Abstract
Objective
High circulating concentrations of parathyroid hormone (PTH) have been associated with increased risks of hypertension, left ventricular hypertrophy, congestive heart failure, and cardiovascular mortality. Impaired arterial function is a potential mechanism for these associations. We tested whether serum PTH concentration is associated with measures of arterial function.
Design
Cross-sectional study.
Participants
6,545 persons without clinical cardiovascular disease participating in the community-based Multi-Ethnic Study of Atherosclerosis.
Measurements
Brachial artery flow-mediated dilation as well as aortic pulse pressure and arterial pulse parameters derived from Windkessel modeling of the radial pressure waveform.
Results
Higher serum PTH concentration was associated with lower brachial artery flow-mediated dilation (mean difference −0.09% per 10 pg/mL PTH), higher aortic pulse pressure (0.53 mmHg per 10 pg/mL), and reduced Windkessel capacitive index C1 (large artery elasticity, −0.12 ml/mmHg X 10 per 10 pg/mL), adjusting for potential confounding variables (all p-values ≤ 0.001). These relationships were independent of serum calcium concentration, serum 25-hydroxyvitamin D concentration, and estimated glomerular filtration rate and were consistent across relevant participant subgroups. Associations of PTH with aortic pulse pressure and capacitive index C1 were attenuated after adjustment for blood pressure. Serum PTH concentration was not associated with the oscillatory index C2 (small artery elasticity).
Conclusions
Higher serum PTH concentration was associated with impaired endothelial function, increased aortic pulse pressure, and decreased capacitive index C1 in a large, diverse, community-based population. These relationships may help explain previously observed associations of elevated PTH with cardiovascular disease.
Keywords: parathyroid hormone, calcium, vitamin D, arterial function, epidemiology
Introduction
High circulating concentrations of parathyroid hormone (PTH) have been associated with hypertension and blood pressure in the National Health and Nutrition Examination Survey (NHANES) and other studies.(1–3) Elevated PTH has further been associated with higher left ventricular mass in primary hyperparathyroidism(4, 5) and with congestive heart failure and cardiovascular mortality in people without known parathyroid disease.(1, 6–10) Mechanisms explaining these associations are not known, but one possibility is that PTH adversely affects arterial function. The PTH receptor has been found in both vascular smooth muscle and endothelial cells,(11, 12) and primary hyperparathyroidism has been associated with impaired brachial artery flow-mediated dilation (FMD) and higher aortic pressure augmentation.(13–15) Prior studies of PTH and arterial function, however, have focused on primary hyperparathyroidism, rather than the broad range of PTH concentrations occurring without a specific disorder, and have not accounted for important potential confounding variables, such as kidney function and 25-hydroxyvitamin D (25(OH)D). In addition, PTH and other biomarkers of mineral metabolism vary substantially by race,(6) and prior studies have been limited largely to Caucasian populations.
We tested associations of PTH with arterial function in the Multi-Ethnic Study of Atherosclerosis (MESA), a racially and ethnically diverse cohort of persons without known cardiovascular disease. Arterial function was assessed as brachial artery FMD as well as aortic pulse pressure and arterial capacitive and oscillatory indices derived from Windkessel modeling of the radial pressure waveform. We hypothesized that higher serum concentrations of PTH would be associated with impaired FMD, higher aortic pulse pressure, and reduced capacitive and oscillatory arterial indices.
Methods
Participants
MESA is a community-based study of subclinical cardiovascular disease. MESA enrolled 6,814 men and women ages 45–84 years without known prevalent clinical cardiovascular disease, defined as myocardial infarction, angina, stroke, transient ischemic attack, heart failure, atrial fibrillation, use of nitroglycerin, prior angioplasty, coronary artery bypass grafting, valve replacement, pacemaker or defibrillator implantation, or any surgery on the heart or arteries. Study recruitment took place from 2000 – 2002 at six study sites (Baltimore, MD; St. Paul, MN; Chicago, IL; Forsyth County, NC; New York, NY; and Los Angeles, CA).(16) Institutional review boards at each site approved the study, and all participants granted written informed consent to participate.
Of the 6,814 MESA participants, we measured serum PTH concentration at baseline for 6,555 (96%) with available stored serum. For this study, we excluded participants with estimated glomerular filtration rate (eGFR) < 15 ml/min/1.73m2 (n = 3), PTH < 1.0 pg/ml (n = 1), or 25(OH)D > 100 ng/ml (suggesting high-dose vitamin D supplementation, n = 6). Of the 6,545 remaining participants, 6,094 (93%) had capacitive and oscillatory index measurements, and 6,053 (92%) had estimates of aortic pulse pressure. FMD was originally measured in 6,489 MESA participants but were reread due to quality concerns. Rereading with excellent quality was performed in a subset, selected using a case-cohort design.(17)
PTH
Serum samples were obtained in the morning after an overnight fast. Samples were stored at −80°C from the baseline study visit (in 2000–2002) until they were thawed for PTH measurement in 2011. Modest reductions in PTH concentration have been reported with long-term storage.(18) Intact PTH concentration was measured at the University of Washington in using the Beckman-Coulter DxI automated two-site immunoassay (Beckman-Coulter, Inc., Brea, CA). Between-lot variations in PTH results were adjusted using repeated measurements of aliquoted serum samples from 20 normal controls. After adjustment, inter-assay coefficient of variation was 6.1% at 30.1 pg/ml and 3.4% at 94.5 pg/ml.
Measurement of flow-mediated dilation
FMD was measured with high-resolution B-mode ultrasonography of the right brachial artery diameter. Ischemia was achieved by inflating a blood pressure cuff on the right forearm 2 inches distal to the antecubital fossa to 50mmHg above systolic blood pressure or 200mmHg, whichever was higher, for 5 minutes. Radial artery diameter was measured at baseline and 60 seconds after cuff deflation.(16, 17, 19) FMD was calculated as the difference between brachial artery diameter before ischemia-induced dilation and brachial artery diameter after ischemia-induced dilation, divided by the pre-ischemia baseline diameter, and then multiplied by 100%. Intraclass correlation coefficients were 0.99, 0.99, and 0.93 for baseline diameter, maximum diameter, and percent FMD intrareader reproducibility, respectively, and 0.90, 0.90, and 0.54 for intrasubject variability, respectively.(17)
Windkessel modeling of the radial pressure waveform
Radial artery pulse wave analysis with the PulseWave CR-2000 Research CardioVascular Profiling Instrument (Hypertension Diagnostics, Inc., Eagan, Minnesota) was used to obtain blood pressure measurements with an oscillometric blood pressure module and radial artery pulse wave forms with a radial artery pulse pressure sensor. With the patient supine, the pulse pressure sensor was placed on the supported wrist of the dominant arm and a blood pressure cuff was placed on the contralateral arm over the brachial artery. Measurements were taken for 30 seconds.
Arterial capacitive (C1) and oscillatory (C2) indices were obtained using a third-order, 6-element Windkessel model that derives from the diastolic pulse contour.(20) The pressure at the radial artery at time (t) after the beginning of diastole is modeled as a decaying exponential function plus a sinusoidal function dampened by a decaying exponential. SVR is calculated as mean arterial pressure divided by cardiac output. Cardiac output (CO) is estimated using heart rate (HR), ejection time (ET), and body surface area (BSA). Estimates combining information from the waveform and SVR variables were used to determine C1 and C2. The terms large artery elasticity (LAE) and small artery elasticity (SAE) are used to describe C1 and C2, respectively as recommended by Hypertension Diagnostics, Inc and as in prior studies.(19–21) Intrasubject correlation was 0.74 for LAE and 0.84 for SAE.(20)
Aortic pulse pressure estimation
Radial artery waveform 30-second recordings from the PulseWave CR-2000 Research CardioVascular Profiling Instrument were digitized at 200Hz and exported for offline-processing using custom-designed software written in Matlab (The Mathworks; Natick, MA). The radial pressure waveform was calibrated with brachial systolic and diastolic pressures, assuming no brachial-to-radial amplification. A generalized transfer function was applied to the radial pressure waveform to obtain a central pressure waveform.(22) Aortic pulse pressure was computed as aortic systolic minus aortic diastolic pressure.
Covariate data
Age, race/ethnicity, and gender were self-identified. Height and weight were measured with participants wearing light clothing.(20) Body mass index (BMI) was defined as weight/height2 (kg/m2). Brachial artery blood pressure was measured in the seated position 3 times with a Dinamap Pro100 (Critikon, Tampa, Florida), and the last two measurements were averaged. Medications were determined by questionnaire and review of pill bottles. Diabetes was defined as fasting blood glucose ≥ 126 mg/dL or use of a glucose-lowering medication. Hypertension was defined as systolic blood pressure ≥ 140mmHg, diastolic blood pressure ≥ 90mmHg, or use of antihypertensive medication. Smoking was categorized as never smoker, former smoker, or current smoker.
Serum calcium was measured by indirect ion selective electrode. Serum phosphorus was measured using a timed-rate calorimetry reaction. Serum 25(OH)D was measured by mass spectrometry using previously described methods.(6) Measurement of cystatin C was by a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Siemens AG, Munich). Estimated glomerular filtration rate (eGFR) was calculated using cystatin C in the formula: eGFRcystatin = 76.7 * (cystatin C)−1.19.(23) We estimated GFR from serum cystatin C instead of serum creatinine because this may increase precision, particularly in the normal or near-normal range of GFR.(24) Plasma triglycerides, high-density lipoprotein cholesterol, and total cholesterol were measured using CDC-standardized methods. Low-density lipoprotein (LDL) cholesterol was estimated using the Friedwald equation.(25)
Statistical Analysis
We examined unadjusted associations of PTH with FMD, aortic pulse pressure, LAE, and SAE using Spearman rank correlation coefficients, scatter plots, and locally weighted scatterplot smoothing (LOWESS). We used multivariable linear regression to test associations of PTH with each arterial outcome. Serum PTH concentration was evaluated in categories and as a continuous variable. We applied a threshold of 65 pg/ml to define elevated PTH because this is the upper limit of normal for our assay and because previous studies observed increased risk of adverse cardiovascular disease outcomes above this concentration.(6, 7, 26) PTH concentrations < 65 pg/mL were divided into tertiles to examine associations within the normal range. In secondary analyses, participants with PTH ≥ 65 pg/ml were subdivided by serum calcium concentration, dichotomized as high (≥ 10.2 mg/dL) or normal (< 10.2 mg/dL) based on the upper limit of normal for the assay and suggesting primary or secondary hyperparathyroidism, respectively. For analyses of PTH as a continuous variable only, participants with PTH > 160 pg/mL were excluded to avoid excessive influence of outliers (n=13).
Our basic model included adjustment for age (categorical), gender, race, and MESA site. Our fully adjusted model additionally adjusted for diabetes, smoking, BMI, LDL-cholesterol (continuous), use of lipid lowering medications, eGFR (continuous), and 25(OH)D (continuous). Missing values for eGFR (n=35) and 25(OH)D (n=611) were imputed using multiple imputation by chained equations. Imputations were based on all covariates included in the models and the outcome measures. Ten imputed data sets were made and then results were combined using Rubin’s rules.(27, 28) We used inverse probability weighting to account for the limited sampling of FMD.
We additionally adjusted for blood pressure in sensitivity analyses. For FMD, we adjusted for systolic blood pressure (SBP) and use of anti-hypertensive medications.(17) For LAE and SAE, we adjusted for mean arterial pressure, heart rate, height, and weight because these are in the formula used to calculate these outcomes. For aortic pulse pressure, we adjusted for mean arterial pressure because pulse pressure is influence by arterial compliance, and arterial compliance is influenced by the underlying distending pressure.
We performed relevant subgroup analyses of the associations of PTH with FMD and aortic pulse pressure using our fully adjusted model. Interactions were tested using the Wald test. In parallel analyses, we also tested associations of serum calcium concentration and 25(OH)D concentration with the four arterial outcomes. Analyses of 25(OH)D included only participants with measured (not imputed) 25(OH)D concentration.
Analyses were performed using Stata version 11.1 (College Station, TX) and R 2.12.1 (R Foundation, Vienna, Austria). All p-values are two-sided, and values below 0.05 were considered statistically significant.
Results
The 6,545 participants included in this study had a mean age of 62 years and 47% were men (Table 1). By design, the cohort was racially diverse (39% white, 12% Chinese, 27% black, and 22% Hispanic). There were 12% of participants with diabetes, and 44% had hypertension. Median PTH was 40.5 pg/ml (interquartile range 31.1–53.2 pg/ml). Participants with higher circulating PTH were more likely to be older, female, and black or Hispanic. Those with higher PTH were also more likely to have higher systolic and diastolic blood pressure and body mass index and lower eGFR and serum 25(OH)D concentration.
Table 1.
Characteristics of 6,545 participants in the Multi-Ethnic Study of Atherosclerosis by quintiles of PTH
| Serum PTH Category (pg/ml)
|
||||
|---|---|---|---|---|
| < 32.8 | 32.9 – 44.2 | 44.3 – 65 | >65 | |
| N | 1926 | 1928 | 1917 | 774 |
| Age, years | 60.9 ±10.2 | 61.6 ±10.4 | 63 ±10.1 | 64.3 ±10.1 |
| Male | ||||
| Race | 937 (49%) | 923 (48%) | 887 (46%) | 314 (41%) |
| White | 922 (48%) | 780 (40%) | 653 (34%) | 188 (24%) |
| Chinese | 318 (17%) | 254 (13%) | 182 (9%) | 38 (5%) |
| Black | 327 (17%) | 481 (25%) | 640 (33%) | 333 (43%) |
| Hispanic | 359 (19%) | 413 (21%) | 442 (23%) | 215 (28%) |
| Diabetes | 258 (13%) | 206 (11%) | 230 (12%) | 114 (15%) |
| Hypertension | 688 (36%) | 783 (41%) | 953 (50%) | 478 (62%) |
| Anti-hypertensive drug | 520 (27%) | 572 (30%) | 698 (36%) | 357 (46%) |
| Lipid lowering drug | 304 (16%) | 286 (15%) | 316 (17%) | 151 (20%) |
| Smoking | ||||
| Never | 927 (48%) | 980 (51%) | 974 (51%) | 417 (54%) |
| Former | 705 (37%) | 683 (36%) | 721 (38%) | 273 (36%) |
| Current | 289 (15%) | 258 (13%) | 220 (11%) | 79 (10%) |
| Systolic blood pressure, mmHg | 121.9 ±20.3 | 124.8 ±20.6 | 129.1 ±21.4 | 135 ±23.4 |
| Diastolic blood pressure, mmHg | 70.4 ±9.8 | 71.4 ±10.1 | 72.7 ±10.2 | 74.2 ±11.4 |
| Body mass index, kg/m2 | 26.7 ±4.6 | 28.1 ±5.2 | 29.1 ±5.6 | 30.7 ±6.4 |
| LDL, mg/dL | 116.9 ±30.6 | 117.5 ±31.9 | 117.9 ±30.9 | 114.5 ±33.9 |
| eGFR, ml/min/1.73m2 | 78.6 ±15 | 78.9 ±15.7 | 78.6 ±16.1 | 74.5 ±19.6 |
| Calcium, mg/dL | 9.7 ±0.4 | 9.7 ±0.4 | 9.6 ±0.4 | 9.6 ±0.5 |
| 25(OH)D, ng/ml | 29.9 ±10.7 | 26.1 ±10.4 | 22.9 ±10.2 | 18.8 ±9.9 |
Values expressed as N (%) or mean ± standard deviation. LDL, low density lipoprotein cholesterol, eGFR, estimated glomerular filtration rate, 25(OH)D, 25-hydroxyvitamin D.
Mean (SD) values were 4.4 (2.8) percent for FMD, 13.3 (5.6) ml/mmHgX10 for LAE, 4.5 (2.8) ml/mmHgX10for SAE, and 53.1 (14.8) mmHg for aortic pulse pressure. LAE and SAE were moderately correlated (R = 0.50), aortic pulse pressure was negatively correlated with LAE (R = −0.59) and SAE (R = −0.48), and FMD was weakly correlated with LAE (R = 0.08), SAE (R = 0.11), and aortic pulse pressure (R = −0.15) (Supplementary Table 1). Each arterial function measurement was correlated with age: Pearson r = −0.31 for FMD, −0.36 for LAE, −0.42 for SAE, and 0.46 for aortic pulse pressure.
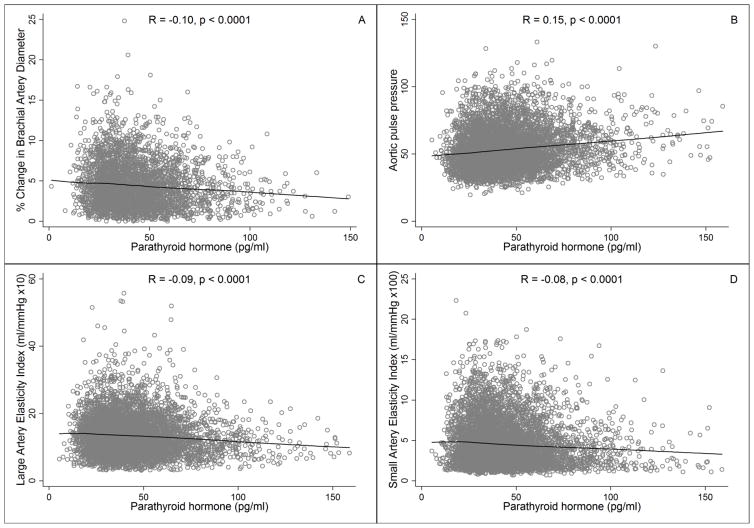
In unadjusted analyses, serum PTH concentration was negatively correlated with FMD (R = −0.10, p < 0.0001), LAE (R = −0.09, p < 0.0001), and SAE (R = −0.08, p < 0.0001) and was positively correlated with aortic pulse pressure (R = 0.15, p < 0.0001). Mean values of FMD, LAE, and SAE decreased and mean values of aortic pulse pressure increased steadily across the full range of PTH in an apparently linear manner (Figure 1, Table 2).
Figure 1.
Associations of PTH concentration with (A) Brachial artery flow mediated dilation, (B) Aortic pulse pressure, (C) Large artery elasticity, and (D) Small artery elasticity. LOWESS smoothed curves on scatter plots. R values are Spearmen correlation coefficients. Analyses included 3,341 participants for flow mediated dilation, 6,042 participants for aortic pulse pressure, and 6,083 participants for large and small artery elasticities.
Table 2.
Association of parathyroid hormone (quintiles and per 10 pg/ml increment) with measures of arterial function.
| Model | Serum PTH Category (pg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| < 32.8 | 32.9 – 44.2 | 44.3 – 65 | > 65 | Continuous PTH, per 10 pg/ml | |||
|
| |||||||
| Beta | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | p | ||
|
| |||||||
| FMD, percent change (N = 3341) | 1 | 0 (Reference) | 0.05(−0.18, 0.29) | −0.17(−0.41, 0.06) | −0.32(−0.64, 0.01) | −0.07 (−0.12, −0.03) | 0.003 |
| 2 | 0 (Reference) | −0.01(−0.25, 0.23) | −0.26(−0.51, −0.02) | −0.39(−0.73, −0.04) | −0.09 (−0.14, −0.04) | 0.001 | |
|
| |||||||
| Aortic PP, mmHg (N = 6042) | 1 | 0 (Reference) | 0.74(−0.08, 1.57) | 1.44(0.61, 2.27) | 3.27(2.16, 4.39) | 0.59 (0.41, 0.77) | <0.001 |
| 2 | 0 (Reference) | 0.68(−0.15, 1.52) | 1.27(0.4, 2.13) | 2.81(1.63, 3.98) | 0.53 (0.34, 0.73) | <0.001 | |
|
| |||||||
| LAE, mL/mmHg X 10 (N = 6083) | 1 | 0 (Reference) | 0.06(−0.26, 0.39) | −0.03(−0.36, 0.29) | −0.49(−0.92, −0.05) | −0.10 (−0.16, −0.03) | 0.003 |
| 2 | 0 (Reference) | 0.02(−0.31, 0.35) | −0.13(−0.47, 0.21) | −0.63(−1.1, −0.17) | −0.12 (−0.19, −0.06) | <0.001 | |
|
| |||||||
| SAE, mL/mmHg X 100 (N = 6083) | 1 | 0 (Reference) | −0.07(−0.23, 0.09) | −0.01(−0.17, 0.15) | −0.06(−0.27, 0.16) | −0.002 (−0.03, 0.03) | 0.912 |
| 2 | 0 (Reference) | −0.12(−0.28, 0.04) | −0.12(−0.28, 0.05) | −0.24(−0.46, −0.01) | −0.03 (−0.06, 0.01) | 0.118 | |
Model 1: Adjusted for age, gender, race, and study site.
Model 2: Adjusted for age, gender, race, study site, diabetes, smoking, BMI, LDL, lipid lowering medications, eGFR, and 25(OH)D.
Beta and 95% confidence interval are differences in mean measure of arterial function (units unique to each outcome) compared to lowest category or per 10 pg/ml increment of parathyroid hormone. PTH, parathyroid hormone, CI, confidence interval, FMD, flow-mediated dilation, Aortic PP, aortic pulse pressure, LAE, large artery elasticity, SAE, small artery elasticity. P-values are for continuous parathyroid hormone. Mean ± standard deviation of each arterial outcome: FMD (4.40 ± 2.81), Aortic PP (53.10 ± 14.83), LAE (13.34 ± 5.60), SAE (4.49 ± 2.83).
In adjusted linear regression models, serum PTH concentration was significantly associated with brachial FMD, aortic pulse pressure, and LAE, whereas no association was observed with SAE (Table 2). On average, each additional 10 pg/ml of PTH was associated with a 0.09 percentage point decrement in FMD, a 0.53 mmHg higher aortic pulse pressure, and a 0.12 ml/mmHgX10 lower LAE (each p ≤ 0.001). Additional adjustment for serum calcium concentration did not significantly alter the associations, with mean differences nearly unchanged (−0.09 percentage points for FMD, 0.52 mmHg for aortic pulse pressure, and −0.12 ml/mmHgX10 for LAE, each p ≤ 0.001). Brachial artery pulse pressure was highly correlated with aortic pulse pressure (R = 0.72, p < 0.0001, Supplementary Table 1), and the association of PTH with brachial pulse pressure was similar to that of PTH with aortic pulse pressure (Supplementary Table 2). Adjusting for SBP and anti-hypertensive medications, each 10 pg/ml increment in PTH was associated with a −0.07 percentage point lower FMD (p = 0.006). Adjusting for mean arterial pressure, PTH was not significantly associated with aortic pulse pressure (mean difference −0.09 mmHg, p = 0.25). Adjusting for height, weight, heart rate, and mean arterial pressure, PTH was not significantly associated with LAE (mean difference −0.04 ml/mmHgX10, p = 0.15).
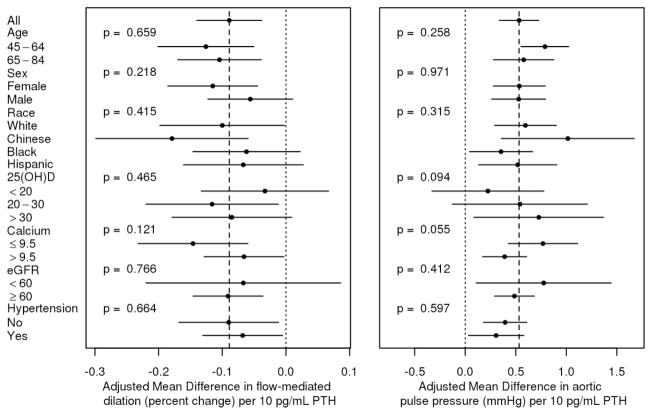
Associations of PTH concentration with FMD, aortic pulse pressure, and LAE were consistent across subgroups defined by age, gender, race, 25(OH)D concentration, calcium concentration, eGFR, and hypertension (Figure 2). Serum PTH concentration ≥ 65 pg/ml, evaluated as a categorical exposure and compared with PTH < 65 pg/ml, was associated with lower FMD, lower LAE, and higher aortic pulse pressure (p<0.05 for LAE and aortic pulse pressure only). These associations were observed whether the serum calcium concentration was above or below 10.2 mg/dL, although beta coefficients were further from zero in those with serum calcium ≥ 10.2 mg/dL (Table 3).
Figure 2.
Stratified analysis of the association of serum parathyroid hormone concentration (PTH) with (A) Brachial artery flow-mediated dilation (FMD) and (B) Aortic pulse pressure. Point estimates are change in mean FMD or mean aortic pulse pressure per 10 pg/ml increment in serum parathyroid hormone concentration. For FMD, left favors stronger association with PTH, and right favors weaker association with PTH. For aortic pulse pressure, left favors weaker association with PTH, and right favors stronger association with PTH. P-values are for interaction of PTH with each subgroup. Analyses included 3,341 participants for flow mediated dilation and 6,042 participants for aortic pulse pressure.
Table 3.
Association of parathyroid hormone and calcium with measures of arterial function
| Mean ± SD | Model | PTH < 65 pg/ml | PTH ≥ 65 pg/ml
|
||
|---|---|---|---|---|---|
| Calcium < 10.2 mg/dL | Calcium ≥ 10.2 mg/dL | ||||
|
| |||||
| Beta | Beta (95% CI) | Beta (95% CI) | |||
|
| |||||
| FMD, percent change (N = 3341) | 4.40 ± 2.81 | 1 | 0 (Reference) | −0.28 (−0.57, 0.02) | −0.52 (−1.18, 0.15) |
| 2 | 0 (Reference) | −0.28 (−0.59, 0.03) | −0.54 (−1.22, 0.13) | ||
|
| |||||
| Aortic PP, mmHg (N = 6042) | 53.11 ± 14.84 | 1 | 0 (Reference) | 2.34 (1.24, 3.45)* | 3.77 (0.79, 6.76)* |
| 2 | 0 (Reference) | 1.82 (0.68, 2.95)* | 3.37 (0.28, 6.46)* | ||
|
| |||||
| LAE, ml/mmHg X 10 (N = 6083) | 13.34 ± 5.61 | 1 | 0 (Reference) | −0.46 (−0.84, −0.07)* | −1.03 (−2.07, 0.01) |
| 2 | 0 (Reference) | −0.50 (−0.90, −0.11)* | −1.14 (−2.20, −0.07)* | ||
|
| |||||
| SAE, ml/mmHg X 100 (N = 6083) | 4.49 ± 2.83 | 1 | 0 (Reference) | −0.02 (−0.22, 0.17) | −0.21 (−0.72, 0.3) |
| 2 | 0 (Reference) | −0.11 (−0.30, 0.08) | −0.34 (−0.86, 0.18) | ||
Model 1: Adjusted for age, gender, race, and study site.
Model 2: Adjusted for age, gender, race, study site, diabetes, smoking, BMI, LDL, lipid lower medications, eGFR, and 25(OH)D.
Beta and 95% confidence interval are differences in mean measure of arterial function (units unique to each outcome) comparing high parathyroid hormone (> 65 pg/ml) categories to normal parathyroid hormone (< 65 pg/ml) category. PTH, parathyroid hormone, SD, standard deviation, CI, confidence interval, FMD, flow-mediated dilation, Aortic PP, aortic pulse pressure, LAE, large artery elasticity, SAE, small artery elasticity.
p < 0.05
Serum calcium concentration was not associated with any measure of arterial function. (Supplementary Table 3) Lower serum 25(OH)D concentration was associated with a modestly lower SAE but not with FMD, aortic pulse pressure, or LAE (Supplementary Table 4).
Discussion
Higher serum PTH concentration was associated with arterial dysfunction as measured by reduced FMD, higher aortic pulse pressure, and lower LAE in a large, generally healthy, and racially diverse cohort of adults. These associations were modest in magnitude, and associations of PTH with aortic pulse pressure and LAE were attenuated after adjustment for mean arterial pressure. Importantly, however, observed associations were independent of potential confounders, including age, race, gender, kidney function, 25(OH)D, and serum calcium; and they were consistent across a range of PTH concentrations, among those with laboratory values suggestive of either primary or secondary hyperparathyroidism, and among subgroups defined by age, gender, race/ethnicity, 25(OH)D concentration, calcium concentration, eGFR, and hypertension. Our findings suggest that PTH may be one of many complex factors affecting arterial function, particularly the function of medium- and large-sized arteries, and that arterial dysfunction may partially explain associations of elevated serum PTH concentration with hypertension, left ventricular mass, congestive heart failure, and cardiovascular mortality.
Brachial artery FMD is generally accepted as a measure of endothelial function.(29) Therefore, our results demonstrate a modest but potentially important association of elevated serum PTH concentration with impaired endothelial function. A mechanism by which PTH could impair endothelial function has not been elucidated, but the PTH receptor has been found on endothelial cells in animals,(11) suggesting a potential direct action of PTH on the endothelium. Work with cell culture and animals suggests that PTH stimulates, rather than inhibits, the endothelium and causes vasodilation acutely.(30, 31) Clinical epidemiologic evidence, however, supports a role for PTH impairing endothelial function. FMD is impaired in persons with primary hyperparathyroidism, and it improves after parathyroidectomy.(13, 14, 32) In dialysis patients, secondary hyperparathyroidism is also associated with lower FMD.(33) Infusion of PTH has shown variable effects, lowering mean arterial pressure in a group of hypertensive men and raising mean arterial pressure in a group of normotensive men, both during 2-hour infusions.(34, 35) Another study found that a 12-day infusion of PTH raised mean arterial pressure in normal subjects.(36) Clinically, factors affecting endothelial dysfunction may be important because decreased FMD is associated with greater incident cardiovascular events and higher left ventricular mass.(17)
While FMD is widely considered to represent nitric oxide-mediated endothelial function, the physiologic interpretation of parameters derived from radial artery pulse wave analysis (aortic pulse pressure, LAE, and SAE) is more complex. Aortic pulse pressure is a measure of the aorta’s ability to dampen pulsatile flow from the left ventricle. Higher aortic pulse pressure represents an impairment of this function. LAE (C1) is related to total arterial compliance, which is influenced predominantly by the large arteries. However, the precise physiologic interpretation of LAE is not defined. Nonetheless, LAE appears to be clinically meaningful as it has been found to associate with incident hypertension, kidney disease, and heart failure.(19–21) In our study, higher circulating PTH concentration was associated with increased aortic pulse pressure and decreased LAE, suggesting impaired large artery function. However, these relationships were attenuated after adjustment for mean arterial pressure, suggesting that blood pressure may mediate this association, or that the previously demonstrated association of PTH with hypertension may be mediated by alterations in arterial function. The latter is consistent with the finding of an association between LAE and incident hypertension in MESA.(21)
Elevated PTH concentration has previously been shown to associate with aortic augmentation index in a small cohort with primary hyperparathyroidism,(15) but to our knowledge, this is the first assessment of an association between PTH concentration and aortic pulse pressure or LAE/C1 in a large community-based population. Similar to our findings, among 2,229 elderly community-dwelling participants of the Health Aging and Body Composition (Health ABC) study, plasma PTH concentration was associated with a trend toward increased arterial pulse wave velocity (a measure of arterial stiffness) before adjustment for blood pressure, but this association was not statistically significant. In comparison to the MESA cohort, the Health ABC cohort was smaller in sample size, older, less racially diverse, and included people with prevalent cardiovascular disease.(37)
Our study has several strengths. It is the first, large community-based study to our knowledge to examine the association of PTH concentration with multiple measures of arterial function. The MESA cohort is racially and ethnically diverse and the absence of prevalent clinical cardiovascular disease combined with adjustment for well-characterized cardiovascular disease risk factors minimizes potential confounding. Our study also has important limitations. First, it is cross-sectional, so causality cannot be inferred from our results. Second, only total serum calcium was measured rather than ionized calcium, and serum albumin was not available for adjustment. Third, our measurements of arterial pressures were all non-invasive rather than directly measured. Finally, the degree to which the observed associations of PTH with impaired arterial function translate to differences in rates of clinical events remains to be determined.
In conclusion, higher serum PTH concentration was associated with decreased brachial FMD, increased aortic pulse pressure, and decreased LAE in a large, racially diverse population. Our findings suggest that previously observed associations of elevated PTH with hypertension, left ventricular mass, congestive heart failure, and cardiovascular mortality may be partially explained by negative effects of PTH on arterial function. Further work is needed to elucidate the effects of PTH on endothelial and vascular smooth muscle cells in states of health and disease and to better define associations of PTH with clinical outcomes such as hypertension, cardiovascular events, and mortality.
Supplementary Material
Acknowledgments
This research was supported by grant R01-HL-096875 from the National Heart, Lung, and Blood Institute (NHLBI), contracts N01-HC-95159 through N01-HC-95169 from the NHLBI, and grants UL1-RR-024156 and UL1-RR-025005 from National Center for Research Resources (NCRR). These data were presented in part as an abstract at the American Society of Nephrology Kidney Week (Philadelphia, PA, November, 2011). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. IHdB receives research funding from Abbott Laboratories.
Contributor Information
Cortney Bosworth, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA.
Michael C. Sachs, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA.
Daniel Duprez, Cardiovascular Division, University of Minnesota, Minneapolis, MN.
Andrew N. Hoofnagle, Departments of Laboratory Medicine and Medicine, University of Washington, Seattle, WA.
Joachim H. Ix, Division of Nephrology, Department of Medicine, University of California San Diego, and Nephrology Section, Veterans Affairs San Diego Healthcare System, San Diego, CA.
David R. Jacobs, Jr, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN.
Carmen A. Peralta, Department of Medicine, University of California, San Francisco, San Francisco, CA.
David S. Siscovick, Cardiovascular Health Research Unit, Departments of Medicine and Epidemiology, University of Washington, Seattle, WA.
Bryan Kestenbaum, Division of Nephrology and Kidney Research Institute, Departments of Medicine and Epidemiology, University of Washington, Seattle, WA.
Ian H. de Boer, Division of Nephrology and Kidney Research Institute, Departments of Medicine and Epidemiology, University of Washington, Seattle, WA.
References
- 1.Zhao G, Ford ES, Li C, Kris-Etherton PM, Etherton TD, Balluz LS. Independent associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with blood pressure among US adults. J Hypertens. 2010 Sep;28(9):1821–8. doi: 10.1097/HJH.0b013e32833bc5b4. [DOI] [PubMed] [Google Scholar]
- 2.Grobbee DE, Hackeng WH, Birkenhäger JC, Hofman A. Raised plasma intact parathyroid hormone concentrations in young people with mildly raised blood pressure. Br Med J (Clin Res Ed) 1988 Mar;296(6625):814–6. doi: 10.1136/bmj.296.6625.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saleh F, Jorde R, Svartberg J, Sundsfjord J. The relationship between blood pressure and serum parathyroid hormone with special reference to urinary calcium excretion: the Tromsø study. J Endocrinol Invest. 2006 Mar;29(3):214–20. doi: 10.1007/BF03345542. [DOI] [PubMed] [Google Scholar]
- 4.Piovesan A, Molineri N, Casasso F, Emmolo I, Ugliengo G, Cesario F, et al. Left ventricular hypertrophy in primary hyperparathyroidism. Effects of successful parathyroidectomy. Clin Endocrinol (Oxf) 1999 Mar;50(3):321–8. doi: 10.1046/j.1365-2265.1999.00651.x. [DOI] [PubMed] [Google Scholar]
- 5.Stefenelli T, Abela C, Frank H, Koller-Strametz J, Globits S, Bergler-Klein J, et al. Cardiac abnormalities in patients with primary hyperparathyroidism: implications for follow-up. J Clin Endocrinol Metab. 1997 Jan;82(1):106–12. doi: 10.1210/jcem.82.1.3666. [DOI] [PubMed] [Google Scholar]
- 6.Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011 Sep;58(14):1433–41. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009 Jun;119(21):2765–71. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 8.Cawthon PM, Parimi N, Barrett-Connor E, Laughlin GA, Ensrud KE, Hoffman AR, et al. Serum 25-hydroxyvitamin D, parathyroid hormone, and mortality in older men. J Clin Endocrinol Metab. 2010 Oct;95(10):4625–34. doi: 10.1210/jc.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorkman M, Sorva A, Tilvis R. Parathyroid hormone as a mortality predictor in frail aged inpatients. Gerontology. 2009;55(6):601–6. doi: 10.1159/000239757. [DOI] [PubMed] [Google Scholar]
- 10.Sambrook PN, Chen JS, March LM, Cameron ID, Cumming RG, Lord SR, et al. Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin d status, bone mass, and renal function in the frail and very old: a cohort study. J Clin Endocrinol Metab. 2004 Nov;89(11):5477–81. doi: 10.1210/jc.2004-0307. [DOI] [PubMed] [Google Scholar]
- 11.Jiang B, Morimoto S, Yang J, Niinoabu T, Fukuo K, Ogihara T. Expression of parathyroid hormone/parathyroid hormone-related protein receptor in vascular endothelial cells. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S142–4. doi: 10.1097/00005344-199800001-00042. [DOI] [PubMed] [Google Scholar]
- 12.Okano K, Wu S, Huang X, Pirola CJ, Juppner H, Abou-Samra AB, et al. Parathyroid hormone (PTH)/PTH-related protein (PTHrP) receptor and its messenger ribonucleic acid in rat aortic vascular smooth muscle cells and UMR osteoblast-like cells: cell-specific regulation by angiotensin-II and PTHrP. Endocrinology. 1994 Sep;135(3):1093–9. doi: 10.1210/endo.135.3.8070351. [DOI] [PubMed] [Google Scholar]
- 13.Baykan M, Erem C, Erdoğan T, Hacihasanoğlu A, Gedikli O, Kiriş A, et al. Impairment of flow mediated vasodilatation of brachial artery in patients with primary hyperparathyroidism. Int J Cardiovasc Imaging. 2007 Jun;23(3):323–8. doi: 10.1007/s10554-006-9166-8. [DOI] [PubMed] [Google Scholar]
- 14.Kosch M, Hausberg M, Vormbrock K, Kisters K, Gabriels G, Rahn KH, et al. Impaired flow-mediated vasodilation of the brachial artery in patients with primary hyperparathyroidism improves after parathyroidectomy. Cardiovasc Res. 2000 Sep;47(4):813–8. doi: 10.1016/s0008-6363(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 15.Smith JC, Page MD, John R, Wheeler MH, Cockcroft JR, Scanlon MF, et al. Augmentation of central arterial pressure in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2000 Oct;85(10):3515–9. doi: 10.1210/jcem.85.10.6880. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002 Nov;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009 Aug;120(6):502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalier E, Delanaye P, Hubert P, Krzesinski JM, Chapelle JP, Rozet E. Estimation of the stability of parathyroid hormone when stored at −80 degrees C for a long period. Clinical journal of the American Society of Nephrology : CJASN. 2009 Dec;4(12):1988–92. doi: 10.2215/CJN.03970609. [Research Support, Non-U.S. Gov’t Validation Studies] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peralta CA, Jacobs DR, Katz R, Ix JH, Madero M, Duprez DA, et al. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012 Jan;59(1):41–9. doi: 10.1053/j.ajkd.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duprez DA, Jacobs DR, Lutsey PL, Bluemke DA, Brumback LC, Polak JF, et al. Association of small artery elasticity with incident cardiovascular disease in older adults: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2011 Sep;174(5):528–36. doi: 10.1093/aje/kwr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peralta CA, Adeney KL, Shlipak MG, Jacobs D, Duprez D, Bluemke D, et al. Structural and functional vascular alterations and incident hypertension in normotensive adults: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2010 Jan;171(1):63–71. doi: 10.1093/aje/kwp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karamanoglu M, O’Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. European heart journal. 1993 Feb;14(2):160–7. doi: 10.1093/eurheartj/14.2.160. [Review] [DOI] [PubMed] [Google Scholar]
- 23.Stevens L, Coresh J, Schmid C, Feldman H, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008 Mar;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012 Jul 5;367(1):20–9. doi: 10.1056/NEJMoa1114248. [Research Support, N.I.H., Extramural Validation Studies] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007 May;192(1):211–7. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, et al. Vitamin D, parathyroid hormone, and sudden cardiac death: results from the Cardiovascular Health Study. Hypertension. 2011 Dec;58(6):1021–8. doi: 10.1161/HYPERTENSIONAHA.111.179135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999 Mar;18(6):681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.Little R, Rubin D. Statistical Analysis with Missing Data. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 29.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011 Jan;300(1):H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashid G, Bernheim J, Green J, Benchetrit S. Parathyroid hormone stimulates the endothelial nitric oxide synthase through protein kinase A and C pathways. Nephrol Dial Transplant. 2007 Oct;22(10):2831–7. doi: 10.1093/ndt/gfm269. [DOI] [PubMed] [Google Scholar]
- 31.Pang PK, Tenner TE, Yee JA, Yang M, Janssen HF. Hypotensive action of parathyroid hormone preparations on rats and dogs. Proc Natl Acad Sci U S A. 1980 Jan;77(1):675–8. doi: 10.1073/pnas.77.1.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekmekci A, Abaci N, Colak Ozbey N, Agayev A, Aksakal N, Oflaz H, et al. Endothelial function and endothelial nitric oxide synthase intron 4a/b polymorphism in primary hyperparathyroidism. J Endocrinol Invest. 2009 Jul;32(7):611–6. doi: 10.1007/BF03346518. [DOI] [PubMed] [Google Scholar]
- 33.Bortotolotto LA, Costa-Hong V, Jorgetti V, Consolim-Colombo F, Rosa K, Silva BC, et al. Vascular changes in chronic renal disease patients with secondary hyperparathyroidism. J Nephrol 2007. 2007 Jan-Feb;20(1):66–72. [PubMed] [Google Scholar]
- 34.Jespersen B, Randløv A, Abrahamsen J, Fogh-Andersen N, Kanstrup IL. Effects of PTH(1–34) on blood pressure, renal function, and hormones in essential hypertension: the altered pattern of reactivity may counteract raised blood pressure. Am J Hypertens. 1997 Dec;10(12 Pt 1):1356–67. doi: 10.1016/s0895-7061(97)00275-6. [DOI] [PubMed] [Google Scholar]
- 35.Fliser D, Franek E, Fode P, Stefanski A, Schmitt CP, Lyons M, et al. Subacute infusion of physiological doses of parathyroid hormone raises blood pressure in humans. Nephrol Dial Transplant. 1997 May;12(5):933–8. doi: 10.1093/ndt/12.5.933. [DOI] [PubMed] [Google Scholar]
- 36.Hulter HN, Melby JC, Peterson JC, Cooke CR. Chronic continuous PTH infusion results in hypertension in normal subjects. J Clin Hypertens. 1986 Dec;2(4):360–70. [PubMed] [Google Scholar]
- 37.Madero M, Wassel CL, Peralta CA, Najjar SS, Sutton-Tyrrell K, Fried LF, et al. Markers of mineral metabolism are not associated with aortic pulse wave velocity in community-living elderly persons: the Health Aging and Body Composition study. Am J Hypertens. 2011 Jul;24(7):755–61. doi: 10.1038/ajh.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.