Abstract
Background
The epidemiology of invasive mold infections (IMI) in transplant recipients differs, based on geography, hosts, preventative strategies, and methods of diagnosis.
Methods
We conducted a retrospective observational study to evaluate the epidemiology of proven and probable IMI, using prior definitions, among all adult hematopoietic stem cell (HSCT) and solid organ transplant (SOT) recipients in the era of “classic” culture-based diagnostics (2000–2009). Epidemiology was evaluated before and after an initiative was begun to increase bronchoscopy in HSCT recipients after 2005.
Results
In total, 106 patients with one IMI were identified. Invasive aspergillosis (IA) was the most common IMI (69; 65.1%), followed by mucormycosis (9; 8.5%). The overall rate of IMI (and IA) was 3.5% (2.5%) in allogeneic HSCT recipients. The overall incidence for IMI among lung, kidney, liver, and heart transplant recipients were 49, 2, 11, and 10 per 1000 person-years, respectively. The observed rate of IMI among HLA-matched unrelated and haploidentical HSCT recipients increased from 0.6% annually to 3.0% after bronchoscopy initiation (P<0.05). The 12-week mortality among allogeneic HSCT, liver, kidney, heart, and lung recipients with IMI was 52.4%, 47.1%, 27.8%, 16.7%, and 9.5%, respectively. Among allogeneic HSCT (odds ratio [OR]: 0.07, P=0.007) and SOT (OR: 0.22, P=0.05) recipients with IA, normal platelet count was associated with improved survival. Male gender (OR: 14.4, P=0.007) and elevated bilirubin (OR: 5.7, P=0.04) were significant predictors of mortality for allogeneic HSCT and SOT recipients with IA, respectively.
Conclusions
During the era of culture-based diagnostics, observed rates of IMI were low among all transplants except lung transplant recipients, with relatively higher mortality rates. Diagnostic aggressiveness and host variables impact the reported incidence and outcome of IMI and likely account for institutional variability in multicenter studies. Definitions to standardize diagnoses among SOT recipients are needed.
Keywords: mold infections, transplant recipients, epidemiology
Background
More than 200,000 solid organ transplants (SOT) and 45,000 allogeneic hematopoietic stem cell transplants (HSCT) have been performed in the United States during the last decade (1). Invasive mold infections (IMI), especially invasive aspergillosis (IA), have been associated with high mortality rates in these patients (2–8). Recent reports suggest that outcomes of IA, especially among HSCT recipients, have significantly improved compared to observations in the 1990s (4–9). However, epidemiology and outcomes from multicenter registries have been conflicting with variability in the reported incidence, even among similar patient groups (9, 10). This variability is thought to be associated with institutional transplant practices, seasonality, geography, differences in hosts, and ways reported rates are calculated and presented (9–13). Variability is also likely a result of diagnostic bias, as diagnostic aggressiveness (e.g., bronchoscopies), empirical treatments, and diagnostic tests performed in the laboratory are not standardized across centers. Historically, diagnosis was largely dependent on culture, with antigen-based assays having been introduced in many institutions in the mid 2000s. Therefore, some of the reported incremental changes may merely be related to changing diagnostic practices.
Considering the increasing numbers of HSCT and SOTs performed since 2000, we sought to review the epidemiology and outcomes of IMI in a contemporary 10-year cohort of adult HSCT and SOT recipients at our center, which had historically relied on culture-based diagnostics alone. In addition, we sought to assess how a more aggressive diagnostic algorithm, implemented among allogeneic HSCT recipients in 2006, impacted the observed rates of IMI.
Methods
Study design
This study was approved by the Institutional Review Board. All adult (≥18 years) patients who received an HSCT or SOT between 1/1/2000 and 12/31/2009 and had a diagnosis of a proven or probable IMI were included. Patients with IMI were identified by review of electronic microbiology, pathology, and infection control databases searching for positive culture results for all molds, except for Penicillium species, and from all specimens, except urine. Following the identification of IMI, this list was cross-matched to a list including all HSCT and SOT recipients. All cases of IMI obtained from this process were then confirmed and coded as proven or probable by detailed chart review. For the purposes of this study the following exclusion criteria were applied: 1) IMI before transplantation, 2) duplicate specimens, and 3) subsequent IMI at any time after the first infection were excluded (only the first IMI identified per patient was included).
Transplant specific diagnostic protocols and antifungal prophylactic strategies
Fluconazole was administered until engraftment in all HSCT recipients. Allogeneic HSCT recipients with graft-versus-host disease (GVHD) were not routinely given anti-mold prophylaxis until 2009. A diagnostic algorithm for allogeneic HSCT recipients with new respiratory tract symptoms and abnormalities on chest computed tomography (CT) was implemented in 2005. Briefly, a bronchoscopy was performed within 24 h of the patient’s admission; a transbronchial biopsy (if feasible) was added beyond day 30 post transplant. Lung transplant recipients had surveillance bronchoscopy performed at 1, 3, 6, 9, 12, 18, and 24 months post transplant and then yearly thereafter. Routine antifungal prophylaxis included aerosolized amphotericin B during the first 30 days post transplant. If Aspergillus species were isolated from respiratory secretions pre-transplant, lung recipients received itraconazole or voriconazole for 6 months post transplant. Liver transplant recipients received fluconazole for 30 days post transplant. Kidney and heart transplant recipients did not receive primary systemic antifungal prophylaxis.
Variables
The following host variables were collected: demographics, underlying disease leading to transplant, and the following baseline (±7 days of the IMI diagnosis) laboratory data: total white blood cell count, absolute lymphocyte count, platelet count, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin. Transplant variables for HSCT patients included stem cell source, stem cell manipulation, donor type (autologous vs. allogeneic: unrelated, human leukocyte antigen (HLA)-matched related, or HLA-mismatched related), conditioning regimens, presence and severity of GVHD (acute grade II to IV and chronic), and associated treatments. Infection-related variables included the site of infection, fungal genus and species (when isolated), diagnostic modalities used, and timing of IMI post transplant. Imaging tests, including chest radiographs, chest and sinus CT, and brain imaging ±7 days of the IMI diagnosis, were reviewed.
Definitions
IMI were defined based on modified consensus guidelines that were originally developed for implementation within the cancer patient population (14). Briefly, the diagnosis of proven IMI required histopathological documentation of infection on a specimen from a normally sterile site. Probable IMI were diagnosed based on the presence of findings from the following 3 categories: host factors, clinical manifestations (symptoms, signs, and radiological features), and microbiological evidence (14). As no specific definitions or classic radiographic findings have been established in non-cancer (e.g., SOT) patients, microbiologic evidence of a mold without an alternative etiology was considered as probable IMI in the setting of dense consolidations or/and infiltrates on chest CT (15–17). Renal and liver impairment were defined as creatinine ≥1.5 mg/dL and either AST or ALT ≥100 units/L, respectively. Acute and chronic GVHD grades were from institutional records, using published guidelines (18, 19).
Statistical analysis
Rates of infection for HSCT recipients were calculated by dividing the number of patients who developed an IMI by the total number of transplants performed in the cohort year. We also reviewed the rates and outcomes of IMI and IA before and after 2005, the year in which a diagnostic algorithm was implemented for allogeneic HSCT recipients. For SOT recipients, the lengths of time to IMI, death, or end of study were used to calculate incidence rates per 1000 person-years for each SOT category.
Predictors of mortality among transplant recipients with IA were identified using multivariable logistic regression models. Because of the small sample sizes for autologous HSCT and SOT recipients receiving >1 organ, these analyses were limited to allogeneic HSCT and SOT recipients who received a single organ only. The multivariable conditional logistic regression models were built in a stepwise fashion using variables from the univariate analyses with a P-value ≤0.10. Two different models were constructed, for (a) allogeneic HSCT, (b) SOT recipients. The 12-week Kaplan-Meier survival curves during the study period were performed based on the type of IMI (IA vs. other molds) and different transplant categories. The log-rank test was used to compare survival distribution between groups.
Results
Between 2000 and 2009, 117 transplant recipients had IMI identified, 106 with 1, and 11 with 2 possible pathogens (the latter were excluded from all subsequent analyses). Characteristics of 44 HSCT and 62 SOT recipients with 1 IMI are presented in Tables 1 and 2, respectively. IA was the most common IMI observed (65.1%), followed by mucormycosis (8.5%) (Table 3). Thirty of the 106 (28.3%) patients with IMI had a “proven” infection, based on consensus definitions (14).
Table 1.
Characteristics of hematopoietic stem cell transplant (HSCT) recipients with diagnosed invasive mold infections (IMI), by type of transplant
| Characteristic | HLA-matched related N= 21 (%) |
Allogeneic, Other* N= 21 (%) |
Autologous N = 2 (%) |
|---|---|---|---|
| Demographics | |||
| Age in years, mean (range) | 53 (32–72) | 62 (20–67) | 51 (44–58) |
| Gender, Female | 7 (33.3) | 7 (33.3) | 2 (100) |
| Race, Caucasian | 19 (90.5) | 19 (90.5) | 1 (50) |
| Underlying disease | |||
| Acute leukemia | 5 (23.8) | 6 (28.6) | 1 (50) |
| Chronic leukemia | 5 (23.8) | 6 (28.6) | 0 |
| Lymphoma | 5 (23.8) | 7 (33.3) | 1 (50) |
| Multiple myeloma | 4 (19.0) | 0 | 0 |
| Myelodysplastic syndrome | 1 (4.8) | 0 | 0 |
| Other | 1 (4.8) | 2 (9.5) | 0 |
| Underlying disease status | |||
| New diagnosis | 6 (28.6) | 6 (28.6) | 0 |
| Relapse | 12 (57.1) | 14 (66.7) | 1 (50) |
| Transplant characteristics | |||
| Stem cell source, Bone marrow1 | 21 (100) | 18 (85.7) | 1 (50) |
| Stem cell manipulation, T-cell depleted | 6 (28.6) | 0 | 0 |
| Conditioning, myeloablative | 15 (71.4) | 9 (42.6) | NA |
| Prior HSCT2 | 0 | 6 (28.6) | 0 |
| Transplant complications | |||
| GVHD, Acute, Grade II–IV | 10 (47.6) | 11 (52.4) | NA |
| GVHD, Chronic | 2 (9.5) | 5 (23.8) | NA |
| Corticosteroid therapy | 14 (66.7) | 14 (66.7) | NA |
| Other Immunosuppressive therapy3 | 14 (66.7) | 14 (66.7) | NA |
| Laboratory variables (Median, range)4 | |||
| White blood cell count, cells/mm3 | 4160 (50–30900) | 4020 (50–14870) | 2956 (12–5900) |
| Absolute lymphocyte count, cells/mm3 | 385 (0–2590) | 415.5 (0–2270) | 225 (0–450) |
| Platelet count, cells/mm3 | 52 (12–356) | 65 (9–145) | 80.5 (31–130) |
| Creatinine, mg/dL | 1.05 (0.4–2.9) | 1.1 (0.4–2) | 0.8 (0.7–1) |
| Total bilirubin, mg/dL | 1.6 (0.2–11.6) | 1.0 (0.4–11) | 0.6 (0.3–0.9) |
| Aspartate aminotransferase, units/L | 32 (10–1927) | 36 (12–71 | 16 (14–18) |
| Chest CT findings for patients with IA5 | 13 | 15 | 0 |
| Nodular lesions | 5 (38.5) | 12 (80.0) | NA |
| Consolidations/Infiltrates | 6 (46.1) | 6 (40.0) | NA |
| Certainty of IMI diagnosis | |||
| Proven | 9 (42.9) | 5 (23.8) | 0 |
| Probable | 12 (57.1) | 16 (76.2) | 2 (100) |
| Site of infection6 | |||
| Lung | 19 (90.5) | 16 (76.2) | 2 (100) |
| Sinus | 5 (23.8) | 7 (33.3) | 0 |
| Brain | 3 (14.3) | 1 (4.8) | 0 |
Allogeneic, Other: 8-HLA matched unrelated and 13-haploidentical HSCT recipients.
The stem cell source for 1 and 2 allogeneic, other HSCT recipients were peripheral blood stem cells and cord blood, respectively. One autologous HSCT recipient received peripheral blood stem cells.
Six allogeneic, other HSCT recipients had a prior HSCT: 3 an autologous and 3 an allogeneic HSCT.
Other Immunosuppressive therapy included ≥1 of the following: sirolimus, tacrolimus, cyclosporine, mycophenolate mofetil, cyclophosphamide, pentostatin, or azathioprine.
Laboratory data were collected within 7 days of the IMI diagnosis. Data were not available for all patients.
Patients with IA per HSCT category listed. Rates are calculated by dividing the number of cases with different imaging findings by the total number of IA cases by HSCT category. Imaging findings were not mutually exclusive (patients could have >1).
Patients could have >1 sites infected.
GVHD, graft-versus-host disease; CT, computed tomography; IA, invasive aspergillosis
Table 2.
Characteristics of solid transplant recipients with invasive mold infections (IMI) by type of transplant*
| Characteristic | Lung N=21 (%) |
Kidney N=18 (%) |
Liver N=17 (%) |
Heart N=6 (%) |
|---|---|---|---|---|
| Demographics | ||||
| Age in years, mean (range) | 46 (21–65) | 69 (33–74) | 46 (22–63) | 46 (20–63) |
| Gender, Female | 9 (42.9) | 9 (50) | 4 (23.5) | 3 (50) |
| Race, Caucasian | 15 (71.4) | 10 (55.6) | 14 (82.3) | 3 (50) |
| Prior transplant | 0 | 2 (11.1) | 3 (17.6) | 0 |
| Underlying disease1 | COPD: 6 (28.6) | DM: 5 (27.8) | HCV: 5 (29.4) | Non-ischemic CMP: 3 (50) |
| IPF: 2 (9.5) | Hypertension: 5 (27.8) | ETOH: 4 (23.5) | Ischemic CMP: 2 (33.3) | |
| Cystic fibrosis: 3 (14.3) | ||||
| Sarcoidosis: 2 (9.5) | ||||
| Transplant characteristics | Single lung: 7 (33.3) | High-risk2 12 (66.7) | NA | NA |
| Laboratory variables, median (range)3 | ||||
| White blood cell count, cells/mm3 | 11130 (3070–22090) | 7630 (650–31370) | 5760 (1080–31640) | 13380 (6540–23440) |
| Absolute lymphocyte count, cells/mm3 | 632 (8–4270) | 283.5 (0–760) | 286 (115–349) | 1270 |
| Platelet count, cells/mm3 | 240 (40–390) | 234 (25–536) | 81 (22–570) | 126 (36–316) |
| Creatinine, mg/dL | 1.2 (0.5–8.1) | 1.6 (0.7–5.1) | 1.5 (0.5–4.4) | 1.6 (1.0–1.8) |
| Total bilirubin, mg/dL | 2.8 (0.2–42) | 0.5 (0.3–2.1) | 1.8 (0.3–28.2) | 1.2 (0.4–8.2) |
| Aspartate aminotransferase, units/L | 32 (9–122) | 25 (12–911) | 58 (12–337) | 24.5 (8–106) |
| Chest CT findings for patients with IA4 | 10 | 10 | 11 | 3 |
| Nodular lesions | 2 (20) | 5 (50) | 0 | 2 (66.7) |
| Consolidations/Infiltrates | 8 (80) | 5 (50) | 8 (72.7) | 1 (33.3) |
| Certainty of IMI diagnosis | ||||
| Proven | 3 (14.3) | 4 (22.2) | 7 (41.2) | 2 (33.3) |
| Probable | 18 (85.7) | 14 (77.8) | 10 (58.8) | 4 (66.7) |
| Site of infection5 | ||||
| Lung | 21 (100) | 15 (85.3) | 12 (70.6) | 4 (66.7) |
| Sinus | 2 (9.5) | 1 (5.6) | 3 (17.6) | 1 (16.7) |
| Skin and soft tissue | 0 | 2 (11.1) | 6 (35.3) | 2 (33.3) |
Only the most frequently observed organ transplant categories with on mould infection are presented in this Table. Four patients who received ≥2 transplants are not included.
Only the most frequently observed comorbidities leading to transplant are included in this Table. Comorbidities were not mutually exclusive (patients could have >1).
High-risk kidney transplants include ABO incompatible or/and positive cross match.
Laboratory data were collected within 7 days of the IMI diagnosis. Data were not available for all patients.
Patients with IA per transplant category listed. Rates are calculated by dividing the number of cases with different imaging findings by the total number of IA cases by transplant category. Imaging findings were not mutually exclusive (patients could have >1); not all imaging findings are included in this table.
Patients could have >1 site infected. All skin and soft tissue infections were biopsy proven. One kidney transplant recipient had an infection affecting the brain as well.
COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; DM, diabetes mellitus; HCV, hepatitis C; ETOH, alcoholic cirrhosis; CMP, cardiomyopathy; NA, not applicable; CT, computed tomography; IA, invasive aspergillosis.
Table 3.
Distribution of 106 invasive mold infections (IMI) among transplant recipients
| IMI | HSCT1 N=44 (%) |
SOT2 N=62 (%) |
Lung N=21 (%) |
Kidney N=18 (%) |
Liver N=17 (%) |
Heart N=6 (%) |
|---|---|---|---|---|---|---|
| Aspergillus species | 28 (64.3) | 41 (66.1) | 17 (80.9) | 10 (55.6) | 11 (64.7) | 3 (50.0) |
| A. flavus | 3 (10.7) | 4 (9.8) | 2 (11.8) | 1 (10.0) | 1 (9.1) | 0 |
| A. fumigatus | 18 (64.3) | 27 (65.9) | 10 (58.8) | 9 (90.0) | 6 (54.5) | 2 (66.7) |
| A. niger | 3 (10.7) | 4 (9.8) | 2 (11.8) | 0 | 1 (9.1) | 1 (33.3) |
| A. terreus | 1 (3.6) | 1 (2.4) | 1 (5.9) | 0 | 0 | 0 |
| A. versicolor | 0 | 1 (2.4) | 0 | 0 | 1 (9.1) | 0 |
| Other3 | 3 (10.7) | 4 (9.8) | 2 (11.8) | 0 | 2 (18.2) | 0 |
| Zygomycetes | 1 (2.3) | 85 (12.9) | 1 (4.8) | 3 (16.7) | 3 (17.5) | 1 (16.7) |
| Rhizopus | 0 | 5 (62.5) | 1 (100) | 1 (33.3) | 2 (66.7) | 1(100) |
| Other4 | 1 | 3 (37.5) | 0 | 2 (66.7) | 1 (33.3) | 0 |
| Fusarium species | 3 (6.8) | 1 (1.6) | 0 | 1 (5.6) | 0 | 0 |
| Scedosporium species | 2 (4.5) | 1 (1.6) | 0 | 0 | 1 (5.9) | 0 |
| Other5 | 10 (22.7) | 7 (11.3) | 3 (14.3) | 1 (5.6) | 1 (5.9) | 2 (33.3) |
Two of 44 patients had an autologous HSCT: one with Scedosporium species and another with other mold infection.
Four patients underwent >1 SOTs, and were not included in this Table.
Other Aspergillus species were not identified.
Other Zygomycetes included: Cunninghamella (n=1), Mucor (n=1), and not speciated Zygomycetes (n=1).
Other molds included: Cladosporium (n=1), Paecilomyces species (n=3), Acremonium (n=1), and other not speciated molds (n=12).
HSCT, hematopoietic stem cell transplant; SOT, solid organ transplant.
HSCT recipients
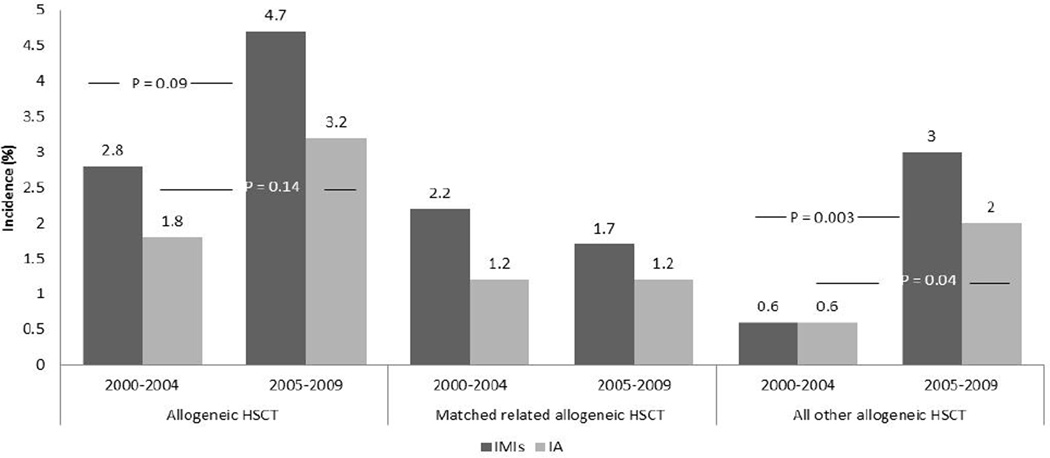
The overall rate of IMI was 0.2% (2 of 874) and 3.8% (42 of 1109) in autologous and allogeneic HSCT recipients, respectively. The overall rate of IA among allogeneic HSCT recipients was 2.5% (N=28). Although the overall rates of IMI and IA did not significantly change during the study period, the rates of IMI and IA among HLA-matched unrelated and haploidentical allogeneic HSCT recipients increased from 0.6% each to 3.0% (P=0.003) and 2.0% (P=0.04) after 2005, respectively (Fig. 1). The mean time-to-diagnosis for IMI and IA was 214.5 and 219.2 days, respectively. The overall 12-week mortality among allogeneic HSCT recipients with IMI and IA was 50% (21 of 42) and 42.9% (12 of 28), respectively. Allogeneic HSCT recipients with an IMI other than IA were more likely to have died by 12 weeks (64.3%, 9 of 14) compared with those who had IA (42.9%; log-rank P= 0.05). No difference in 12-week mortality was found, based on the type of allogeneic HSCT (HLA-matched related vs. other) or time of transplant (2000–2004 vs. 2005–2009) for IMI and IA. Based on stepwise multivariable logistic regression model, among allogeneic HSCT recipients with IA, male gender (OR: 14.4, P=0.007) was a significant predictor of mortality, while normal platelet count (OR: 0.07, P=0.007) was significantly less associated with mortality (Table 4).
Fig. 1.
Rates of invasive mold infections (IMI) overall and invasive aspergillosis (IA) by type of hematopoietic stem cell, between 2000–2004 and 2005–2009. Sites of infections were not mutually exclusive. Patients could have >1 site infected. HSCT, hematopoietic stem cell transplant; MR, matched related.
Table 4.
Univariate and multivariable analysis for 12-week mortality predictors for hematopoietic stem cell (HSCT) and solid organ transplant (SOT) recipients with invasive aspergillosis (IA)*
| Allogeneic HSCT recipients with IA (N=28) |
SOT1 recipients with IA (N=41) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| Variable | OR | P-value | OR | P-value | OR | P-value | OR | P-value |
| Demographics | ||||||||
| Gender (male vs. female) | 5.3 | 0.04 | 14.4 | 0.007 | 0.9 | 0.8 | ||
| Race (Caucasian vs. other) | 1 | NA | 2.3 | 0.2 | ||||
| Age (≥ vs. <50 years) | 2.7 | 0.15 | 1.5 | 0.5 | ||||
| Host-related variables | ||||||||
| ALC (≥ vs. <1000 cells/mm3) | 0.70 | 0.64 | 0.74 | 0.73 | ||||
| Platelet count (≥ vs. <100,000 cells/mm3) | 0.15 | 0.02 | 0.07 | 0.007 | 0.16 | 0.01 | 0.22 | 0.05 |
| AST (≥ vs. <100 units/L) | 1 | NA | 2.4 | 0.3 | ||||
| ALT (≥ vs. <100 units/L) | 0.66 | 0.62 | 2.8 | 0.12 | ||||
| Total bilirubin (≥ vs. <3.0 mg/dL) | 6.07 | 0.12 | 6.5 | 0.01 | 5.2 | 0.04 | ||
| Creatinine (≥ vs. <1.5 mg/dL) | 6.70 | 0.03 | 1.8 | 0.34 | ||||
| Renal or/and liver impairment2 | 3.60 | 0.08 | 5.4 | 0.04 | ||||
| Transplant-related variables | ||||||||
| SOT, other vs. lung | NA | NA | NA | NA | 8.3 | 0.07 | ||
| HSCT, Matched-related vs. other | 1.72 | 0.42 | NA | NA | ||||
| HSCT, Conditioning regimen (myeloablative vs. non) | 0.50 | 0.26 | NA | NA | ||||
| HSCT, GVHD3 (Yes vs. No) | 1.62 | 0.53 | NA | NA | ||||
| HSCT, Steroids4 (Yes vs. No) | 0.50 | 0.37 | NA | NA | ||||
| IA-related variables | ||||||||
| Year of IA diagnosis (2005–2009 vs. 2000–2004) | 0.66 | 0.56 | 0.53 | 0.31 | ||||
| Days of diagnosis post transplant5 | 1 | 0.17 | 0.76 | 0.7 | ||||
| Primary antifungal treatment (VOR vs. other) | 0.30 | 0.11 | 0.14 | 0.08 | ||||
Multivariable analyses were performed with univariate predictors (P-value ≤0.10) introduced into a regression model in a stepwise fashion.
Only SOT recipients that received a single organ transplant were included in the analyses.
Renal or/and liver impairment were defined as creatinine ≥1.5 mg/dL and either aspartate or alanine aminotransferase ≥100 units/L, respectively.
Presence of chronic or/and acute (grade ≥2) GVHD within 6 months prior to IA diagnosis.
Steroids as treatment for GVHD.
Days to IA diagnosis post-transplant: <40 days vs. ≥40 days.
OR, odds ratio; NA, not applicable; ALC, absolute lymphocyte count; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GVHD, graft-versus-host disease; VOR, voriconazole.
SOT recipients
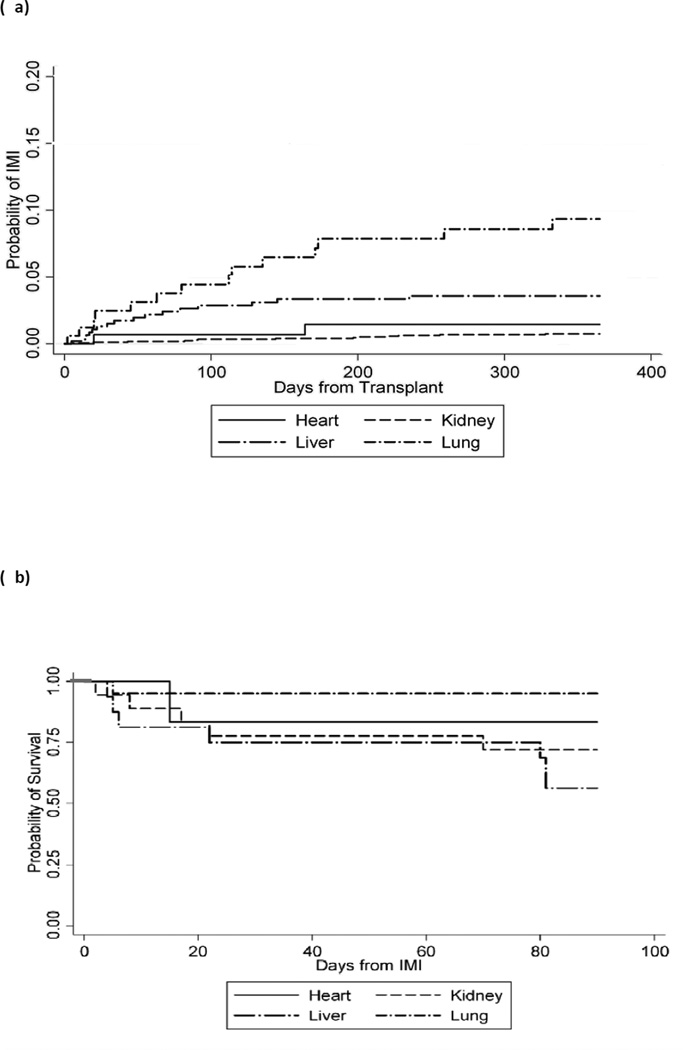
The overall incidence rates for IMI among 168 lung, 1748 kidney, 485 liver, and 156 heart transplant recipients during the study period were 49 (95% confidence interval [CI]: 31.7, 74.8), 2 (95% CI: 1.6, 4.0), 11 (95% CI: 7.1, 18.3), and 10 (95% CI: 4.6, 22.8) per 1000 person-years, respectively. The 1-year cumulative incidence of IA for heart, kidney, liver, and lung transplant recipients is presented in Figure 2a. IA and IMI were late diagnoses in all SOT recipients except in liver transplant recipients, and had a median time to diagnosis of 34 (interquartile range [IQR]: 108) and 48 days (IQR: 60) post transplant, respectively (P<0.001). IA and IMI occurred particularly late in heart transplant recipients, with a median 2514 (IQR: 2620) and 771 days (IQR: 2350), respectively. Liver transplant recipients had the highest 12-week mortality after the diagnosis of IMI (47.1%, 8 of 17; log-rank P<0.001) compared with kidney (27.8%, 5 of 18), heart (16.7%, 1 of 6), and lung (9.5%, 2 of 21) (Fig. 2b). Similarly, 12-week mortality for patients with IA was 54.6% (6 of 11) for liver vs. 40.0% (4 of 10) for kidney, 33.3% (1 of 3) heart, and 11.8% (2 of 17) lung transplant recipients (log-rank P=0.05). Based on stepwise multivariable logistic regression model, in SOT recipients with IA, normal platelet counts (OR: 0.22, P=0.05) was associated with improved survival, while elevated bilirubin was a significant predictor of mortality (OR: 5.7, P=0.04; Table 4).
Fig. 2.
(a) Probability of invasive mold infection (IMI) among solid organ transplant (SOT) recipients (by organ type). Four patients who received ≥2 SOTs are not included. (b) The 12-week survival probability for SOT categories post diagnosis of IMI: liver transplant recipients were the most likely to have died by 12 weeks compared to other SOT recipients (log-rank P=0.05).
Discussion
This retrospective, single-center study describes the epidemiology and outcomes in a contemporary cohort of adult HSCT and SOT recipients with a proven or probable IMI between 2000 and 2009. Low rates of IMI were observed in all except lung transplant recipients and were associated with high 12-week mortality. Using the existing definitions, lung transplant recipients had the highest rates of IMI with low mortality, which illustrates the need to standardize IMI definitions among SOT recipients.
High rates of IMI were observed in lung transplant recipients, as previously reported (11, 20–22). A proportion of IMI defined here may represent airway colonization, rather than invasive disease. Notably, only 3 (14.3%) of these patients had a biopsy-proven diagnosis and 2 (11.8%) of 17 IA cases were due to Aspergillus niger, organism with typically low pathogenicity. High rates may be associated with multiple routine surveillance bronchoscopies in this patient population. Most importantly, the definitions used here were established to predict disease in patients with hematologic malignancies or/and HSCT recipients as underlying conditions (14). Lung transplant recipients represent a unique category of immunocompromised hosts, by virtue of their underlying structural abnormalities associated with surgical anastomoses and direct contact of the transplanted organ with the environment. As a result, imaging changes may be challenging to interpret, as they could represent postoperative changes and/or a variety of infectious complications. In addition, retrieval of molds, particularly in clinical conditions in which the significance of airway colonization is not entirely clear, frequently results in “empirical” treatment, because of implications with other secondary manifestations, such as bronchiolitis obliterans. Definitions of IMI in this patient group should be revisited, to consider associated biases and risks in an effort to create a common, efficient, and meaningful diagnostic standard for study enrollment and clinical practice considerations.
A critical review of recent data coming from 2 multicenter registries on the epidemiology and outcomes of invasive fungal infections (IFIs) in transplant recipients emphasizes that there is variability in the reported rates (9–12). For instance, the diagnosis of IFI among HSCT recipients was proven in 56% and 11.5% in the TRANSNET (including IC cases) and PATH Alliance Registry cohorts, respectively (9, 10). The latter group reported that the use of galactomannan enzyme immunoassay (GM EIA; in serum and/or bronchoalveolar lavage [BAL]) contributed to 73% and 16% of IA diagnoses in HSCT and SOT recipients, respectively (9, 12). A large variation existed in reported rates of IFI between centers in the PATH Alliance cohort, ranging from 2 to 93, even among similar host groups (9). The above facts suggest that results are subject to multiple variables, including transplant practices, geography, seasonality, hosts, and diagnostic methods applied at different centers. Nevertheless, rates of IMI among transplant recipients appear to have increased, with some of the reported incremental changes potentially related to more aggressive and sensitive diagnostic practices, such as antigen-based or/and molecular assays (2, 4, 7, 10, 11).
The exclusively culture-based diagnostic methods applied in our center during the last decade likely explain the low rates of IMI observed in all but lung transplants. Rates of IFI are subject to multiple variables, including transplant practices, geography, seasonality, hosts, and diagnostic methods applied at different centers. Lack of aggressive procedures and initiation of empirical or pre-emptive treatment based on host factors, clinical suspicion, and imaging findings were common practices, particularly in the HSCT service, before 2006. Hence, a significant number of patients were treated for a possible IMI, a significant proportion of which, we believe, represented real infections, not confirmed microbiologically. Initiation of a more aggressive diagnostic algorithm, to include BAL, in 2005 led to higher rates of IMI in allogeneic HSCT recipients. Notably, higher numbers of haploidentical HSCT with low-intensity conditioning regimens were also performed at our institution after 2005, which could account for the significantly higher numbers of IMI diagnosed during the latter part of the study. However, detailed evaluation of this cohort did not reveal particularly increased risks for infections (23).
In addition to lack of aggressive bronchoscopy, the use of non-culture based diagnostic tests was limited. Importantly, the diagnosis of IMI was exclusively culture- or biopsy-driven in this series. The sensitivity rates of sputum, BAL, and tissue cultures for the diagnosis of pulmonary IA have ranged between 15–69%, 0–67%, and 30–52%, respectively (24–26). Thus, a significant proportion of patients with IA might have been missed due to lack of use of non-culture based diagnostic tools. The use of more sensitive diagnostic practices, such as antigen-based or/and molecular assays may significantly increase the diagnostic yield and quicken the diagnosis of IA (27, 28). The use of the GM EIA in our center was limited until late 2009 and diagnosis was based on non-antigen, culture-based data. Since then, we have implemented the routine use of GM EIA in the serum and BAL of immunocompromised hosts, which may help identify a significant proportion of patients with IA that could otherwise be missed or simply empirically treated for a possible “fungal pneumonia.”
Host-related variables appeared to be the most important predictors of mortality in this cohort of transplant recipients with IA. Normal platelet count was associated with reduced risk for death among all transplant recipients with IA. This association may, in part, represent thrombocytopenia signifying underlying disease severity. Similarly, patients with normal platelet counts might have been more likely to undergo a diagnostic bronchoscopy sooner, thus leading to earlier diagnosis, initiation of appropriate targeted treatment, and better outcomes. Finally, recent data suggest that platelets may affect the virulence and viability of Aspergillus species, hence enhancing the activity of antifungal agents and, perhaps, leading to improved outcomes (29, 30). Baseline elevated bilirubin was strongly associated with 12-week mortality in SOT. We believe that this likely represents a surrogate of disease severity, suggesting that sicker patients are more likely to die. Gender was found to be a significant mortality predictor in HSCT recipients with IA. Whether this is an indicator of other possible risk factors, such as severity of GVHD, is not entirely clear, but warrants further investigation.
In conclusion, during the years in which we relied on culture-driven diagnoses exclusively, we observed lower than reported rates of IMI among all except lung transplant recipients, with relatively high mortality rates. Multiple host variables impacted the overall survival among the different transplant cohorts. These observations, including host-derived variations in survival and diagnostic aggressiveness, impact the reported incidence of IMI and should be taken into consideration when interpreting epidemiology and outcome studies, especially from multiple centers that utilize different diagnostic approaches. Finally, definitions to standardize diagnoses of IMI among SOT recipients should be revisited.
Acknowledgments
Thanks: The authors would like to thank Nicole Kwiatkowski, Ramon Konewko, Brigitte Sullivan, Sean Zhang, Karen Carroll, Richard Ambinder, and Carol-Ann Huff for their assistance with data acquisition.
Support: The study was supported by a research grant (WS484583) from Pfizer and a National Institute of Health K24 grant, #AI85118.
Footnotes
Presentation: This study was presented, in part, at the 49th Annual Meeting of the Infectious Diseases Society of America (IDSA), October 20–23, 2011, Abstract: 30071.
Conflicts of interest: D.N. has received research grants from Pfizer and has served on advisory boards for Roche. K.A.M. has received grant support from Astellas, Merck and Pfizer and served on advisory boards for Astellas, Basilea, Merck, and Pfizer. All other authors: No conflicts of interest.
References
- 1. [last accessed June 30, 2011]; www.ustransplant.org. [Google Scholar]
- 2.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: Changes in epidemiology and risk factors. Blood. 2002;100(13):4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 3.Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 2000;30(6):851–856. doi: 10.1086/313803. [DOI] [PubMed] [Google Scholar]
- 4.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 5.Cordonnier C, Ribaud P, Herbrecht R, et al. Prognostic factors for death due to invasive aspergillosis after hematopoietic stem cell transplantation: a 1-year retrospective study of consecutive patients at French transplantation centers. Clin Infect Dis. 2006;42(7):955–963. doi: 10.1086/500934. [DOI] [PubMed] [Google Scholar]
- 6.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Vidal C, Upton A, Kirby K, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–1050. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nivoix Y, Velten M, Letscher-Bru V, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47:1176–1184. doi: 10.1086/592255. [DOI] [PubMed] [Google Scholar]
- 9.Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48:265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 10.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 11.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50(8):1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 12.Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12(3):220–229. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 13.Panackal AA, Li H, Kontoyiannis DP, Mori M. Geoclimatic influences on invasive aspergillosis after hematopoietic stem cell transplantation. Clin Infect Dis. 2011;50(12):1588–1597. doi: 10.1086/652761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kojima R, Tateishi U, Kami M, et al. Chest computed tomography of late invasive aspergillosis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):506–511. doi: 10.1016/j.bbmt.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Kim SH, Choi SH, et al. Clinical and radiological features of invasive pulmonary aspergillosis in transplant recipients and neutropenic patients. Transpl Infect Dis. 2010;12(4):309–315. doi: 10.1111/j.1399-3062.2010.00499.x. [DOI] [PubMed] [Google Scholar]
- 17.Nucci M. Probable invasive aspergillosis without pre-specified radiologic findings: proposal for inclusion of a new category of aspergillosis and implications for studying novel therapies. Clin Infect Dis. 2010;51(11):1281–1283. doi: 10.1086/657065. [DOI] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Baddley JW, Andes DR, Marr KA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis. 2010;50(12):1559–1567. doi: 10.1086/652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson JE. Epidemiology of fungal infections in solid organ transplant patients. Transpl Infect Dis. 1999;1(4):229–236. doi: 10.1034/j.1399-3062.1999.010402.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Husain S. Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for management. J Heart Lung Transplant. 2003;22(3):258–266. doi: 10.1016/s1053-2498(02)00477-1. [DOI] [PubMed] [Google Scholar]
- 23.Luznik L, O’Donnell P, Symons H, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodey G, Bueltmann B, Duguid W, et al. Fungal infections in cancer patients: an international autopsy survey. Eur J Clin Microbiol Infect Dis. 1992;11(2):99–109. doi: 10.1007/BF01967060. [DOI] [PubMed] [Google Scholar]
- 25.Tarrand JJ, Lichterfeld M, Warraich I, et al. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol. 2003;119(6):854–858. doi: 10.1309/EXBV-YAUP-ENBM-285Y. [DOI] [PubMed] [Google Scholar]
- 26.Reichenberger F, Habicht J, Matt P, et al. Diagnostic yield of bronchoscopy in histologically proven invasive pulmonary aspergillosis. Bone Marrow Transplant. 1999;24(11):1195–1199. doi: 10.1038/sj.bmt.1702045. [DOI] [PubMed] [Google Scholar]
- 27.Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J Infect Dis. 2004;190(3):641–649. doi: 10.1086/422009. [DOI] [PubMed] [Google Scholar]
- 28.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42(10):1417–1427. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 29.Perkhofer S, Kehrel BE, vonEiff C, et al. Human platelets attenuate virulence of Aspergillus spp. via granule dependent mechanisms. J Infect Dis. 2008;198:1243–1246. doi: 10.1086/591458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkhofer S, Trappl K, Striessnig B, et al. Platelets enhance activity of antimycotic substances against non-Aspergillus fumigatus Aspergillus species in vitro. Med Mycol. 2011;49:157–166. doi: 10.3109/13693786.2010.510150. [DOI] [PubMed] [Google Scholar]