Abstract
NF-κB transcription factors are pivotal regulators of innate and adaptive immune responses, and perturbations of NF-κB signaling contribute to the pathogenesis of immunological disorders. NF-κB is a well-known proinflammatory mediator, and its deregulated activation is associated with the chronic inflammation of autoimmune diseases. Paradoxically, NF-κB plays a crucial role in the establishment of immune tolerance, including both central tolerance and the peripheral function of regulatory T (Treg) cells. Thus, defect or deregulated activation of NF-κB may contribute to autoimmunity and inflammation, highlighting the importance of tightly controlled NF-κB signaling. This review will focus on the recent progress regarding NF-κB regulation and its association with autoimmunity.
Keywords: NF-κB, autoimmunity, inflammation, ubiquitination, regulatory T cells, alternative NF-κB, p100 processing, NIK, TRAF3, TRAF2, cIAP
NF-κB signaling pathways
Nuclear factor κB (NF-κB) represents a family of structurally related transcription factors, including RelA (also called p65), RelB, c-Rel, NF-κB1 (also called p50), and NF-κB2 (also called p52) [1]. The NF-κB members function as various hetero- or homo-dimers that transactivate a large number of genes via binding to a kB enhancer. The NF-κB target genes are involved in different aspects of immune functions, ranging from the development, activation, and differentiation of lymphocytes to the maturation and inflammatory functions of innate immune cells. The NF-κB factors are normally sequestered in the cytoplasm via association with a family of inhibitory proteins, including IκBα and related ankyrin repeat-containing proteins. In addition, the IκB family also includes the precursor proteins of NF-κB1 and NF-κB2, p105 and p100, which contain a C-terminal IκB-like structure and inhibit the nuclear translocation of specific NF-κB members. Proteasome-mediated processing of p105 and p100 involves selective degradation of their C-terminal IκB-like structure, leading to the generation of respective mature NF-κB subunits, p50 and p52, and the nuclear translocation of sequestered NF-κB proteins. The latent NF-κB complexes can be activated by various immune stimuli, which involves two major signaling pathways: the canonical and noncanonical pathways [2] (Box1). Both the canonical and noncanonical NF-κB pathways play a critical role in regulating immune activation and tolerance. Recent studies have emphasized diverse mechanisms that negatively regulate NF-κB activation in immune cells. It is also increasingly clear that NF-κB has paradoxical roles in the regulation of autoimmunity. While deregulated NF-κB activation contributes to the chronic inflammation and tissue damages of various autoimmune diseases, NF-κB is also important for preventing the development of autoimmunity through regulation of immune tolerance. In this review, we highlight the recent progress regarding the molecular mechanisms underlying NF-κB regulation and discuss how NF-κB exerts paradoxical roles in the regulation of autoimmunity.
NF-κB in autoimmune inflammation
Autoimmunity occurs as a result of immune reaction against self-antigens, which leads to chronic inflammation and tissue destructions [3]. NF-κB has been implicated in the pathogenesis of a number of autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, type I diabetes, multiple sclerosis, inflammatory bowel disease [4]. Whereas NF-κB activation occurs transiently during a normal immune response, it is chronically activated in the affected tissues of autoimmune diseases. A well-recognized pathological action of NF-κB is induction of proinflammatory cytokines and chemokines, which mediate the recruitment of immune cells and the establishment of inflammation. In addition, NF-κB promotes the activation of autoimmune T cells either directly or indirectly through modulating the function of dendritic cells (DCs).
Regulation of T-cell activation and homeostasis
The canonical NF-κB pathway has a central role in mediating T-cell activation. Optimal NF-κB activation requires both the TCR signal and the CD28 costimulatory signal [5]. The T-cell costimulation serves as a mechanism to prevent the induction of T-cell anergy, a major mechanism of peripheral T-cell tolerance that occurs when naïve T cells are stimulated through the TCR without adequate costimulation. Attenuated NF-κB activation is associated with T-cell anergy [5, 6], and conversely, deregulated NF-κB signaling can cause aberrant T-cell activation, thus reducing the sensitivity of T cells to anergy induction [7]. Emerging evidence suggests that properly regulated NF-κB signaling is also important for T-cell homeostasis. It is generally thought that the maintenance of T-cell homeostasis requires weak TCR signals elicited from contact with semi-self ligands [8]. Deregulated TCR signaling may perturb the homeostatic signaling threshold, resulting in the expansion of certain autoimmune T-cell clones and the development of systemic autoimmunity [8]. Consistent with the critical role of NF-kB in mediating TCR signaling, deregulated NF-κB activation is associated with impaired T-cell homeostasis and systemic autoimmunity in animal models [7, 9, 10].
Regulation of inflammatory T-cell differentiation
NF-κB also regulates the differentiation of CD4+ T cells, particularly the T helper 17 (Th17) cells, which have a central role in the pathogenesis of autoimmunity and inflammation [11]. The differentiation of Th17 cells is induced by TCR stimulation in the presence of specific cytokines, such as IL-6, TGFβ, IL-1, and IL-23, which promote the expression of lineage-specific transcription factors, RORγt and RORα, and epigenetic changes [11]. The role of NF-κB pathway in Th17 responses was first suggested by the finding that T cell-specific ablation of IKKβ renders mice highly resistant to the induction of a T cell-dependent autoimmunity, experimental autoimmune encephalomyelitis (EAE), coupled with a reduction in Th17 cells [12]. More recent work suggests that c-Rel and RelA directly regulate Th17 cell differentiation by inducing the expression of RORγt [13, 14]. In addition to its T cell-intrinsic function, NF-κB also regulates Th17 responses via induction of proinflammatory cytokines, such as IL-6 and IL-23, in DCs and macrophages.
The noncanonical NF-κB pathway also plays a role in regulating T-cell responses to self-antigens and the production of autoimmune inflammatory T cells. T cells lacking the central signaling component of this pathway, NIK, are tolerant in a model of graft-versus-host disease (GVHD), whereas NIK overexpression in T cells induces fatal autoimmunity [15]. Furthermore, genetic deficiency in a major negative regulator of NIK, TRAF2, causes aberrant T-cell activation and elevated production of Th17 cells, coupled with fatal autoimmune inflammation [16]. Conversely, NIK deficiency attenuates the differentiation of Th17 cells and renders animals refractory to the induction of the T cell-dependent autoimmunity EAE [17]. It is important to note that NIK may regulate autoimmunity and inflammation via mechanisms that are dependent or independent of the noncanonical NF-κB pathway [16-18]. The latter mechanism appears to involve regulation of STAT3 activation that is synergistically stimulated by the TCR and IL-6 receptor signals [17]. Moreover, the function of NIK and noncanonical NF-κB in autoimmunity regulation may involve both T cell-intrinsic and indirect mechanisms. In particular, NIK has a crucial role in regulating the maturation of DCs, which in turn contributes to T-cell activation and autoimmunity [19].
Regulation of DC function
DCs are the primary antigen-presenting cells (APCs) that not only mediate T-cell activation and initiate protective immune responses but also play a critical role in the induction of immune tolerance [20]. The activation versus tolerogenic functions of DCs largely depends on their state of maturation. During an infection, microbial components induce the maturation of DCs via stimulating pattern-recognition receptors, such as the toll-like receptors (TLRs). The mature DCs express costimulatory molecules and proinflammatory cytokines and are competent in the activation of naïve T cells. In the absence of a microbial trigger, immature DCs induce T-cell anergy or indirectly induce immune tolerance via expanding Treg cells. The NF-κB pathway has a pivotal role in mediating the maturation of DCs, and deregulated activation of NF-κB in DCs is associated with development of autoimmunity [21, 22]. Along the same line, a recent study has identified NF-κB1 as an essential factor for maintaining the resting state and tolerogenic function of DCs [23]. Although NF-κB1 p50 dimerizes with RelA and RelB to form DC activators under stimulated conditions [24], the p50 homodimer may be formed predominantly in unstimulated DCs and serve as a transcriptional repressor [23].
NF-κB as a mediator of immune tolerance
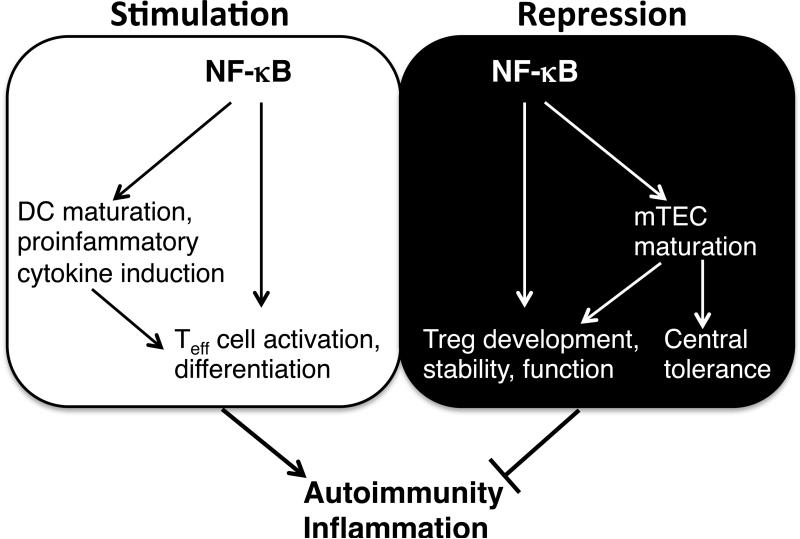
It is increasingly clear that NF-κB has paradoxical roles in the regulation of autoimmunity and inflammation (Fig. 1). In addition to its well-known functions in promoting inflammation and autoimmunity, NF-κB is crucial for the induction of immune tolerance.
Fig. 1. Paradoxical roles of NF-κB in the regulation of autoimmunity and inflammation.
NF-κB is involved in both the stimulation (Yang) and repression (Yin) of autoimmunity and inflammation. NF-κB promotes autoimmunity and inflammation by mediating the activation and differentiation of autoimmune and inflammatory T cells, such as Th17 cells. This is achieved either directly through a T cell-intrinsic function or indirectly via promoting the maturation and proinflammatory cytokine production of DCs. NF-κB suppresses autoimmunity and inflammation through mediating the development and immunosuppressive function of Treg cells. Canonical NF-κB pathway directly mediates this function, whereas noncanonical NF-κB pathway functions indirectly through mediating mTEC maturation, which is also crucial for central tolerance.
NF-κB in central tolerance
Immune tolerance consists of central and peripheral mechanisms. Central tolerance involves deletion or functional inactivation of self-reacting T cells during their development in the thymus, a process known as negative selection. The negative selection of thymocytes occurs in the medulla of the thymus and critically requires medullary epithelial cells (mTEC) and thymic DCs. Thymocytes that recognize self-antigens displayed on the MHC molecules of mTECs or DCs are deleted. A well-known function of the noncanonical NF-κB pathway is regulation of mTEC development [25]. Mutant mice lacking the noncanonical NF-κB members (RelB or NF-κB2) or upstream signaling molecules, such as NIK, IKKα, and LTβR, have impaired mTEC formation, coupled with autoimmune symptoms [25].
NF-κB in Treg cell development
Despite the powerful role of central tolerance, some self-reactive T cells can escape the negative selection and provoke autoimmunity in the periphery unless tightly controlled by the peripheral mechanisms of tolerance. Treg cells form a major family of immunosuppressive T cells that is essential for the maintenance of peripheral immune homeostasis and tolerance [26]. The immunosuppressive function of Treg cells relies on a master transcription factor, Foxp3, which controls a Treg cell-specific gene expression program. Foxp3+CD4+ Treg cells are mainly developed in the thymus and referred to natural Treg (nTreg) cells. In addition, inducible Treg (iTreg) cells can be generated in the periphery from the CD4+Foxp3– peripheral T cells when activated in the presence of TGFβ. Like thymocyte selection, the generation of nTreg cells requires mTECs [27]. Given the crucial role of the noncanonical NF-κB pathway in mTEC development [25], it is not surprising to see the requirement of this pathway for nTreg cell development. Indeed, the alymphoplasia (aly) mice, which carry a mutation in the NIK gene, have a reduced frequency of Treg cells. Ablation of other noncanonical NF-κB signaling components also impairs Treg cell development [25].
The canonical NF-κB pathway has a cell-intrinsic role in driving the development of Treg cells. Early studies detected reduced frequency of Treg cells in mutant mice lacking IKKβ or the NF-κB subunits p50 and c-Rel [25]. Genetic deficiencies in upstream signaling molecules of the canonical NF-κB pathway, including protein kinase C theta (PKCθ), Bcl-10, CARMA1, and Tak1 also impair Treg cell development [28-33]. The cell-intrinsic role of NF-κB in Treg cell generation has recently been demonstrated by several studies, which suggest the predominant involvement of c-Rel [34-36]. C-Rel mediates the TCR signal that synergizes with the cytokine signals in the induction of Foxp3 gene expression, a process that involves c-Rel-mediated formation of a Foxp3-specific enhanceosome [36].
NF-κB pathway in Treg cell stability and function
Treg cells are generally considered a stable lineage of immunosuppressive T cells, although a small population of Treg cells may display plasticity and lose Foxp3 expression under lymphopenic or inflammatory conditions [37]. The lineage stability of Treg cells appears to be regulated by epigenetic events induced by the TCR signal during Treg cell development [38]. However, the role of TCR signal in committed Treg cells is still poorly understood. As the TCRs of Treg cells recognize self-antigens and are thought to be constantly stimulated, it is conceivable that some of the TCR-stimulated signaling pathways may contribute to the stability and/or immunosuppressive functions of Treg cells. Recent evidence indicates a role for the canonical NF-κB pathway in the regulation of Treg stability and in vivo function [39]. Treg-specific ablation of a K63-specific ubiquitin-conjugating enzyme, Ubc13, compromises the in vivo function of Treg cells, leading to impaired T-cell homeostasis and development of autoimmunity. In conventional T cells, Ubc13 is essential for TCR-stimulated activation of IKK as well as MAPKs [40]. The Treg-specific function of Ubc13 is crucially dependent on the IKK pathway, since the functional defect of the Ubc13-defective Treg cells can be largely rescued by a transgene encoding a constitutively active IKKβ (IKK2ca) [39]. Moreover, Treg-specific ablation of IKKβ also impairs the function of Treg cells and causes autoimmunity. It remains to be examined which of the NF-κB members are involved in Treg regulation.
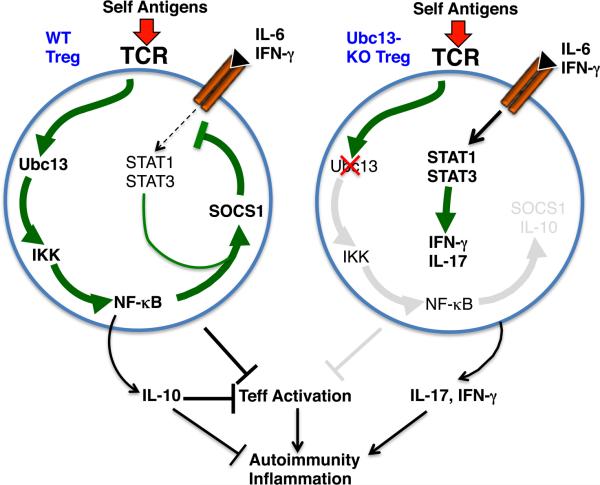
Ubc13 and IKK are not required for the expression of Foxp3 or the in vitro suppressing activity of Treg cells [39]. Instead, this signaling axis is crucial for maintaining the in vivo stability and function of Treg cells under lymphopenic conditions. When adoptively transferred into Rag1-KO mice, a large proportion of the Ubc13-deficient Treg cells acquire Th1- and Th17-like effector functions despite their stable expression of Foxp3. The Ubc13-IKK signaling axis is required for expression of specific genes, including those encoding IL-10 and SOCS1 [39]. IL-10 is important for Treg-mediated inhibition of Th17 cells, whereas SOCS1 regulates Treg cell stability by repressing the signaling of proinflammatory cytokine receptors [41]. The promoter region of SOCS1 contains an evolutionally conserved sequence element that covers binding sites for STAT and NF-κB, and this composite sequence is crucial for the synergistic activation of SOCS1 promoter by the TCR and cytokine signals [39]. The reduced SOCS1 expression in Ubc13-deficient Treg cells contributes to their instability, since a SOCS1 mimetic peptide partially blocks the conversion of Ubc13-deficient Treg cells to Th1- and Th17-like effector T cells [39]. Based on these data, a model can be proposed to address the potential mechanism by which the Ubc13-IKK signaling axis regulates Treg cell stability and function (Fig. 2). This model suggests a central role for the NF-κB pathway in mediating TCR-stimulated expression of specific genes involved in the maintenance of Treg cell stability and function.
Fig. 2. A model of Treg cell regulation by the Ubc13-IKK signaling axis.
Constant stimulation of the TCR by self-antigens may lead to chronic NF-κB activation via the Ubc13-IKK signaling axis, which in turn promotes the expression of SOCS1, IL-10, and possibly additional factors involved in the function and stability of Treg cells. The NF-κB-mediated SOCS1 induction also requires the synergy from STAT proteins, and the induced SOCS1 in turn restrains the signaling from receptors of IL-6 and IFNγ. Ablation of Ubc13, or IKKβ, disrupts the activation of NF-κB and attenuates the expression SOCS1 and IL-10. The reduced SOCS1 expression may cause heightened IL-6 and IFNγ signaling and induction of IFNγ and IL-17, thereby promoting autoimmunity and inflammation. The impaired expression of IL-10 and possibly other factors may also compromise the in vivo suppressive function of Treg cells.
Mechanisms of NF-κB regulation
Given the important role of NF-κB in regulating immune activation and tolerance, the NF-κB signaling must be tightly controlled to prevent immunological disorders. Indeed, it is now clear that NF-κB is subject to regulation at multiple levels.
Feedback regulation by IκBα
A fundamental mechanism of NF-κB negative regulation is IκBα-mediated feedback inhibition, which was discovered about two decades ago [1]. Following its depletion by phosphorylation-dependent degradation, IκBα is rapidly replenished via NF-κB-mediated IκBα gene induction. The physiological significance of the IκBα feedback has recently been demonstrated using an IκBα knockin mouse (IκBαM/M) harboring mutated kB enhancers in the promoter of the IκBα gene [9]. As expected, cells derived from these mutant mice have impaired induction of IκBα gene expression and perturbed NF-κB activation. Most prominent is the chronic activation of NF-κB in T cells, coupled with impaired T-cell homeostasis and elevated frequency of activated T cells [9]. These mutant animals spontaneously develop autoimmunity with features of Sjögren's syndrome [9]. These findings suggest that the IκBα-mediated NF-κB feedback mechanism is crucial for regulating the T-cell homeostasis and preventing autoimmunity.
Regulation by deubiquitinases
Ubiquitination is a crucial mechanism that mediates activation of NF-κB signaling (Box 2). The NF-κB pathway is negatively regulated by a number of deubiquitinases (DUBs), the best studied of which are CYLD and A20 [42, 43] (Box 2). CYLD negatively regulates NF-κB signaling by cleaving the K63-linked, ubiquitin chains from various NF-κB signaling components [44]. Animal studies suggest a role for CYLD in the regulation of colonic inflammation [10, 45]. Moreover, the CYLD gene is located adjacent to a well-known IBD-susceptibility gene, NOD2, in both the human and the mouse chromosomes [10], and genome-wide association studies have identified CYLD as one of the IBD-susceptibility genes [46]. The association of CYLD with inflammation has also been demonstrated using mice harboring liver-specific CYLD inactivation [47]. As shown in T cells [10], the loss of CYLD function in hepatocytes leads to activation of TAK1 and JNK. Interestingly, however, the consequence of TAK1/JNK activation in this case is hepatocyte cell death, which triggers inflammation, fibrosis, as well as late onset of carcinogenesis [47].
A20 functions as a ubiquitin-editing enzyme that is essential for terminating NF-κB signaling [43] (Box 2). A crucial role for A20 in suppressing autoimmunity and inflammation was first demonstrated using global A20-deficient mice [48]. Recent studies further suggest that A20 functions in multiple cell types to control NF-κB activation and, thereby, maintain tissue homeostasis and restricts autoimmunity and inflammation [21, 22, 49-53]. Consistent with animal studies, A20 polymorphisms and mutations have been linked to human autoimmune and inflammatory diseases as well as lymphoid malignancies [48]. In addition to its ubiquitin-editing function, A20 may also inhibit IKK activation in a mechanism that does not involve deubiquitination or modulation of ubiquitination enzymes [54]. A20 binds to NEMO via its C-terminal zinc fingers (ZnFs), particularly ZnF7, in a manner that is dependent on free K63 polyubiquitin chains. Several other studies have also demonstrated the ZnF7-dependent ubiquitin-binding activity of A20 in the negative regulation of NF-κB [23, 55, 56]. However, in the latter case, A20 was found to preferentially bind to linear ubiquitin chains and inhibit IKK activation by the linear ubiquitin chain assembly complex (LUBAC). Further studies are warranted to assess the physiological significance of these new findings using animal models. The function of A20 may also require ABIN (A20-binding inhibitor of NF-κB) family of proteins, characterized by the possession of a ubiquitin-binding domain termed UBAN (ubiquitin-binding domain in ABIN and NEMO) [57]. It is thought that ABINs inhibit NF-κB signaling by recruiting A20 to K63-polyubiquitinated signaling proteins, such as NEMO, thereby terminating the activation of IKK. It is also possible that ABINs may act through interfering the activation of IKK by linear ubiquitin chains, which are bound by the UBAN with high affinity [58-60]. A recent knockin mouse study suggests that the ubiquitin-binding activity of ABIN1 is crucial for negatively regulating TAK1 and its downstream signaling factors, IKK, JNK, and p38, and for suppressing the development of autoimmunity [61]. ABIN1 also possesses NF-κB-independent functions, including suppression of TNFα-stimulated caspase 8 activation and apoptosis and negative regulation of the transcription factor C/EBPβ[62, 63].
Regulation by E3 ubiquitin ligases
While ubiquitination is a well-recognized mechanism mediating IKK activation, emerging evidence suggests the involvement of specific ubiquitination events in the termination or negative control of NF-κB signaling. In particular, ubiquitination of nuclear NF-κB serves as a mechanism to eliminate activated NF-κB members via the proteasome degradation pathway [64]. Nuclear RelA is ubiquitinated by a multi-subunit ubiquitin ligase, ECSSOCS1, which interacts with RelA via its substrate-binding subunit SOCS1 (suppressor of cytokine 1). A copper metabolism murr1 domain-containing (COMMD) protein, COMMD1, physically interacts with ECSSOCS1 and RelA and promotes the ubiquitination and degradation of RelA [64]. The in vivo role of COMMD1 and ECSSOCS1 in RelA regulation remains unclear. SOCS1-deficient mice have lethal inflammation and enhanced sensitivity to LPS-induced septic shock [65]. However, since the anti-inflammatory function of SOCS1 involves targeting of proinflammatory receptors and the receptor-associated kinase JAK [65], further studies are required to determine the specific function of SOCS1 in NF-κB regulation. Another possible RelA E3 ubiquitin ligase is PDLIM2 (also called SLIM), a nuclear protein containing both PDZ and LIM domains and initially found to mediate ubiquitination of STATs [64]. In response to LPS stimulation, the PDLIM2-deficient DCs have impaired RelA ubiquitination, coupled with accumulation of nuclear RelA and hyper production of proinflammatory cytokines [64]. Consistently, the PDLIM2 KO mice display enhanced sensitivity to LPS-stimulated septic shock. PDLIM2 also inhibits CD4 T-cell differentiation into Th1 and Th17 lineages and, thereby, negatively regulates bacterium-induced granulomatous inflammation and the T cell-dependent autoimmunity EAE, although this function of PDLIM2 appears to primarily involve regulation of STAT3 ubiquitination [66, 67].
A recent study suggests a role for ubiquitin-dependent c-Rel degradation in the regulation of T-cell tolerance [7]. Among the NF-κB members, c-Rel is particularly important for T-cell activation and differentiation and for prevention of T-cell tolerance. Compared to RelA, c-Rel is relatively insensitive to IκBα-mediated feedback regulation, suggesting the involvement of additional mechanisms in c-Rel negative regulation. Interestingly, activated c-Rel undergoes ubiquitin-dependent degradation in T cells, which requires a newly characterized E3 ubiquitin ligase, Peli1 (also called Pellino 1) [7, 68]. Consistent with the crucial role of c-Rel in T-cell activation, the Peli1-deficient T cells are hyper-responsive to TCR/CD28 stimulation in vitro and display activated phenotypes in vivo [7]. Moreover, the loss of Peli1 also renders T cells less sensitive to suppression by Treg cells and TGFβ. Consequently, the Peli1-KO mice develop autoimmune symptoms, characterized by lymph node enlargement, multiorgan inflammation, and enhanced serum levels of autoantibodies [7]. In addition to RelA and c-Rel, p50 is regulated by ubiquitin-dependent degradation. In LPS-stimulated macrophages, p50 undergoes ubiquitin-dependent degradation, a process that is particularly disseminated in cells lacking the p50 partner protein Bcl3 [64]. Since the p50 homodimer serves as a transcriptional repressor of NF-κB-target genes, the uncontrolled degradation of p50 in Bcl3-deficient cells is associated with aberrant production of proinflammatory cytokines.
Regulation by microRNAs
MicroRNAs (miRNAs) form a family of small non-coding RNAs that regulates gene expression by base-pairing with the 3’ untranslated region of target mRNAs, which causes their destabilization or attenuated translation. Early work revealed that the TLR4 ligand LPS stimulates the expression of several miRNAs, two of which, miR-146a and miR-155, are induced in a NF-κB-dependent manner [69]. More recently, a number of other miRNAs have been implicated as target genes of the NF-κB pathway [69]. Interestingly, several of these NF-κB-induced miRNAs function as negative or positive regulators of the NF-κB signaling pathway, suggesting feedback regulatory roles. MiR-146a is one of the miRNAs that negatively regulate NF-κB signaling. Upon induction by innate immune receptors, miR-146a targets the degradation of TRAF6 and IRAK1, central signaling components mediating activation of NF-κB and mitogen-activated kinases (MAPKs) by IL-1R and TLRs [69]. The miR-146a KO mice are hypersensitive to LPS-induced septic shock, coupled with overproduction of proinflammatory cytokines [70]. At older ages, the miR-146a KO mice develop autoimmunity, characterized by splenomegaly and lymphadenopathy, multiorgan inflammation, and accumulation of autoantibodies in the serum [70]. MiR-146a appears to regulate immune homeostasis in different cell types, including myeloid cells and T cells. Compared to naïve T cells, Treg cells express much higher levels of miR-146a, which is crucial for the function of Treg cells to suppress Th1 responses [71]. A major target of miR-146a in Treg cells is STAT1, although its role in NF-κB regulation in this cell type is unclear. A more recent study demonstrates a T cell-autonomous function of miR-146a in the control of T-cell homeostasis and tolerance [72]. Although the expression level of miR-146a is normally low in naïve T cells, it is markedly upregulated following T-cell activation. The inducible expression of miR-146a is dependent on NF-κB and serves as a feedback mechanism for negatively regulating TCR/CD28-stimulated activation of canonical NF-κB members [72]. As seen in macrophages, miR-146a suppresses the expression of TRAF6 and IRAK1 in T cells. However, it is possible that miR-146a also targets other NF-κB signaling factors, since the in vivo role of TRAF6 and IRAK1 in TCR-stimulated NF-κB activation is still obscure. MiRNAs, including miR-15a, miR-16, and miR-223, have also been found to regulate the noncanonical NF-κB signaling component IKKα, although their connection with autoimmunity is unclear.
Regulation of the noncanonical NF-κB pathway
A central mechanism of noncanonical NF-κB activation is stabilization of NIK, a kinase that induces phosphorylation-dependent processing of p100 together with IKKα [2]. NIK is a constitutively active kinase [73, 74], but its signaling function is restricted through its degradation by a TRAF3-dependent mechanism [2]. TRAF3, together with TRAF2, functions to recruit NIK to the E3 ubiquitin ligase c-IAP1 (or c-IAP2) for ubiquitination [2]. Genetic deficiencies in TRAF2, TRAF3, or c-IAP1/2 lead to NIK accumulation and constitutive activation of p100 processing [2]. NIK is also accumulated in cells lacking its downstream kinase IKKα, which phosphorylates NIK and promotes NIK degradation [75]. Thus, under steady-state conditions, NIK is controlled by both the TRAF3-dependent and IKKα-dependent degradation mechanisms.
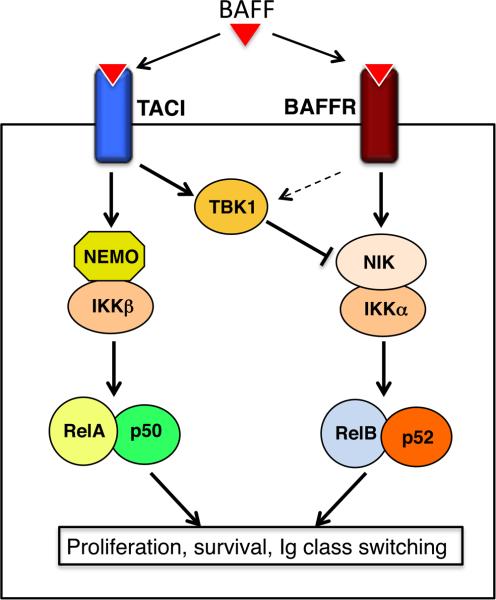
The signal-stimulated noncanonical NF-κB activation involves degradation of TRAF3 and TRAF2 and the concomitant accumulation of NIK. Recent work has shed light onto the negative regulation of signal-induced noncanonical NF-κB signaling and identified the IKK-related kinase TBK1 as a pivotal player in this function [76]. TBK1, as well as its homolog IKKe, are known as kinases that mediate induction of type I interferons in response to viral infection or TLR stimulation [77]. However, the in vivo function of TBK1 has been poorly studied due to the embryonic lethality of the global TBK1 KO mice [78]. Interestingly, B cell-specific TBK1 ablation greatly promotes the activation of noncanonical NF-κB activation in B cells stimulated by an agonistic anti-CD40 antibody or BAFF [76]. TBK1 is activated by both anti-CD40 and BAFF, and the activated TBK1 mediates phosphorylation-dependent NIK degradation. It appears that BAFF stimulates TBK1 activation predominantly via TACI, whereas another BAFF receptor, BAFFR, mediates the induction of noncanonical NF-κB signaling (Fig. 3). This finding provides an explanation to the negative role of TACI in BAFFR-mediated noncanonical NF-κB activation [79]. The deregulated activation of noncanonical NF-κB signaling in TBK1 B cell-conditional KO mice is associated with aberrant production of systemic IgA and autoantibodies and development of nephropathy-like symptoms [76].
Fig. 3. Negative regulation of BAFF-stimulated noncanonical NF-κB signaling by TBK1.
BAFF stimulates both TACI and BAFFR, which mediate the canonical and noncanoncal NF-κB pathways, respectively. TACI, and to a lesser extent BAFFR, activates TBK1, which in turn negatively regulates noncanonical NF-κB signaling by promoting NIK degradation.
Concluding remarks
It is now clear that NF-κB has a crucial and paradoxical role in the regulation of autoimmunity. On the one hand, it promotes inflammation associated with autoimmune diseases, and on the other hand, it mediates immune tolerance through promoting the development and stability of Treg cells and the deletion of self-reacting T cells in the thymus. Thus, efficient and properly controlled NF-κB signaling is important for mediating normal immune homeostasis and function and for preventing autoimmunity. Not surprisingly, the NF-κB signaling pathways are subject to negative regulation by multiple mechanisms. E3 ubiquitin ligases and DUBs form a major class of NF-κB regulators, which function to control the upstream signaling events or the fate of nuclear NF-κB. KO mouse models have proved to be valuable tools in assessing the physiological roles of E3s and DUBs in the regulation of NF-κB signaling and immune homeostasis. Equally important is the genome-wide association studies using human autoimmune patients. However, while future studies should strengthen these lines of physiological studies, in vitro work is also warranted for dissecting the biochemical mechanism by which specific E3s and DUBs regulate NF-κB signaling.
MiRNAs are emerging as another major class of NF-κB regulators. A hallmark of these miRNAs is that they are often induced by NF-κB or related signaling factors and, thus, involved in NF-κB feedback regulation or the crosstalk between NF-κB and related pathways. A major challenge in this area of research is to determine the target specificity and in vivo function of individual miRNAs. Since each miRNA usually has multiple targets, it is often hard to clearly dissect the relative contributions of NF-κB and other targets to the pathological phenotypes associated with ablation of a specific miRNA. Conditional miRNA KO mouse models, combined with genetic rescuing approaches, are useful tools for addressing this problem.
Compared to the canonical NF-κB pathway, relatively little is known regarding the regulation of the noncanonical NF-κB pathway. To date, much of our knowledge is on the steady-state regulation of NIK. Nevertheless, recent work has shed light onto the regulation of signal-induced noncanonical NF-κB activation. It is clear that NIK is a major target of regulation under both steady-state and signal-induced conditions. Since activation of noncanonical NF-κB critically involves ubiquitin-dependent degradation of TRAF3, regulation of TRAF3 ubiquitination may serve as another major mechanism that controls noncanonical NF-κB signaling. Better understanding of the mechanisms that underlie NF-κB regulation and the paradoxical roles of NF-κB in mediating immune activation and tolerance will be very important for developing therapeutic strategies.
Box 1. Canonical and noncanonical NF-κB pathways.
The canonical pathway of NF-κB activation centers on the activation of an IκB kinase (IKK) complex, composed of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit termed NF-κB-essential modulator (NEMO) or IKKγ [1]. Upon activation, IKK phosphorylates IκBα and triggers ubiquitin-dependent degradation of this major inhibitor of NF-κB and the nuclear translocation of canonical NF-κB members, predominantly the p50/RelA and p50/c-Rel dimers. IKK activation by different stimuli may involve different upstream factors, but a common signaling mechanism is conjugation of lysine (K)63-linked and linear ubiquitin chains that facilitate the assembly of signaling complexes and the catalytic activation of IKK and its activating kinase, Tak1 [80]. The noncanonical pathway of NF-κB activation is based on the inducible processing of the NF-κB2 precursor protein p100 [2]. A central signaling component of this pathway is the NF-κB-inducing kinase (NIK), which induces p100 phosphorylation via a downstream kinase, IKKα. The phosphorylation of p100 triggers its ubiquitin-dependent processing, leading to the production of the mature NF-κB2 protein p52 and nuclear translocation of p52/RelB heterodimer [2]. Of note, p100 also functions as an inhibitor of RelA [81], and the inducible processing or degradation of p100 contributes to the activation of RelA-containing NF-κB complexes [2, 82]. In contrast to the canonical NF-κB pathway, which responds to diverse stimuli, the noncanonical NF-κB pathway selectively responds to signals elicited by a subset of tumor necrosis factor receptors (TNFRs), including CD40, B-cell activating factor belonging to TNF family (BAFF) receptor (BAFFR), lymphotoxin beta receptor (LTβR), and receptor activator for nuclear factor κB (RANK) [2].
Box 2. Regulation of NF-κB signaling by CYLD and A20.
Ubiquitination is a central mechanism mediating NF-κB signaling. While the lysine (K)48-linked ubiquitin chains target proteins for degradation in the proteasome, such as degradation of IκBα and p100 processing, the K63-linked and linear ubiquitin chains mediate nondegradative processes, including the activation of IKK and its activating kinase Tak1 [80]. Like phosphorylation, ubiquitination is a reversible process with the reverse reaction being catalyzed by a subfamily of cysteine proteases, termed deubiquitinases (DUBs) [42]. Recent studies have led to the identification of specific DUBs with pivotal roles in the negative regulation of NF-κB signaling. CYLD is a K63-specific DUB originally found to be mutated in familial cylindromatosis, a genetic condition that predisposes patients for the development of benign tumors of skin appendages [44]. CYLD negatively regulates NF-κB activation by deubiquitinating a number of NF-κB signaling factors, including NEMO, TAK1, RIP1, TRAF2, TRAF6, and TRAF7 [44]. In particular, CYLD is crucial for preventing spontaneous activation of NF-κB in T cells [10]. The CYLD deficiency causes constitutive activation of NF-κB, as well as JNK, in both peripheral T cells and thymocytes [10, 83, 84]. The deregulated NF-κB activation may contribute to impaired development of T and natural killer T (NKT) cells and aberrant activation of peripheral T cells, the latter of which appears to contribute to the colonic inflammation [10, 83, 84].
A20 is a zinc-finger (ZnF) protein originally identified as a TNFα-induced factor and therefore also known as TNFα-induced protein 3 (TNFAIP3) [43]. A20 inhibits the activation of NF-κB by various innate immune receptors as well as antigen receptors [43]. A unique feature of A20 is its ability to mediate both deubiquitination and ubiquitination and, thereby, function as a ubiquitin-editing enzyme [43]. In the TNFR pathway, A20 negatively regulates NF-κB activation by removing K63-linked ubiquitin chains from RIP1 and then mediates K48 ubiquitination of RIP1, thereby both inhibiting RIP1-mediated IKK activation and triggering RIP1 degradation [43]. The E3 ligase function of A20 may also involve its association with other proteins within a so-called A20 ubiquitin-editing complex that is composed of the adaptor protein Tax1-binding protein 1 (TAX1BP1) and two E3 ubiquitin ligases, the ring finger protein 11 (RNF11) and ITCH [85]. The assembly of the A20 complex occurs in a signal-dependent manner, which is triggered through IKKα-mediated phosphorylation of TAX1BP1, a finding that assigns a negative role for IKKα in regulating the canonical NF-κB pathway [43]. At least in the IL-1R and TLR pathways, A20 suppresses the K63 ubiquitination of TRAF6 by disrupting its association with Ubc13 and UbcH5c and by mediating the degradation of these E2s [43].
Acknowledgements
Work performed in the author's laboratory is supported by National Institutes of Health Grants R01 AI064639, R01 AI057555, and R01 CA94922, the MD Anderson G.S. Hogan Gastrointestinal Cancer Research Fund and Sister Institution Network Fund, and a pilot grant from the MD Anderson Center for Inflammation and Cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun SC. The noncanonical NF-kappaB pathway. Immunol. Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theofilopoulos AN. The basis of autoimmunity: Part I. Mechanisms of aberrant self-recognition. Immunol. Today. 1995;16:90–98. doi: 10.1016/0167-5699(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 4.Pai S, Thomas R. Immune deficiency or hyperactivity-Nf-kappab illuminates autoimmunity. J. Autoimmun. 2008;31:245–251. doi: 10.1016/j.jaut.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz ML, Krappmann D. Controlling NF-kappaB activation in T cells by costimulatory receptors. Cell. Death Differ. 2006;13:834–842. doi: 10.1038/sj.cdd.4401845. [DOI] [PubMed] [Google Scholar]
- 6.Clavijo PE, Frauwirth KA. Anergic CD8+ T lymphocytes have impaired NF-kappaB activation with defects in p65 phosphorylation and acetylation. J. Immunol. 2012;188:1213–1221. doi: 10.4049/jimmunol.1100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang M, et al. The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat. Immunol. 2011;12:1002–1009. doi: 10.1038/ni.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theofilopoulos AN, et al. T cell homeostasis and systemic autoimmunity. J. Clin. Invest. 2001;108:335–340. doi: 10.1172/JCI12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng B, et al. Defective feedback regulation of NF-kappaB underlies Sjogren's syndrome in mice with mutated kappaB enhancers of the IkappaBalpha promoter. Proc. Natl. Acad. Sci. U S A. 2010;107:15193–15198. doi: 10.1073/pnas.1005533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiley WW, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J. Exp. Med. 2007;204:1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 12.Greve B, et al. I kappa B kinase 2/beta deficiency controls expansion of autoreactive T cells and suppresses experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:179–185. doi: 10.4049/jimmunol.179.1.179. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, et al. The NF-kappaB transcription factor c-Rel is required for Th17 effector cell development in experimental autoimmune encephalomyelitis. J. Immunol. 2011;187:4483–4491. doi: 10.4049/jimmunol.1101757. [DOI] [PubMed] [Google Scholar]
- 14.Ruan Q, et al. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J. Exp. Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray SE, et al. NF-kappaB-inducing kinase plays an essential T cell-intrinsic role in graft-versus-host disease and lethal autoimmunity in mice. J Clin Invest. 2011;121:4775–4786. doi: 10.1172/JCI44943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin WJ, et al. Crucial role for TNF receptor-associated factor 2 (TRAF2) in regulating NFkappaB2 signaling that contributes to autoimmunity. Proc. Natl. Acad. Sci. U S A. 2011;108:18354–18359. doi: 10.1073/pnas.1109427108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin W, et al. Regulation of Th17 cell differentiation and EAE induction by the MAP3K NIK. Blood. 2009;113:6603–6610. doi: 10.1182/blood-2008-12-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hacker H, et al. NIK prevents the development of hypereosinophilic syndrome-like disease in mice independent of IKKalpha activation. J. Immunol. 2012;188:4602–4610. doi: 10.4049/jimmunol.1200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann J, et al. NIK signaling in dendritic cells but not in T cells is required for the development of effector T cells and cell-mediated immune responses. J. Exp. Med. 2011;208:1917–1929. doi: 10.1084/jem.20110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer CT, et al. Layers of dendritic cell-mediated T cell tolerance, their regulation and the prevention of autoimmunity. Front. Immunol. 2012;3:183. doi: 10.3389/fimmu.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammer GE, et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat. Immunol. 2011;12:1184–1193. doi: 10.1038/ni.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kool M, et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity. 2011;35:82–96. doi: 10.1016/j.immuni.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Dissanayake D, et al. Nuclear factor-kappaB1 controls the functional maturation of dendritic cells and prevents the activation of autoreactive T cells. Nat. Med. 2011;17:1663–1667. doi: 10.1038/nm.2556. [DOI] [PubMed] [Google Scholar]
- 24.Shih VF, et al. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-kappaB pathways. Nat. Immunol. 2012;13:1162–1170. doi: 10.1038/ni.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu M, Fu Y. The complicated role of NF-kappaB in T-cell selection. Cell. Mol. Immunol. 2010;7:89–93. doi: 10.1038/cmi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakaguchi S, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Supprian M, et al. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc. Natl. Acad. Sci. U S A. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato S, et al. TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int. Immunol. 2006;18:1405–1411. doi: 10.1093/intimm/dxl082. [DOI] [PubMed] [Google Scholar]
- 30.Gupta S, et al. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol. Immunol. 2008;46:213–224. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes MJ, et al. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol. 2009;7:e51. doi: 10.1371/journal.pbio.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medoff BD, et al. Differential requirement for CARMA1 in agonist-selected T-cell development. Eur. J. Immunol. 2009;39:78–84. doi: 10.1002/eji.200838734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molinero LL, et al. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J. Immunol. 2009;182:6736–6743. doi: 10.4049/jimmunol.0900498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isomura I, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J. Exp. Med. 2009;206:3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long M, et al. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 36.Ruan Q, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hori S. Regulatory T cell plasticity: beyond the controversies. Trends Immunol. 2011;32:295–300. doi: 10.1016/j.it.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Ohkura N, et al. T cell receptor stimulation-induced epigenetic changes and foxp3 expression are independent and complementary events required for treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Chang JH, et al. Ubc13 maintains the suppressive function of regulatory T cells and prevents their conversion into effector-like T cells. Nat. Immunol. 2012;13:481–490. doi: 10.1038/ni.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto M, et al. Cutting Edge: Pivotal function of Ubc13 in thymocyte TCR signaling. J. Immunol. 2006;177:7520–7524. doi: 10.4049/jimmunol.177.11.7520. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi R, et al. SOCS1 is essential for regulatory T cell functions by preventing loss of Foxp3 expression as well as IFN-{gamma} and IL-17A production. J. Exp. Med. 2011;208:2055–2067. doi: 10.1084/jem.20110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun SC. Deubiquitylation and regulation of the immune response. Nat. Rev. Immunol. 2008;8:501–511. doi: 10.1038/nri2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harhaj EW, Dixit VM. Regulation of NF-kappaB by deubiquitinases. Immunol. Rev. 2012;246:107–124. doi: 10.1111/j.1600-065X.2012.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-κB activation. Cell Death Differ. 2010;17:25–34. doi: 10.1038/cdd.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J. Clin. Invest. 2006;116:3042–3049. doi: 10.1172/JCI28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cleynen I, et al. Genetic evidence supporting the association of protease and protease inhibitor genes with inflammatory bowel disease: a systematic review. PLoS One. 2011;6:e24106. doi: 10.1371/journal.pone.0024106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikolaou K, et al. Inactivation of the deubiquitinase CYLD in hepatocytes causes apoptosis, inflammation, fibrosis, and cancer. Cancer Cell. 2012;21:738–750. doi: 10.1016/j.ccr.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 48.Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 2012;12:774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tavares RM, et al. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity. 2010;33:181–191. doi: 10.1016/j.immuni.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu Y, et al. B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood. 2011;117:2227–2236. doi: 10.1182/blood-2010-09-306019. [DOI] [PubMed] [Google Scholar]
- 51.Hovelmeyer N, et al. A20 deficiency in B cells enhances B-cell proliferation and results in the development of autoantibodies. Eur. J. Immunol. 2011;41:595–601. doi: 10.1002/eji.201041313. [DOI] [PubMed] [Google Scholar]
- 52.Lippens S, et al. Keratinocyte-specific ablation of the NF-kappaB regulatory protein A20 (TNFAIP3) reveals a role in the control of epidermal homeostasis. Cell Death Differ. 2011;18:1845–1853. doi: 10.1038/cdd.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matmati M, et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat. Genet. 2011;43:908–912. doi: 10.1038/ng.874. [DOI] [PubMed] [Google Scholar]
- 54.Skaug B, et al. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol. Cell. 2011;44:559–571. doi: 10.1016/j.molcel.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tokunaga F, et al. Specific recognition of linear polyubiquitin by A20 zinc finger 7 is involved in NF-kappaB regulation. EMBO J. 2012;31:3856–3870. doi: 10.1038/emboj.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verhelst K, et al. A20 inhibits LUBAC-mediated NF-kappaB activation by binding linear polyubiquitin chains via its zinc finger 7. EMBO J. 2012;31:3845–3855. doi: 10.1038/emboj.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verstrepen L, et al. ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling. Biochem. Pharmacol. 2009;78:105–114. doi: 10.1016/j.bcp.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Rahighi S, et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell. 2009;136:1098–1109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Ivins FJ, et al. NEMO oligomerization and its ubiquitin-binding properties. Biochem. J. 2009;421:243–251. doi: 10.1042/BJ20090427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lo YC, et al. Structural basis for recognition of diubiquitins by NEMO. Mol. Cell. 2009;33:602–615. doi: 10.1016/j.molcel.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nanda SK, et al. Polyubiquitin binding to ABIN1 is required to prevent autoimmunity. J. Exp. Med. 2011;208:1215–1228. doi: 10.1084/jem.20102177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oshima S, et al. ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development. Nature. 2009;457:906–909. doi: 10.1038/nature07575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou J, et al. A20-binding inhibitor of NF-kappaB (ABIN1) controls Toll-like receptor-mediated CCAAT/enhancer-binding protein beta activation and protects from inflammatory disease. Proc. Natl. Acad. Sci. U S A. 2011;108:E998–1006. doi: 10.1073/pnas.1106232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Natoli G, Chiocca S. Nuclear ubiquitin ligases, NF-kappaB degradation, and the control of inflammation. Sci. Sigal. 2008;1(1):pe1. doi: 10.1126/stke.11pe1. [DOI] [PubMed] [Google Scholar]
- 65.Yoshimura A, et al. SOCS, Inflammation, and Autoimmunity. Front. Immunol. 2012;3:20. doi: 10.3389/fimmu.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka T, et al. PDLIM2 inhibits T helper 17 cell development and granulomatous inflammation through degradation of STAT3. Sci. Signal. 2011;4:ra85. doi: 10.1126/scisignal.2001637. [DOI] [PubMed] [Google Scholar]
- 67.Qu Z, et al. PDLIM2 restricts Th1 and Th17 differentiation and prevents autoimmune disease. Cell Biosci. 2012;2:23. doi: 10.1186/2045-3701-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin W, et al. Peli: a family of signal-responsive E3 ubiquitin ligases mediating TLR signaling and T-cell tolerance. Cell. Mol. Immunol. 2012;9:113–122. doi: 10.1038/cmi.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boldin MP, Baltimore D. MicroRNAs, new effectors and regulators of NF-kappaB. Immunol. Rev. 2012;246:205–220. doi: 10.1111/j.1600-065X.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 70.Boldin MP, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu LF, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142:914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang L, et al. miR-146a controls the resolution of T cell responses in mice. J. Exp. Med. 2012;209:1655–1670. doi: 10.1084/jem.20112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Leon-Boenig G, et al. The Crystal Structure of the Catalytic Domain of the NF-kappaB Inducing Kinase Reveals a Narrow but Flexible Active Site. Structure. 2012;20:1704–1714. doi: 10.1016/j.str.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, et al. Structure of the nuclear factor kappaB-inducing kinase (NIK) kinase domain reveals a constitutively active conformation. J. Biol. Chem. 2012;287:27326–27334. doi: 10.1074/jbc.M112.366658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Razani B, et al. Negative feedback in non-canonical NF-κB signaling modulates NIK stability through IKKα-mediated phosphorylation. Sci. Sig. 2010;3(123):ra41. doi: 10.1126/scisignal.2000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin J, et al. The kinase TBK1 controls IgA class switching by negatively regulating noncanonical NF-kappaB signaling. Nat. Immunol. 2012;13:1101–1109. doi: 10.1038/ni.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niederberger E, et al. The non-canonical IkappaB kinases IKKepsilon and TBK1 as potential targets for the development of novel therapeutic drugs. Curr. Mol. Med. 2012 doi: 10.2174/1566524011313070004. [DOI] [PubMed] [Google Scholar]
- 78.Bonnard M, et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-κB-dependent gene transcription. EMBO J. 2000;19:4976–4985. doi: 10.1093/emboj/19.18.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanno Y, et al. TACI induces cIAP1-mediated ubiquitination of NIK by TRAF2 and TANK to limit non-canonical NF-kappaB signaling. J. Recept. Signal. Transduct. Res. 2010;30:121–132. doi: 10.3109/10799891003634509. [DOI] [PubMed] [Google Scholar]
- 80.Chen ZJ. Ubiquitination in signaling to and activation of IKK. Immunol. Rev. 2012;246:95–106. doi: 10.1111/j.1600-065X.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun S-C, et al. Autoregulation of the NF-κB transactivator Rel A (p65) by multiple cytoplasmic inhibitors containing ankyrin motifs. Proc. Natl. Acad. Sci. USA. 1994;91:1346–1350. doi: 10.1073/pnas.91.4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Basak S, et al. A fourth IkappaB protein within the NF-kappaB signaling module. Cell. 2007;128:369–381. doi: 10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee AJ, et al. Regulation of natural killer T-cell development by deubiquitinase CYLD. EMBO J. 2010;29:1600–1612. doi: 10.1038/emboj.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsagaratou A, et al. Thymocyte-specific truncation of the deubiquitinating domain of CYLD impairs positive selection in a NF-kappaB essential modulator-dependent manner. J Immunol. 2010;185:2032–2043. doi: 10.4049/jimmunol.0903919. [DOI] [PubMed] [Google Scholar]
- 85.Shembade N, Harhaj EW. Regulation of NF-kappaB signaling by the A20 deubiquitinase. Cell. Mol. Immunol. 2012;9:123–130. doi: 10.1038/cmi.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]