Abstract
We investigated longitudinal relations between children’s sleep and symptoms of depression, anxiety, and anger/aggression. We expected that initial sleep problems and increases in these problems over time would be associated with worse adjustment outcomes. The study had three waves with one year lags. At T1, 128 girls and 123 boys (M age = 8.23 years, SD = 0.73) participated; M ages at T2 and T3 = 9.31 years (SD = 0.79) and 10.28 (SD = 0.99). The sample was diverse in relation to economic adversity and ethnicity (66% European and 34% African American). Higher initial levels and increases in sleep/wake problems or sleepiness over three years predicted higher levels of depression and anxiety symptoms at T3, controlling for T1 levels. These associations were more pronounced for girls, African-American children, and children from lower SES homes. Findings build on a small body of literature addressing links between sleep and adjustment longitudinally and highlight the importance of adequate sleep for children’s optimal development, especially within the broader sociocultural milieu.
Keywords: adjustment, sleep problems, depression, anxiety, individual growth modeling
Child sleep problems (Meltzer & Mindell, 2006) are highly prevalent and are associated with adjustment problems (El-Sheikh, Kelly, Buckhalt, & Hinnant, 2010). Research is clarifying relations between sleep and adjustment yet several important gaps remain. Longitudinal studies with more than two waves are scarce, not much is known about sleep trajectories of school-age children or their sequelae and studies of the impact of persistent sleep problems are scant (Jansen et al., 2011). Using data collected over three years, we examined whether initial levels and rates of change in children’s sleep problems predicted T3 adjustment. We also assessed the moderating role of child sex, ethnicity, and family socioeconomic status (SES) in these links. We examined sleep problems along a continuum in typically developing children, which were indicated by increased sleepiness and sleep/wake problems (S/WP) (i.e., insufficient sleep and difficulty initiating and maintaining sleep). We examined the effects of these sleep measures on children’s cognitive functioning in Bub, El-Sheikh, & Buckhalt (2011) using the same sample.
Child sleep problems including duration, quality, and daytime sleepiness are associated with symptoms of depression and anxiety (Chorney, Detweiler, Morris, & Kuhn, 2008) as well as anger and aggression (Chervin, Dillon, Archbold, & Ruzicka, 2003). Across two waves of data, parent-reported child sleep problems at age four years predicted an increase in depression, anxiety and aggression during mid-adolescence (Gregory & O’Connor, 2002). With an independent sample from that utilized in the present study, more S/WP were associated with increases in internalizing and externalizing symptoms two years later (El-Sheikh et al. 2010). Sleep problems can disrupt processes mediated in the prefrontal cortex (PFC), including executive functioning needed for emotion regulation (Jones & Harrison, 2000), which in turn put children at risk for both internalizing and externalizing problems (Dahl, 1996).
Researchers have begun to use individual or latent growth modeling to examine cross-domain associations but work in this area is relatively nascent. Adolescents who experienced a faster decline in sleep duration over time were more likely to exhibit increases in depression symptoms over three years (Fredriksen, Rhodes, Reddy & Way, 2004). Further, children whose sleep problems (mother report) increased at a faster rate also exhibited increasing externalizing behaviors (teacher report) (Goodnight, Bates, Staples, Pettit, & Dodge, 2007).
The prevalence of children’s sleep problems may be higher among African (AAs) than European Americans (EAs), and among those from lower SES backgrounds (Buckhalt, El-Sheikh, Keller & Kelly, 2009). Examination of sociocultural moderators of the link between children’s sleep and their adjustment is critical for identifying for whom and under which conditions sleep has a protective or vulnerability function. Consistent with views of cumulative risk (e.g., Buckhalt, 2011), ethnic minority or economically disadvantaged children may be especially vulnerable to the negative effects of sleep problems and emerging evidence supports this proposition for children’s adjustment (El-Sheikh et al., 2010).
Findings pertaining to sex differences in children’s sleep are inconsistent and little work has examined child sex as a moderator of the sleep-adjustment connection. Girls may have better sleep quality (Buckhalt et al., 2009) or longer duration (Fredriksen et al., 2004); opposite findings have been reported (Gaina et al., 2007). In their 3-wave study with adolescents, Meijer, Reitz, Dekovic, van den Wittenboer, and Stoel (2010) found that sleep problems predicted increased adjustment problems more robustly for boys. Further, because girls compared to boys are more susceptible to internalizing symptoms in adolescence (Hankin & Abramson, 2001; Van Oort et al., 2009), we investigated child sex as a moderator of the sleep-adjustment link.
This study builds on the literature in important ways. We examined several outcomes including depression symptoms, anxiety, and anger/aggression (referred to as anger for brevity). Building on the scarce longitudinal literature most of which is with two study waves, research questions were examined using three waves of data, a requirement for linear growth modeling, thereby allowing for a more thorough explication of associations. With an independent sample, we found that S/WP predicted increases in internalizing and externalizing symptoms two years later (El-Sheikh et al., 2010). The present investigation utilizes three waves of data and examines not only initial S/WP but also changes in sleep as predictors of adjustment. We expected that children with more sleep problems at T1 and increases in sleep problems over time would have poorer adjustment at T3 and expected more pronounced relations for AA children, those from lower SES homes, and girls (similar to findings reported by Bub et al., 2011 in relation to cognitive outcomes).
Method
Participants
The study consisted of three waves around one year apart (more detail is provided in Bub et al., 2011). Children were recruited from public schools and did not have chronic illnesses or diagnosed developmental delays or learning disabilities. At T1, participants were 128 girls and 123 boys (M age = 8.23 years, SD = 0.73); 66% European (EA) and 34% African American (AA). We oversampled to include EAs and AAs from a wide range of SES backgrounds. The SES raw score was derived based on education and occupation (Hollingshead, 1975) and was used as a continuous variable in analyses. Based on mother-report (Petersen, Crockett, Richards, & Boxer, 1988), 94% of children were classified as prepubertal at T1. There was an 85% retention rate between T1 and T2, and 77% retention between T1 and T3. At T2, 214 children (106 boys; M age = 9.31 years, SD = .79) participated; 194 (92 boys; M age = 10.28 years, SD = .99) participated at T3. There were no significant differences between participants (n =194) and non-participants (n = 57) on child or demographic characteristics.
Procedure and Measures
At each study wave, children and their parents visited our lab. Consent and assent were obtained and the study was approved by the Institutional Review Board. Children completed questionnaires via interview. All measures were completed during each study wave.
Children reported on depression symptoms over the past two weeks using the Children’s Depression Inventory (CDI; Kovacs, 1985); αs for this study = .71 to .95 across waves. Children completed the Revised Children’s Manifest Anxiety Scale (RCMAS; Reynolds & Richmond, 1978) regarding symptoms during the past year; αs = .91 to .92. They also completed the Anger/Agression scale of the Trauma Symptoms Checklist for Children (TSCC; Briere, 1996), which is composed of nine items (e.g., getting mad and can’t calm down, getting into fights). The TSCC was completed in general and not in the context of a specified trauma (Briere, 1996). Children were told: “The items in this survey describe things that kids sometimes think, feel, or do.” Children rated how frequently angry/aggressive thoughts, feelings, and behaviors occurred within the last year on a scale ranging from never (0) to almost all the time (4); αs = .80 to .85.
Sleep problems
Children reported on their sleep within the last two weeks using the Sleep Habits Survey (SHS; Wolfson & Carskadon, 1998), which is widely used (Buckhalt et al., 2009) and has established reliability and validity (Wolfson et al., 2003). The Sleepiness Scale is composed of 9 items and assesses whether children fell asleep or struggled to stay awake while performing daily activities; an item regarding driving a car was omitted. Sleepiness was rated during various activities from never (1) to every day/night (5); “never” was given if the child did not engage in the activity. The Sleep/Wake Problems Scale assesses difficulty initiating and maintaining sleep, oversleeping, insufficient sleep, and feeling dissatisfied with sleep. This scale is comprised of 10-items and choices range from never (1) to every day/night (5). In our sample, α = .70 to .74 for Sleepiness and .73 – .83 for S/WP.
Analysis Plan
To determine whether there was significant growth in children’s adjustment and sleep problems, we fit an unconditional growth model for each of the adjustment and sleep problems variables (Singer & Willett, 2003). We represented change by linear growth and examined estimates of the population average initial level and population average rate of change. We centered time at the first assessment. Because there was no statistically significant growth in any adjustment index, for all subsequent analyses we used children’s T3 adjustment scores as our outcome variable and controlled for T1 scores. Children’s sleepiness changed linearly over time, while children’s S/WP did not. Nevertheless, for consistency and because S/WP evidenced relations with cognitive outcomes in the same sample (Bub et al., 2011), we estimated growth parameters for both sleep indices.
To examine changes in sleep problems as a predictor of T3 adjustment, we needed variability in our estimates of intercept and rate of change, which is not provided by the population average intercept and rate of change. We thus estimated an intercept and rate of change for each child in the analytic sample using ordinary least squares (OLS) regression (Willett, 1997). Although these estimates are based on three data points for each individual and are thus may be more unstable than the population estimates, this approach is a more precise measure of change over time than a difference score (T3-T1) and is a common way to obtain growth parameters in one domain that can then be used to predict outcomes in other domains (Willett, 1997). Four estimates for each child were generated: initial level and rate of change for sleepiness and S/WP. We then fit a set of multiple regression models in which we regressed T3 adjustment on these estimates of initial level and rate of change in sleep problems, controlling for T1 adjustment and a common set of child and family characteristics (i.e., child sex (1 = boy; 0 = girl); ethnicity (1 = AA; 0 = EA); age in months, pubertal status, and family SES at T3). We controlled for T3 anger in our models predicting depression and anxiety symptoms and vice versa. Because child age and pubertal status were not strongly correlated with our adjustment or sleep variables and did not emerge as predictors of adjustment in our control models, we removed them from our models and present below the analyses without these controls.
To investigate socio-demographic effects, we added to our models interactions between children’s initial level and rate of change in sleepiness or S/WP and sex, ethnicity, and family SES at T3. Because SES was highly correlated across waves (.94 – .98) and for parsimony, we used SES measured at the same point as our outcome (T3). We assessed model fit by comparing the total R2 statistics across model specifications; larger values indicate that more variation in the outcome is explained and suggest a better fit. To illustrate the magnitude and direction of the moderated associations between children’s adjustment and changes in sleep, we constructed a set of plots for prototypical children with low (−1 SD) and high (+ 1 SD) initial levels and rates of change in sleep problems (Singer & Willett, 2003). That is, following well-established procedures for constructing prototypical plots (Singer & Willett, 2003), we selected particular values of these predictors (i.e., the sleep problems growth parameters) and calculated predicted outcome values for that prototypical individual at each age. Note that low and high sleepiness trajectories are separated by just under 10 points at the intercept and just under 7 points on the slope; low and high sleep/wake problems trajectories are separated by just under 12 points at the intercept and just under 8 points on the slope. This strategy provides trajectories that would be typical for individuals in the population with the same characteristics.
A multiple imputation (MI) procedure was used to impute data on key demographic control variables and predictors. MI assumes that data are missing at random (MAR) and generates a representative value for each data point that preserves the multivariate structure of relations among variables (Wideman, 2006). The percentage of missing data across variables was less than 5% based on sample sizes for the T3 adjustment variables. Five imputed datasets were created and estimates for each predictor were generated using the following variables: OLS estimates of both sleepiness and S/WP intercepts and rates of change, sex, ethnicity, T1 – T3 family SES, age, and pubertal status. OLS estimates of the growth parameters for sleep problems were calculated prior to the MI procedure. We did not impute any of our outcome variables nor did we use the outcome variables to generate estimates of our predictor variables. Parameter estimates and goodness of fit statistics presented in Tables 4 and 5 reflect the average associations across the five imputed datasets (standard errors are adjusted for the multiple imputations). All analyses were conducted in Stata version 10.
Table 4.
Predicting Depression symptoms, Anxiety and Anger/Aggression at Time 3 from Initial Level and Rate of Change in Sleepiness
| T3 Child-Reported Depression Symptoms (n = 168)
|
T3 Child-Reported Anxiety (n = 179)
|
T3 Child-Reported Anger/Aggression (n = 163)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 | Model 10 | Model 11 | Model 12 | |
| Constant | −2.92 (2.07) | −6.47** (2.28) | −3.25 (2.32) | −11.67* (5.39) | −3.78 (2.71) | −3.87 (3.03) | −3.81 (3.10) | −8.60 (6.57) | 2.08 (1.27) | 4.17** (1.43) | 1.50 (1.43) | 7.54* (3.21) |
| Main Effects | ||||||||||||
| T1 Adjustment | .11 (.07) | .11 (.06) | .11 (.07) | .11 (.07) | .16* (.07) | .13* (.07) | .15* (.07) | .16* (.07) | .24*** (.04) | .22*** (.04) | .24*** (.04) | .23*** (.04) |
| Sleepiness Intercept | .54*** (.11) | .75*** (.13) | .55*** (.14) | 1.20** (.36) | .60*** (.14) | .61*** (.17) | .62** (.19) | .97* (.44) | −.23** (.07) | −.38*** (.08) | −.19* (.09) | −.60** (.22) |
| Sleepiness Slope | .51** (.17) | 1.15*** (.21) | .30 (.24) | 1.81** (.62) | .81*** (.21) | 1.16*** (.28) | 1.04** (.33) | 1.75* (.77) | −.16 (.11) | −.49** (.14) | −.18* (.16) | −.42 (.40) |
| Boy | −.07 (.67) | 5.44 (2.85) | −.22 (.68) | −.06 (.66) | −.82 (.84) | −2.15 (3.60) | −.68 (.86) | −.83 (.85) | .72 (.40) | −3.29 (1.78) | .70 (.40) | .73 (.39) |
| African American | −2.71*** (.75) | −2.16** (.72) | −1.44 (3.35) | −2.86*** (.75) | .63 (.95) | .91 (.94) | .27 (3.99) | .54 (.95) | 1.46** (.46) | 1.24** (.45) | 3.41 (2.00) | 1.53** (.46) |
| T3 SES | −.06 (.03) | −.04 (.03) | −.06 (.03) | .19 (.14) | −.03 (.04) | −.02 (.04) | −.03 (.04) | .11 (.17) | .02 (.02) | .02 (.02) | .02 (.02) | −.13 (.09) |
| T3 Depression Symptoms | -- | -- | -- | -- | -- | -- | -- | -- | .23*** (.05) | .27*** (.05) | .22*** (.05) | .24*** (.05) |
| T3 Anxiety | -- | -- | -- | -- | -- | -- | -- | -- | .12** (.04) | .12** (.04) | .12** (.04) | .11** (.04) |
| T3 Anger/ Aggression | .66*** (.11) | .69*** (.10) | .64*** (.11) | .68*** (.11) | .69*** (.12) | .70*** (.12) | .70*** (.12) | .69*** (.12) | -- | -- | -- | -- |
| Interactions | ||||||||||||
| Sleepiness_I* Boy | −.46* (.20) | .04 (.24) | .31* (.12) | |||||||||
| Sleepiness_S* Boy | −1.40*** (.31) | −.74 (.40) | .71** (.20) | |||||||||
| Sleepiness_I* AA | −.06 (.22) | −.001 (.27) | −.12 (.13) | |||||||||
| Sleepiness_S* AA | .32 (.34) | −.37 (.44) | .01 (.21) | |||||||||
| Sleepiness_I* SES | −.02 (.01) | −.01 (.01) | .01 (.01) | |||||||||
| Sleepiness_S* SES | −.04* (.02) | −.03 (.02) | .01 (.01) | |||||||||
| Model Fit Statistics | ||||||||||||
| F-statistic | 14.37*** | 14.97*** | 11.57*** | 11.98*** | 15.47*** | 13.17*** | 12.16*** | 12.18*** | 18.98*** | 17.42*** | 15.38*** | 15.68*** |
| R2 | .3861 | .4602 | .3972 | .4056 | .3877 | .4122 | .3930 | .3935 | .4965 | .5339 | .5030 | .5078 |
| ΔR2 | -- | .07 | .01 | .02 | -- | .02 | .01 | .01 | -- | .04 | .01 | .01 |
| Model Comparison | -- | 1 | 1 | 1 | -- | 5 | 5 | 5 | -- | 9 | 9 | 9 |
Note. T1 = Standard errors appear within parentheses. First assessment; T3 = Third Assessment; AA = African-American; SES = Socioeconomic status; I = Initial Level; S = Rate of Change. Models 1, 5, and 9 are the main effects models; Models 2, 6, and 10 are the sleepiness by child sex interaction models; Model 3, 7, and 11 are the sleepiness by child ethnicity models; and Models 4, 8, and 12 are the sleepiness by family SES interaction models. Parameter estimates and fit statistics presented represent the average estimate across the five imputations (with standard errors corrected for the multiple imputations).
p < .05;
p < .01;
p < .001.
Table 5.
Predicting Depression symptoms, Anxiety and Anger/Aggression at Time 3 from Initial Level and Rate of Change in Sleep/Wake Problems
| T3 Child-Reported Depression Symptoms (n=168)
|
T3 Child-Reported Anxiety (n=179)
|
T3 Child-Reported Anger/Aggression (n=163)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 | Model 10 | Model 11 | Model 12 | |
| Constant | 3.70* (1.88) | 3.82 (2.26) | 4.25* (1.89) | 5.61** (1.87) | .76 (2.33) | 1.68 (2.78) | 1.26 (2.34) | 1.78 (2.36) | −1.43 (1.16) | −.71 (1.41) | −1.92 (1.13) | −2.35* (1.17) |
| Main Effects | ||||||||||||
| T1 Adjustment | .24*** (.07) | .24** (.07) | .20** (.07) | .19** (.07) | .19** (.07) | .18** (.07) | .18** (.07) | .15* (.07) | .21*** (.04) | .22*** (.04) | .22*** (.04) | .22*** (.04) |
| Sleep/Wake_I | .05 (.07) | .04 (.10) | .01 (.07) | −.04 (.07) | .27** (.09) | .23 (.13) | .20* (.10) | .21* (.10) | .02 (.05) | −.02 (.07) | .06 (.05) | .06 (.05) |
| Sleep/Wake_S | .48*** (.12) | .53** (.16) | .43** (.12) | .37** (.12) | .75*** (.15) | .94*** (.21) | .68*** (.16) | .63*** (.16) | −.01 (.08) | −.09 (.11) | .02 (.08) | .03 (.08) |
| Boy | −.08 (.68) | −.39 (2.61) | −.15 (.68) | .07 (.66) | −1.22 (.84) | −2.81 (3.16) | −1.23 (.84) | −1.08 (.83) | .73 (.41) | −.63 (1.60) | .70 (.40) | .64 (.41) |
| African American | −1.80* (.73) | −1.79* (.73) | −8.13** (2.97) | −2.40** (.73) | 1.24 (.90) | 1.24 (.90) | −5.10 (3.24) | .75 (.91) | 1.02* (.46) | 1.01* (.46) | 6.29*** (1.74) | 1.30** (.46) |
| T3 SES | −.07* (.03) | −.07* (.03) | −.06 (.03) | −.22*** (.05) | −.06 (.04) | −.06 (.04) | −.04 (.04) | −.18** (.06) | .03 (.02) | .03 (.02) | .02 (.02) | .10** (.03) |
| T3 Depression Symptoms | -- | -- | -- | -- | -- | -- | -- | -- | .19*** (.05) | .19*** (.05) | .21*** (.05) | .23*** (.05) |
| T3 Anxiety | -- | -- | -- | -- | -- | -- | -- | -- | .10* (.04) | .10* (.04) | .10* (.04) | .10* (.04) |
| T3 Anger/ Aggression | .57*** (.11) | .57*** (.11) | .62*** (.11) | .60*** (.10) | .62*** (.13) | .62*** (.13) | .62*** (.13) | .61*** (.12) | -- | -- | -- | -- |
| Interactions | ||||||||||||
| Sleep/Wake_I* Boy | .01 (.14) | .07 (.16) | .07 (.08) | |||||||||
| Sleep/Wake_S* Boy | −.14 (.25) | −.44 (.29) | .16 (.15) | |||||||||
| Sleep/Wake_I* AA | .41* (.18) | .41* (.20) | −.33** (.11) | |||||||||
| Sleep/Wake_S* AA | .44 (.25) | .41 (.29) | −.18 (.15) | |||||||||
| Sleep/Wake_I* SES | .01** (.003) | .01* (.004) | −.01** (.001) | |||||||||
| Sleep/Wake_S* SES | .01 (.005) | .01* (.01) | −.003 (.003) | |||||||||
| Model Fit Statistics | ||||||||||||
| F-statistic | 13.20*** | 10.24*** | 10.99*** | 12.69*** | 16.49*** | 13.49*** | 13.45*** | 13.90*** | 16.03*** | 12.90*** | 14.95*** | 14.45*** |
| R2 | .3662 | .3683 | .3851 | .4196 | .4031 | .4180 | .4173 | .4254 | .4544 | .4307 | .4958 | .4873 |
| ΔR2 | -- | .00 | .02 | .05 | -- | .01 | .01 | .02 | -- | .00 | .04 | .03 |
| Model Comparison | -- | 1 | 1 | 1 | -- | 5 | 5 | 5 | -- | 9 | 9 | 9 |
Note. T1 = Standard errors appear within parentheses. First assessment; T3 = Third Assessment; AA = African American; SES = Socioeconomic status; I = Initial Level; S = Rate of Change. Models 1, 5, and 9 are the main effects models; Models 2, 6, and 10 are the sleepiness by child sex interaction models; Model 3, 7, and 11 are the sleepiness by child ethnicity models; and Models 4, 8, and 12 are the sleepiness by family SES interaction models. Parameter estimates and fit statistics presented represent the average estimate across the five imputations (with standard errors corrected for the multiple imputations).
p < .05;
p < .01;
p < .001.
Results
Preliminary Analyses
Means and standard deviations for main variables are presented in Table 1 and correlations are presented in Table 2. On average, mean levels of children’s depression, anxiety, and anger decreased between T1 and T3. Mean sleepiness also decreased over time. In contrast, the mean level of S/WP at T2 was higher than at T1 or T3 (Table 1). There were no significant associations between children’s adjustment or sleep problems and their pubertal status and few associations with their age, sex, ethnicity, and SES (Table 2). However, AA children reported more sleep problems, on average, than did EA children.
Table 1.
Descriptive Statistics and Sample Ranges for Outcome, Key Predictor, and Control Variables by Assessment
| T1 | T2 | T3 | Other | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean/% (SD) | Range | Mean/% (SD) | Range | Mean/% (SD) | Range | Mean/% (SD) | Range | |
| Outcome Variables | ||||||||
| Child Reported Depression Symptoms (CDI) | 7.90 (6.14) | 0–33 | 7.15 (6.67) | 0–32 | 4.85 (5.19) | 0–31 | ||
| Child Reported Anxiety | 11.52 (6.93) | 0–28 | 9.84 (7.14) | 0–26 | 7.64 (6.83) | 0–28 | ||
| Child Reported Anger/Aggression | 5.34 (5.36) | 0–27 | 4.14 (4.23) | 0–19 | 3.72 (3.65) | 0–17 | ||
| Predictor Variables | ||||||||
| Sleepiness | 15.67 (5.00) | 9–31 | 14.21 (4.73) | 2–34 | 13.66 (4.20) | 9–33 | ||
| Sleepiness Intercept | 15.54 (4.85) | 7.5–31.17 | ||||||
| Sleepiness Slope | −.946 (3.41) | −13–18 | ||||||
| Sleep/Wake Problems | 18.78 (6.02) | 9–42 | 19.79 (7.58) | 10–48 | 17.87 (6.01) | 10–36 | ||
| Sleep/Wake Problems Intercept | 19.15 (5.73) | 9–44.3 | ||||||
| Sleep/Wake Problems Slope | −.312 (3.92) | −14–21 | ||||||
| Moderator & Control Variables | ||||||||
| Sex (boys) | 49.00% | 0–1 | 48.85% | 0–1 | 48.85% | 0–1 | ||
| Ethnicity (African American) | 35.46% | 0–1 | 35.94% | 0–1 | 35.94% | 0–1 | ||
| Socioeconomic Status | 37.38 (9.92) | 12–66 | 37.73 (10.03) | 19–66 | 37.87 (10.40) | 11–66 | ||
| Child Age (in Months) | 98.71 (8.64) | 80–128 | 111.70 (9.46) | 91–143 | 123.30 (11.99) | 87–219 | ||
| Puberty Status | 1.39 (.33) | 1–2.4 | 1.56 (.44) | 1–4 | 1.72 (.56) | 1–3.8 | ||
Table 2.
Intercorrelations Among Predictor, Outcome and Control Variables Across Waves
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | 17. | 18. | 19. | 20. | 21. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Depression Symptoms T1 | -- | ||||||||||||||||||||
| 2. Depression Symptoms T2 | .42*** | -- | |||||||||||||||||||
| 3. Depression Symptoms T3 | .39*** | .37*** | -- | ||||||||||||||||||
| 4. Anxiety T1 | .45*** | .28*** | .28*** | -- | |||||||||||||||||
| 5. Anxiety T2 | .34*** | .44*** | .34*** | .56*** | -- | ||||||||||||||||
| 6. Anxiety T3 | .20** | .25*** | .52*** | .37*** | .54*** | -- | |||||||||||||||
| 7. Anger/Aggression T1 | .46*** | .19** | .31*** | .38*** | .31*** | .19* | -- | ||||||||||||||
| 8. Anger/Aggression T2 | .38*** | .43*** | .33*** | .32*** | .51*** | .29*** | .46*** | -- | |||||||||||||
| 9. Anger/Aggression T3 | .41*** | .24** | .47*** | .28*** | .32*** | .46*** | .46*** | .49*** | -- | ||||||||||||
| 10. Sleepiness Intercept | .30*** | .20** | .30*** | .32*** | .37*** | .31*** | .32*** | .22** | .18* | -- | |||||||||||
| 11. Sleepiness Slope | −.001 | .08 | −.09 | −.03 | −.03 | .04 | −.24*** | .03 | −.06 | −.63*** | -- | ||||||||||
| 12. Sleep/Wake Intercept | .26*** | .06 | .05 | .36*** | .30*** | .23** | .35*** | .23*** | .31*** | .39*** | −.21*** | -- | |||||||||
| 13. Sleep/Wake Slope | .01 | .16* | .23** | .04 | .14* | .25*** | −.07 | .14* | −.01 | −.06 | .29*** | −.41*** | -- | ||||||||
| 14. Boys | −.04 | .05 | .05 | −.14* | −.05 | −.06 | .06 | .11 | .15* | −.05 | −.07 | .03 | −.06 | -- | |||||||
| 15. African American | .08 | −.05 | −.01 | .12* | .13 | .25*** | .01 | .09 | .17* | .26*** | −.05 | .15* | .01 | −.04 | -- | ||||||
| 16. SES T3 | −.04 | .01 | −.09 | −.04 | −.04 | −.16* | .02 | .06 | .01 | −.14 | −.06 | −.09 | −.03 | .11 | −.28*** | -- | |||||
| 17. Age T1 | −.09 | −.08 | −.15 | −.16* | −.16* | −.23** | .01 | .01 | −.10 | −.17** | .10 | .01 | −.02 | .20** | −.11 | .06 | -- | ||||
| 18. Age T2 | −.04 | −.12 | −.17* | −.17* | −.17* | −.27*** | .02 | .002 | −.09 | −.15* | .10 | −.01 | −.01 | .19** | −.11 | .04 | .93*** | -- | |||
| 19. Age T3 | .02 | −.11 | −.01 | −.08 | −.08 | −.04 | .05 | .03 | −.06 | .02 | −.02 | .07 | .04 | .11 | .03 | .05 | .65*** | .62*** | -- | ||
| 20. Pubertal Status T1 | .03 | .03 | −.08 | .06 | .06 | .12 | −.06 | .03 | −.03 | .08 | .10 | .09 | .00 | −.19** | .23*** | −.07 | .05 | .05 | −.05 | -- | |
| 21. Pubertal Status T2 | .08 | .01 | −.02 | .07 | .07 | .07 | −.02 | .06 | −.13 | .07 | .02 | −.03 | .09 | −.30*** | .19** | −.14 | .11 | .12 | .02 | .64*** | -- |
| 22. Pubertal Status T3 | .04 | −.15* | −.02 | .09 | .09 | −.01 | −.07 | −.04 | −.04 | .02 | .02 | −.01 | .06 | −.29*** | .30*** | −.13 | .17* | .25*** | .08 | .54*** | .76*** |
Note. T1 = First assessment; T2 = Second assessment; T3 = Third Assessment; SES = Socioeconomic status
p < .05;
p < .01;
p < .001.
Although there were mean level differences in adjustment over time, results from unconditional growth models (not presented) indicated that the rate at which depression symptoms, anxiety, and anger declined over time was not statistically significant. Thus, although the sample means decreased somewhat across assessments, the population average rate at which this occurred was not significant. The population average rate of decline in sleepiness from T1 to T3, however, was significant (β = −1.01, p < .001), with lower levels of sleepiness reported as children got older. Children’s S/WP changed over time, suggesting there may be considerable stability within individuals over time or that change is non-linear.
Sleep Problems and Adjustment
Given the complexity of the analyses and the numerous findings across our predictor and outcome variables, we created a visual summary of the significant associations between the sleep growth parameters and adjustment as well as the significant interaction effects involving child sex, ethnicity, and family SES (Table 3). Parameter estimates and model fit statistics from the fitted multiple regression models predicting the residualized change in children’s adjustment from growth in sleep variables are presented in Tables 4 and 5.
Table 3.
Summary of direct and moderated associations between changes in sleep problems and children’s adjustment.
| T3 Child-Reported Depressive Symptoms (n=168)
|
T3 Child-Reported Anxiety (n = 179)
|
T3 Child-Reported Anger/Aggression (n = 163)
|
||||
|---|---|---|---|---|---|---|
| Sleepiness | Sleep/Wake | Sleepiness | Sleep/Wake | Sleepiness | Sleep/Wake | |
| Main effectsa | ||||||
| Intercept | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Slope | ✓ | ✓ | ✓ | ✓ | ||
| Interactionsb | ||||||
| Intercept*Sex | ✓ | ✓ | ||||
| Slope*Sex | ✓ | ✓ | ||||
| Intercept*Ethnicity | ✓ | ✓ | ✓ | |||
| Slope*Ethnicity | ||||||
| Intercept*SES | ✓ | ✓ | ✓ | |||
| Slope*SES | ✓ | ✓ | ||||
Statistically significant main effects were identified using Models 1, 4, and 9 in Tables and 4
Statistically significant interactions were identified using Models 2, 6, and 10 for child sex, Models 3, 7, and 11 for child race/ethnicity, and Models 4, 8, and 12 for family SES.
Sleepiness
Controlling for within- and cross-domain adjustment, children’s initial level and rate of change in sleepiness were positively associated with their depression symptoms and anxiety scores at T3 (see Table 4, Models 1 and 5, respectively). Children who had higher levels of sleepiness at T1 exhibited more depression and anxiety symptoms at T3 compared with their peers who reported lower initial levels of sleepiness. Similarly, children whose sleepiness decreased less rapidly (i.e., shallower slope) or even increased over time, reported more depression and anxiety symptoms at T3, compared with children whose sleepiness decreased more rapidly or remained stable over time. Children who reported higher levels of sleepiness at T1 also reported lower levels of anger at T3 (see Table 4, Model 9).
Effect sizes differed by outcome. T3 depression symptoms score for children who reported more sleepiness (i.e., initial level and rate of change + 1 SD) was ~ 9 points higher than the predicted score for children with lower sleepiness (10.4 vs. 1.7 out of a possible score of 54). The association was even stronger for anxiety, with children reporting higher initial levels and increases in sleepiness having an estimated T3 anxiety score of 12.7 points (out of a total possible score of 28), compared with a score of 1.4 for children reporting lower initial levels and decreases in sleepiness. Children with lower sleepiness reported higher anger scores than their counterparts with higher sleepiness, although the difference was small (over 3 points). These models accounted for around 39% of the variation in depression symptoms and anxiety at T3, and 50% of the variation in anger scores. This, in combination with the significant F-Statistics (see Table 4, Models 1, 5, and 9), suggests that our main-effects models fit our data well.
Sleep/Wake Problems
Children’s initial level of S/WP was positively associated with their anxiety scores at T3 (see Table 5, Model 5). Rate of change in S/WP was a positive predictor of depression and anxiety symptoms at T3 (See Table 5, Models 1 and 5). On average, children who experienced less rapid decreases (e.g., whose slopes were negative and shallow) or even increases (e.g., whose slopes were positive and shallow or steep) in S/WP over time had higher levels of depression or anxiety symptoms at T3 than did children whose S/WP remained stable or decreased more rapidly across the study period.
Effect sizes varied by outcome. The T3 depression score for children with higher initial and increasing levels of S/WP was more than 10 points higher than that for children with lower and decreasing levels of sleep problems (13.4 vs. 3.2). The pattern was similar for children’s anxiety, with children reporting higher sleep problems having a predicted score of 16.4 and those reporting lower levels of problems having a score of 3.1. As evidenced by the significant F-statistics and the small to moderate R2 statistics, the predictor and moderator variables do a reasonable job of explaining variation in children’s adjustment at T3 (see Models 1, 5, and 9 in Table 4): 37% for depression, 40% for anxiety, and 45% for anger.
Because our measures of depression and anxiety included items pertaining to sleep, we removed the four items from the CDI and the two items from the RCAMS that were related to sleep and re-calculated the total score. We then recomputed the total scores and conducted a set of sensitivity analyses. That is, we re-fit our regression models with the new composites (i.e., those withouth the sleep related items). Although the effect sizes were slightly smaller, the pattern of findings was identical and thus we retained the original depression and anxiety composites based on the validated scales for our analyses.
Child Sex, Ethnicity, and Family SES as Moderators of Sleep Problems
To investigate moderation effects, we added to our models a series of interactions between children’s initial level and rate of change in sleep problems (either sleepiness or S/WP) and child sex, ethnicity, and family SES at T3 (see Tables 4 and 5, Models 2–4 for depression symptoms, 6–8 for anxiety, and 10–12 for anger).
Sleepiness
Child sex moderated the association between children’s initial level and rate of change in sleepiness and their depression symptoms at T3 such that sleepiness appears to be a considerable risk factor for girls but not for boys (Table 4, Model 2). To illustrate this moderation effect, we present in Figure 1a the sleepiness growth trajectories and their associated depression symptom scores for three prototypical children: 1) those with an initial level and rate of change in sleepiness 1 SD above the mean (labeled “high” in the figure); 2) those with an initial level and rate of change in sleepiness at the mean (labeled “average” in the figure); and 3) those with an initial level and rate of change in sleepiness 1 SD below the mean (labeled “low” in the figure). These three prototypical trajectories reflect only a fraction of the possible trajectories we could have constructed from our fitted model. Recall that low and high sleepiness trajectories are separated by just under 10 points at the intercept (out of a possible 31) and just under 7 points on the slope (out of a possible 18). Controlling for children’s depression symptoms at T1 (set to the mean), their anger scores at T3 (set to the mean), child ethnicity [(set to 0 (EA)], and family SES at T3 (set to the mean), boys reported few differences in depression symptoms at T3 regardless of their sleepiness trajectory. In contrast, girls who reported higher initial levels of sleepiness and increases in sleepiness over time also reported higher levels of depression symptoms at T3 on average than did girls who reported lower initial levels of sleepiness at T1 and decreases in sleepiness over time. An increase in the F-statistic, coupled with a ΔR2 statistic from Model 1 to Model 2, suggests that the inclusion of the sex by sleepiness growth parameter interactions improved model fit.
Figure 1.
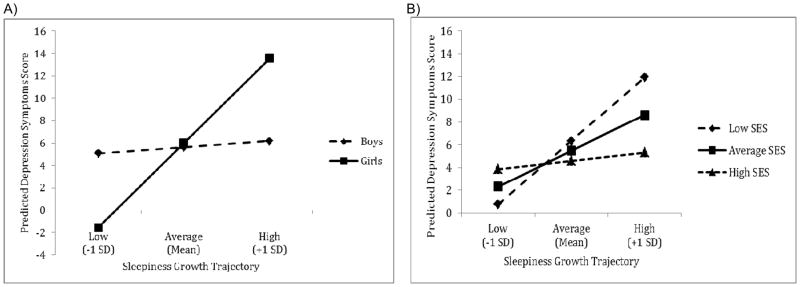
Predicted differences in adjustment between T1 and T3 associated with (a) lower sleepiness growth trajectories (i.e., initial level and rate of change are 1 SD below the mean), (b) average sleepiness growth trajectories (i.e., initial level and rate of change are at the mean), and (c) higher sleepiness growth trajectories (initial level and rate of change are 1SD above the mean). Sleepiness growth trajectories are represented by points on the plot. That is, each point represents the predicted difference score in the outcome for boys and girls (or low, average, and high SES children) whose sleepiness growth trajectories were low (intercept and slope 1 SD below the mean), average (intercept and slope at the mean), or high (intercept and slope 1 SD above the mean). Panel A illustrates moderation by child sex and Panel B illustrates moderation by family socioeconomic status at T3.
Sex moderated the association between changes in sleepiness (initial level and rate of change) and children’s anger scores at T3. Girls whose sleepiness trajectories were considered high (+1 SD) reported lower predicted means for their anger at T3 compared to those with low sleepiness (− 1 SD). There were few differences in boys’ anger scores regardless of sleepiness.
Finally, family SES at T3 moderated the association between rate of change in sleepiness and children’s depression symptoms at T3 (Table 4, Model 4; see Figure 1b). For children from higher SES backgrounds, sleepiness appears to have a minimal effect on their T3 depression. For children from lower SES backgrounds, however, sleepiness has a clear negative association with depression symptoms such that higher sleepiness predicted higher depression. When comparing children from higher and lower SES homes, the difference in depression scores for children characterized by a lower sleepiness trajectory was only 3 points; in contrast, the depression score for children characterized by a high sleepiness trajectory was around 6 points higher for children from low than high SES backgrounds.
Sleep/Wake Problems
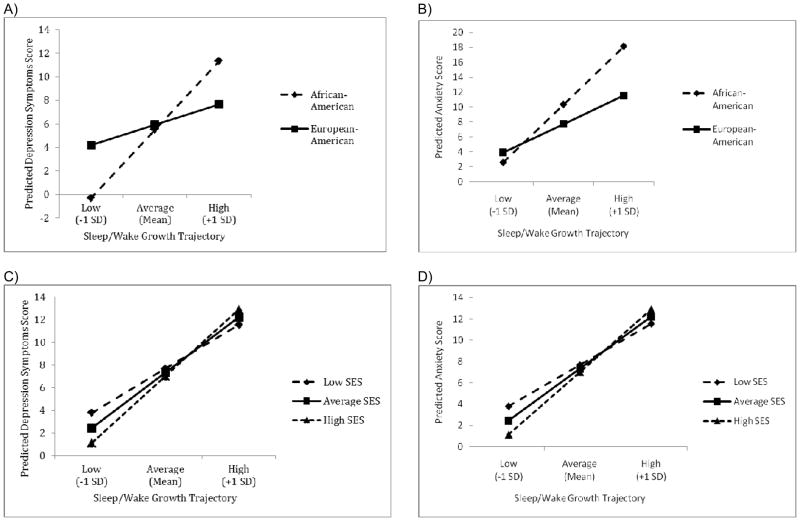
Ethnicity moderated the link between children’s initial level of S/WP and their T3 depression and anxiety (Figures 2a and 2b; see also Table 5, Models 3 and 7). Recall that low and high sleep/wake problems trajectories are separated by just under 12 points at the intercept (out of a possible 44) and under 8 points on the slope (out of a possible 21). For AA children, those whose S/WP trajectories were classified as high had higher levels of depression and anxiety at T3 than those with lower initial levels of sleep problems. The depression score for an AA child who was characterized by a high S/W problem trajectory was more than 11 points higher than that for an AA child who was characterized by a low sleep problem trajectory, compared to a 3-point difference for EAs. Similarly, for AAs, the anxiety score for those with high S/WP was around 16 points higher than for those with low sleep problems. There was little difference in anxiety scores between AA and EA children with low sleep problems (2.6 vs. 3.9, respectively). Although the interaction term was significant, the F-statistic declined from the models with no interactions (i.e., Models 1 and 5) to the models with the ethnicity interactions (i.e., Models 4 and 7) and the R2 statistics increased by only about 1%.
Figure 2.
Predicted differences in adjustment between T1 and T3 associated with (a) lower (1SD below the mean) initial levels and rates of change in sleep/wake problems (b) average (at the mean) initial levels and rates of change in sleep/wake problems and (c) higher (1SD above the mean) initial levels and rates of change in sleep/wake problems. Sleep/wake growth trajectories are represented by points on the plot. That is, each point represents the predicted difference score in the outcome for boys and girls (or low, average, and high SES children) whose sleep/wake growth trajectories were low (intercept and slope 1 SD below the mean), average (intercept and slope at the mean), or high (intercept and slope 1 SD above the mean). Panels A and B illustrate moderation by child ethnicity and Panels C and D illustrate moderation by family socioeconomic status.
Finally, family SES moderated the association between S/WP and children’s depression and anxiety symptoms at T3 (see Table 5, Models 4 and 8). On average, children with high initial levels of sleep problems reported more depression symptoms than did their peers with average or low initial levels of sleep problems across all levels of family SES (see Figure 2c). Further, for children whose S/W trajectories were characterized as low, children from low SES backgrounds reported somewhat greater depression symptoms at T3 than children from high SES backgrounds; there were few differences in depression scores for children with average or high sleep problems across levels of SES. The pattern of findings for children’s anxiety was quite similar (see Figure 2d); note, however, that SES was a significant moderator of the links between both the intercept and the slope of S/WP and children’s anxiety. High S/WP appears to serve as a vulnerability factor for children across a range of SES levels while low S/WP in conjunction with high SES appear to serve as protective factors against children’s anxiety.
Discussion
Toward a better understanding of relations between a primary biological regulatory system and children’s development, we examined relations between children’s sleep trajectories and adjustment. Higher initial levels and increases in sleep problems over three years predicted children’s internalizing problems at T3. Consistent with a health disparities perspective (Buckhalt, 2011), sleep problems had their most pronounced negative effects on the adjustment of AA and lower SES children, highlighting the importance of the broader socio-cultural milieu when considering children’s adjustment in the context of biological dysregulation.
Results build on a scant body of research on relations between children’s sleep trajectories and adjustment. Fredricksen et al. (2004) found that faster decreases in sleep duration predicted increases in adolescents’ depression. Goodnight et al. (2007) found that children whose sleep problems increased at a faster rate also exhibited externalizing behaviors that increased at a faster rate. We found ample evidence for longitudinal links between children’s sleep trajectories and depression and anxiety symptoms. Higher initial levels and greater increases in sleepiness across three years were significant predictors of elevated levels of depression and anxiety at T3. Similarly, higher initial levels of S/WP were associated with greater anxiety three years later; increases in S/WP over time were related to higher depression and anxiety symptoms at T3.
We addressed a call in the literature to examine the consequences of persistent sleep loss during childhood (Jan et al., 2010). Sleep disruptions are linked to compromised functioning of the prefrontal cortex (PFC; Jones & Harrison, 2000), which in turn may put children at risk for adjustment problems (Dahl, 1996). The PFC develops rapidly during early adolescence, a substantial part of this development occurs while asleep, and thus, the effects of sleep problems may be pronounced for youth (Jan et al., 2010). Findings provide support for the hypothesis that sleep problems during childhood may have long lasting effects on emotional functioning.
Girls but not boys who reported higher initial levels and increases in sleepiness over time had higher levels of depression symptoms at T3. There were large differences in depression for girls with higher and lower levels of sleepiness indicating that girls are more vulnerable than boys in this context. Few studies have investigated sex as a moderator of these effects and among existing studies, findings have been mixed. Using a mid-adolescent aged sample, Meijer et al. (2010) found that a shorter time in bed was related to a faster increase in boys’ versus girls’ externalizing and internalizing symptoms, which is not consistent with our results. Yet, another study based on this sample found that higher sleepiness predicted worse cognitive functioning (verbal comprehension) over time especially for girls (Bub et al., 2011).
The relation between sleepiness and depression symptoms was more evident for girls. In adolescence, girls are more vulnerable to depression (Hankin & Abramson, 2001) and it is plausible that the observed gender-related effects are due at least in part to this differential susceptibility. Risk factors for escalating depression among preadolescent girls are not well understood and findings suggest that consideration of sleep in this context is warranted. Given the restricted range of age and puberty in our sample, developmental effects are likely attenuated and assessments over a longer time frame should provide more clarity.
Support was also found for our proposition that sleep problems would predict higher levels of depression and anxiety symptoms most robustly for AAs and children from lower SES homes. Our sample consisted of both EAs and AAs from a wide range of economic backgrounds and in analyses of SES, ethnicity was controlled and vice versa. Whereas both AA and EA children were impacted by S/WP, the former’s anxiety and depression symptoms were more affected by poor sleep. Further, the combination of high sleepiness (slope) and lower SES status was especially predictive of increased depression symptoms over time. Children from lower SES homes who had lower sleepiness were especially protected against depression symptoms at T3. Further, SES moderated the association between initial levels of S/WP and children’s depression and anxiety symptoms at T3; changes in S/WP over time (slope) were also significant predictors of anxiety. Consistent with expectations, children from lower SES backgrounds were especially at risk for internalizing symptoms when their sleep was disrupted.
Results build on previous two-wave longitudinal work with an independent sample in which sleep problems had a greater impact on adjustment (El-Sheikh et al., 2010) and cognitive functioning (Buckhalt et al., 2009) for AAs (compared to EAs) and for children from lower SES families. Further, findings are consistent with those reported in a recent study with the current sample in which increases in sleep problems over three years had a greater impact on cognitive problems for AA children compared to EA children (Bub et al., 2011). Findings suggest that persistent sleep problems over three years may have an especially strong influence on later internalizing symptoms for children who face cultural and economic disadvantage.
Towards greater understanding of links between sleep and “pure” symptomatology, we controlled for anger while examining depression and anxiety symptoms and vice versa. Results were in expected directions for internalizing but not for anger; a higher level of sleep problems predicted lower levels of anger at the end of the study. These findings are contradictory to those in which sleep problems predicted increases in externalizing symptoms (e.g., Meijer et al., 2010) even when controlling for internalizing symptoms (Coulombe, Reid, Boyle, & Racine, 2011). However, in studies that control for comorbidity (e.g., Coulombe et al., 2011), several of the associations between sleep problems and externalizing behaviors that were originally significant when internalizing behaviors were not controlled, were no longer significant when the latter were controlled. In fact, we explored this possibility in our study and found the expected relations between both sleepiness and S/WP and increases in anger when internalizing symptoms were not controlled. Another potential explanation for this unexpected finding is that relatively low levels of anger were reported, which may have attenuated possible effects. Thus, care should be exercised in inferring relations between sleep and anger pending further assessments.
Findings should be interpreted within the study’s context. We examined subjective sleep parameters and objective measures (e.g., actigraphy) were not obtained. We did not examine variables associated with ethnicity or SES and important questions remain about why AA and lower SES children are more vulnerable to the effects of sleep problems. Further, non-significant moderation effects may have been due to reduced power. Moreover, more than three waves of data would allow for testing non-linear effects and the long-term effects of periodic and chronic sleep problems.
Acknowledgments
This research was supported by a National Institute of Health Grant R01-HD046795. We acknowledge contributions made by the staff of our Research Laboratory for data collection and management. We also thank the school personnel, children and parents who participated.
References
- Briere J. Trauma Symptom Checklist for Children (TSCC): Professional Manual. Odessa, FL: Psychological Assessment Resources; 1996. http://www4.parinc.com/products/product.aspx?Productid=TSCC. [Google Scholar]
- Bub KL, Buckhalt J, El-Sheikh M. Children’s sleep and cognitive performance: A cross-domain analysis of change over time. Developmental Psychology. 2011;47:1504–1514. doi: 10.1037/a0025535. doi: 10.1a0025535037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhalt JA. Insufficient sleep and the socioeconomic status achievement gap. Child Development Perspectives. 2011;5:59–65. doi: 10.1111/j.1750-8606.2010.00151.x. [DOI] [Google Scholar]
- Buckhalt JA, El-Sheikh M, Keller PS, Kelly RJ. Concurrent and longitudinal relationships between children’s sleep and cognitive functioning: The moderating role of parent education. Child Development. 2009;80:875–892. doi: 10.1111/j.1467-8624.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Chervin RD, Dillon JE, Archbold KH, Ruzicka DL. Conduct problems and symptoms of sleep disorders in children. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:201–208. doi: 10.1097/00004583-200302000-00014. [DOI] [PubMed] [Google Scholar]
- Chorney DB, Detweiler MF, Morris TL, Kuhn BR. The interplay of sleep disturbance, anxiety, and depression in children. Journal of Pediatric Psychology. 2008;33:339–348. doi: 10.1093/jpepsy/jsm105. [DOI] [PubMed] [Google Scholar]
- Coulombe JA, Reid GJ, Boyle MH, Racine Y. Sleep problems, tiredness, and psychological symptoms among healthy adolescents. Journal of Pediatric Psychology. 2011;36:25–35. doi: 10.1093/jpepsy/jsq028. [DOI] [PubMed] [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: Development and psychopathology. Development and Psychopathology. 1996;8:3–27. http://journals.cambridge.org/action/displayJournal?jid=DPP. [Google Scholar]
- El-Sheikh M, Kelly RJ, Buckhalt JA, Hinnant JB. Children’s sleep and adjustment over time: The role of socioeconomic context. Child Development. 2010;81:870–883. doi: 10.1111/j.1467-8624.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- Fredriksen K, Rhodes J, Reddy R, Way N. Sleepless in Chicago: Tracking the effects of adolescent sleep loss during the middle school years. Child Development. 2004;75:84–95. doi: 10.1111/j.1467-8624.2004.00655.x. [DOI] [PubMed] [Google Scholar]
- Gaina A, Sekine M, Hamanishi S, Chen X, Wang H, Kagamimori S. Daytime sleepiness and associated factors in Japanese school children. The Journal of Pediatrics. 2007;151:518–522. doi: 10.1016/j.jpeds.2007.04.036. [DOI] [PubMed] [Google Scholar]
- Goodnight JA, Bates JE, Staples AD, Pettit GS, Dodge KA. Temperamental resistance to control increases the association between sleep problems and externalizing behavior development. Journal of Family Psychology. 2007;21:39–48. doi: 10.1037/0893-3200.21.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, O’Connor TG. Sleep problems in childhood: A longitudinal study of developmental change and association with behavioral problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Unpublished manuscript 1975 [Google Scholar]
- Jan JE, Reiter RJ, Bax MCO, Ribary U, Freeman RD, Wasdell MB. Long-term sleep disturbances in children: A cause of neuronal loss. European Journal of Paediatric Neurology. 2010;14:380–390. doi: 10.1016/j.ejpn.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Jansen PW, Saridjan NS, Hofman A, Jaddoe VWV, Verhulst FC, Tiemeier H. Does disturbed sleeping precede symptoms of anxiety and depression in toddlers? The generation R study. Psychosomatic Medicine. 2011;73:242–249. doi: 10.1097/PSY.0b013e31820a4abb. [DOI] [PubMed] [Google Scholar]
- Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Medicine Reviews. 2001;5:463–475. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. http://www.medworksmedia.com/ [PubMed] [Google Scholar]
- Meijer AA, Reitz E, Dekovic M, van den Wittenboer GLH, Stoel RD. Longitudinal relations between sleep quality, time in bed and adolescent problem behavior. Journal of Child Psychology and Psychiatry. 2010;51:1278–1286. doi: 10.1111/j.1469-7610.2010.02261.x. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Mindell JA. Sleep and sleep disorders in children and adolescents. Psychiatric Clinics of North America. 2006;29:1059–1076. doi: 10.1016/j.psc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1037/0012-1649.40.6.1188. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. What I think and feel: A revised measure of children’s manifest anxiety. Journal of Abnormal Child Psychology. 1978;6:271–280. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. http://www.oup.com/us/catalog/general/subject/Education/?view=usa&ci=9780195152968. [Google Scholar]
- Van Oort FVA, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC. The developmental course of anxiety symptoms during adolescence: The TRAILS study. Journal of Child Psychology and Psychiatry. 2009;50:1209–1217. doi: 10.1111/j.1469-7610.2009.02092.x. [DOI] [PubMed] [Google Scholar]
- Wideman K. Missing data: What to do with or without them. In: McCartney K, Burchinal M, Bub KL, editors. Best Practices in Quantitative Methods for Developmentalists, Monographs of the Society for Research in Child Development. 3. Vol. 71. Boston, MA: Blackwell Publishing; 2006. http://www.wiley.com/WileyCDA/WileyTitle/productCd-1405169419,descCd-tableOfContents.html. [DOI] [PubMed] [Google Scholar]
- Willett JB. Measurement of change. In: Keeves JP, editor. Educational research, methodology and measurement: An international handbook. 2. Oxford, England: Pergamon Press; 1997. [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69:875–887. http://onlinelibrary.wiley.com/journal/10.1111/%28ISSN%291467-8624. [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, Martin JL. Evidence for the validity of a sleep habits survey of adolescents. Sleep. 2003;26:213–216. doi: 10.1093/sleep/26.2.213. http://www.journalsleep.org/Default.aspx. [DOI] [PubMed] [Google Scholar]