Abstract
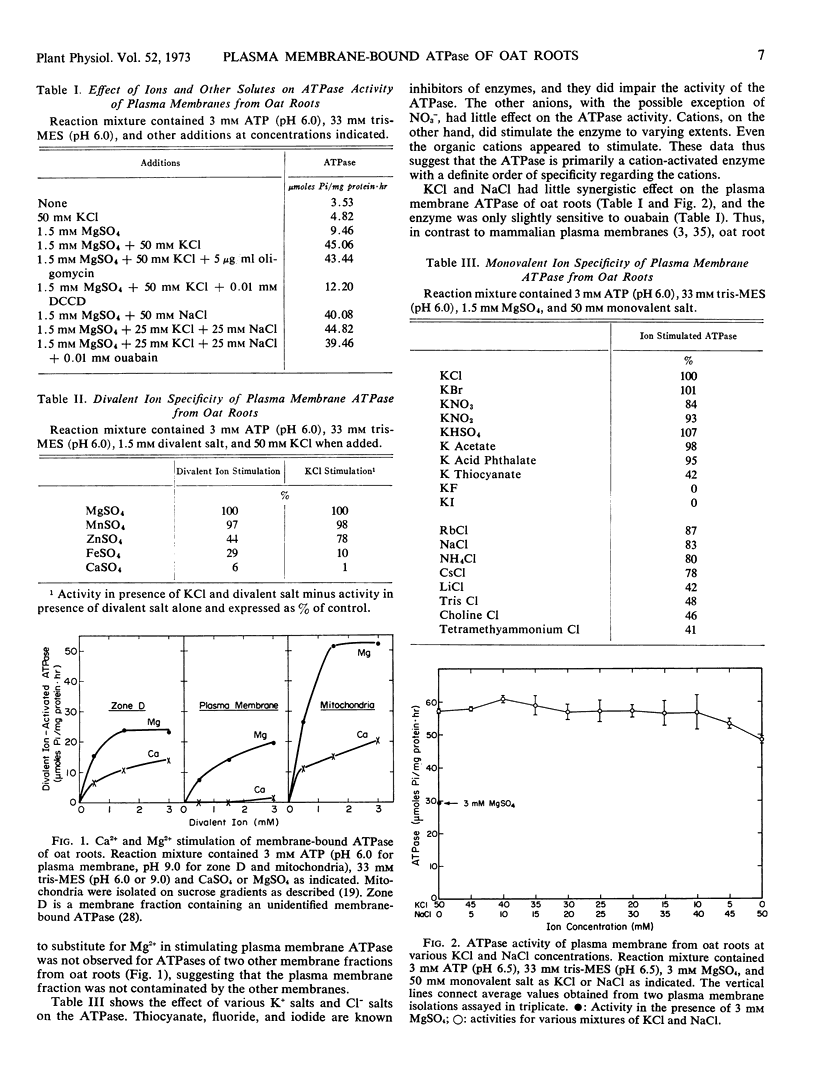
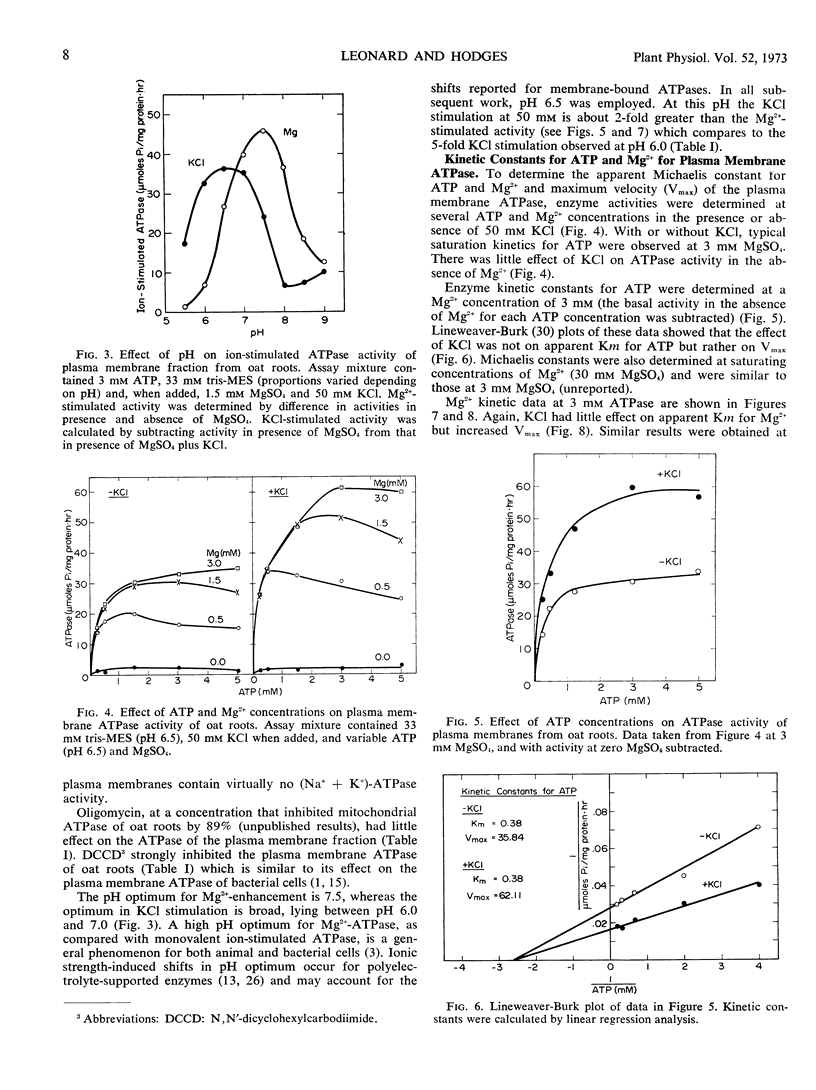
ATPase activity of plasma membranes isolated from oat (Avena sativa L. cv. Goodfield) roots was activated by divalent cations (Mg2+ = Mn2+ > Zn2+ > Fe2+ > Ca2+) and further stimulated by KCl and a variety of monovalent salts, both inorganic and organic. The enzyme exhibited greater specificity for cations than anions. The presence of Mg2+ was necessary for KCl stimulation. Ca2+ was ineffective in replacing Mg2+ for activation of plasma membrane ATPase, but it did activate other membrane-bound ATPases. The pH optima for Mg2+ activation and KCl stimulation of the plasma membrane ATPase were 7.5 and 6.5, respectively.
The plasma membrane ATPase showed little synergistic effects of K+ and Na+, and it was only slightly sensitive to ouabain. Oligomycin did not inhibit the ATPase, while N,N′-dicyclohexylcarbodiimide was a potent inhibitor of the enzyme.
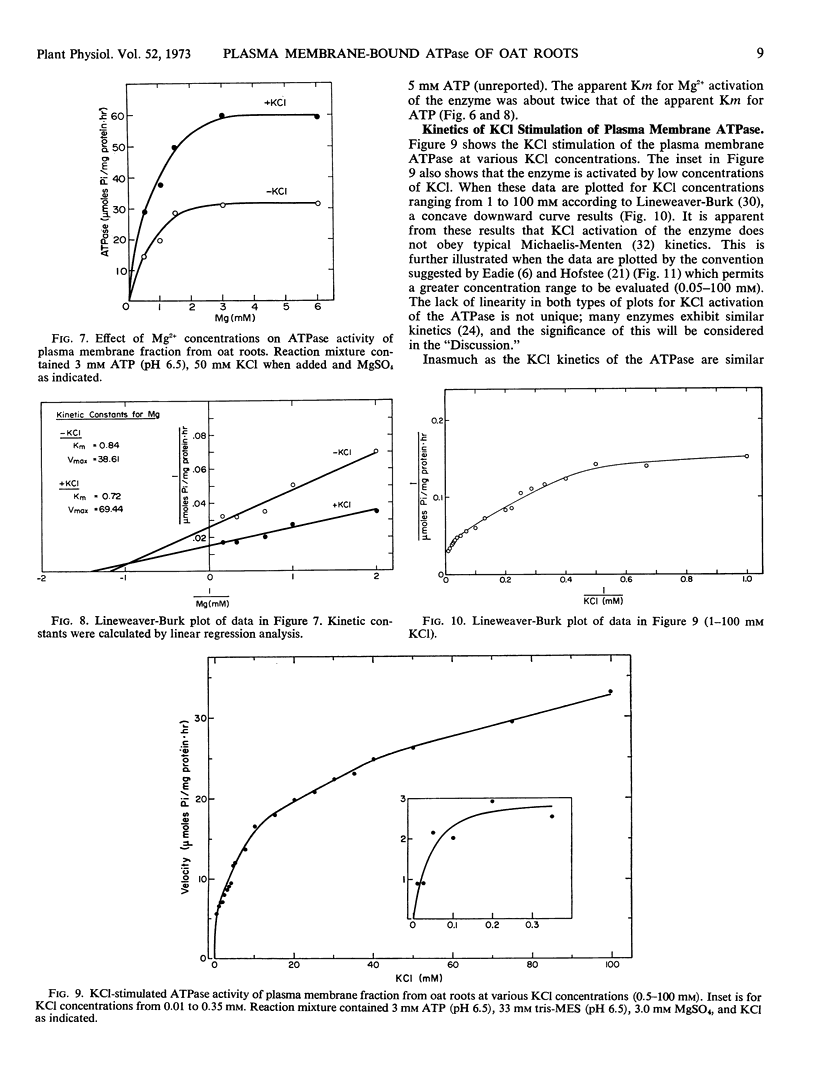
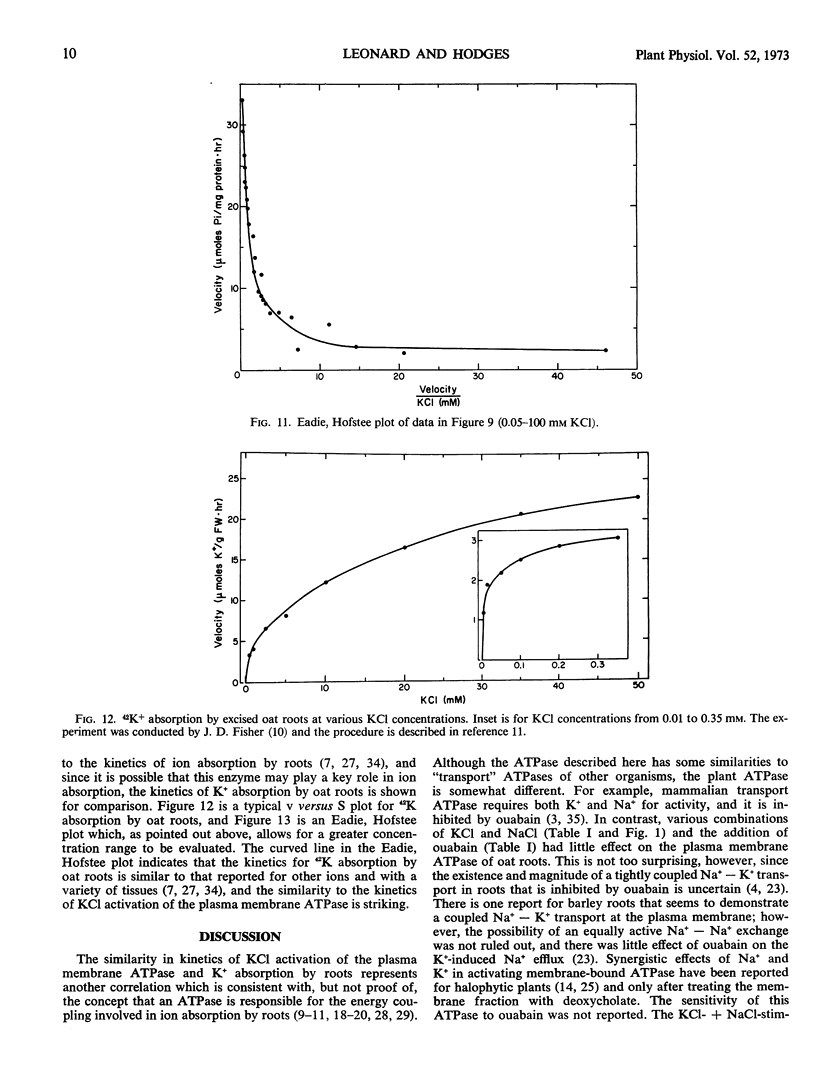
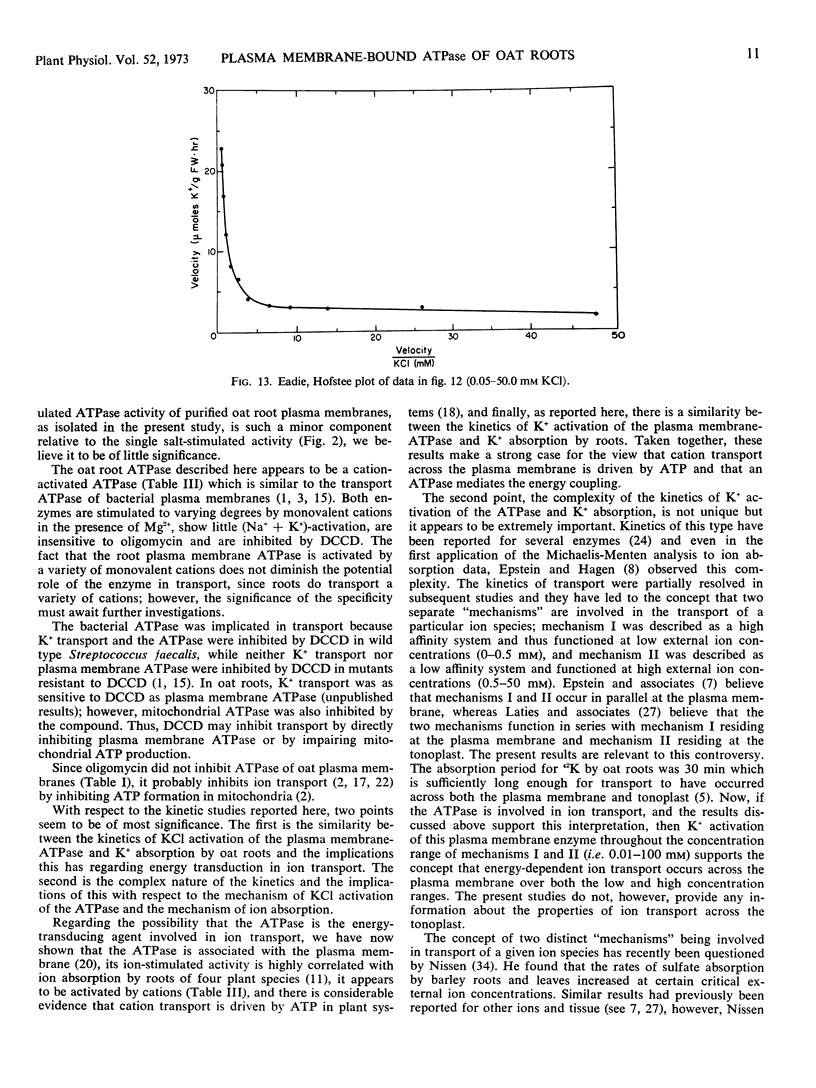
The apparent Km for Mg2+ activation (0.84 mm) of the ATPase was about twice that of the apparent Km for ATP (0.38 mm). The effect of KCl in stimulating the enzyme was not on the apparent Km values for ATP and Mg2+ but rather on maximum velocity. The kinetics of KCl stimulation of the plasma membrane ATPase were similar to the kinetics of 42K+ influx into oat roots and neither followed the Michaelis-Menten equation but rather were best described by a single activity curve with continually changing kinetic parameters. These results support the concept that cation transport at the plasma membrane of root cells is coupled to a cation-activated ATPase which is functional from low (0.01 mm) to high (50.0 mm) concentrations of KCl.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A., Smith J. B., Baron C. Carbodiimide-resistant membrane adenosine triphosphatase in mutants of Streptococcus faecalis. I. Studies of the mechanism of resistance. J Biol Chem. 1972 Mar 10;247(5):1484–1488. [PubMed] [Google Scholar]
- Bledsoe C., Cole C. V., Ross C. Oligomycin inhibition of phosphate uptake and ATP labeling in excised maize roots. Plant Physiol. 1969 Jul;44(7):1040–1044. doi: 10.1104/pp.44.7.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Hagen C. E. A KINETIC STUDY OF THE ABSORPTION OF ALKALI CATIONS BY BARLEY ROOTS. Plant Physiol. 1952 Jul;27(3):457–474. doi: 10.1104/pp.27.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. D., Hansen D., Hodges T. K. Correlation between ion fluxes and ion-stimulated adenosine triphosphatase activity of plant roots. Plant Physiol. 1970 Dec;46(6):812–814. doi: 10.1104/pp.46.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Hodges T. K. Monovalent ion stimulated adenosine triphosphatase from oat roots. Plant Physiol. 1969 Mar;44(3):385–395. doi: 10.1104/pp.44.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylin A., Gee R. Adenosine Triphosphatase Activities in Leaves of the Mangrove Avicennia nitida Jacq: Influence of Sodium to Potassium Ratios and Salt Concentrations. Plant Physiol. 1970 Feb;45(2):169–172. doi: 10.1104/pp.45.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]


