Abstract
Indirect immunofluorescence antinuclear antibodies (IIF-ANA) are detected in approximately 90% of scleroderma patients, and the staining pattern correlates with scleroderma-specific antibody subsets. Solid-phase ANA assays that are dependent on multiplex bead technology (MULTIPLEX-ANA) are replacing immunofluorescence in many commercial labs; however, performance of these assays has not been compared to IIF-ANA in scleroderma. The purpose of this study was to evaluate whether a proportion of scleroderma patients have negative testing on MULTIPLEX-ANA assays and demonstrate whether negative MULTIPLEX-ANA is associated with particular scleroderma-specific autoantibodies. A retrospective chart review was completed on all 238 scleroderma patients evaluated in the Georgetown scleroderma clinic between June 1, 2008 and May 31, 2009. Autoantibody results, demographics, and scleroderma features were collected. Data were analyzed using unpaired t test and Mann–Whitney U test for continuous variables, and Fisher’s exact test for dichotomous variables. Simple kappa coefficient was used to measure the level of agreement between MULTIPLEX-ANA and IIF-ANA results. Two-tailed p values <0.05 were considered significant. MULTIPLEX-ANA testing was available in 57 patients and only 29 (51%) tested positive. In contrast, IIF-ANA was positive in 91% of these patients. Using simple kappa coefficient, there was a good agreement between the MULTIPLEX-ANA, and presence of Scl70, RNP, and centromere antibodies (0.76; 95% CI 0.59, 0.92), but there was no agreement between MULTIPLEX-ANA and presence of other IIF-ANA patterns including nucleolar ANA (−0.40; 95% CI −0.64, −0.16). Because RNA polymerase III and nucleolar antibodies are seen in 43% of the entire scleroderma population, we are concerned that these false-negative tests could result in delays in referral and diagnosis. Until the MULTIPLEX-ANA assays can be modified to include the antigens for RNA polymerase III and the nucleolar ANA subsets, IIF-ANA remains the recommended screening test for ANA in suspected scleroderma.
Keywords: Antinuclear antibody, Autoantibody, IF-ANA, Immunofluorescence, Multiplex, Scleroderma
Introduction
Detection of antinuclear autoantibodies (ANA) is an important screening tool when evaluating patients with possible scleroderma (SSc) [1]. Autoantibodies are not known to have a pathologic role in the disease development, but they are important markers of clinical disease subgroups [2, 3]. Using the HEp-2 cell line, approximately 95% of scleroderma patients test positive with immunofluorescence ANA (IIF-ANA) [2]. HEp-2 cells contain 100–150 autoantigens and therefore false-negative results in patients with clinically apparent connective tissue disease are rare [4].
More recently, new, high throughput, solid-phase assays often based on multiplex technology (MULTIPLEX-ANA) have been introduced to replace IIF-ANA in clinical practice. The advantages of these methodologies are that they are easier to perform with reduced labor costs and variability in interpretation, allowing screening for several defined autoantibodies in large numbers of patients simultaneously [5, 6]. Many commercial laboratories have adopted these methods as their primary method of ANA screening. However, as these assays have gained more widespread use, many clinicians have raised concerns that solid-phase assays are not as sensitive as IIF-ANA for detection of autoantibodies. An ad hoc committee of the American College of Rheumatology found that up to 35% of patients with known systemic lupus erythematosus and positive IIF-ANA have negative testing using the MULTIPLEX-ANA techniques [7]. Furthermore, there is significant inter-assay variability in sensitivity between the various solid-phase commercial assays [8–10].
To date, no studies have evaluated the use of MULTIPLEX-ANA assays in scleroderma. Many of the antigens, particularly the nucleolar antigens, which are targeted by autoantibodies in scleroderma, are missing from the MULTIPLEX-ANA assay. Therefore, we hypothesize that the MULTIPLEX-ANA assay will perform poorly in scleroderma. The purpose of this study was to use commercial laboratory results obtained during routine clinical care to test the hypothesis that a significant proportion of scleroderma patients test negative using MULTIPLEX-ANA assays. While costs prohibit a prospective direct comparison of MULTIPLEX-ANA and IIF-ANA in our scleroderma cohort, using data from our cohort, we also sought to establish whether false-negative testing on MULTIPLEX-ANA assays corresponded to particular scleroderma-specific antibody subsets.
Methods
This study was approved by the Biomedical Institutional Review Board at Georgetown University Hospital.
Subjects
Charts were reviewed on all 238 scleroderma patients evaluated at the Georgetown University scleroderma clinic between June 1, 2008 and May 31, 2009. All subjects had a confirmed diagnosis of scleroderma based on accepted criteria [11].
Data collection
Using the electronic medical record (Centricity, GE), data were abstracted on demographics, scleroderma phenotype, and autoantibody profile based on commercial testing. Results of the following autoantibodies were recorded: IIF-ANA with titer and pattern (titer of >1:160 considered positive), MULTIPLEX-ANA, Scl-70, Anti-centromere, RNA polymerase III (Pol3), and U1-RNP. An IIF-ANA with nucleolar pattern was also noted if present, since this staining pattern corresponds to U3-RNP and anti-TH-TO antibodies which were not commercially available at the time of this study. Finally, we also noted the presence or absence of SSA and B antibodies since as many as 15% of scleroderma patients have these antibodies.
Antibody testing
In our practice, two commercial labs (Laboratory Corporation of America and Quest Diagnostics) are used depending on the patient’s insurance requirements, and both rely on the BioPlex 2200 method for MULTIPLEX-ANA. Although it is our routine practice to request IIF-ANA for evaluation of scleroderma patients, several patients had the MULTIPLEX-ANA performed due to clerical issues. This was a random and unpredictable event; but as a result, a subset of patients had both MULTIPLEX-ANA, IIF-ANA, and scleroderma-specific antibody profile performed, affording us an opportunity to assess performance of these tests and to correlate this with clinical phenotype. No independent testing was performed on these samples
Scleroderma-specific antibodies were all performed through commercial laboratories by immunoassay, and included anti-centromere antibody, Scl-70 antibody, RNP antibody, THTO antibody, U3 RNP antibody, polymerase III antibody, or PM-Scl antibody. Again it should be noted that owing to the retrospective nature of this study, testing was performed according to clinical need and not all subjects had all antibodies tested. Autoantibodies previously obtained on established patients were recorded to allow assessment of antibody profile across our scleroderma cohort. This is valid assessment since antibody profile in scleroderma generally does not change over time [12].
Data analysis
Data were analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Unpaired t test and Mann–Whitney U test were used for continuous variables, and chi-square test was used for dichotomous variables. Simple kappa coefficient was calculated using SAS version 9.1 (SAS institute, Cary, NC). Two-tailed p values <0.05 was considered statistically significant.
Results
Between June 1, 2008 and May 31, 2009, 238 patients with a confirmed diagnosis of scleroderma were evaluated in the Georgetown scleroderma clinic.
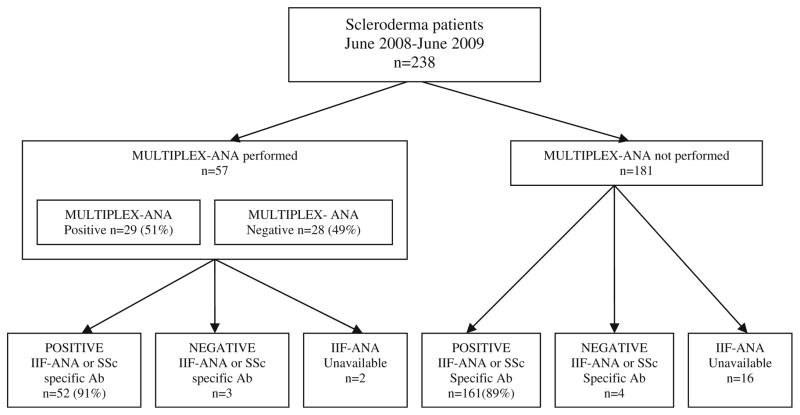
Due to the retrospective nature of this study, only 220 of the 238 patients had IIF-ANA or scleroderma-specific antibody results available (Fig. 1). Of these 220 patients, 213 (97%) had either a positive IIF-ANA or positive scleroderma-specific autoantibodies. Results from MULTIPLEX-ANA testing were available in 57 patients, while 181 had no MULTIPLEX-ANA results available. Of these 57 patients, 41 had concomitant MULTIPLEX-ANA and IIF-ANA results (both tests performed within a 12-month period), 14 additional patients had scleroderma-specific antibody profiles, and 2 patients did not have sufficient data in the medical record to verify their antibody profile.
Fig. 1.
Flowchart outlining autoantibody results on the 238 patients evaluated. Of the 57 patients with MULTIPLEX-ANA results available, 29 tested positive (51%) while 28 tested negative (49%). The numbers of patients with positive results on IIF-ANA or positive SSc-specific autoantibodies are shown. (Scleroderma-specific antibodies included anti-centromere antibody, Scl-70 antibody, RNP antibody, THTO antibody, U3 RNP antibody, polymerase III antibody, or PM-Scl antibody, and were measured through commercial laboratories using immunoassay techniques)
Demographics
The demographics of the groups with and without MULTIPLEX-ANA testing are shown in Table 1. Notably the group in whom MULTIPLEX-ANA results were available had a significantly higher proportion of patients with diffuse scleroderma (61% compared to 41%, p=0.009). This reflected a greater proportion of newly evaluated patients with diffuse scleroderma coinciding with the time period of the study. Age, sex, and race were not significantly different between the two groups.
Table 1.
Demographic and antibody characteristics of the entire Georgetown Scleroderma Clinic population, compared to the groups with and without MULTIPLEX-ANA test results, demonstrating that despite the higher than expected proportion of subjects with diffuse scleroderma in the cohort with MULTIPLEX-ANA results available, the frequency of nucleolar ANA, pol3 antibodies, and nonspecific ANA patterns in the two cohorts was similar
| Total Georgetown SSc population n=238 | MULTIPLEX-ANA cohort n=57 | No MULTIPLEX- ANA cohort n=181 | p value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 212 (89%) | 48 (84%) | 164 (91%) | 0.22 |
| Male | 26 (11%) | 9 (16%) | 17 (9%) | |
| Age | ||||
| Mean (±SEM) | 53.73 (±0.86) | 52.16 (±1.81) | 54.23 (±0.97) | 0.30 |
| SSc type | ||||
| Diffuse | 109 (45.8%) | 35 (61%) | 74 (41%) | 0.009 |
| Limited | 130 (54.6%) | 22 (39%) | 107 (59%) | |
| Race | ||||
| Caucasian | 146 (61.3%) | 32 (56%) | 114 (63%) | 0.35 |
| African American | 68 (28.6%) | 21 (37%) | 47 (26%) | 0.13 |
| Asian | 12 (5%) | 1 (2%) | 11 (6%) | 0.30 |
| Other | 12 (5%) | 3 (5%) | 9 (5%) | 1.00 |
| Autoantibodies | ||||
| Centromere | 40 (16.8%) | 4 (7%) | 36 (19.9%) | 0.02 |
| Scl-70 | 49 (20.6%) | 12 (21%) | 37 (20.4%) | 0.92 |
| RNP | 26 (10.9%) | 8 (14.0%) | 18 (10%) | 0.39 |
| SSA | 23 (9.7%) | 6 (10.5%) | 17 (9.4%) | 0.99 |
| SSB | 5 (2.1%) | 1 (1.7%) | 4 (2.2%) | 0.83 |
| Nucleolar | 47 (19.7%) | 9 (15.8%) | 38 (21%) | 0.39 |
| Polymerase III | 26 (10.9%) | 9 (15.8%) | 18 (9.9%) | 0.22 |
| Nonspecific ANA | 29 (12.2%) | 7 (12.2%) | 22 (12.1%) | 0.98 |
| Total nucleolar, Pol3, and nonspecific ANA | 102 (42.9%) | 25 (43.8%) | 78 (43.1%) | 0.92 |
The p value refers to comparison of the MULTIPLEX-ANA cohort to the no MULTIPLEX-ANA cohort
Comparison of MULTIPLEX-ANA and IIF-ANA
As shown in Fig. 1, while 91% of the 57 patients with MULTIPLEX-ANA results available had positive IIF-ANA or other scleroderma-specific antibody, only 51% (29 out of 57) tested positive on the MULTIPLEX-ANA assay. Concomitant results from both MULTIPLEX-ANA and IIF-ANA were available in 41 patients. Only a small subset had both tests performed since generally, patients were not subjected to additional IIF-ANA testing if sufficient data were available to confirm the diagnosis of scleroderma (for example, if one of the scleroderma-specific antibodies was positive). However, in the subset who had both tests performed, all patients with a positive MULTIPLEX-ANA also had a positive IIF-ANA (MULTIPLEX-ANA specificity 100%, 95% CI 0.48–1.00), but 75% of patients with negative MULTIPLEX-ANA had positive IIF-ANA (MULTIPLEX-ANA sensitivity 57%, 95% CI 0.39–0.74).
Comparison of MULTIPLEX-ANA and scleroderma antibodies
Using simple kappa coefficient, we found good agreement between positive MULTIPLEX-ANA and presence of RNP, Scl-70, or centromere antibodies. This is as expected since these antibodies are detected by the MULTIPLEX-ANA assay. In contrast there was no agreement between the MULTIPLEX-ANA and nucleolar or pol3 antibodies (Table 2). Of the seven patients with positive MULTIPLEX-ANA but without RNP, Scl-70, or centromere antibodies, three tested positive for SSA, an antibody that would be expected to be detected on the multiplex assay. In the remaining four patients, the antibody that triggered the positive MULTIPLEX-ANA testing is unclear.
Table 2.
Simple kappa coefficient suggests good agreement between multiplex and RNP/Scl-70, but no agreement between multiplex and nucleolar ANA and Pol3 antibodies
| Multiplex positive | Multiplex negative | Total | Simple kappa coefficient (95% CI) | |
|---|---|---|---|---|
| RNP, Scl-70, or centromere positive | 23 | 1 | 24 | 0.76 (0.59, 0.92) |
| RNP, Scl-70, or centromere negative | 6 | 27 | 33 | |
| Total | 29 | 28 | 57 | |
| Nucleolar ANA or Pol3 positive | 7 | 18 | 25 | −0.40 (−0.64, −0.16) |
| Nucleolar ANA or Pol3 negative | 22 | 10 | 32 | |
| Total | 29 | 28 | 57 |
Kappa coefficient <0 as indicating no agreement and 0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as good, and 0.81–1 as very good agreement
Subset with MULTIPLEX-ANA was representative of entire scleroderma cohort
Given that this was a retrospective study, there was concern that the patients who had MULTIPLEX-ANA performed were not representative of the general scleroderma population. This was particularly important given the higher than expected representation of the diffuse scleroderma phenotype in this group. Table 1 shows the results of the scleroderma-specific antibodies in the total scleroderma population and the two subpopulations (those with and without MULTIPLEX-ANA results available). The frequency of nucleolar, pol3, and nonspecific ANA patterns was 43% in the whole population and 44% in MULTIPLEX-ANA group, indicating that the subset of patients who had MULTIPLEX-ANA testing was representative of the entire population. We therefore postulate that the MULTIPLEX-ANA fails to identify up to 43% of scleroderma patients who would have positive testing using IIF-ANA.
Discussion
Despite their widespread use in commercial laboratories, there have been no studies directly comparing MULTIPLEX-ANA and IIF-ANA in the scleroderma population. The current study had significant limitations due to MULTIPLEX-ANA data only being available on a small subset of the scleroderma population. However, extrapolating to the entire scleroderma population, we predict that MULTIPLEX-ANA assays likely miss up to 43% of scleroderma patients who would be expected to test positive on IIF-ANA.
While costs prohibit prospective comparison of MULTIPLEX-ANA with IIF-ANA in our cohort, one of the strengths of this study was the use of data collected through commercial laboratories and during the course of routine clinical care rather than comparing commercially available tests to those obtained in research laboratories. Both of the commercial labs used by patients in our cohort use the BioPlex 2200 platform. This method uses individual magnetic 8-μm beads, each of which is coated with a single antigen. Beads coated with different antigens are then mixed together such that in one assay, responses to up to 13 different antigens can be tested. Personal communication with our two commercial laboratories confirmed that the antigens included in this multiplex assay are double-stranded DNA, chromatin, ribosomal P, SSA and B, Smith, RNP, Scl-70, Jo-1, and centromere B. Notably while Scl-70 and centromere B are included in the assay, none of the other known scleroderma-specific antibody targets are included. It is therefore not surprising that this test performs poorly as a diagnostic tool in our scleroderma population.
Patients in our cohort had testing performed according to their clinical needs. MULTIPLEX-ANA and IIF-ANA testing was rarely performed on the same specimen, and most patients did not have repeat testing. While this is a theoretical concern since some patients without autoimmune disease will have low-titer IIF-ANA that becomes negative over time, in patients with known scleroderma, we would not expect antibody profile to change with time [12] and therefore this is unlikely to account for the differences seen.
In scleroderma patients, the presence of individual subtypes of scleroderma-specific autoantibodies are generally mutually exclusive [12] and the autoantibody profile can be extremely helpful in predicting disease manifestations and prognosis [2, 13]. Failure to detect positive ANA using solid-phase and MULTIPLEX-ANA assays is particularly important in regards to certain antibody subsets. Scleroderma patients with Pol3 antibodies often present with “arthritis” and swollen hands without Raynaud’s. Relying on a negative MULTIPLEX-ANA early in disease may delay diagnosis of scleroderma. This subgroup of patients develops severe skin thickening and is at high risk of renal crisis [14], so accurate ANA testing is critical to ensure appropriate follow-up and management. Likewise, the failure to detect nucleolar ANA can also result in delays in diagnosis. Nucleolar antibodies are associated with limited cutaneous disease, but a high risk of severe interstitial lung disease which needs to be identified and treated early in disease. Obtaining a negative MULTIPLEX-ANA may prevent physicians from entertaining a diagnosis of scleroderma, and thereby could potentially delay diagnosis and intervention for this serious complication.
With the introduction of the new ELISA assay for detection of Pol3 antibodies [15, 16], it should be possible to include these antigens in multiplex assays, thereby improving the performance of these tests for detection of scleroderma. Immunoassays are also available in commercial use for the Pm-Scl antibody. However, the other nucleolar antibodies require more intensive immunoprecipitation assays, and the antigens for these antibodies have not yet been isolated, so it is not yet feasible to incorporate them in solid-phase assays.
In response to recommendations from the American College of Rheumatology, physicians are now able to request commercial laboratories to perform IIF-ANA rather than MULTIPLEX-ANA using appropriate test codes [7]. It is essential that internists and rheumatologists are aware of the discrepancy between MULTIPLEX-ANA and IIF-ANA so that IIF-ANA can be requested when a diagnosis of scleroderma is being entertained.
Conclusions
In this cohort of scleroderma patients, 51% had negative results on the MULTIPLEX-ANA assays and false-negative MULTIPLEX-ANA was strongly associated with nucleolar IIF-ANA and Pol3 antibodies. Based on the prevalence of these antibodies in our population, we predict that as many as 40% of scleroderma patients will test negative on MULTIPLEX-ANA assays. This may result in delays in diagnosis and treatment. At this time, IIF-ANA using HEp-2 cells should be the screening ANA for patients in whom scleroderma is suspected.
Acknowledgments
Dr. Shanmugam’s work is supported by award number KL2RR031974 and UL1RR031975 from the National Center for Research Resources.
Footnotes
Disclosures None.
Contributor Information
Victoria K. Shanmugam, Email: vks4@gunet.georgetown.edu, Division of Rheumatology, Immunology and Allergy, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007, USA
Donna R. Swistowski, Division of Rheumatology, Martinsburg Veterans Affairs Medical Center, 510 Butler Avenue, Martinsburg, WV 25405, USA
Nicole Saddic, Division of Rheumatology, Immunology and Allergy, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007, USA.
Hong Wang, Department of Biostatistics and Epidemiology, MedStar Health Research Institute, 6525 Belcrest Road, Suite 700, Hyattsville, MD 20782, USA.
Virginia D. Steen, Division of Rheumatology, Immunology and Allergy, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007, USA
References
- 1.Lyons R, Narain S, Nichols C, Satoh M, Reeves WH. Effective use of autoantibody tests in the diagnosis of systemic autoimmune disease. Ann N Y Acad Sci. 2005;1050(1):217–228. doi: 10.1196/annals.1313.023. [DOI] [PubMed] [Google Scholar]
- 2.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35(1):35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Koenig M, Dieudé M, Senécal J-L. Predictive value of antinuclear autoantibodies: the lessons of the systemic sclerosis autoantibodies. Autoimmun Rev. 2008;7(8):588–593. doi: 10.1016/j.autrev.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Eissfeller P, Sticherling M, Scholz D, Hennig K, Lüttich T, Motz M, Kromminga A. Comparison of different test systems for simultaneous autoantibody detection in connective tissue diseases. Ann N Y Acad Sci. 2005;1050(1):327–339. doi: 10.1196/annals.1313.035. [DOI] [PubMed] [Google Scholar]
- 5.Shovman O, Gilburd B, Barzilai O, Shinar E, Larida B, Zandman-Goddard G, Binder SR, Shoenfeld Y. Evaluation of the BioPlex™ 2200 ANA screen: analysis of 510 healthy subjects: incidence of natural/predictive autoantibodies. Ann N Y Acad Sci. 2005;1050(1):380–388. doi: 10.1196/annals.1313.120. [DOI] [PubMed] [Google Scholar]
- 6.Shovman O, Gilburd B, Zandman-Goddard G, Yehiely A, Langevitz P, Shoenfeld Y. Multiplexed AtheNA multilyte immunoassay for ANA screening in autoimmune diseases. Autoimmunity. 2005;38(1):105–109. doi: 10.1080/08916930400022707. [DOI] [PubMed] [Google Scholar]
- 7.ACR. [Accessed March 2011];Methodology of testing for antinuclear antibodies. 2009 http://www.rheumatology.org/practice/ana_position_stmt.pdf.
- 8.Hanly JG, Su L, Farewell V, Fritzler MJ. Comparison between multiplex assays for autoantibody detection in systemic lupus erythematosus. J Immunol Meth. 2010;358(1–2):75–80. doi: 10.1016/j.jim.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Copple SS, Martins TB, Masterson C, Joly E, Hill HR. Comparison of three multiplex immunoassays for detection of antibodies to extractable nuclear antibodies using clinically defined sera. Ann N Y Acad Sci. 2007;1109(1):464–472. doi: 10.1196/annals.1398.052. [DOI] [PubMed] [Google Scholar]
- 10.Martins TB, Burlingame R, von Muhlen CA, Jaskowski TD, Litwin CM, Hill HR. Evaluation of multiplexed fluorescent microsphere immunoassay for detection of autoantibodies to nuclear antigens. Clin Diagn Lab Immunol. 2004;11(6):1054–1059. doi: 10.1128/cdli.11.6.1054-1059.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeRoy EC, Medsger TA. Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28(7):1573–1576. [PubMed] [Google Scholar]
- 12.Okano Y. Antinuclear antibody in systemic sclerosis (scleroderma) Rheum Dis Clin North Am. 1996;22(4):709–735. doi: 10.1016/s0889-857x(05)70297-0. [DOI] [PubMed] [Google Scholar]
- 13.Hamaguchi Y. Autoantibody profiles in systemic sclerosis: predictive value for clinical evaluation and prognosis. J Dermatol. 2010;37(1):42–53. doi: 10.1111/j.1346-8138.2009.00762.x. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen B, Assassi S, Arnett FC, Mayes MD. Association of RNA polymerase III antibodies with scleroderma renal crisis. J Rheumatol. 2010;37(5):1068. doi: 10.3899/jrheum.091048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker J, Burlingame R, Webb T, Bunn C. Anti-RNA polymerase III antibodies in patients with systemic sclerosis detected by indirect immunofluorescence and ELISA. Rheumatology. 2008;2008 (47):976–979. doi: 10.1093/rheumatology/ken201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwana M, Okano Y, Pandey JP, Silver RM, Fertig N, Medsger TA. Enzyme-linked immunosorbent assay for detection of anti-RNA polymerase III antibody: analytical accuracy and clinical associations in systemic sclerosis. Arthritis Rheum. 2005;52(8):2425–2432. doi: 10.1002/art.21232. [DOI] [PubMed] [Google Scholar]