Abstract
Seizures are a serious and debilitating co-morbidity of primary brain tumors that affect most patients, yet their etiology is poorly understood. In many CNS pathologies, including epilepsy and brain injury, high levels of extracellular glutamate have been implicated in seizure generation. It has been shown that gliomas release neurotoxic levels of glutamate through their high expression of system xc-. More recently it was shown that the surrounding peritumoral cortex is spontaneously hyperexcitable. In this review, we discuss how gliomas induce changes in the surrounding environment that may further contribute to elevated extracellular glutamate and tumor-associated seizures. Peritumoral astrocytes become reactive and lose their ability to remove glutamate, while microglia, in response to signals from glioma cells, may release glutamate. In addition, gliomas increase blood brain barrier permeability, allowing seizure-inducing serum components, including glutamate, into the peritumoral region. These factors, working together or alone, may influence the frequency and severity of tumor-associated epilepsy.
Keywords: glioma, seizures, astrocytes, gliosis, hyperexcitability, glutamate
Introduction
Seizures, focal, partial, or generalized, are experienced by 60–80% of glioma patients during the course of their disease (Lynam et al. 2007; Oberndorfer et al. 2002; Hildebrand et al. 2005; Kurzwelly et al. 2010). Primary brain tumors have an incidence rate of 18 per 100,000 by year in the United States (Porter et al. 2010). Approximately 60% of those tumors are gliomas that originate from astrocytes or other glial cells. Astrocytic tumors are clinically categorized as either low-grade or high-grade astrocytomas based on their specific histological features (Furnari et al. 2007). Brain tumors that originate from metastasizing peripheral cancers also induce seizures in approximately 25% of patients (Davis et al. 2012), and while some of the etiology of these seizures may be similar, for this review, we will focus on the mechanisms causing glioma-associated seizures.
The incidence of tumor-associated seizures in glioma patients appears to correlate with the histological grade of the tumor and the tumor’s location within the brain; patients with lower grade gliomas in cortical areas of the temporal lobe are more than two times as likely to present with seizures, compared to patients with high-grade gliomas (Lee et al. 2010; Hildebrand et al. 2005), and those with tumors located in the insular cortex are more likely to present with seizures, especially if the tumor is on the right side. Interestingly, tumors located within the right hemisphere have the highest correlation to seizures as the presenting symptom (Hildebrand et al. 2005). Several studies have determined that seizures originate close to the tumor mass. Patt et al. (2000) used EEG dipole localization techniques to identify the foci of epileptic activity in glioma patients and found that within a 15-minute recording period, all patients exhibited abnormal activity consisting of sharp waves, spikes and/or polyspikes. They found a strong correlation between tumor histological grade and distance between the seizure foci and the tumor border; in general, the foci in low grade glioma patients was closest to the tumor border, while most of the foci in high grade glioma patients were found to be more distant from the border, particularly in those with glial-derived tumors (Patt et al. 2000). Surgical resection of the tumor leads to seizure-free periods, however ultimately the majority of patients will again experience seizures that coincide with tumor recurrence.
Tumor-associated Seizures
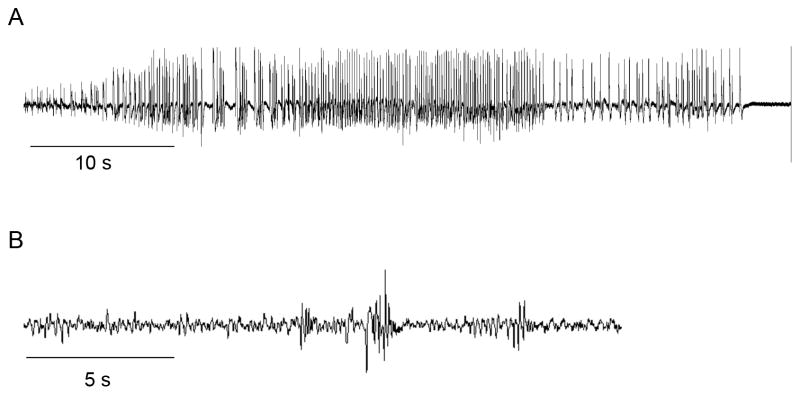
A seizure may be the first unmistakable co-morbidity that leads to tumor diagnosis, but changes in cortical excitability likely occur well before the first convulsion. Evidence for this can be found in some of the few studies that have attempted to model peritumoral seizures in rodents. Studies have been limited, in part due to the difficulty of extensively monitoring glioma-implanted animals for spontaneously-occurring events, most of which can only be detected through EEG. In addition, rodents are thought to be far more resistant to seizure development compared to humans. However, in 2006, Kohling et al (2006) observed that rats, implanted intracranially with rodent C6 glioma cells and monitored by EEG, exhibited spontaneous epileptiform discharges throughout the 12– 15 day recording period; this activity consisted of spikes and slow waves or sharp waves lasting for several seconds without any phenotypic convulsive behavior by the animals. In this study, epileptiform activity was found to originate outside the tumor mass but within the peritumoral area, identified as within 1–2 mm from the tumor border (Kohling et al. 2006). A more recent study by Buckingham et al (2011) used continuous EEG/video recording to monitor mice intracranially implanted with human glioma cells. They found that 37% of these animals showed spontaneous spiking that increased over time and that this spiking was associated with a subtle behavioral phenotype (Fig. 1A; Buckingham et al. 2011).
Fig 1. Animals implanted with human glioma cells show spontaneous epileptic activity.
A) EEG recording of a seizure from a mouse intracranially-implanted with GBM22 human glioma cells, 15 d after implantation, and B) EEG recording showing typical spontaneous spiking in a mouse intracranially-implanted with the human glioma cell line U251
Since these studies used well-established cell lines maintained in vitro, the absence of a convulsive phenotype in glioma-implanted rodents may reflect the nature of how tumor cells are maintained and propagated. Recent data from our lab shows that mice intracranially implanted with human glioma cells, maintained as orthotopic tumors on the flanks of nude mice, exhibit grand-mal seizures evident both in EEG recordings and in their convulsive behavior (Fig. 1B). Orthotopically passaged xenografts, initiated at the Mayo Clinic from patient biopsies, do not lose expression of key genes and proteins, such as epidermal growth factor receptor (EGFR) and platelet derived growth factor receptor (PDGFRα), and they maintain similar intracranial growth characteristics as human gliomas (Giannini et al. 2005; Sarkaria et al. 2007). Interestingly, mice implanted with these tumors show periods of increased spike activity for several days preceding seizures (unpublished data). This finding is corroborated in a human study that used EEG to detect brain wave abnormalities in glioma patients who had no seizure history; those who exhibited irregularities later developed seizures whereas those with normal EEGs remained seizure-free (Lynam et al. 2007). Tumor-associated seizures may also result from alterations in many factors and processes within the brain environment; however, recent experimental evidence indicates that in the tumor and in peritumoral brain, prolonged increases in extracellular glutamate may play a pivotal role in seizure generation.
Elevated Peritumoral Glutamate
Prolonged elevations of extracellular glutamate, dysfunctional glutamate receptor signaling, or glutamate processing, are all implicated in the development of cortical hyperexcitability, seizures, and ultimately, neuronal death that has been noted in epilepsy, stroke, and brain trauma, as well as in chronic neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease (Wang and Qin 2010). Elevated levels of extracellular glutamate causes a prolonged activation of neuronal NMDA receptors that, in turn, induces the sustained influx of Ca2+ into the cell, and ultimately Ca2+-mediated excitotoxicity. In a recent study, sustained activation of NMDARs by glutamate was found to significantly alter the phosphorylation state of a GABABR subunit, resulting in this subunit’s degradation and the loss of GABABRs inhibitory function (Terunuma et al. 2010). In addition, prolonged glutamate-activation of NMDARs has been shown to cause a reduction in KCC2, a neuron-specific potassium-chloride co-transporter involved in the proper functioning of GABAAR, thereby disrupting GABAergic transmission (Lee et al. 2011a). Peritumoral cortical hyperexcitability has been found to result, in part, from excessive glutamate-driven NMDAR activation in conjunction with reduced inhibitory GABAergic transmission (Campbell et al. 2012).
Glutamate concentrations measured in microdialysates from tumor and peritumoral brain of high-grade astrocytoma patients were significantly elevated, especially proximal to tumors that contained areas of necrosis (Roslin et al. 2003). Further evidence of high extracellular glutamate in peritumoral brain can be found in a study that used magnetic resonance spectroscopy to assess the metabolic profiles of oligodendrogliomas in patients. The authors found that glutamate concentrations were significantly increased in peritumoral regions compared to uninvolved brain (Rijpkema et al. 2003). In an animal model of glioma, tumor-associated glutamate measured by microdialysis was increased almost 4-fold within the tumor and 2-fold within peritumoral brain compared to surrounding cortex; the authors speculate that although glutamate levels are known to be higher after an acute brain injury from stroke or trauma, this increase is transient, whereas elevated tumor-associated glutamate is likely to persist throughout tumor growth (Behrens et al. 2000). And finally, in a recent study using an animal model of glioma, glutamate release from tumor-containing acute brain slices was measured by HPLC-mass spectrometry and glutamate release from tumor-containing slices was significantly greater compared to control, non-tumor-bearing slices (Buckingham et al. 2011). A number of studies investigating the source of peritumoral glutamate have found that glioma cells, unable to take up glutamate due to their lack of functional astrocyte-specific transporters GLT-1 and GLAST, highly express the cystine-glutamate exchanger, system xc- through which they release cytotoxic levels of glutamate into the extracellular space (Ye and Sontheimer 1999; Takano et al. 2001; de Groot et al. 2005).
System xc- is a Na+-independent cystine/glutamate exchanger composed of a regulatory subunit, CD98, and a catalytic subunit, xCT; it serves as a key regulator of intracellular glutathione by importing one molecule of extracellular cystine, a precursor required in glutathione synthesis, in exchange for the obligatory release of one molecule of glutamate (Bannai 1986; Sato et al. 1999; for a thorough review of system xc- and it’s role in pathological conditions, see Bridges et al. 2012). Expression of the xCT subunit is significantly increased in human glioma cell lines and patient biopsies, and cultured glioma cells release large amount of glutamate through system xc- (Savaskan et al. 2008; Lyons et al. 2007; Ye and Sontheimer 1999). Glioma cells with experimentally inhibited xCT expression released significantly less glutamate; after these cells were intracranially implanted in rodents, these animals lived longer and showed delayed symptom development compared to animals implanted with control cells (Savaskan et al. 2008). Interestingly, animals implanted with xCT-silenced cells had similar tumor volumes as animals implanted with unaltered gliomas, yet they showed significantly less tumor-associated edema, indicating that system xc- has a role in the disruption of the blood brain barrier. Biophysical recordings conducted on peritumoral neurons in acute brain slices from glioma-bearing animals determined that neurons located closest to the tumor mass were more excitable with a reduced latency for development of epileptiform activity that was inhibited by application of a system xc- specific blocker, sulfasalazine (SAS), a drug that is used in the treatment of Crohn’s disease; SAS treatment also reduced the number of spontaneous epileptic events in glioma-implanted mice, further implicating system xc- mediated glutamate release in the generation of peritumoral seizures (Buckingham et al. 2011; for a review of SAS in the treatment of primary brain tumors, see Sontheimer and Bridges 2012).
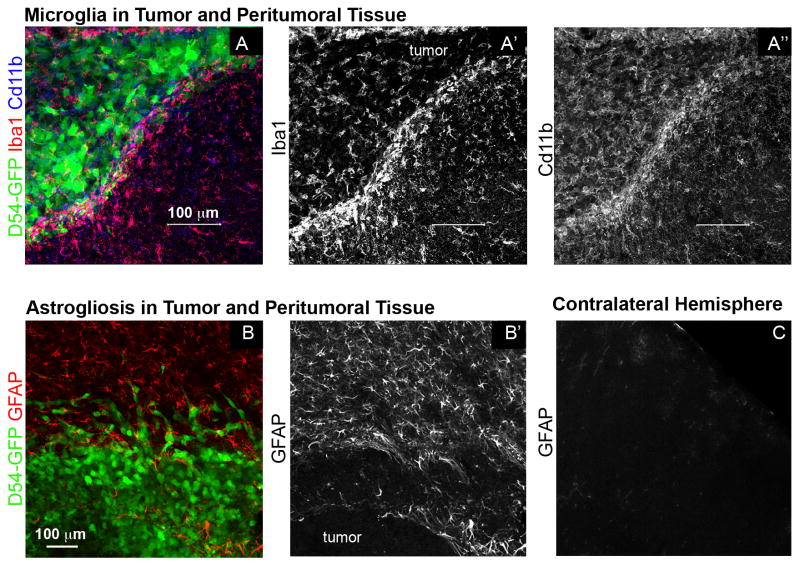
Additional sources of elevated tumor-associated extracellular glutamate may be activated microglia (Fig. 2A–A″), which also express system xc-, and in pathological conditions, release glutamate either through system xc- or through reverse sodium-dependent glutamate transport (for review, see Kettenmann et al. 2011). Activated microglia have been shown to take up glutamate in response to injury in vitro (Shaked et al. 2005) through a mechanism that involves a TNF-α-dependent upregulation of the glutamate transporter Glt-1, whose expression is normally restricted to astrocytes in the healthy brain (Persson et al. 2005; Persson et al. 2007). However, glioma cells may inhibit the microglial response by inducing an alternatively activated, anti-inflammatory phenotype, M2, without stimulating the production of TNF- α and other pro-inflammatory cytokines (for a recent review on the interactions between gliomas and microglia, see Li and Graeber 2012). Thus, it is conceivable that invading microglia surrounding the tumor mass do not take up glutamate, but instead may actually contribute to the high extracellular glutamate levels found in peritumoral brain.
Fig 2. Peritumoral astrocytes and microglia are activated.
A, A′ and A″) Microglia of tumor-implanted animals are positive for Iba1 and Cd11b. They are enriched at the tumor border and enter the GFP-positive tumor mass. B and B′) Gray matter astrocytes surrounding a glioma 3 wk after D54 tumor cell implantation become hypertroph and upregulate the intermediate filament GFAP. C) Unaffected astrocytes of the contralateral hemisphere are negative for GFAP.
Astrocytic Glutamate Homeostasis
The concept that astrocytes, specifically reactive astrocytes, are involved in epilepsy has been hypothesized for more than 40 years (Pollen and Trachtenberg 1970). In the healthy brain, astrocytes are almost exclusively responsible for the removal of extracellular glutamate and potassium to maintain very low extracellular concentrations. Parenchymal astrocytes are arranged in equally spaced, non-overlapping domains for optimal coverage (Bushong et al. 2002; Distler et al. 1991), and they possess a highly branched morphology with their finest processes enwrapping synapses to quickly uptake glutamate and avoid the spread of glutamate into neighboring areas. However, pathologic conditions can disrupt the ability of astrocytes to maintain glutamate/potassium homeostasis which may ultimately result in seizure generation (for review see Seifert et al. 2010).
Astrocytes express two electrogenic high-affinity sodium-dependent glutamate transporters, Glt-1 (EAAT2) and GLAST (EAAT1) (Chaudhry et al. 1995; Rothstein et al. 1994), both of which take up glutamate from the extracellular space after synaptic activity (Rothstein et al. 1996). Studies using transgenic mice have revealed that both transporters are highly expressed in the cerebral cortex at postnatal stages. Interestingly, at that developmental period there seems to be remarkably little overlap between GLAST and Glt-1 within individual astrocytes, suggesting that only one transporter at a time is active (Regan et al. 2007). However the mechanisms regulating expression in individual cells remain obscure. In the mouse, Glt-1 promoter activity remains high or even increases in regions such as hippocampal gray matter, but by postnatal day 25, cortical GLAST promoter activity is significantly reduced, remaining active in the forebrain only in hippocampus and white matter (Regan et al. 2007). The timing and mechanisms involved in the switch between GLAST and Glt-1 are not clear. Fate mapping studies using a knock-in mouse line that expresses tamoxifen-inducible Cre recombinase under the endogenous GLAST promoter (Glast::CreERT2) crossed to a GFP-reporter line, show successful recombination of cortical gray matter astrocytes after tamoxifen administration to adult mice. These results suggest that GLAST is expressed, at least at the transcriptome level, even in adult cortical gray matter astrocytes (Mori et al. 2006). Genetic deletion of Glt-1 in mice results in development of spontaneous seizures and increased susceptibility to subconvulsive doses of the GABA antagonist pentylenetetrazole (PTZ). The most likely cause for this increased excitability is that glutamate uptake in cerebral synaptosomes is significantly diminished, leading to increased levels of free glutamate in the synaptic cleft and a subsequent degeneration of hippocampal CA1 neurons (Tanaka et al. 1997; Rothstein et al. 1996). Spontaneous seizures have not been reported in mice that do not express GLAST, however these animals also have an increased susceptibility to PTZ-induced seizures (Watanabe et al. 1999), indicating that GLAST is functionally important in adult mice, albeit to a lesser degree than Glt-1.
Once glutamate is taken up by astrocytes, it can be released back into the extracellular space to act as a gliotransmitter for modulation of neuronal synaptic activity (Agulhon et al. 2012). Glutamate can also be metabolized in the TCA cycle or enzymatically converted into glutamine by glutamine synthetase (GS). Glutamine can then be shuttled back into neurons as a precursor for either glutamate or the inhibitory neurotransmitter GABA. In the CNS, GS is exclusively expressed in astrocytes, where it is important for glutamate metabolism and ammonium detoxification (for an extensive review on glutamate metabolism in astrocytes, see Coulter and Eid 2012). Ortinski et al (2010) found that in mouse hippocampus, the loss of GS function following experimental adenovirus-induced astrogliosis resulted in disruption of glutamine-glutamate cycling and a rapid depletion of synaptic GABA. They reported that GABA depletion did not affect excitatory synaptic currents in CA1 pyramidal neurons but did reduce inhibitory currents; the subsequent hyperexcitability of the hippocampal circuit was reversed following glutamine application (Ortinski et al. 2010).
Astrocytic potassium homeostasis
In the healthy CNS, one of the major functions of astrocytes is to optimize synaptic transmission by tightly controlling extracellular ionic homeostasis. A single action potential can increase the concentrations of extracellular potassium (K+) surrounding synapses by almost 1 mM (Ransom et al. 1995). Quiescent astrocytes maintain a strongly negative resting membrane potential and a high resting permeability to K+ ions. The negative resting membrane potential, near to the K+ equilibrium potential of −85mV, is largely established and maintained by the inward rectifying astrocytic K+ channel Kir4.1 (Olsen et al., 2007; Olsen and Sontheimer, 2008). Kir4.1 acts to support spatial K+ buffering and homeostasis in response to elevated extracellular K+ following neuronal activity. Mice with experimentally deleted Kir4.1 channel expression show prolonged elevations in extracellular K+ after neuronal activity; in addition, they have hyperexcitable neurons and develop seizures (Djukic et al., 2007). Kir4.1 function and potassium uptake are also impaired in the hippocampal CA1 region of patients with temporal lobe epilepsy (Bordey and Sontheimer 1998; Seifert et al. 2009; Hinterkeuser et al. 2000)
The major driving force for glutamate transport across the astrocytic membrane is the electrochemical gradient for sodium and K+ (Coulter 2012). Glt-1 and GLAST transporters take up glutamate more efficiently at negative resting potentials (for review, see Olsen and Sontheimer 2008). Since the resting membrane potential is mainly established by Kir4.1, the loss of this channel should also compromise glutamate uptake. The genetic knockdown or pharmacologic block of Kir4.1 channel function indeed reduces glutamate uptake in cultured astrocytes (Kucheryavykh et al. 2007). These experiments clearly demonstrate that glutamate and K+ homeostasis are closely linked. The disruption of proper Kir4.1 functioning in astrocytes can result in increased levels of extracellular K+ and interfere with effective glutamate removal, both of which would lead to neuronal hyperexcitability and the generation of seizures.
Astrogliosis in the Peritumoral Brain
Astrogliosis is a normal reaction of the CNS to diverse insults, and is commonly found in epileptic animal brains as well as in brain tissue from epilepsy patients (for recent reviews, see Wetherington et al. 2008; Seifert et al. 2010; Coulter and Eid 2012). In animal models of neuronal hyperexcitability, reactive astrocytes often exhibit an elongated morphology and increased overlap of their extended processes (Oberheim et al. 2008). Upregulated expression of the intermediate filament glial fibrillary acidic protein (GFAP) is an accepted marker of astrocyte reactivity. In the rodent pilocarpine model of epilepsy, and in brain samples from patients with drug resistant epilepsy, induction of GFAP gene expression was mediated by phospho-STAT3 (Xu et al. 2011), a transcriptional regulator involved in regulating GFAP expression in astrocytes during development and after injury. In mouse models of glioma (Fig. 2B–C; Lee et al. 2011b), as well as in tissue of glioma patients (Burel-Vandenbos et al. 2011; Raore et al. 2011), GFAP expression is upregulated. The extent of GFAP increase seems to positively correlate with glioma size (Lee et al. 2011b). Although peritumoral astrocytes also increased GFAP expression and are morphologically reactive, their physiological changes are not well characterized. The precise changes that occur for astrocytes to become reactive and their related functional consequences seem to be distinct for different pathologies and need to be evaluated separately for every condition (Sofroniew and Vinters 2010). It remains to be seen whether glutamate uptake in peritumoral astrocytes is impaired, as it is in astrocytes within the epileptic brain.
Tumor-associated Changes of the Blood-brain Barrier
There are other factors in the peritumoral brain microenvironment that have been shown to trigger seizures in animals, including exposure to serum components from blood-brain barrier (BBB) disruption. In a study by Marchi et al. (2007), 25% of cancer patients injected with Mannitol to temporarily open the BBB for chemotherapeutic drug delivery to the brain, experienced focal motor seizures (Marchi et al. 2007). Glioma growth is accompanied by a breakdown of the BBB (for reviews, see Lee et al. 2009; Wolburg et al. 2009), exposing the peritumoral brain to blood serum components such as albumin and glutamate, which may not only induce seizures acutely, but may also initiate astrogliosis (for a review of signaling pathways that induce gliosis, see Robel et al. 2011). The serum component fibrinogen has been shown to induce reactive astrogliosis through activation of TGF-β signaling (Schachtrup et al. 2010). TGF-β binds to its receptor, which leads to upregulation of intermediate filament expression and secretion of extracellular matrix components such as neurocan. In addition, following experimental focal BBB disruption in the rat cortex, TGF-βRs were found to mediate serum albumin uptake by astrocytes (Ivens et al. 2007). In this model, activation of astrocytes was followed by a lasting neuronal hypersynchrony in the absence of neuronal loss. In contrast, acute treatment of brain slices with albumin did not affect neuronal excitability (Seiffert et al. 2004; Ivens et al. 2007), indicating that astrogliosis precedes and is causative for neuronal hyperexcitability after BBB opening.
Serum-exposure can also lead to the direct down regulation of Kir4.1 astrocytic potassium channels that then increases extracellular potassium concentrations (David et al. 2009; Ivens et al. 2007), which might very well contribute to the development of hyperexcitability in these models. Strikingly, experimentally induced BBB disruption in the mouse cortex induced neuronal hyperexcitability, epileptiform activity, and neuronal network synchronization that was not seen in animals that were treated with a TGF-β receptor antagonist at the time of BBB breach (David et al. 2009; Ivens et al. 2007; Seiffert et al. 2004). Taken together, these studies indicate that reactive astrocytes undergo numerous morphological and physiological changes that ultimately interfere with glutamate and potassium homeostasis and result in neuronal hyperexcitability.
Conclusions
Animal models and patient studies that look at other forms of epilepsy provide valuable information to help us understand the underlying mechanisms of the serious and debilitating seizures associated with gliomas. The heterogeneity of glioma cell types, grades, and the affected brain regions all influence cortical hyperexcitability to a certain extent. On the cellular level, there are numerous factors that might work together or alone to create the hyperexcitable peritumoral environment. High levels of glutamate caused by gliomas and their microenvironment play an important role in peritumoral seizure generation. Glutamate can act acutely on peritumoral neurons by binding directly to NMDA receptors, but can also work through more circuitous routes, all of which lead to hyperexcitability and seizures. Glutamate is released by glioma cells via system xc- and possibly, by activated microglia. Glutamate concentrations may also be augmented by its entry from the blood stream through the disrupted blood brain barrier. In addition, peritumoral glutamate concentrations may remain elevated due to the loss of glutamate and potassium uptake capabilities in neighboring reactive astrocytes. Clearly, in this context, the many facets of glutamate signaling and metabolic/catabolic processes present numerous therapeutic targets for treatment of this devastating disease.
seizures are a common comorbidity of primary brain tumors in men and mice
extracellular glutamate concentrations are increased in peritumoral tissue
glioma cells release glutamate through the cystine/glutamate exchanger system xc-
astrocytes are responsible for glutamate homeostasis in the healthy brain
peritumoral astrocytes are reactive and might be impaired in glutamate uptake
Acknowledgments
We are grateful to Harald Sontheimer for his helpful comments on the manuscript. Work in the laboratory was supported by the German Research foundation (grant # RO 4224/1-1), the Epilepsy Foundation (grant # 222427), the National Institute of Health (grant # 5 RO1-NS052634), and by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (grant # 5P30HD038985). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Agulhon C, Sun MY, Murphy T, Myers T, Lauderdale K, Fiacco TA. Calcium Signaling and Gliotransmission in Normal vs. Reactive Astrocytes. Front Pharmacol. 2012;3:139. doi: 10.3389/fphar.2012.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. [PubMed] [Google Scholar]
- Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J. Extracellular glutamate and other metabolites in and around RG2 rat glioma: an intracerebral microdialysis study. J Neurooncol. 2000;47:11–22. doi: 10.1023/a:1006426917654. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. doi: 10.1016/s0920-1211(98)00059-x. [DOI] [PubMed] [Google Scholar]
- Bridges R, Lutgen V, Lobner D, Baker DA. Thinking Outside the Cleft to Understand Synaptic Activity: Contribution of the Cystine-Glutamate Antiporter (System xc-) to Normal and Pathological Glutamatergic Signaling. Pharmacol Rev. 2012;64:780–802. doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, Sontheimer H. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17:1269–1274. doi: 10.1038/nm.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burel-Vandenbos F, Benchetrit M, Miquel C, Fontaine D, Auvergne R, Lebrun-Frenay C, Cardot-Leccia N, Michiels JF, Paquis-Flucklinger V, Virolle T. EGFR immunolabeling pattern may discriminate low-grade gliomas from gliosis. J Neurooncol. 2011;102:171–178. doi: 10.1007/s11060-010-0308-4. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SL, Buckingham SC, Sontheimer H. Human glioma cells induce hyperexcitability in cortical networks. Epilepsia. 2012 doi: 10.1111/j.1528-1167.2012.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Eid T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia. 2012;60:1215–1226. doi: 10.1002/glia.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D, Friedman A. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J Neurosci. 2009;29:10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012;14:1171–1177. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JF, Liu TJ, Fuller G, Yung WK. The excitatory amino acid transporter-2 induces apoptosis and decreases glioma growth in vitro and in vivo. Cancer Res. 2005;65:1934–1940. doi: 10.1158/0008-5472.CAN-04-3626. [DOI] [PubMed] [Google Scholar]
- Distler C, Dreher Z, Stone J. Contact spacing among astrocytes in the central nervous system: an hypothesis of their structural role. Glia. 1991;4:484–494. doi: 10.1002/glia.440040508. [DOI] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, Schroeder MA, James CD. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J, Lecaille C, Perennes J, Delattre JY. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. 2005;65:212–215. doi: 10.1212/01.wnl.0000168903.09277.8f. [DOI] [PubMed] [Google Scholar]
- Hinterkeuser S, Schröder W, Hager G, Seifert G, Blümcke I, Elger CE, Schramm J, Steinhäuser C. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Europ J Neurosci. 2000;12:2087–2096. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kohling R, Senner V, Paulus W, Speckmann EJ. Epileptiform activity preferentially arises outside tumor invasion zone in glioma xenotransplants. Neurobiol Dis. 2006;22:64–75. doi: 10.1016/j.nbd.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, Skatchkov SN, Eaton MJ. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia. 2007;55:274–281. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- Kurzwelly D, Herrlinger U, Simon M. Seizures in patients with low-grade gliomas--incidence, pathogenesis, surgical management, and pharmacotherapy. Adv Tech Stand Neurosurg. 2010;35:81–111. doi: 10.1007/978-3-211-99481-8_4. [DOI] [PubMed] [Google Scholar]
- Lee HH, Deeb TZ, Walker JA, Davies PA, Moss SJ. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat Neurosci. 2011a;14:736–743. doi: 10.1038/nn.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Borboa AK, Baird A, Eliceiri BP. Non-invasive quantification of brain tumor-induced astrogliosis. BMC Neurosci. 2011b;12:9. doi: 10.1186/1471-2202-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lund-Smith C, Borboa A, Gonzalez AM, Baird A, Eliceiri BP. Glioma-induced remodeling of the neurovascular unit. Brain Res. 2009;1288:125–134. doi: 10.1016/j.brainres.2009.06.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Wen PY, Hurwitz S, Black P, Kesari S, Drappatz J, Golby AJ, Wells WM, III, Warfield SK, Kikinis R, Bromfield EB. Morphological characteristics of brain tumors causing seizures. Arch Neurol. 2010;67:336–342. doi: 10.1001/archneurol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012 doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynam LM, Lyons MK, Drazkowski JF, Sirven JI, Noe KH, Zimmerman RS, Wilkens JA. Frequency of seizures in patients with newly diagnosed brain tumors: a retrospective review. Clin Neurol Neurosurg. 2007;109:634–638. doi: 10.1016/j.clineuro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67:9463–9471. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, Hallene K, Diglaw T, Franic L, Najm I, Janigro D. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M. Inducible gene deletion in astroglia and radial glia--a valuable tool for functional and lineage analysis. Glia. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberndorfer S, Schmal T, Lahrmann H, Urbanits S, Lindner K, Grisold W. The frequency of seizures in patients with primary brain tumors or cerebral metastases. An evaluation from the Ludwig Boltzmann Institute of Neuro-Oncology and the Department of Neurology, Kaiser Franz Josef Hospital, Vienna. Wien Klin Wochenschr. 2002;114:911–916. [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 2008;107:589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt S, Steenbeck J, Hochstetter A, Kraft R, Huonker R, Haueisen J, Haberland N, Ebmeier K, Hliscs R, Fiehler J, Nowak H, Kalff R. Source localization and possible causes of interictal epileptic activity in tumor-associated epilepsy. Neurobiol Dis. 2000;7:260–269. doi: 10.1006/nbdi.2000.0288. [DOI] [PubMed] [Google Scholar]
- Persson M, Brantefjord M, Hansson E, Ronnback L. Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-alpha. Glia. 2005;51:111–120. doi: 10.1002/glia.20191. [DOI] [PubMed] [Google Scholar]
- Persson M, Brantefjord M, Liljeqvist JA, Bergstrom T, Hansson E, Ronnback L. Microglial GLT-1 is upregulated in response to herpes simplex virus infection to provide an antiviral defence via glutathione. Glia. 2007;55:1449–1458. doi: 10.1002/glia.20560. [DOI] [PubMed] [Google Scholar]
- Pollen DA, Trachtenberg MC. Neuroglia: gliosis and focal epilepsy. Science. 1970;167:1252–1253. doi: 10.1126/science.167.3922.1252. [DOI] [PubMed] [Google Scholar]
- Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010;12:520–527. doi: 10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom CB, Ransom BR, Sontheimer H. Activity-dependent K+ accumulation in the rat optic nerve is cleared by temperature-sensitive and temperature-insensitive mechanisms. Soc Neurosci Abs. 1995;21(Part 1) [Google Scholar]
- Raore B, Schniederjan M, Prabhu R, Brat DJ, Shu HK, Olson JJ. Metastasis infiltration: an investigation of the postoperative brain-tumor interface. Int J Radiat Oncol Biol Phys. 2011;81:1075–1080. doi: 10.1016/j.ijrobp.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpkema M, Schuuring J, van der MY, van der GM, Bernsen H, Boerman R, van der KA, Heerschap A. Characterization of oligodendrogliomas using short echo time 1H MR spectroscopic imaging. NMR Biomed. 2003;16:12–18. doi: 10.1002/nbm.807. [DOI] [PubMed] [Google Scholar]
- Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Roslin M, Henriksson R, Bergstrom P, Ungerstedt U, Bergenheim AT. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J Neurooncol. 2003;61:151–160. doi: 10.1023/a:1022106910017. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang YF, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Yang L, Grogan PT, Kitange GJ, Carlson BL, Schroeder MA, Galanis E, Giannini C, Wu W, Dinca EB, James CD. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6:1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Eyupoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med. 2008;14:629–632. doi: 10.1038/nm1772. [DOI] [PubMed] [Google Scholar]
- Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Carmignoto G, Steinhauser C. Astrocyte dysfunction in epilepsy. Brain Res Rev. 2010;63:212–221. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Seifert G, Huttmann K, Binder DK, Hartmann C, Wyczynski A, Neusch C, Steinhauser C. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci. 2009;29:7474–7488. doi: 10.1523/JNEUROSCI.3790-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked I, Tchoresh D, Gersner R, Meiri G, Mordechai S, Xiao X, Hart RP, Schwartz M. Protective autoimmunity: interferon-gamma enables microglia to remove glutamate without evoking inflammatory mediators. J Neurochem. 2005;92:997–1009. doi: 10.1111/j.1471-4159.2004.02954.x. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H, Bridges RJ. Sulfasalazine for brain cancer fits. Expert Opin Investig Drugs. 2012;21:575–578. doi: 10.1517/13543784.2012.670634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter glt-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Vargas KJ, Wilkins ME, Ramirez OA, Jaureguiberry-Bravo M, Pangalos MN, Smart TG, Moss SJ, Couve A. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc Natl Acad Sci U S A. 2010;107:13918–13923. doi: 10.1073/pnas.1000853107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15:1382–1402. doi: 10.1007/s10495-010-0481-0. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Morimoto K, Hirao T, Suwaki H, Watase K, Tanaka K. Amygdala-kindled and pentylenetetrazole-induced seizures in glutamate transporter GLAST-deficient mice. Brain Res. 1999;845:92–96. doi: 10.1016/s0006-8993(99)01945-9. [DOI] [PubMed] [Google Scholar]
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Noell S, Wolburg-Buchholz K, Mack A, Fallier-Becker P. Agrin, aquaporin-4, and astrocyte polarity as an important feature of the blood-brain barrier. Neuroscientist. 2009;15:180–193. doi: 10.1177/1073858408329509. [DOI] [PubMed] [Google Scholar]
- Xu Z, Xue T, Zhang Z, Wang X, Xu P, Zhang J, Lei X, Li Y, Xie Y, Wang L, Fang M, Chen Y. Role of signal transducer and activator of transcription-3 in up-regulation of GFAP after epilepsy. Neurochem Res. 2011;36:2208–2215. doi: 10.1007/s11064-011-0576-1. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. [PubMed] [Google Scholar]