Abstract
Objectives
Stage III melanoma is associated with an increased risk of recurrence and death. Complete surgical resection remains the best chance for cure. Unfortunately, no adjuvant therapy has demonstrated a consistent improvement in melanoma-specific survival. We hypothesize that adjuvant GM-CSF may improve clinical outcomes.
Patients and Methods
Retrospective cohort study of 317 surgically resected stage III melanoma patients managed with observation or adjuvant GM-CSF at a single institution from 2001 to 2010.
Results
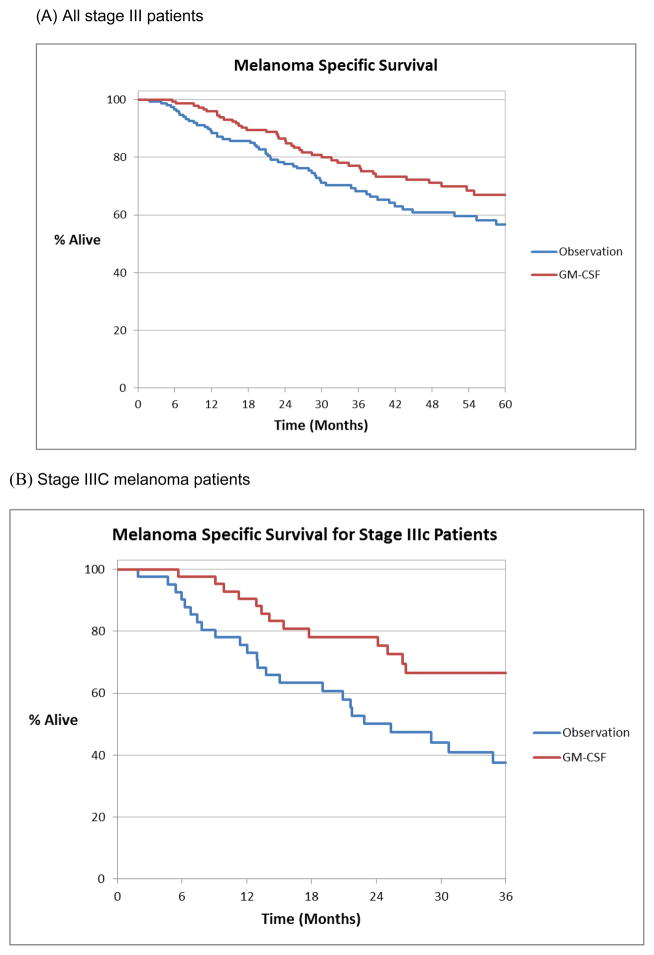
Of the 317 stage III patients 165 (52%) were observed and 152 (48%) were treated with GM-CSF, with a median follow-up of 34 months. Patients treated with GM-CSF tended to be younger (p < 0.0001), had more advanced stage disease (p = 0.002) and were more likely to have had a recurrence prior to initiation of adjuvant therapy than the observation group (p < 0.0001). Adjuvant GM-CSF appeared to be associated with improved MSS but this did not reach statistical significance (p = 0.08). Patients with stage IIIC melanoma derived a substantial benefit from adjuvant GM-CSF, with a 52% risk reduction in melanoma-specific death (HR 0.48, 95% CI 0.27–0.87, p = 0.02).
Conclusion
Despite selecting patients with more advanced stage and a higher incidence of regional relapse, adjuvant GM-CSF was associated with an improved MSS but not DFS in patients with stage IIIC disease. In patients not otherwise eligible for clinical trials adjuvant GM-CSF treatment is a reasonable option for individuals with resected high risk melanoma.
Keywords: immunotherapy, recurrence, postoperative treatment
Introduction
Lymphatic metastases are the single most important prognostic factor in patients with melanoma. The 5-year overall survival rate for patients with stage III disease is approximately 50% 1. Survival decreases with increasing nodal tumor burden, measured as an increasing number of lymph nodes involved and increasing size of metastasis 1. When possible, surgical resection remains the most promising opportunity for long-term survival. Despite having (clinical or measurable) disease confined to the primary site and regional basin, this population is at very high risk of distant relapse and death secondary to clinically occult micrometastatic disease present at the time of diagnosis, that progresses during follow-up. The ability to prevent further progression and control these unrecognized disseminated tumor cells is the goal of adjuvant therapy, ultimately culminating in improved patient survival.
Melanoma impairs the anti-tumor immune response within nearby lymph nodes. Tumor draining lymph nodes contain fewer and less mature or activated dendritic cells suggesting poor antigen presentation capabilities. Furthermore, there is a reduction in T cell number and function, leading to melanoma progression and dissemination 2. Restoration of the locoregional and systemic immunity to a level that controls the progression of melanoma may lead to prolonged survival. Interferon alpha (IFN-α) is currently the only FDA approved adjuvant therapy for stage III melanoma and offers a modest improvement in disease-free survival and minimal, if any, improvement in overall survival. IFN-α is poorly tolerated and the optimal dose, treatment duration and subset of patients most likely to benefit from therapy have not been elucidated 3. Granulocyte-macrophage colony-stimulating factor (GM-CSF) activates the innate immune system by stimulating the cytotoxicity of peripheral blood monocytes 4,5 and is the principle mediator of dendritic cell proliferation, maturation and migration 6–8. GM-CSF also stimulates tumor infiltrating macrophages to produce more angiostatin which suppresses angiogenesis, a critical component in the metastatic cascade 9. This restoration of the innate immune system may be beneficial for patients rendered surgically NED and at high risk for recurrence and death.
Despite the limited data on GM-CSF and lack of FDA approval, our institution has been utilizing adjuvant GM-CSF for the past decade because of the poor tolerability, poor response and relative lack of survival benefit of IFN-α. This study reports on the largest clinical experience with adjuvant GM-CSF in patient’s with melanoma. The aim of this study is to compare outcomes of two different postoperative management regimens in patients with stage III melanoma; specifically, observation or adjuvant GM-CSF in a retrospective cohort study.
Materials and Methods
After institutional IRB approval we performed a retrospective cohort study of all adult patients with stage III melanoma treated at the Mayo Clinic Rochester from January 1st, 2001 to December 30th, 2010. Clinical and demographic data was collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Mayo Clinic 10. Patients were staged according to the American Joint Commission on Cancer (AJCC) 7th edition. Adjuvant treatment after complete surgical resection and staging was divided into expectant observation and GM-CSF according to the Spitler et al. regimen11 (GM-CSF administered at 250 mcg/day subcutaneous for 14 days of a 28 day cycle) given for 1 to 3 years or until recurrence11. Patients treated with IFN-α were excluded from analysis. Patients treated in clinical trials with vaccine therapy (n=43) were included in the observation cohort as the group was too small for separate analysis and no vaccine therapy has been demonstrated to be superior to observation. Patients who received at least one dose of GM-CSF within 90 days of their definitive surgical procedure were included using an intention-to-treat analysis. Patients with initial stage III disease as well as those with earlier stage melanoma who subsequently developed a nodal recurrence were included. However, patients who had recurrent disease were only included if they had the recurrence completely excised and were observed or began adjuvant GM-CSF therapy within 90 days of the surgery for stage III disease. The date of complete resection of all disease was utilized as the start date for all patients and patients who were lost to follow up or died secondary to unrelated causes were censored at time of last follow up.
Statistical Analysis
Descriptive statistics are reported as median and interquartile range (IQR) or number and frequency. Clinical and pathological characteristics for the two cohorts were compared using the Wilcoxon rank sum test or student’s t-test as appropriate. Patient survival and recurrence survivals at 1, 3, 5, and 10 years were estimated using the Kaplan-Meier method. Univariate and multivariate associations between observation and adjuvant GM-CSF and locoregional or distant recurrence and melanoma-specific survival were assessed using Cox proportional hazards regression. The magnitude of associations is reported as hazard ratios and 95% confidence interval (CI). The following variables were included in univariate analysis for each outcome; treatment, age, sex, Breslow depth, ulceration, location, recurrence or primary, AJCC stage (IIIA, IIIB and IIIC), ECOG status (0 vs. ≥ 1) The multivariate model was chosen using a backward selection method, though forward and stepwise selection procedures also happen to have yielded the same final models. All statistical tests were two-sided, with a p-values of ≤.05 were considered statistically significant. All statistical analysis was performed using SAS software.
Results
Patient Characteristics
Over the 10 year study period 317 patients with stage III disease were followed for a median of 34 months. Survivors were followed for a median of 44 months. There were 165 (52%) patients observed expectantly with history and physical exam every 3–6 months, imaging as per physician discretion and at minimum annual dermatological examinations including the skin and lymph node basins. There were 152 (48%) patients treated with adjuvant GM-CSF in addition to routine surveillance. The clinical and pathological characteristics of the two cohorts are illustrated in Table 1. Briefly, the group of patients treated with GM-CSF tended to be younger (p < 0.0001), had more advanced stage disease (p = 0.002) and were more likely to have had a recurrence prior to initiation of adjuvant therapy than the observation group (p < 0.0001).
Table 1.
Comparison of clinical and pathological characteristics of each adjuvant treatment cohort following complete resection of AJCC stage III melanoma.
| GMCSF (N=152) | Observation (N=165) | Total (N=317) | p value | |
|---|---|---|---|---|
| Sex | 0.50851 | |||
| Female | 57 (38%) | 56 (34%) | 113 (36%) | |
| Male | 95 (63%) | 109 (66%) | 204 (64%) | |
| Age | <0.00012 | |||
| Median (Q1,Q3) | 52 (39, 59) | 59 (47, 68) | 55(44, 66) | |
| Location | 0.62421 | |||
| Unknown | 27 (18%) | 21 (13%) | 48 (15%) | |
| Head or Neck | 35 (23%) | 42 (25%) | 77 (24%) | |
| Trunk | 36 (24%) | 38 (23%) | 74 (23%) | |
| Extremity | 54 (36%) | 64 (39%) | 118 (37%) | |
| Breslow | 0.66832 | |||
| Median(Q1,Q3) | 2.3(1.3, 4.0) | 2.2(1.4, 4.0) | 2.3(1.3, 4.0) | |
| Ulceration | 41 (27%) | 40 (24%) | 81 (26%) | 0.57751 |
| Stage | 0.00222 | |||
| IIIA | 31 (20%) | 70 (42%) | 101 (32%) | |
| IIIB | 75 (49%) | 52 (32%) | 127 (40%) | |
| IIIC | 46 (30%) | 43 (26%) | 89 (28%) | |
| ECOG Category | 0.14861 | |||
| 0 | 122 (86%) | 145 (91%) | 267 (89%) | |
| ≥1 | 20 (14%) | 14 (9%) | 34 (11%) | |
| Recurrence prior to adjuvant therapy | 52 (34%) | 13 (8%) | 65 (21%) | <0.00011 |
| Radiation | 28 (18%) | 25 (15%) | 53 (17%) | 0.43581 |
Chi-Square
Kruskal Wallis
Disease-free survival
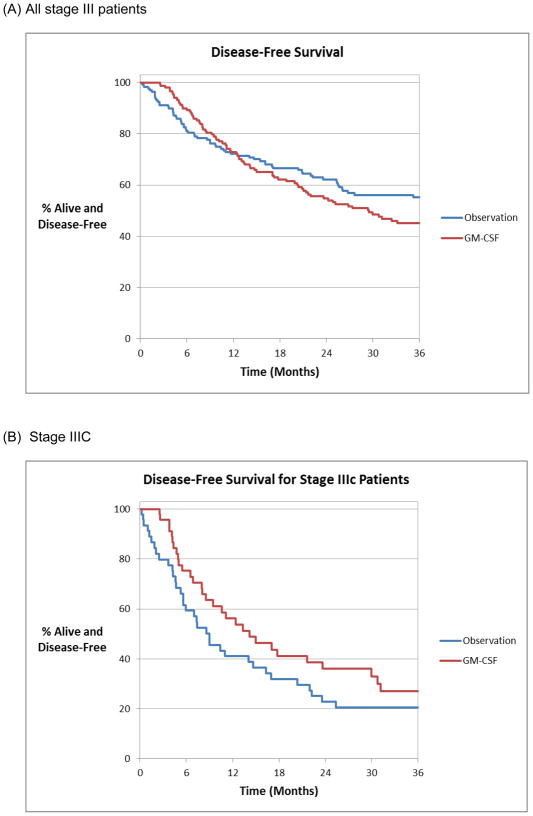
The median disease-free survival (DFS) was 36 months with a 1, 3 and 5-year disease-free survival of 72%, 50% and 40% respectively. Nearly half (49%) of the recurrences occurred within the first year of follow up. The median DFS for patients who were observed expectantly was 47 months compared to 29 months for those treated with GM-CSF (Figure 1a). There was no significant difference in DFS for the different treatment groups as a whole, as well as within each stage separately (IIIA, IIIB and IIIC). Adjuvant GM-CSF appeared to prolong DFS within the 89 patients with stage IIIC melanoma; however, this did not reach significance (p = 0.14) (Figure 1b). In univariate analysis sex, age, Breslow depth, AJCC stage (IIIA, IIIB and IIIC), recurrence and adjuvant regional radiation were significantly associated with DFS; however, type of adjuvant systemic treatment was not associated with DFS (p = 0.38). To correct for significant clinical and demographic prognostic factors, a Cox proportional hazards model that included the variables from the univariate analysis that remained after backward selections was applied. This analysis confirmed that male sex (Males HR 2.38, 95% CI 1.56–3.64, p < 0.0001), older age (per year, HR 1.01 95% CI 1.00–1.02, p = 0.047), increased Breslow depth (HR 1.06 per mm, 95% CI 1.01–1.13, p = 0.03) and advanced AJCC stage (IIIB HR 2.2 95% CI 1.43–3.4, p < 0.001 and IIIC HR 3.96 95% CI 2.48–6.33, p < 0.0001) remained independently associated with a worse DFS (Table 2). Treatment type was not associated with disease-free survival in multivariate analysis of the entire cohort.
Figure 1.
Disease-free survival estimate using Kaplan Meier method for (A) all stage III and (B) all stage IIIC patients treated with adjuvant GM-CSF or observation.
Table 2.
Multivariate analysis using a Cox proportional Hazards Model for (A) Disease-free and (B) Melanoma-specific survival.
| (A)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Level | Total (Events) | Univariate Hazard Ratio | Univariate 95% CI | Univariate p-value | Total (Events) | Multivariable Hazard Ratio | Multivariable 95% CI | Multivariable p-value |
| Treatment | GMCSF | 152 (87) | 1.14 | (0.84,1.55) | 0.384 | ||||
| Observation | 165 (80) | 1.0 (Ref) | - | - | |||||
| Sex | Female | 113 (47) | 1.0 (Ref) | - | - | 85 (29) | - | - | |
| Male | 204 (120) | 1.72 | (1.23,2.42) | 0.002 | 181 (108) | 2.38 | (1.56,3.64) | <0.001 | |
| Age | per year | 317 (167) | 1.01 | (1.00,1.02) | 0.008 | 266 (137) | 1.01 | (1.00,1.02) | 0.047 |
| Stage | IIIA | 101 (35) | 1.0 (Ref) | - | - | 97 (33) | - | - | |
| IIIB | 127 (67) | 1.89 | (1.25,2.85) | 0.002 | 103 (58) | 2.20 | (1.43,3.40) | <0.001 | |
| IIIC | 89 (65) | 3.81 | (2.52,5.77) | <0.001 | 66 (46) | 3.96 | (2.48,6.33) | <0.001 | |
| ECOG Category | 0 | 267 (132) | 1.0 (Ref) | - | - | ||||
| ≥1 | 34 (21) | 1.50 | (0.94,2.38) | 0.087 | |||||
| Breslow Depth | per mm | 266 (137) | 1.11 | (1.06,1.17) | <0.001 | 266 (137) | 1.06 | (1.01,1.13) | 0.031 |
| Recurrence prior to adjuvant therapy | No | 252 (122) | 1.0 (Ref) | - | - | ||||
| Yes | 65 (45) | 1.59 | (1.13,2.24) | 0.008 | |||||
| Radiation | No | 264 (132) | 1.0 (Ref) | - | - | ||||
| Yes | 53 (35) | 1.72 | (1.18,2.50) | 0.005 | |||||
| (B)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Level | Total (Events) | Univariate Hazard Ratio | Univariate 95% CI | Univariate p-value | Total (Events) | Multivariable Hazard Ratio | Multivariable 95% CI | Multivariable p-value |
| Treatment | GMCSF | 152 (42) | 0.70 | (0.47,1.04) | 0.079 | ||||
| Observation | 165 (58) | 1.0 (Ref) | - | - | |||||
| Sex | Female | 113 (21) | 1.0 (Ref) | - | - | 85 (14) | - | - | |
| Male | 204 (79) | 2.56 | (1.58,4.15) | <0.001 | 181 (71) | 2.45 | (1.35,4.43) | 0.003 | |
| Age | per year | 317 (100) | 1.02 | (1.01,1.04) | <0.001 | 266 (85) | 1.03 | (1.01,1.04) | 0.002 |
| Stage | 3A | 101 (23) | 1.0 (Ref) | - | - | 97 (21) | - | - | |
| 3B | 127 (31) | 1.17 | (0.68,2.00) | 0.576 | 103 (30) | 1.37 | (0.78,2.42) | 0.278 | |
| 3C | 89 (46) | 3.28 | (1.98,5.41) | <0.001 | 66 (34) | 3.29 | (1.87,5.77) | <0.001 | |
| ECOG Category | 0 | 267 (73) | 1.0 (Ref) | - | - | ||||
| ≥1 | 34 (14) | 1.88 | (1.06,3.34) | 0.032 | |||||
| Breslow Depth | per mm | 266 (85) | 1.14 | (1.07,1.21) | <0.001 | 266 (85) | 1.1 | (1.02,1.18) | 0.012 |
| Recurrence prior to adjuvant therapy | No | 252 (81) | 1.0 (Ref) | - | - | ||||
| Yes | 65 (19) | 0.85 | (0.51,1.40) | 0.520 | |||||
| Radiation | No | 264 (80) | 1.0 (Ref) | - | - | ||||
| Yes | 53 (20) | 1.60 | (0.98,2.62) | 0.060 | |||||
Melanoma-specific survival
The median melanoma-specific survival (MSS) for the entire cohort was 103 months with a 3, 5 and 10-year melanoma-specific survival of 73%, 62% and 43%, respectively. The median MSS for patients who were observed expectantly was 99 months compared to 102 months for those treated with GM-CSF. The 3, 5 and 10-year MSS for patients treated with adjuvant GM-CSF was 77%, 67% and 49%, respectively and 68%, 57% and 39% for those who were observed, respectively. This improvement in MSS with adjuvant GM-CSF over observation approached but did not reach significance (p = 0.08) (Figure 2a). In univariate analysis sex, age, Breslow depth, ulceration, ECOG status and AJCC stage (IIIA, IIIB and IIIC) were significant predictors of MSS. In multivariate analysis sex (Males HR 2.45 95% CI 1.35–4.43, p = 0.003), older age (per year HR 1.03 95% CI 1.01–1.04, p = 0.002), increasing Breslow depth (per mm OR 1.1 95% CI 1.02–1.18, p = 0.012) and stage IIIC (OR 3.29 95% CI 1.87–5.77, p < 0.001) remained independently associated with a worse MSS (Table 2). Adjuvant GM-CSF was not independently associated with improved MSS in multivariate analysis (p = 0.19) compared with observation. However, within the subgroup of stage IIIC patients, GM-CSF relative to observation was significantly associated with a 52% lower risk of melanoma-specific mortality (HR 0.48, 95% CI 0.27–0.87, p = 0.02) (Figure 2b). This improvement in MSS was not seen in patients with less advanced stage III disease (IIIA and IIIB).
Figure 2.
Melanoma-specific survival estimate using Kaplan Meier method for (A) all stage III patients and (B) patients with stage IIIC melanoma treated with adjuvant GM-CSF or observation.
Discussion
We report the largest experience of adjuvant GM-CSF in patients with melanoma. In our study of patients with surgically resected stage III melanoma we observed that the stage IIIC population receiving adjuvant GM-CSF experienced a greater than 50% relative reduction in melanoma-specific deaths compared with the observation group. This benefit was only seen in those with advanced stage IIIC disease and not the stage IIIA or stage IIIB cohorts. Our results are similar to preliminary studies done by Spitler et al. who first reported prolonged overall and disease-free survival in forty-eight patients with stage III or IV melanoma treated with adjuvant GM-CSF compared to matched historical controls11. It is worth highlighting that the stage III inclusion criteria for Splitler et al’s phase II clinical trial included more than four positive nodes or a nodal mass > 3-cm in diameter suggesting that most of the patients were stage IIIC; the same cohort we found derived the most benefit from adjuvant GM-CSF. A subsequent single arm clinical trial by Spitler et al. of 98 patients with stage II, III and IV melanoma reported 5-year melanoma-specific survival for stage III and IV patients of 67% and 40%, respectively, which compare favorably to historical controls12. The largest group in their study consisted of patients with stage IIIC disease and over half of the study population was stage IIIB or IIIC, with only 5% stage II.
Similarly, multiple phase II clinical trials including patients with stage II, III and IV melanoma treated with sequential GM-CSF and IL-2 with or without autologous vaccine reported benefit for high-risk patients with surgically resected melanoma,13–15 though it is not clear how much each individual component provided benefit. Together, these results suggest that adjuvant GM-CSF is effective in prolonging melanoma-specific survival in a subset of patients with high risk melanoma.
In our study, we did not see an improvement in DFS; however, the Kaplan Meier graph in Figure 1b suggests that there could potentially be a delay in recurrences in the stage IIIC patients treated with adjuvant GM-CSF. The delay in recurrence was not significant, although this may be due to the small sample size. It is reasonable to postulate that the activated macrophages and dendritic cells from GM-CSF treatment alone may not be able to completely eliminate microscopic residual disease, but in a subset of patients suppresses the growth of these malignant cells while therapy is ongoing. Interestingly, Spitler et al. also observed a greater effect of adjuvant therapy with GM-CSF on overall survival than on disease-free survival, as well as an increase in disease recurrences when patients stopped treatment after 1 year11. She subsequently has increased the duration of treatment with GM-CSF to three years and has reported a longer median DFS and a longer median survival which has not yet been reached after 5.3 years of follow up12. Since her publication we have since gone to a longer 3-year course of GM-CSF. In our patients who develop local-regional or oligometasatic distant recurrence and undergo ablation or resection, we also resume GM-CSF once rendered NED. Our clinical outcome data, though intriguing and confirmatory of other smaller series, remains hypothesis generating in terms of mechanistic explanations of the findings.
Our study confirms the heterogeneous nature of stage III melanoma as the prognosis varied from 80% 5-year MSS for stage IIIA melanoma to 22% 5-year MSS for stage IIIC melanoma. As such, patients with stage IIIA melanoma appear to do well regardless of treatment type and therefore may be best served with close surveillance. The low number of events in this cohort likely diluted the beneficial effects of our analysis when combining all the stage III patients into one group. In contrast, patients with stage IIIC melanoma are at high risk of subsequent relapse and death and the high number of events allow for the benefit from adjuvant GM-CSF to be demonstrated in spite of the study size. The prognosis for stage IIIB patients is more intermediate and therefore the benefit from adjuvant GM-CSF may be smaller, requiring a much larger sample size to demonstrate, if indeed a difference does exist. Better prognostic markers are needed in order to clarify the stage IIIB patients at highest risk of relapse who may benefit from adjuvant therapy.
This retrospective cohort study is limited by its very design, in that there is inherent selection bias in the clinical decision to observe or provide adjuvant therapy, and if adjuvant therapy is to be administered, which type. As illustrated in Table 1 patients with more advanced stage and recurrent disease are more likely to receive intervention, in this case adjuvant GM-CSF. Expectant observation was most frequently favored for patients at lower risk for recurrence and this group was also on average older. These differences are likely to underestimate the benefit of adjuvant GM-CSF and we have attempted to control for this by multivariate analysis using a Cox proportional hazards model. Another weakness of this study is the small sample size that limits our ability to derive conclusions from subgroup analysis. We also chose to include vaccine treated patients in the observation arm. Though there is some data to suggest patients may have done worse on vaccine arms16. We found no significant difference in DFS or MSS between the observed cohort and the cohort receiving vaccine (data not shown) and therefore did not exclude these patients. This study represents the largest reported experience with adjuvant GM-CSF and provides additional support for its use in high risk patients.
In conclusion, despite its inherent limitations the current study has expanded the preliminary evidence on GM-CSF as an adjuvant therapy for patients with melanoma who are at high risk for recurrence and death. Our study is consistent with previous studies by Spitler11,12, demonstrating an improvement in MSS for advanced stage III patients and lends further support for the use of adjuvant GM-CSF in the highest risk cohort. Additional evidence regarding the efficacy of adjuvant GM-CSF therapy will await the results of the E4697, a phase III clinical trial which randomizes stage IIIB, IIIC and IV melanoma to GM-CSF or placebo for one year. This trial has completed accrual and has been presented in abstract form 17.
Acknowledgments
Grant Support
The work was supported by NIH/NCRR/NCATS CTSA Grant Number UL1 TR000135. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
The authors individually or collectively have no significant financial conflicts that need disclosing.
References
- 1.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 2.Grotz TE, Mansfield AS, Jakub JW, et al. Regional lymphatic immunity in melanoma. Melanoma Res. 2011 doi: 10.1097/CMR.0b013e32834e1f33. [DOI] [PubMed] [Google Scholar]
- 3.Mocellin S, Pasquali S, Rossi CR, et al. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 4.Grabstein KH, Urdal DL, Tushinski RJ, et al. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986;232:506–8. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- 5.Thomassen MJ, Barna BP, Rankin D, et al. Differential effect of recombinant granulocyte macrophage colony-stimulating factor on human monocytes and alveolar macrophages. Cancer Res. 1989;49:4086–9. [PubMed] [Google Scholar]
- 6.Young JW, Szabolcs P, Moore MA. Identification of dendritic cell colony-forming units among normal human CD34+ bone marrow progenitors that are expanded by c-kit-ligand and yield pure dendritic cell colonies in the presence of granulocyte/macrophage colony-stimulating factor and tumor necrosis factor alpha. J Exp Med. 1995;182:1111–9. doi: 10.1084/jem.182.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabolcs P, Avigan D, Gezelter S, et al. Dendritic cells and macrophages can mature independently from a human bone marrow-derived, post-colony-forming unit intermediate. Blood. 1996;87:4520–30. [PubMed] [Google Scholar]
- 8.Szabolcs P, Moore MA, Young JW. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol. 1995;154:5851–61. [PubMed] [Google Scholar]
- 9.Dong Z, Kumar R, Yang X, et al. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell. 1997;88:801–10. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spitler LE, Grossbard ML, Ernstoff MS, et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18:1614–21. doi: 10.1200/JCO.2000.18.8.1614. [DOI] [PubMed] [Google Scholar]
- 12.Spitler LE, Weber RW, Allen RE, et al. Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF, sargramostim) administered for 3 years as adjuvant therapy of stages II(T4), III, and IV melanoma. J Immunother. 2009;32:632–7. doi: 10.1097/CJI.0b013e3181a7d60d. [DOI] [PubMed] [Google Scholar]
- 13.Elias EG, Zapas JL, Beam SL, et al. GM-CSF and IL-2 combination as adjuvant therapy in cutaneous melanoma: early results of a phase II clinical trial. Oncology (Williston Park) 2005;19:15–8. [PubMed] [Google Scholar]
- 14.Block MS, Suman VJ, Nevala WK, et al. Pilot study of granulocyte-macrophage colony-stimulating factor and interleukin-2 as immune adjuvants for a melanoma peptide vaccine. Melanoma Res. 2011;21:438–45. doi: 10.1097/CMR.0b013e32834640c0. [DOI] [PubMed] [Google Scholar]
- 15.Elias EG, Zapas JL, McCarron EC, et al. Sequential administration of GM-CSF (Sargramostim) and IL-2 +/− autologous vaccine as adjuvant therapy in cutaneous melanoma: an interim report of a phase II clinical trial. Cancer Biother Radiopharm. 2008;23:285–91. doi: 10.1089/cbr.2007.0438. [DOI] [PubMed] [Google Scholar]
- 16.Morton DL, Mozzillo N, Thompson JF, et al. An international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. J Clin Oncol. 2007;25(18S):Abstract 8508. [Google Scholar]
- 17.Lawson DH, Lee SJ, Tarhini AA, Margolin KA, Ernstoff MS, Kirkwood JM. E4697: Phase III cooperative group study of yeast-derived granulocyte macrophage colony-stimulating factor (GM-CSF) versus placebo as adjuvant treatment of patients with completely resected stage III–IV melanoma. J Clin Oncol. 2010;28:15s. (suppl; abstr 8504) [Google Scholar]