program contacts that provided helpful information regarding state DBS policies and/or assistance in obtaining DBS.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest regarding this research.
References
- 1.Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. Journal of Nutrition. 2001;131:1631S–1636S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- 2.Hannon WH, Whitley RJ, Davin B, Fernhoff P, Halonen T, Lavochkin M, Miller J, Ojodu J, Therrell BL. Clinical and Laboratory Standards Institute (CLSI) Document LA4-A5. 5. Wayne, PA: CLSI; 2007. Blood Collection on Filter Paper for Newborn Screening Programs; Approved Standard. [Google Scholar]
- 3.National Newborn Screening and Genetics Resource Center. [Accessed January 27, 2010];National Newborn Screening Status Report. Updated 12/17/09. http://genes-r-us.uthscsa.edu/nbsdisorders.pdf.
- 4.Therrell BL, Johnson A, Williams D. Status of newborn screening programs in the United States. Pediatrics. 2006;117:S212–252. doi: 10.1542/peds.2005-2633C. [DOI] [PubMed] [Google Scholar]
- 5.Olney RS, Moore CA, Ojodu JA, Lindegren ML, Hannon WH. Storage and use of residual dried blood spots from state newborn screening programs. Journal of Pediatrics. 2006;148:618–622. doi: 10.1016/j.jpeds.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 6.Warner JA, Jones CA, Jones AC, Warner JO. Prenatal origins of allergic disease. Journal of Allergy and Clinical Immunology. 2000;105:S493–498. doi: 10.1016/s0091-6749(00)90049-6. [DOI] [PubMed] [Google Scholar]
- 7.Gale KB, Ford AM, Repp R, Borkhardt A, Keller C, Eden OB, et al. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13950–13954. doi: 10.1073/pnas.94.25.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taub JW, Ge Y. The prenatal origin of childhood acute lymphoblastic leukemia. Leukemia & Lymphoma. 2004;45:19–25. doi: 10.1080/1042819031000149403. [DOI] [PubMed] [Google Scholar]
- 9.Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, et al. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. Journal of Toxicology and Environmental Health. 2008;11:373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- 10.Shankaran S, Das A, Bauer CR, Bada H, Lester B, Wright L, et al. Fetal origin of childhood disease: intrauterine growth restriction in term infants and risk for hypertension at 6 years of age. Archives of Pediatrics & Adolescent Medicine. 2006;160:977–981. doi: 10.1001/archpedi.160.9.977. [DOI] [PubMed] [Google Scholar]
- 11.Spector LG, Hecht SS, Ognjanovic S, Carmella SG, Ross JA. Detection of cotinine in newborn dried blood spots. Cancer Epidemiology, Biomarkers and Prevention. 2007;16:1902–1905. doi: 10.1158/1055-9965.EPI-07-0230. [DOI] [PubMed] [Google Scholar]
- 12.Olshan AF. Meeting report: the use of newborn blood spots in environmental research: opportunities and challenges. Environmental Health Perspectives. 2007;115:1767–1779. doi: 10.1289/ehp.10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44:899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- 14.Skogstrand K, Ekelund CK, Thorsen P, Vogel I, Jacobsson B, Norgaard-Pedersen B, et al. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. Journal of Immunological Methods. 2008;336:78–84. doi: 10.1016/j.jim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Searles Nielsen S, Mueller BA, De Roos AJ, Checkoway H. Newborn screening archives as a specimen source for epidemiologic studies: feasibility and potential for bias. Annals of Epidemiology. 2008;18:58–64. doi: 10.1016/j.annepidem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klotz J, Bryant P, Wilcox HB, Dillon M, Wolf B, Fagliano J. Population-based retrieval of newborn dried blood spots for researching paediatric cancer susceptibility genes. Paediatric and Perinatal Epidemiology. 2006;20:449–452. doi: 10.1111/j.1365-3016.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- 17.Loffredo CA, Ewing CK. Use of stored newborn blood spots in research on birth defects: variation in retrieval rates by type of defect and infant characteristics. American Journal of Medical Genetics. 1997;69:85–88. doi: 10.1002/(sici)1096-8628(19970303)69:1<85::aid-ajmg16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 18.Steele JR, Wellemeyer AS, Hansen MJ, Reaman GH, Ross JA. Childhood cancer research network: a North American Pediatric Cancer Registry. Cancer Epidemiology, Biomarkers and Prevention. 2006;15:1241–1242. doi: 10.1158/1055-9965.EPI-06-0447. [DOI] [PubMed] [Google Scholar]
- 19.Ross JA, Severson RK, Pollock BH, Robison LL. Childhood cancer in the United States. A geographical analysis of cases from the Pediatric Cooperative Clinical Trials groups. Cancer. 1996;77:201–207. doi: 10.1002/(SICI)1097-0142(19960101)77:1<201::AID-CNCR32>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Krailo M, Reaman GH, Bernstein L. Childhood cancer patients’ access to cooperative group cancer programs: a population-based study. Cancer. 2003;97:1339–1345. doi: 10.1002/cncr.11192. [DOI] [PubMed] [Google Scholar]
- 21.Slattery ML, Edwards SL, Caan BJ, Kerber RA, Potter JD. Response rates among control subjects in case-control studies. Annals of Epidemiology. 1995;5:245–249. doi: 10.1016/1047-2797(94)00113-8. [DOI] [PubMed] [Google Scholar]
- 22.Neidich AB, Joseph JW, Ober C, Ross LF. Empirical data about women’s attitudes towards a hypothetical pediatric biobank. American Journal of Medical Genetics Part A. 2008;146:297–304. doi: 10.1002/ajmg.a.32145. [DOI] [PubMed] [Google Scholar]
- 23.Thompson HS, Valdimarsdottir HB, Jandorf L, Redd W. Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: differences across African American, Latina and Caucasian women. Patient Education and Counseling. 2003;51:217–227. doi: 10.1016/s0738-3991(02)00219-7. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg AJ, Hull SC, Botkin JR, Wilfond BS. Pediatric biobanks: approaching informed consent for continuing research after children grow up. Journal of Pediatrics. 2009;155:578–583. doi: 10.1016/j.jpeds.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therrell BL, Hannon WH, Pass KA, Lorey F, Brokopp C, Eckman J, et al. Guidelines for the retention, storage, and use of residual dried blood spot samples after newborn screening analysis: statement of the Council of Regional Networks for Genetic Services. Biochemical and Molecular Medicine. 1996;57:116–124. doi: 10.1006/bmme.1996.0017. [DOI] [PubMed] [Google Scholar]
- 26.Pelias MK, Markward NJ. Newborn screening, informed consent, and future use of archived tissue samples. Genetic Testing. 2001;5:179–185. doi: 10.1089/10906570152742218. [DOI] [PubMed] [Google Scholar]
- 27.Tarini BA, Goldenberg A, Singer D, Clark SJ, Butchart A, Davis MM. Not without my Permission: Parents’ Willingness to Permit Use of Newborn Screening Samples for Research. Public Health Genomics. 2010;13:125–130. doi: 10.1159/000228724. [DOI] [PubMed] [Google Scholar]
- 28.Fleck L, Mongoven A, Marzec S. Stored Blood Spots: Ethical and Policy Challenges. East Lansing, MI: Institute for Public Policy and Social Research, College of Social Science, Michigan State University; 2008. [Accessed May 11, 2010]. Informing the Debate. www.ippsr.msu.edu/Publications/HPFleck.pdf. [Google Scholar]
- 29.Rothwell E, Anderson R, Botkin J. Policy Issues and Stakeholder Concerns Regarding the Storage and Use of Residual Newborn Dried Blood Samples for Research. Policy, Politics & Nursing Practice. 2010;11:5–12. doi: 10.1177/1527154410365563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American College of Medical Genetics. Position statement on importance of residual newborn screening dried blood spots. Bethesda, MD: Apr 29, 2009. [Accessed May 10, 2010]. http://www.acmg.net/StaticContent/NewsReleases/Blood_Spot_Position_Statement2009.pdf. [Google Scholar]
- 31.Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Briefing Paper: Considerations and Recommendations for National Guidance Regarding the Retention and Use of Residual Dried Blood Spot Specimens after Newborn Screening. Washington, DC: 2010. [Accessed May 11, 2010]. http://www.hrsa.gov/heritabledisorderscommittee/RBSBriefingPaperFINALDraft42310.pdf. [Google Scholar]
- 32.Institute of Medicine. Challenges and Opportunities in Using Residual Newborn Screening Samples for Translational Research: Workshop Summary. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 33.President’s Council on Bioethics. The changing moral focus of newborn screening. Washington, DC: 2008. [Accessed May 10, 2010]. http://bioethics.georgetown.edu/pcbe/reports/newborn_screening/ [Google Scholar]
- 34.Annas GJ. Privacy rules for DNA databanks. Protecting coded ‘future diaries’. Journal of the American Medical Association. 1993;270:2346–2350. [PubMed] [Google Scholar]
- 35.Kharaboyan L, Avard D, Knoppers BM. Storing newborn blood spots: modern controversies. Journal of Law, Medicine and Ethics. 2004;32:741–748. doi: 10.1111/j.1748-720x.2004.tb01979.x. [DOI] [PubMed] [Google Scholar]
- 36.State sued over blood storage. St Paul Pioneer Press; Mar 11, 2009. [Google Scholar]
- 37.Roser MA. State sued over babies’ blood. Austin American-Statesman; Mar 13, 2009. p. B01. [Google Scholar]
- 38.Roser MA. Samples of newborns’ blood to be destroyed. Austin American-Statesman; Dec 23, 2009. p. A01. [Google Scholar]
- 39.Roser MA. State illegally traded, sold newborns’ blood to private companies, suit alleges. Austin American-Statesman; Dec 9, 2010. p. B03. [Google Scholar]
- 40.Hennepin County District Court rules MDH not in violation of privacy laws. Minnesota Lawyer; Dec 7, 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.