Abstract
Binge eating disorder is an addiction-like disorder characterized by excessive food consumption within discrete periods of time. This study was aimed at understanding the role of the opioid system within the mPFC in the consummatory and motivational aspects of binge-like eating. For this purpose, we trained male rats to obtain either a sugary, highly palatable diet (Palatable rats) or a chow diet (Chow rats) for 1h a day. We then evaluated the effects of the opioid receptor antagonist, naltrexone, given either systemically or site-specifically into the NAcc or the mPFC on a fixed ratio 1 (FR1) and a progressive ratio schedule of reinforcement for food. Finally, we assessed the expression of the genes preOpioMelanoCortin (POMC), Pro-Dynorphin (PDyn), and Pro-Enkephalin (PEnk), coding for the opioids peptides in the NAcc and the mPFC in both groups. Palatable rats rapidly escalated their intake by 4 times. Naltrexone, when administered systemically and into the NAcc, reduced FR1 responding for food and motivation to eat under a progressive ratio in both Chow and Palatable rats; conversely, when administered into the mPFC, the effects were highly selective for binge eating rats. Furthermore, we found a two-fold increase in POMC and a ~50% reduction in PDyn gene expression in the mPFC of Palatable rats, when compared to control rats; however, no changes were observed in the NAcc. Our data suggest that neuroadaptations of the opioid system in the mPFC occur following intermittent access to highly palatable food, which may be responsible for the development of binge-like eating.
Keywords: addiction, binge eating disorder, nucleus accumbens, opioid, palatability, prefrontal cortex
Introduction
Binge eating disorder is characterized by excessive and uncontrollable food consumption of highly palatable foods within a short period of time (Avena, Rada and Hoebel, 2008; Corwin, 2006; Cottone et al, 2012). Subjects experiencing binge eating describe it as a loss of control during an over-consumption of food, which leads to an uncomfortable fullness and intense feelings of disgust and embarrassment (Stein et al, 2007). Binge eating disorder often occurs in comorbidity with several diseases such as obesity, diabetes, cardiovascular diseases, as well as other psychiatric disorders (Javaras et al, 2008; Wilfley et al, 2011).
Significant effort has been made in attempting to isolate the factors which contribute to the development of binge eating (Cottone et al, 2008b; Hagan and Moss, 1997). A widely accepted hypothesis on the etiology of binge eating is based on the qualitative alternation of food palatability. Indeed, restriction to low-palatable, “safe” foods, typically driven by perceived cultural norms for thinness or health, may induce craving for more appetitive palatable foods and promotes overeating. The systematical alternation in palatability, therefore, results in a self-perpetuating vicious circle of binge/restriction pattern consumption (Laessle et al, 1989; Polivy and Herman, 1985), raising the question of whether binge eating disorder can be considered an addiction-‘like’ disorder (Corwin and Grigson, 2009; Cottone, Sabino, Steardo et al, 2008b; Cottone, Wang, Park et al, 2012; Epstein and Shaham, 2010).
No effective pharmacotherapies for binge eating disorder are currently available, although different experimental targets have been proposed (Cottone, Sabino, Steardo et al, 2008b; Cottone, Wang, Park et al, 2012). The opioid system has been considered to be one of the main targets for the treatment of eating disorders since the 1970s, due to early observations that opioid antagonists such as naltrexone and naloxone were able to reduce food intake (Holtzman, 1974). Later evidence showed that the opioid system was involved in the bidirectional modulation of feeding behavior, given the ability of morphine to increase appetite in food deprived and non-deprived rats (Sanger and McCarthy, 1980). Since these initial observations, a prominent role of the opioid system in mediating food palatability has been clarified and extensive evidence has indicated that the nucleus accumbens (NAcc) represents a key region mediating these effects (Le Merrer et al, 2009). More recent studies have suggested that the opioid modulation of hedonic food consumption in the NAcc is part of a more complex network, which involves several brain structures including the prefronto-cortical regions (Mena, Sadeghian and Baldo, 2011).
Even though an extensive line of research emphasizes the primary role of the opioid system in the modulation of palatability and hedonic feeding, the specific brain area in which the opioid system mediates binge-like eating is still unknown.
Therefore, the aim of this study was to determine whether the opioid antagonist naltrexone, administered systemically, was able to suppress the consumption of and the motivation to obtain highly palatable food in a rat binge-like eating model. For this purpose, we used a newly developed operant model where rats self-administer a highly palatable, sugary diet under limited access conditions (1 h/day), mimicking the consummatory and motivational features observed in binge eating disorder (Cottone, Wang, Park et al, 2012). We then went on to determine which brain area was responsible for naltrexone’s systemic effects in suppressing the consumption of and the motivation to obtain the sugary, highly palatable diet. For this purpose, we microinfused site specifically the opioid antagonist into the NAcc shell and mPFC. Finally, we evaluated the expression of the genes preOpioMelanoCortin (POMC), Pro-Dynorphin (PDyn), and Pro-Enkephalin (PEnk), coding for the opioids peptides in the NAcc and the mPFC following a history of binge-like eating.
Material and Methods
Subjects
Male Wistar rats (n=70), 41–47 days old upon arrival (Charles River, Wilmington, MA) were housed in wire-topped, standard plastic cages in a 12:12 h reverse light cycle (lights off at 10:00 h), in a humidity- (60%) and temperature-controlled (22 °C) vivarium. Upon arrival, rats had access to corn-based chow (Harlan Teklad LM-485 Diet 7012 (65% (kcal) carbohydrate, 13% fat, 21% protein, 341 cal/100 g); Harlan, Indianapolis, IN) and water ad libitum at all times. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85-23, revised 1996) and the Principles of Laboratory Animal Care (http://www.nap.edu/readingroom/bookslabrats), and were approved by Boston University Institutional Animal Care and Use Committee (IACUC). The experimental procedures did not involve food or water restriction/deprivation, unless otherwise specified.
Drugs
Naltrexone, (5α)-17-(Cyclopropylmethyl)-4,5-epoxy-3,14-dihydromorphinan-6-one hydrochloride, was purchased from Abcam (Cambridge, MA). Naltrexone was freshly dissolved in isotonic filtered saline (0.9%) on the day of the test. Naltrexone was administered subcutaneously (0, 0.03, 0.1 and 0.3 mg/kg, 1 ml/kg) 30 minutes before the session and site-specifically into the NAcc shell and mPFC (0, 5 and 25 ug/side, bilateral) immediately before the session. Doses and pretreatment times were based on the literature (Bodnar et al, 1995; MacDonald, Billington and Levine, 2003; Williams and Broadbridge, 2009).
Operant binge-like eating procedure
Subjects were trained to self-administer food and water in individual operant test chambers described in details in (Blasio et al, 2012; Cottone, Wang, Park et al, 2012). For further details, see Supplementary Materials and Methods.
Training
The operant model of binge-like eating was performed as previously described (Cottone, Wang, Park et al, 2012). Rats (n=70) were fed the standard Harlan Teklad diet in their home cage. After a period of acclimation, food was replaced with an AIN-76A-based diet, hereafter referred to as ‘Chow A/I’ (5TUM diet formulated as 4–5 g extruded pellets, 65.5% (kcal) carbohydrate, 10.4% fat, 24.1% protein, 330 cal/100 g; TestDiet, Richmond, IN). Rats were trained to acquire operant self-administration for food (45-mg precision food pellets (Chow A/I)) and water (100 µl) under a fixed ratio 1 schedule of reinforcement (Cottone et al, 2009). Dispenser delivered a 45-mg precision pellet which is identical to the home cage ~5g extruded diet, in order to ensure that Chow rats’ food intake was driven only by homeostatic needs (Cottone, Sabino, Roberto et al, 2009; Cottone et al, 2008a). Daily sessions were performed before the dark cycle onset and were 1 h in duration.
Testing
After stable baseline performances in the 1 h self-administration sessions, the testing procedure was initiated. Rats, matched for body weight, daily food intake, feed efficiency, as well as water and food responding in the self-administration, were assigned to either a “Chow” control group, which in the operant boxes received the same 45-mg chow pellets offered in the training phase, or a “Palatable” group, which instead received a nutritionally complete, chocolate-flavored, high sucrose (50% kcal) AIN-76A-based diet, comparable in macronutrient composition and energy density to the chow diet (chocolate-flavored Formula 5TUL: 66.7% [kcal] carbohydrate, 12.7% fat, 20.6% protein, metabolizable energy 344 cal/100g; formulated as 45 mg precision food pellets; TestDiet, Richmond, IN). This chocolate-flavored diet is strongly preferred by all rats (Cottone, Sabino, Steardo et al, 2008a, b). Sessions were conducted daily.
Progressive ratio schedule of reinforcement for food
Following stabilization of intake in the binge-like eating procedure, rats (n=53) were trained in progressive ratio for food reinforcers (45-mg chow-precision pellets for Chow group and 45-mg chocolate-flavored, high sucrose-precision pellet for Palatable group). Progressive ratio schedule of reinforcement for food was performed as described previously (Cottone, Sabino, Roberto et al, 2009; Cottone, Sabino, Steardo et al, 2008a; Sabino et al, 2011). For further details, see Supplementary Materials and Methods. At the end of each session, subjects were returned to their home cage where Chow A/I was always available ad libitum; sessions were conducted daily.
Intracranial surgeries and microinfusion procedure
Intracranial surgeries
Following stabilization of intake in the operant sessions, rats were implanted with intracranial cannulas. Stereotaxic surgeries were performed as previously described (Cottone et al, 2007; Iemolo et al, 2012; Sabino et al, 2007). For further details, see Supplementary Materials and Methods. The cannula coordinates used for NAcc shell and mPFC were A/P +1.06 mm, M/L ± 0.75 mm, D/V −6.0 mm and A/P +3.2 mm, M/L ±0.65 mm, D/V −3.5 mm, respectively. The interaural bar was set at flat skull (dorsal/ventral: bregma = lambda); coordinates were based on the atlas of Paxinos & Watson (Paxinos, 1986). A stainless steel dummy stylet (Plastics One, Inc., Roanoke, VA, USA) maintained patency. After surgery, rats were allowed to recover from the surgical procedure for 1 week before the experimental procedure began.
Microinfusion procedure
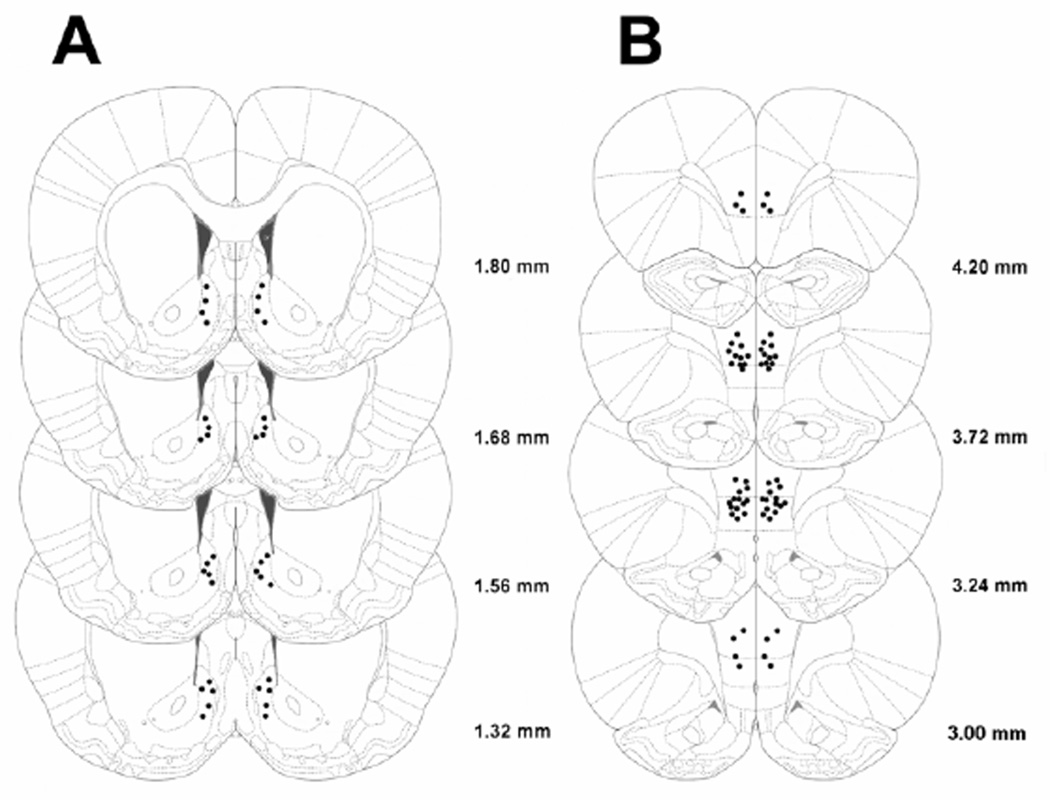
For the microinfusion of naltrexone, the dummy stylet was removed from the guide cannula and replaced with a stainless steel injector projecting 1.0 mm for NAcc shell and 1.5 mm for mPFC beyond the tip of the guide cannula; the injector was connected via a PE 20 tubing to a Hamilton microsyringe driven by a microinfusion pump (Kd Scientifics/Biological Instruments, Holliston, MA, USA). Microinfusions delivered a 0.5 µl volume over 2 min; injectors were left in place for an additional minute to minimize backflow. Treatments were given in full Latin square designs, with 1–3 intervening treatment-free test days, in which the food intake returned to baseline levels. Rats were given 3 days of acclimation to daily sham injections, prior to the beginning of drug treatments. Cannula placement (Fig. 4) was verified at the conclusion of all testing by microinfusing India Ink (0.5 µl over 2 minutes). Slices of 40 µm were collected using a cryostat and placements were verified under a microscope. Of 68 rats used for the microinfusion studies, 3 were excluded for procedural reasons, which included loss or occlusion of cannulae, or inability to maintain stable performance. In 14 of the remaining 65 rats, the position of the cannula was outside the target site.
Figure 4.
Drawing of coronal rats’ brain slices. Dots represent the injection sites in (A) NAcc shell and (B) mPFC included in the data analysis.
Quantitative Real-Time PCR
One cohort of Chow and Palatable rats (n=15) was used for the quantification of the POMC, PDyn, and PEnk peptides mRNA. Animals were sacrificed 24 h after the last daily binge-like eating session. Punches of the medial prefrontal cortex include both the prelimbic and the infralimbic regions. Punches of the nucleus accumbens include both shell and core regions. Procedures were performed as described previously (Sabino et al, 2009). For details, see Supplementary Materials and Methods.
Statistical Analysis
The effects of naltrexone on food intake, water intake, and breakpoint were analyzed using two-way mixed design ANOVAs, with Diet History as a between-subjects factor and Treatment as a within-subject factor, followed by repeated measures one-way ANOVAs. The effects of Diet History on mRNA levels were analyzed using unpaired Student’s t-tests. Variables that failed the test for normality were analyzed as ranked (Akritas, 1990). The statistical packages used were Instat 3.0 (GraphPad, San Diego, CA) and Systat 11.0 (SPSS, Chicago, IL).
Results
Effects of systemic administration of the opioid antagonist naltrexone on operant binge-like eating
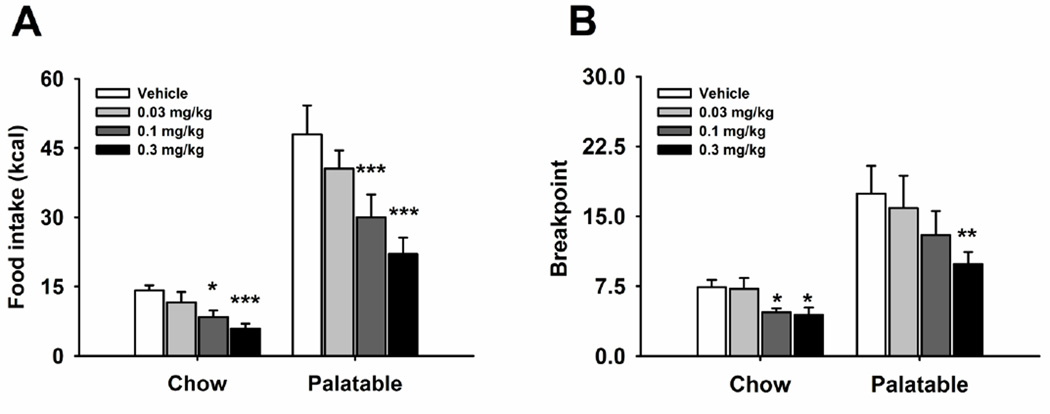
Systemic administration of naltrexone dose-dependently reduced FR1 responding for food in both Chow and Palatable groups (Figure 1A; Treatment: F(3,54)=25.00, p<0.001; Diet History X Treatment: F(3,54)=0.64, n.s.) One-way ANOVAs confirmed the effect of drug treatment in both Chow (Treatment: F(3,27)=7.62, p<0.0008) and Palatable rats (Treatment: F(3,27)=16.78 p<0.0001). Post hoc analysis revealed that, while the 0.03 mg/kg dose was ineffective, both the 0.1 and 0.3 mg/kg doses significantly reduced food self-administration in the two groups, when compared to vehicle condition. Moreover, water intake was affected by the treatment (Table 1; Treatment: F(3,54)=8.46, p<0.0001; Diet History X Treatment: F(3,54)=0.76, n.s.). One-way ANOVAs confirmed the drug treatment effect in both Chow (Treatment: F(3,27)=4.97, p<0.007) and Palatable (Treatment: F(3,27)=3.76, p<0.022) rats. Post hoc analysis revealed a significant water intake reduction following the administration of the 0.1 and 0.3 mg/kg doses in Chow rats and the 0.3 mg/kg dose in Palatable rats, when compared to vehicle condition.
Figure 1.
Effects of systemic treatment with naltrexone (0, 0.03, 0.1, 0.3 mg/kg, subcutaneously) on (A) food self-administration (n=20) and (B) breakpoint on a progressive ratio schedule of reinforcement (n=19) in male Wistar rats. Panels represent M±SEM. Symbols denote: * significant difference from vehicle condition p<0.05, ** p<0.01, *** p<0.001 (Student Newman–Keuls test).
Table 1. Effects of naltrexone administration on water intake.
Effects of naltrexone on water intake following subcutaneous (n=20), NAcc shell (n=18) or mPFC (n=17) administration in male Wistar rats. Values show log-normal values of water intake espressed as M±SEM. Bracketed values show the backtransformed M±SEM.
| Water intake, ln(ml) [ml] | |||
|---|---|---|---|
| Treatment | Chow | Palatable | |
| Systemic | |||
| Vehicle | 1.95-0.06 [7.0±0.5] | 1.70-0.14 [5.4±0.8] | |
| 0.03 mg/kg | 1.60-0.17 [5.0±0.9] | 1.45-0.17 [4.3±0.8] | |
| 0.1 mg/kg | 0.99-0.41 [2.7±1.4] * | 1.24-0.21 [3.5±0.8] | |
| 0.3 mg/kg | 0.82-0.22 [2.3±0.6] * | 0.99-0.32 [2.7±1.0] * | |
| NAcc Shell | |||
| Vehicle | 1.99-0.10 [7.3±6.1] | 2.08-0.19 [8.0±1.7] | |
| 5 ug | 1.80-0.13 [6.1±0.9] | 1.51-0.42 [4.5±2.3] | |
| 25 ug | 1.90-0.15 [6.7±1.1] | 1.79-0.16 [6.0±1.1] | |
| mPFC | |||
| Vehicle | 1.87-0.18 [6.5±1.3] | 1.89-0.15 [6.6±1.1] | |
| 5 ug | 1.87-0.14 [6.5±0.9] | 1.63-0.16 [5.1±0.9] | |
| 25 ug | 1.55-0.13 [4.7±0.6] | 1.64-0.19 [5.2±1.1] | |
Symbols denote: * significant difference from vehicle condition p<0.05 (Student Newman–Keuls test).
Effects of systemic administration of the opioid antagonist naltrexone on a progressive ratio schedule of reinforcement for food
Naltrexone, administered systemically, dose-dependently decreased the breakpoint in both Chow and Palatable rats (Figure 1B; Treatment: F(3,51)=41.31, p<0.0001; Diet History X Treatment: F(3,51)=1.96, n.s.). Following a significant main effect of treatment (Chow group; Treatment: F(3,27)=5.99, p<0.003; Palatable group; Treatment: F(3,24)=6.87, p<0.002), post hoc analysis revealed that the 0.3 mg/kg dose significantly reduced the breakpoint in both groups.
Effects of naltrexone microinfusion into the NAcc shell on operant binge-like eating
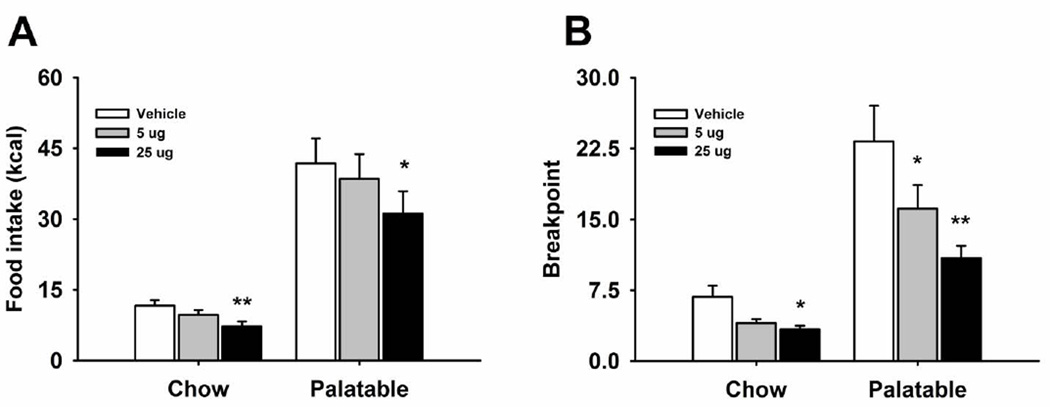
Naltrexone microinfusion into the NAcc dose-dependently decreased responding for food in both the Chow and Palatable group (Figure 2A; Treatment: F(2,32)=10.76, p<0.0001; Diet History X Treatment: F(2,32)=4.36, p<0.02). One-way ANOVAs revealed a drug treatment effect in both Chow (Treatment: F(2,18)=5.72, p<0.01) and Palatable rats (Treatment: F(2,18)=5.344, p<0.02) respectively. Furthermore, post hoc analysis revealed significant reductions in food self-administration following treatment with the highest dose (25 µg) in both Chow and Palatable groups. Water consumption was not affected by the drug treatment (Table 1; Treatment: F(2,32)=2.48, n.s.; Diet History X Treatment: F(2,32)=0.65, n.s.).
Figure 2.
Effect of microinfusion with naltrexone (0, 5, 25 µg/side) in NAcc shell on (A) food self-administration (n=18) and (B) breakpoint on a progressive ratio schedule of reinforcement (n=17) in male Wistar rats. Panel represents M±SEM. Symbols denote: * significant difference from vehicle condition p<0.05, ** p<0.01 (Student Newman–Keuls test).
Effects of naltrexone microinfusion into the NAcc shell on a progressive ratio schedule of reinforcement for food
When naltrexone was site-specifically microinfused into the NAcc, a significant overall reduction of the breakpoint in both the Chow and Palatable group was observed (Figure 2B; Treatment: F(2,30)=16.72, p<0.0001; Diet History X Treatment: F(2,30)=5.22, p<0.01). This result was confirmed by the one way ANOVAs performed individually on each of the two groups (Chow group; Treatment: F(2,16)=6.11, p<0.01; Palatable group; Treatment: F(2,14)=10.62, p<0.001). Post-hoc analysis revealed a significant reduction of the breakpoint in both groups when the 5 and 25 µg doses were microinfused. The reduction of the breakpoint was comparable in magnitude between Chow and Palatable rats (50.8% and 53.2%, when compared to vehicle conditions, respectively).
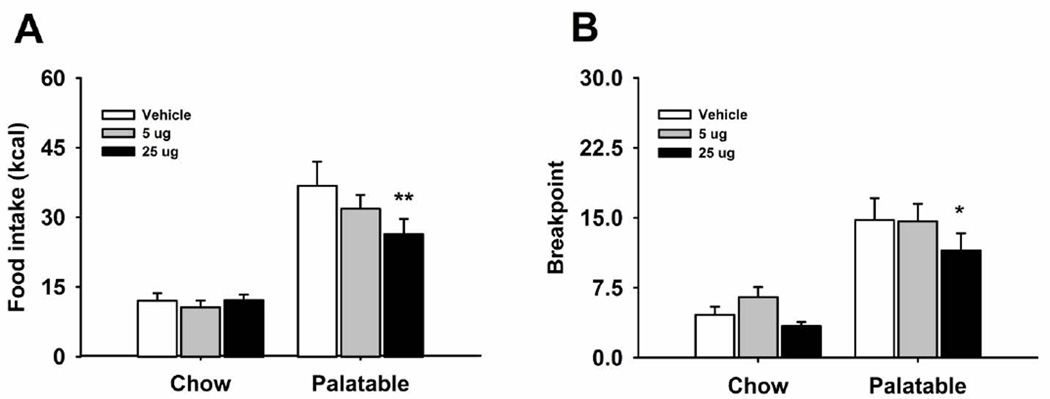
Effects of naltrexone microinfusion into the mPFC on operant binge-like eating
Naltrexone site-specifically microinfused into the mPFC differentially affected food responding in Chow and Palatable rats, as revealed by the significant interaction (Figure 3A; Treatment: F(2,30)=4.77, p<0.02; Diet History X Treatment: F(2,30)=5.08, p<0.01). Indeed, while naltrexone did not affect responding for chow in Chow rats (Treatment: F(2,12)=0.68, n.s.), it dose-dependently reduced binge-like eating in Palatable rats (Treatment: F(2,18)=9.25, p<0.002), with post hoc analysis showing significant reduction following the 25 µg dose, when compared to vehicle condition. Therefore, naltrexone, microinfused into the mPFC, selectively affected binge-like eating in Palatable rats, without affecting feeding in control rats. In addition, naltrexone had no effect on water intake (Table 1; Treatment: F(2,30)=1.89, n.s.; Diet History X Treatment: F(2,30)=0.69, n.s.).
Figure 3.
Effect of microinfusion with naltrexone (0, 5, 25 µg/side) in mPFC on (A) food self-administration (n=17) and (B) breakpoint on a progressive ratio schedule of reinforcement (n=17) in male Wistar rats. Panel represents M±SEM. Symbols denote: * significant difference from vehicle condition p<0.05, ** p<0.01 (Student Newman–Keuls test).
Effects of naltrexone microinfusion into the mPFC on a progressive ratio schedule of reinforcement for food
A two-way ANOVA performed on the breakpoint of Chow and Palatable rats following microinfusion of naltrexone into the mPFC revealed a main effect of drug treatment (Figure 3B; Treatment: F(2,30)=9.057, p<0.001; Diet History X Treatment: F(2,30)=1.84, n.s.). However, although the one-way ANOVA analysis revealed an effect of drug treatment in the Chow group (Treatment: F(2,18)=4.43, p<0.027), following post-hoc analysis neither the 5 µg nor the 25 µg dose significantly differed from the vehicle condition. Likely, the treatment’s significant effect indicated by the ANOVA was driven by a trend toward an increased breakpoint following the 5 µg dose, when compared to vehicle condition. On the other hand, in the Palatable group a significant main effect of drug treatment could be observed (Treatment: F(2,12)=5.31, p<0.02), and the highest dose microinfused into the mPFC significantly reduced the breakpoint, when compared to vehicle condition.
Quantitative Real-Time PCR
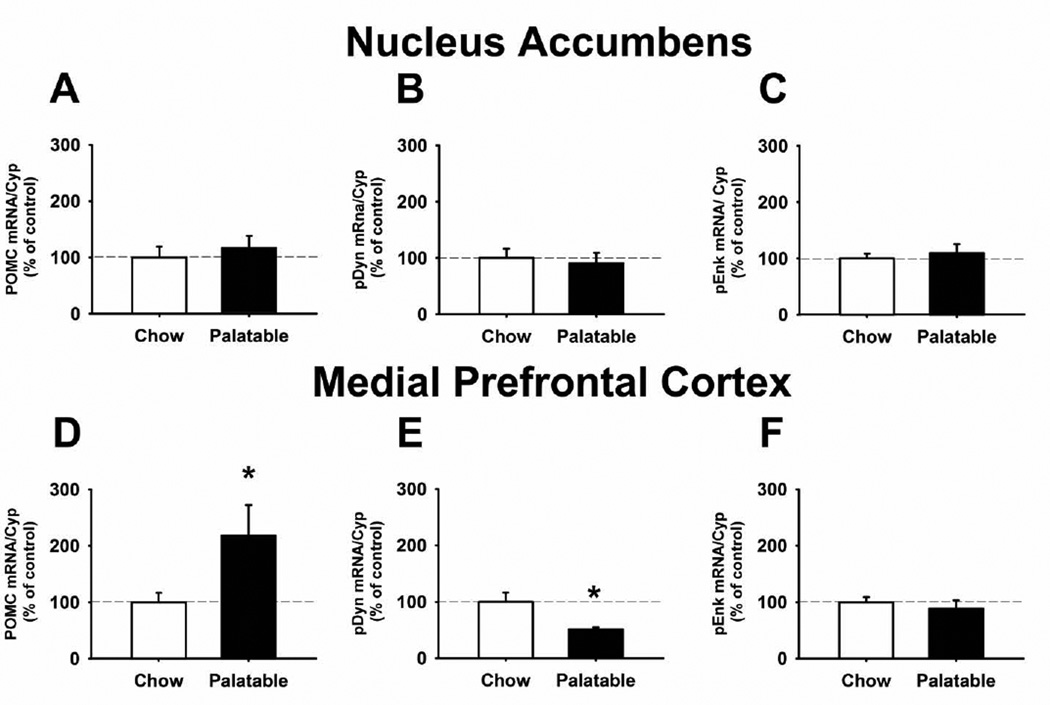
Quantitative real-time PCR showed that, 24 h after the last food self-administration session, no significant differences in POMC, PDyn, and PEnk expression between Chow and Palatable rats were observed in the NAcc (Figures 5A, 5B, and 5C). However, POMC mRNA levels were significantly higher in the PFC of Palatable rats, when compared to Chow rats (117.9% increase; Figure 5D). In addition, PDyn expression levels in the PFC of Palatable rats were significantly lower in comparison to Chow rats (49.3% reduction; Figure 5E). No difference between the two groups was observed in the PEnk mRNA levels in the PFC (Figure 5F).
Figure 5.
POMC, PDyn, and PEnk mRNA expression in the NAcc (A, B and C) and in the mPFC (D, E and F) of male Wistar rats. Brain areas were collected 24 h after the last daily binge-like eating session. Data represent M±SEM expressed as percent of Chow group; * p<0.05 vs Chow group (Student’s t-test).
Discussion
In this study we show that the opioid antagonist naltrexone, systemically administered, non-specifically decreased the consumption and the motivation to obtain food, as well as reduced the intake of water in both rats self-administering a regular chow diet and rats bingeing on a highly palatable diet. Importantly, while the effects on both chow and palatable food intake were maintained when naltrexone was microinfused into the NAcc shell, the opioid antagonist selectively decreased the consumption and the motivation to obtain highly palatable food, but not regular chow, when microinfused into the mPFC. Furthermore, confirming the selectivity of the behavioral effects observed following the microinfusion of naltrexone into the mPFC, the mRNA expression of POMC and PDyn was dysregulated in the mPFC, but not in the NAcc, of binge eating rats in comparison to control rats. No effect was observed in the gene expression of PEnk in either area.
Systemically administered naltrexone, therefore, dose-dependently decreased food consumption of both Palatable and Chow rats. Systemic drug treatment also reduced the motivation to work, in order to obtain both the chow and the palatable diet in a progressive ratio schedule of reinforcement, a validated behavioral paradigm utilized to assess the motivational strength to acquire reinforcers (Cottone, Sabino, Roberto et al, 2009; Cottone, Sabino, Steardo et al, 2008a). Following subcutaneous administration of the highest dose naltrexone, the magnitude of the maximal effects in the reduction of FR1 responding and the breakpoint of a progressive ratio schedule of reinforcement was similar in the two groups (FR1: 58.2% and 54.0%; progressive ratio: 40.5% and 43.3%, when compared to vehicle conditions in Chow and Palatable rats, respectively). Therefore, the effects of systemic naltrexone on food intake likely involved a suppression of both homeostatic and hedonic feeding behavior (Le Merrer, Becker, Befort et al, 2009). Interestingly, the effects of subcutaneous administration of naltrexone were not selective for food, since drug treatment also decreased water intake in both control and binge eating rats. Altogether, these initial observations were suggesting a general suppressive effect on ingestive behavior, following systemic administration with the opioid antagonist (Frenk and Rogers, 1979).
In this study we wanted to determine whether opioid receptors in the NAcc shell mediated the consummatory and motivational aspects of binge-like eating. Indeed, the opioid system in this area has been proposed to be involved in the modulation of the rewarding properties of food (Carlezon, Devine and Wise, 1995). Here we show that naltrexone microinfused into the NAcc shell decreased not only binge-like eating of the highly palatable diet, but also the intake of regular chow. A similar outcome was obtained when we tested the effects of naltrexone microinfusion into the NAcc shell on the breakpoint of a progressive ratio schedule of reinforcement for food. Indeed, drug treatment indiscriminately decreased the motivation to obtain food in both binge eating and control rats. These findings suggest that opioid receptors within the NAcc shell exert a general modulation of feeding behavior and reinforcing efficacy of food, independently from the type or from the incentive salience of the diet. In support of this hypothesis, Kelley and colleagues have shown that blockade of μ-opioid receptors within the NAcc decreases the intake of both a standard chow diet and a sucrose solution (Kelley, Bless and Swanson, 1996). Contrarily to what we observed following systemic administration of naltrexone, NAcc shell microinfusion of the drug did not affect water intake, suggesting that opioid receptors in this brain region are specifically involved in the modulation of feeding behavior, instead of more generally in ingestive behavior, or that higher doses are needed to suppress water intake.
We also investigated whether the opioid system within the mPFC was important in mediating binge-like eating of highly palatable food. In our study, mPFC microinfusion of the opioid antagonist selectively and dose-dependently decreased both the consumption and the motivation to obtain the highly palatable diet in binge eating rats, without affecting the intake of regular chow in control rats. Water intake was not affected by drug treatment in either group, suggesting that the effects are selective for feeding behavior. Fronto-cortical areas of the brain have been implicated in the modulation of feeding behavior (Moran and Westerterp-Plantenga, 2012). A recent report has also shown that μ-opioid receptors within the mPFC play an important role in driving overeating (Mena, Sadeghian and Baldo, 2011).
It is important to discuss the alternative interpretation that the effects of naltrexone may result from its rapid diffusion throughout the brain and periphery, contrarily to other quaternary derivatives opiate antagonists (Vaccarino, Bloom and Koob, 1985; Vaccarino et al, 1985) which have a low diffusion rate (Schroeder et al, 1991). Contrary to this interpretation there is the evidence that, in this study, naltrexone microinfused into two different brain areas (NAcc and PFC) in Chow rats exerted differential effects. Moreover, time course analysis of food responding revealed that naltrexone injected into the mPFC decreased food responding in the Palatable rats after only 6 minutes following microinfusion (M±SEM: 84.3±7.5 vs. 75.3±6.6, veh vs. 25 µg/side, respectively, p<0.05). Because of the short period of time, the alternative interpretation that the observed effect could result either from a CNS-wide or peripheral blockade of opioid receptors is highly unlikely. Furthermore, in support of the validity of the data obtained in this study, literature extensively reports the effects of naltrexone microinfused in specific areas of the brain (Bodnar et al, 2005). Nonetheless, the hypothesis that the effect of naltrexone may be also dependent on a slight diffusion in brain areas contiguous to Nacc shell or PFC, cannot be excluded.
An alternative interpretation of the lack of effects on food intake following naltrexone microinfusion into the prefrontal cortex of Chow rats is that the observed effect could result from a concomitant blockade of mu and kappa opioid receptors. Although the two systems have been demonstrated to exert opposite modulatory effects in multiple processes including reward, they have been shown to modulate homeostatic feeding (like the food intake in Chow animals of this study) in a similar manner. Both mu and kappa opioid receptor activation has been demonstrated to increase food intake, whereas their blockade has been shown to exert anorectic effects (Cooper, Jackson and Kirkham, 1985; Gosnell, Levine and Morley, 1986) Therefore, our findings suggest a differential role in the modulation of eating behavior exerted by the opioid system in the NAcc and the mPFC; while NAcc opioid receptors seem to be involved in a general modulation of feeding, independently from the type of food ingested (Kelley, Bless and Swanson, 1996), the mPFC opioid system only seems to be recruited following a history of limited access to a sugary, palatable diet, when rats lose inhibitory control over food. This hypothesis is in accordance with the higher cognitive function and complex control of reward evaluation exerted by the mPFC.
The hypothesis of a more generalized, not food specific role of NAcc opioid receptors in the mediation of feeding behavior, and the selective recruitment of the mPFC was supported by gene expression analysis of POMC, PDyn, and PEnk. No significant difference in the expression of the three genes was observed in the NAcc, when comparing the binge eating and control rats. Conversely, in the mPFC, binge eating rats showed a more than two-fold increase in the POMC mRNA levels, accompanied by a ~50% reduction in the mRNA levels of PDyn, when compared to control chow rats.
POMC and PDyn genes have been shown to be expressed in both of these brain areas (Leriche, Cote-Velez and Mendez, 2007; Taqi et al, 2011; Ziolkowska et al, 2006). (However, evidence demonstrates that opioid peptides released in mPFC can also originate from cell bodies projecting from different brain regions (i.e. ventral tegmental area (Garzon and Pickel, 2004)), which raises the possibility that the effects observed in this study following naltrexone microinfusion within the mPFC may be unrelated to the variation in POMC and Pdyn expression in that same brain region. POMC is the precursor of endorphins which preferentially bind μ (but also δ) opioid receptors (Mansour et al, 1995), while PDyn is the precursor of dynorphins which preferentially bind κ-opioid receptors (Day et al, 1998). Extensive evidence has suggested an opposing role of μ- and κ-opioid receptors in the modulation of a variety of processes in the brain including analgesia, tolerance, memory processes, and reward (Pan, 1998; Woolley et al, 2007). In particular and consistent with this hypothesis, μ-opioid receptors have been extensively proven to mediate the rewarding properties of food and some drugs of abuse (Zhang and Kelley, 2000); on the other hand, κ-opioid receptors have been demonstrated to mediate their aversive and dysphoric effects, and have been proposed as a part of an “anti-reward” system (Koob and Le Moal, 2001). More importantly, in the context of this study, the pharmacological activation of either μ- or κ-opioid receptors within the prefrontal cortex has been demonstrated to exert opposing rewarding effects: microinfusions of a selective μ-opioid receptor agonist induce place preference, whereas microinfusions of a κ-opioid receptor agonist produce place aversion (Bals-Kubik et al, 1993).
An important point of discussion is whether changes in the mRNA expression observed in this study are dependent on differences in cumulative caloric intake or body weight between Chow and Palatable rats. Although food intake and body weight were not recorded in the cohort of animals used for the quantitative Real-Time PCR, we have previously demonstrated that the binge-like eating procedure used here does not influence cumulative food intake nor body weight. Indeed, binge eating rats show excessive intake during the 1h access to the highly palatable diet, but compensate during the remaining 23 hours of the day by undereating the regular chow diet (Cottone, Wang, Park et al, 2012). This aberrant pattern of intake therefore does not result in differing cumulative caloric intake or body weight between binge eating and control rats (Cottone, Wang, Park et al, 2012).
Although similarities between the overeating/restriction pattern in binge eating disorder and the intoxication/withdrawal pattern of drug abuse have been proposed (Epstein and Shaham, 2010), whether the animal model used in this study could also be useful for the of study the negative symptomatology associated with withdrawal from the highly palatable food is unknown. The increased expression of POMC (“pro-reward” system) and the decreased expression of Pdyn (“anti-reward” system) observed here following 24h withdrawal from the palatable diet suggest that the animals likely do not experience a negative emotional state. However, to address this important aspect of diet cycling, further studies assessing emotionality and the potential involvement of stress systems (e.g. corticotropin-releasing factor) will be needed. Therefore, the differential alterations in the gene expression of POMC and Pdyn observed in mPFC may indeed be interpreted as a general potentiation of the rewarding properties of palatable food, which may be the consequence of or drive binge eating in these subjects.
Altogether, the results of this study support the hypothesis that the opioid system in prefronto-cortical regions of the brain is involved in the control of feeding behavior and expand it to the specific context of the maladaptive excessive intake of highly palatable food observed in binge eaters (Mena, Sadeghian and Baldo, 2011). Fronto-cortical regions of the brain play a major role in reward evaluation and decision making (Clark et al, 2008); extensive literature demonstrates that subjects afflicted by addiction and binge eating disorder show dysfunctions in the mPFC, which are associated with altered reward evaluation (Boeka and Lokken, 2011). Our behavioral, pharmacological, and molecular observations, therefore, support the hypothesis that the opioid system is a key mediator of hedonic feeding (Nathan and Bullmore, 2009) and suggest that neuroadaptations of the opioid system in the mPFC may be responsible for the hyper-evaluation of highly palatable foods, leading to the loss of control over eating.
Supplementary Material
Acknowledgments
We thank Stephen St. Cyr, Aditi R. Narayan, Vamsee Neerukonda, Noah Kelley, Jin Won Park, Anoop Ravilla, and Sishir Yeety for the technical assistance, as well as Tamara Zeric for editorial assistance. This publication was made possible by grant numbers DA023680, DA030425, MH091945, MH093650A1, and AA016731 from the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), by the Peter Paul Career Development Professorship (P.C.) and by Boston University's Undergraduate Research Opportunities Program (UROP). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Authors contribution
AB, PC and VS were responsible for the study concept and design. AB, PC, and VS performed behavioral and molecular experiments. AB and PC performed data analysis. PC, VS and LS assisted with interpretation of findings. AB and PC drafted the manuscript. PC, VS and LS provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Akritas MG. The rank transform method in some two-factor designs. Journal of the American Statistical Association. 1990;85(409):73–78. [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264(1):489–495. [PubMed] [Google Scholar]
- Blasio A, Narayan AR, Kaminski BJ, Steardo L, Sabino V, Cottone P. A modified adjusting delay task to assess impulsive choice between isocaloric reinforcers in non-deprived male rats: effects of 5-HT(2)A/C and 5-HT(1)A receptor agonists. Psychopharmacology (Berl) 2012;219(2):377–386. doi: 10.1007/s00213-011-2517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1995;700(1–2):205–212. doi: 10.1016/0006-8993(95)00957-r. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Lamonte N, Israel Y, Kandov Y, Ackerman TF, Khaimova E. Reciprocal opioid-opioid interactions between the ventral tegmental area and nucleus accumbens regions in mediating mu agonist-induced feeding in rats. Peptides. 2005;26(4):621–629. doi: 10.1016/j.peptides.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Boeka AG, Lokken KL. Prefrontal systems involvement in binge eating. Eat Weight Disord. 2011;16(2):e121–e126. doi: 10.1007/BF03325317. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology (Berl) 1995;122(2):194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131(Pt 5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ, Jackson A, Kirkham TC. Endorphins and food intake: kappa opioid receptor agonists and hyperphagia. Pharmacol Biochem Behav. 1985;23(5):889–901. doi: 10.1016/0091-3057(85)90088-7. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Bingeing rats: a model of intermittent excessive behavior? Appetite. 2006;46(1):11–15. doi: 10.1016/j.appet.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Grigson PS. Symposium overview--Food addiction: fact or fiction? J Nutr. 2009;139(3):617–619. doi: 10.3945/jn.108.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Nagy TR, Coscina DV, Zorrilla EP. Feeding microstructure in diet-induced obesity susceptible versus resistant rats: central effects of urocortin 2. J Physiol. 2007;583(Pt 2):487–504. doi: 10.1113/jphysiol.2007.138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009;106(47):20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol Regul Integr Comp Physiol. 2008a;295(4):R1066–R1076. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008b;33(3):524–535. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- Cottone P, Wang X, Park JW, Valenza M, Blasio A, Kwak J, Iyer MR, Steardo L, Rice KC, Hayashi T, Sabino V. Antagonism of Sigma-1 Receptors Blocks Compulsive-Like Eating. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R, Lazure C, Basak A, Boudreault A, Limperis P, Dong W, Lindberg I. Prodynorphin processing by proprotein convertase 2. Cleavage at single basic residues and enhanced processing in the presence of carboxypeptidase activity. J Biol Chem. 1998;273(2):829–836. doi: 10.1074/jbc.273.2.829. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Shaham Y. Cheesecake-eating rats and the question of food addiction. Nat Neurosci. 2010;13(5):529–531. doi: 10.1038/nn0510-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenk H, Rogers GH. The suppressant effects of naloxone on food and water intake in the rat. Behav Neural Biol. 1979;26(1):23–40. doi: 10.1016/s0163-1047(79)92855-3. [DOI] [PubMed] [Google Scholar]
- Garzon M, Pickel VM. Ultrastructural localization of Leu5-enkephalin immunoreactivity in mesocortical neurons and their input terminals in rat ventral tegmental area. Synapse. 2004;52(1):38–52. doi: 10.1002/syn.20000. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Levine AS, Morley JE. The stimulation of food intake by selective agonists of mu, kappa and delta opioid receptors. Life Sci. 1986;38(12):1081–1088. doi: 10.1016/0024-3205(86)90243-2. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord. 1997;22(4):411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Behavioral effects of separate and combined administration of naloxone and d-amphetamine. J Pharmacol Exp Ther. 1974;189(1):51–60. [PubMed] [Google Scholar]
- Iemolo A, Valenza M, Tozier L, Knapp CM, Kornetsky C, Steardo L, Sabino V, Cottone P. Withdrawal from chronic, intermittent access to a highly palatable food induces depressive-like behavior in compulsive eating rats. Behav Pharmacol. 2012;23(5–6):593–602. doi: 10.1097/FBP.0b013e328357697f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaras KN, Pope HG, Lalonde JK, Roberts JL, Nillni YI, Laird NM, Bulik CM, Crow SJ, McElroy SL, Walsh BT, Tsuang MT, Rosenthal NR, Hudson JI. Co-occurrence of binge eating disorder with psychiatric and medical disorders. J Clin Psychiatry. 2008;69(2):266–273. doi: 10.4088/jcp.v69n0213. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278(3):1499–1507. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Laessle RG, Tuschl RJ, Kotthaus BC, Pirke KM. Behavioral and biological correlates of dietary restraint in normal life. Appetite. 1989;12(2):83–94. doi: 10.1016/0195-6663(89)90098-6. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89(4):1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leriche M, Cote-Velez A, Mendez M. Presence of pro-opiomelanocortin mRNA in the rat medial prefrontal cortex, nucleus accumbens and ventral tegmental area: studies by RT-PCR and in situ hybridization techniques. Neuropeptides. 2007;41(6):421–431. doi: 10.1016/j.npep.2007.08.004. [DOI] [PubMed] [Google Scholar]
- MacDonald AF, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R999–R1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18(1):22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci. 2011;31(9):3249–3260. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH, Westerterp-Plantenga M. The potential role of and deficits in frontal cortical brain areas implicated in executive control of food intake. Int J Obes (Lond) 2012;36(5):625–626. doi: 10.1038/ijo.2011.249. [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Bullmore ET. From taste hedonics to motivational drive: central mu-opioid receptors and binge-eating behaviour. Int J Neuropsychopharmacol. 2009;12(7):995–1008. doi: 10.1017/S146114570900039X. [DOI] [PubMed] [Google Scholar]
- Pan ZZ. mu-Opposing actions of the kappa-opioid receptor. Trends Pharmacol Sci. 1998;19(3):94–98. doi: 10.1016/s0165-6147(98)01169-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Second edn. Orlando: Academic Press; 1986. [Google Scholar]
- Polivy J, Herman CP. Dieting and binging. A causal analysis. Am Psychol. 1985;40(2):193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36(6):1207–1218. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Steardo L, Schmidhammer H, Zorrilla EP. 14-Methoxymetopon, a highly potent mu opioid agonist, biphasically affects ethanol intake in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2007;192(4):537–546. doi: 10.1007/s00213-007-0746-7. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Jr, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology. 2009;34(6):1482–1493. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ, McCarthy PS. Differential effects of morphine on food and water intake in food deprived and freely-feeding rats. Psychopharmacology (Berl) 1980;72(1):103–106. doi: 10.1007/BF00433813. [DOI] [PubMed] [Google Scholar]
- Schroeder RL, Weinger MB, Vakassian L, Koob GF. Methylnaloxonium diffuses out of the rat brain more slowly than naloxone after direct intracerebral injection. Neurosci Lett. 1991;121(1–2):173–177. doi: 10.1016/0304-3940(91)90678-m. [DOI] [PubMed] [Google Scholar]
- Stein RI, Kenardy J, Wiseman CV, Dounchis JZ, Arnow BA, Wilfley DE. What's driving the binge in binge eating disorder?: A prospective examination of precursors and consequences. Int J Eat Disord. 2007;40(3):195–203. doi: 10.1002/eat.20352. [DOI] [PubMed] [Google Scholar]
- Taqi MM, Bazov I, Watanabe H, Nyberg F, Yakovleva T, Bakalkin G. Prodynorphin promoter SNP associated with alcohol dependence forms noncanonical AP-1 binding site that may influence gene expression in human brain. Brain Res. 2011;1385:18–25. doi: 10.1016/j.brainres.2011.02.042. [DOI] [PubMed] [Google Scholar]
- Vaccarino FJ, Bloom FE, Koob GF. Blockade of nucleus accumbens opiate receptors attenuates intravenous heroin reward in the rat. Psychopharmacology (Berl) 1985;86(1–2):37–42. doi: 10.1007/BF00431681. [DOI] [PubMed] [Google Scholar]
- Vaccarino FJ, Pettit HO, Bloom FE, Koob GF. Effects of intracerebroventricular administration of methyl naloxonium chloride on heroin self-administration in the rat. Pharmacol Biochem Behav. 1985;23(3):495–498. doi: 10.1016/0091-3057(85)90027-9. [DOI] [PubMed] [Google Scholar]
- Wilfley D, Berkowitz R, Goebel-Fabbri A, Hirst K, Ievers-Landis C, Lipman TH, Marcus M, Ng D, Pham T, Saletsky R, Schanuel J, Van Buren D. Binge eating, mood, and quality of life in youth with type 2 diabetes: baseline data from the today study. Diabetes Care. 2011;34(4):858–860. doi: 10.2337/dc10-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Broadbridge CL. Potency of naltrexone to reduce ethanol self-administration in rats is greater for subcutaneous versus intraperitoneal injection. Alcohol. 2009;43(2):119–126. doi: 10.1016/j.alcohol.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Lee BS, Kim B, Fields HL. Opposing effects of intra-nucleus accumbens mu and kappa opioid agonists on sensory specific satiety. Neuroscience. 2007;146(4):1445–1452. doi: 10.1016/j.neuroscience.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99(2):267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Ziolkowska B, Stefanski R, Mierzejewski P, Zapart G, Kostowski W, Przewlocki R. Contingency does not contribute to the effects of cocaine self-administration on prodynorphin and proenkephalin gene expression in the rat forebrain. Brain Res. 2006;1069(1):1–9. doi: 10.1016/j.brainres.2005.11.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.