Abstract
Previous studies indicate that green tea extract may inhibit breast cancer progression by blocking angiogenesis, although the molecular mechanisms are not well defined. We demonstrate that administration of Polyphenon E (Poly E), a standardized green tea extract, inhibited MDA-MB231 breast cancer and human dermal microvascular endothelial (HMVEC) cell migration and the expression of vascular endothelial growth factor (VEGF) and matrix metalloproteinase 9 (MMP9). In addition, Poly E inhibited VEGF-induced neovascularization in vivo. We also demonstrate that Poly E blocked signal transducers and activators of transcription (STAT) signaling by suppressing interferon-gamma (IFN-γ)-induced gene transcription via IFN-γ-activating sequence (GAS) elements and downstream STAT3 activation by inhibiting STAT1 and STAT3 dimerization in MDA-MB231 cells. Transient expression of constitutively active STAT3 significantly reduced the inhibitory effect of Poly E on cell migration and VEGF and MMP9 expression. Taken together, these observations indicate that green tea extract inhibits angiogenesis partly through the disruption of STAT3-mediated transcription of genes, including VEGF.
Keywords: Green tea, Breast cancer, STAT3, Angiogenesis
Introduction
Angiogenesis, the development of new capillaries from preexisting blood vessels, is required in physiological processes such as wound healing and pathological conditions including tumor growth and metastases [1]. Tumor angiogenesis is a complex process that consists of several steps including the secretion of angiogenic factors by tumor and host cells, activation of proteolytic enzymes, endothelial cell migration and invasion, and endothelial cell proliferation and capillary formation [2, 3]. These events are regulated by several antiangiogenic and proangiogenic factors, of which vascular endothelial growth factor (VEGF) is most potent. VEGF exerts its biological activities through three receptors including VEGFR-1 (Flt-1), VEGFR-2 (Flk-1), and VEGFR-3 (Flt-4) [3]. VEGF and its receptors are commonly overexpressed in several types of human cancers including breast cancer. Overexpression is an event that frequently occurs during the early stages of breast cancer progression and is associated with poor clinical outcome and resistance to hormonal and chemotherapy in patients with invasive breast cancer [4, 5]. Thus, VEGF and its receptors are attractive therapeutic targets for breast cancer treatment.
Multiple agents that target the VEGF pathway are currently being studied for cancer therapy. Among these agents are monoclonal VEGF antibodies, small-molecule inhibitors of VEGFR, and several natural products that exhibit antiangiogenic properties including milk thistle, turmeric, ginger, and green tea [6–11]. Unlike single antiangiogenic agents, natural products often exert actions on several signaling pathways besides angiogenesis, including proliferation and apoptosis. Due to such combinatorial effects, natural products may potentially enhance the efficacy of conventional cancer therapies. For example, green tea extract, derived from the tea plant Camelia sinensis, exerts diverse biological activities including the inhibition of tumor growth, induction of apoptosis, and inhibition of angiogenesis [12]. Studies demonstrate that a major component in green tea improves the sensitivity of tumor cells to chemotherapeutic agents such as Sulindac and Taxol [13–15].
Next to water, tea is the most popular beverage consumed in the world. Epidemiological data suggest that the consumption of green tea may reduce the risk of several cancer types including cancers of the lung, breast, and prostate [16–18]. The health benefits of green tea are attributed to its epicatechin-derived polyphenolic components, which include (–)-epicatchin (EC), (–)-epigallocatechin (EGC), (–)-epicatechingallate (ECG), and (–)-epigallocatechin-3-gallate (EGCG) [13]. EGCG, the main polyphenolic constituent, mediates the majority of anticarcinogenic effects of green tea and has been shown to effectively inhibit several angiogenic processes. For example, EGCG has been shown to inhibit endothelial cell growth, VEGF expression and binding to its receptor, and VEGFR expression and phosphorylation [19–23]. In addition, EGCG has been shown to inhibit the expression of matrix metalloproteinases (MMPs) such as MMP9, which play a role in angiogenesis by degrading the extracellular matrix [24]. Studies also indicate that EGCG may suppress signaling proteins downstream of VEGFR activation, including VE-cadherin and Akt [21, 23, 25]. Although mechanistic studies demonstrate that EGCG may decrease VEGF expression through the inhibition of NF-kB activity, the exact mechanism by which green tea inhibits VEGF-induced angiogenesis remains unclear [21]. In the present study, we demonstrate that green tea inhibits VEGF signaling in breast cancer cells through the inhibition of STAT3 activation. We demonstrate that STAT3 inactivation is required for the inhibitory effects of green tea on angiogenesis including cell migration, interferon-induced VEGF expression and secretion, and the expression and activity of MMP-9.
Materials and methods
Materials
Polyphenon E (Poly E), generously supplied by Mitsui Norin Co. Ltd. (Shizuoka, Japan), is a de-caffeinated green tea extract containing 65% EGCG, 10% EC, 5% ECG, 5% EGC, and 0.5% caffeine. Recombinant IFN-α and -γ were obtained from Chemicon (Temecula, CA). The pCMV-Tag 1 mammalian expression vector, and pGL13-GAS and pGL13-VEGF reporter constructs were obtained from Stratagene (La Jolla, CA), and the pRc/CMV-STAT3C expression plasmid was obtained from Dr. Bromberg [26].
Cell culture
MDA-MB231 and MCF-7 human breast adenocarcinoma cells were routinely cultured in phenol red-free Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). For all experiments unless otherwise noted, cells were grown in DMEM supplemented with 5% charcoal/dextran stripped FBS (Atlanta Biologicals, Lawrenceville, GA). Human dermal microvascular endothelial (HMVEC) cells were cultured in EGM-MV medium (Cambrex, Charles City, IO).
Transient transfection and luciferase assays
Cells were seeded in 48-well plates and transfected the next day with Polyfect (Qiagen) according to the manufacturer’s instructions. Transfections contained 300 ng of pGAS-Luc or pVEGF-Luc, 50 ng of the control β-galactosidase expression plasmid, and where indicated, 50 ng of expression or control vectors. Treatments were reconstituted in sterile PBS and administered one day following transfection as a 100–1,000-fold dilution in fresh media for the indicated time period. Luciferase activity was measured from harvested cells and normalized for transfection using β-galactosidase as an internal control. All values are representative of at least three independent experiments and are reported as a fold induction over controls.
Coimmunoprecipitation and western blot analysis
Coimmunoprecipitation (coIP) studies were performed using antibodies against STAT1 and STAT3 (Santa Cruz Biotechnology, Santa Cruz, CA) as previously described [27]. For non-coIP studies, whole cell extracts were obtained using 2× SDS–PAGE sample buffer. Equal amounts of lysates were passed through a 27 gauge needle three times, resolved on gradient Tris–glycine polyacrylamide gels, and transferred to nitrocellulose membrane. Equal protein loading was confirmed by Ponceau staining. Blots were blocked in 5% nonfat milk in TBST for at least 3 h and incubated with phospho-STAT1, phospho-STAT3, STAT1 (Upstate, Charlottsville,VA), STAT3 (BD Biosciences, San Jose, CA), or actin (Upstate, Charlottsville, VA) antibodies at a 1:1000 dilution overnight. Blots were then washed and incubated with the appropriate infrared-labeled secondary antibody (Li-COR, Lincoln, NE) for 1 h and detected with the Odyssey Infrared Imaging System (Li-COR, Lincon, NE).
Enzyme-linked immunosorbent assay
VEGF levels in conditioned medium from MCF-7 and MDA-MB231 cells were measured using the human VEGF QuantiGlo Kit (R&D Systems, Minneapolis, MN) as previously described [28].
In vivo matrigel plug assay
Female C57BL/6 mice were purchased from Jackson Laboratory and maintained in the Animal Resource Center at the University of Chicago on a 14 h on/10 h off light cycle. Care of the animals was in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. Following acclimation to a soy free diet for 7 days, female C57BL/6 mice (8 weeks of age) were lightly anesthetized with sodium pentobarbital (50 mg/kg, i.p.). Matrigel (BD Biosciences, Bedford, MA) was gently mixed with 100 ng/ml mouse VGEF (VEGF, R&D Systems, Minneapolis, MN) and injected subcutaneously into the bilateral flanks of the mice in a final volume of 0.5 ml/site. Beginning the day of matrigel implantation, mice were subcutaneously injected five times weekly with 0.75, 1.5, or 2.5 mg of Poly E in PBS vehicle per animal. Control animals were injected with an equivalent volume of PBS. Each experimental group contained five animals. Mice were sacrificed after 2 weeks and matrigel plugs were harvested. Plugs from each animal were divided and either fixed in formalin for histological analysis or frozen for hemoglobin analysis. To determine hemoglobin content, plugs were homogenized in Drabkin’s reagent (Sigma– Aldrich, St. Louis, MO) and the resultant supernatant following centrifugation was measured at a wavelength of 540 nm according to the manufacturer’s instructions.
Immunohistochemistry
For histological analysis, paraffin embedded sections (5 µm) prepared from matrigel plugs were subjected to H&E staining according to standard procedures by the Human Tissue Research Center at the University of Chicago. CD31 (Santa Cruz, San Jose, CA) immunostaining was also performed to determine vascularity as previously described [28]. All stained sections were scanned using the Automated Cellular Imaging System (ACIS, Chroma Vision, San Juan Capistrano, CA). Ten selected areas at 200 µm from the edge of each matrigel plug were acquired and scored to assess cellularity (H&E) or microvascular density (microvessel number per unit area). CD31-stained clusters of cells or single cells were defined as microvessels.
Cell migration assay
Cell migration assays were performed using matrigelcoated Boyden chambers (Chemicon, Temecula, CA) according to the manufacturer’s instructions. Prior to plating, MDA-MB231 and HMVEC cells were cultured in serum and supplement-free DMEM and EBM (Cambrex, Charles City, IO), respectively, for at least 6 h. Cell suspensions, in the presence or absence of the indicated treatments, were added to the upper chambers and incubated overnight at 37°C. For all cells, DMEM containing 10% FBS was used as the chemoattractant in the lower chamber. Migrated cells were stained with crystal violet, imaged, eluted, and transferred to a 96-well plate for absorbance readings at 595 nm. All results are representative of at least three independent experiments.
Capillary tube formation
The In Vitro Angiogenesis Assay Kit (Chemicon, Temecula, CA) was used to monitor capillary tube formation in HMVEC cells. According to the manufacturer’s instructions, cells were plated in 96-well microtiter plates coated with matrigel in the presence or absence of the indicated concentrations of Poly E. Four hours after plating, tube formation was determined under an inverted light microscope and photographed. Images are representative of three independent experiments with triplicate samples.
Gelatin zymography
MMP-9 activity from MDA-MB231 culture medium was determined by gelatin zymography. Cells were plated into 6-well plates in DMEM supplemented with 10% FBS and treated with the indicated compounds for 48 h. Conditioned medium was collected, concentrated 20-fold using Centricon filters (Millipore, Bedford, MA) according the manufacturer’s instructions, and electrophoresed on polyacrylamide gels containing gelatin (Biorad, Hercules, CA). Human fibroblast MMP-9 (Sigma–Aldrich, St. Louis, MO) was also loaded as a positive control. Gels were incubated twice in renaturing buffer (2.5% Triton X-100) for 30 min, washed in developing buffer (Biorad, Hercules, CA) for 30 min, and further incubated in fresh developing buffer overnight. Gels were stained for 30 min with 0.5% (w/v) Coomassie Brilliant Blue R-250 in 80% methanol/20% acetic acid, and destained with 50% methanol/10% acetic acid.
Quantitative reverse transcriptase-PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. RNA quality and quantity were measured using the Nanodrop ND-1000 (NanoDrop, Wilmington, DE) and reverse transcribed into cDNA with random primers using the SuperScript III First Strand Synthesis System (Invitrogen, Carlsbad, CA). Real-time quantitative reverse transcriptase-PCR was performed using the QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA) with primers targeting VEGF-A, MMP-9, or RPL13A (Qiagen, Valencia, CA) as an internal control for RNA input on the ABI 7300 Sequence Detector (Applied Biosystems) according to the manufacturer’s instructions. Standard curves were generated for each primer set using QPCR Reference Total Human RNA (Stratagene, La Jolla, CA) to determine gene expression levels. All reactions were performed in triplicate for samples and in duplicate for standards.
Results
Effects of green tea on angiogenesis
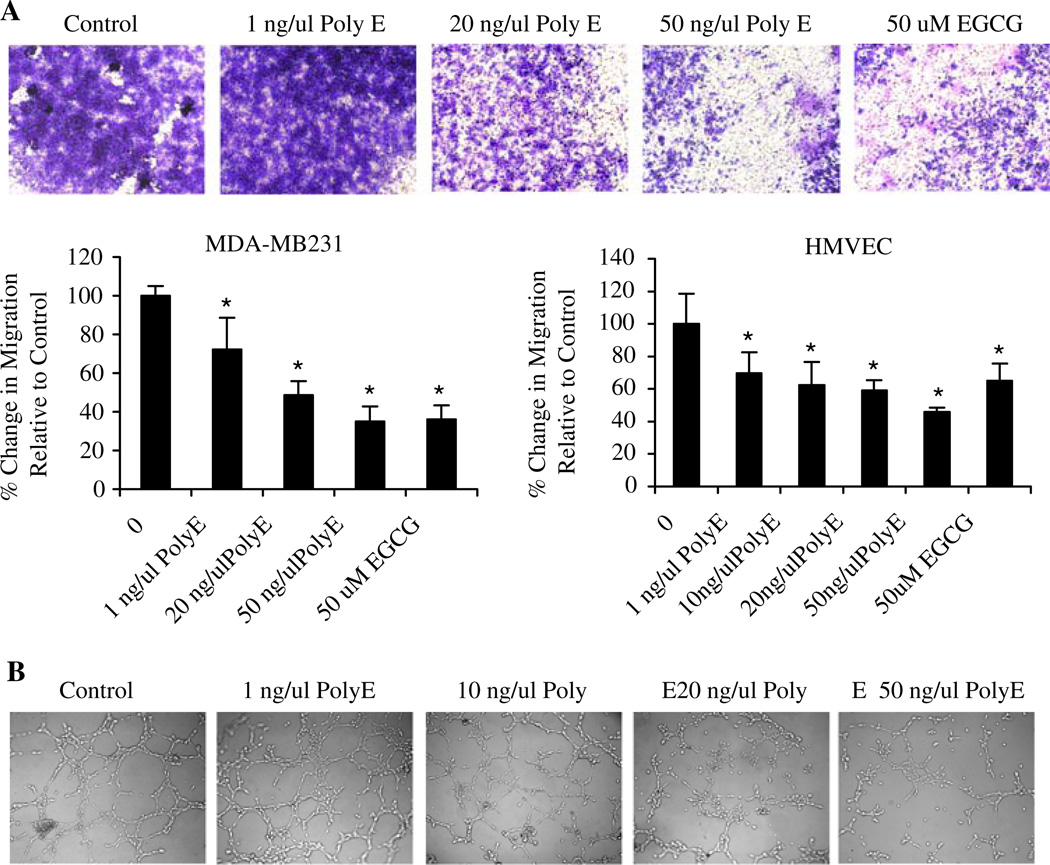
Angiogenesis and tumor metastases require the migration and invasion of cells through surrounding extracellular matrix. Thus, we investigated the effect of green tea on the invasive behavior of both MDA-MB231 breast cancer and HMVEC endothelial cells. Cells were plated in Boyden chambers with matrigel-coated membranes. Using 10% FBS as a chemoattractant, the number of invasive cells that migrated to the underside of the membranes were photographed and counted after 24 h. Treatment with Poly E decreased the invasive behavior of cells from 1 to 50 ng/µl in a concentration-dependent manner (Fig. 1a). Poly E inhibited the invasion of both MDA-MB231 and HMVEC cells by up to ~40% compared to controls. EGCG inhibited invasion to a similar extent, suggesting that EGCG is the active compound in Poly E.
Fig. 1.
Poly E inhibits invasive behavior and capillary-like structure formation. a Treatment of MDA-MB231 and HMVEC cells by Poly E (>10 ng/µl) and 50 µM EGCG significantly reduced cell invasive behavior by Boyden chamber assay. Microscopic images reflect representative crystal violet staining of migrated MDA-MB231 cells 24 h after catechin treatment. Data points indicate an average of triplicate samples. Bars represent SD. * P < 0.05 by Student’s t test compared to the control group. b Poly E treatment (20–50 ng/µl) inhibited morphologic differentiation of HMVEC cells into capillary-like structures. Microscopic images reflect representative reorganization of endothelial cells into vascular networks 6 h after plating and catechin treatment
The angiogenic effects of Poly E were also determined by examining the effects on morphologic differentiation of HMVEC cells into capillary-like structures using the matrigel assay. Untreated endothelial cells showed reorganization and formation of typical networks containing distinct rings of cells after 6 h of plating (Fig. 1b). Administration of Poly E at concentrations of 20 ng/µl or greater significantly decreased the formation of vascular structures, indicating that green tea interferes with endothelial cell differentiation.
Green tea inhibits neovascularization in vivo
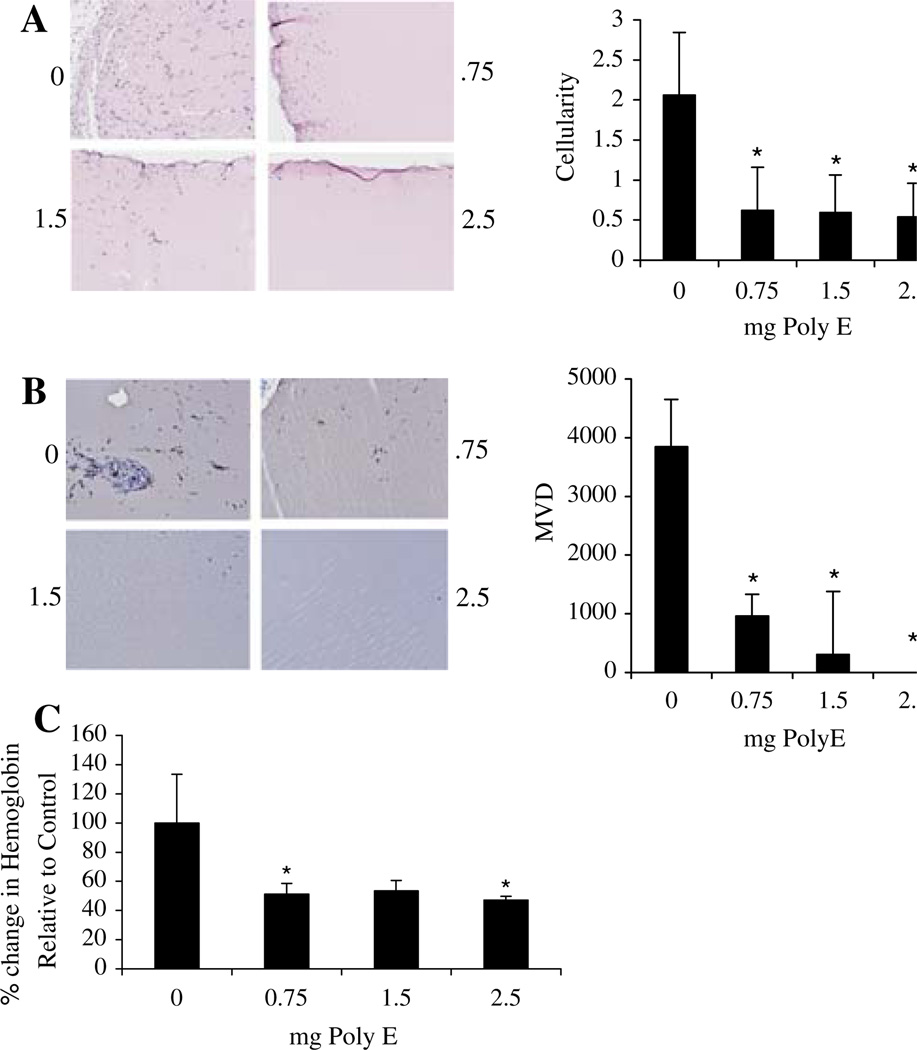
The effects of green tea on vascularity were further evaluated using the matrigel implant model. C57BL/6 mice were implanted with matrigel and treated subcutaneously with Poly E five times weekly. Following 14 days of treatment, matrigel plugs were harvested and assessed for neovascularization. Histochemical analysis revealed that all administered concentrations of Poly E markedly decreased cellularity by ~75% compared to controls (Fig. 2a). Migration of endothelial cells into the matrigel plugs of Poly E-treated animals was significantly reduced, as confirmed by CD31 staining (Fig. 2b). Quantification of microvessel density (MVD) also indicated that Poly E drastically reduced angiogenesis by up to 100% in a dose-dependent manner (Fig. 2a, b). To determine the functionality of migrated endothelial cells, the hemoglobin content was measured within the matrigel plugs. Compared to controls, all doses of Poly E reduced hemoglobin content by ~50%, demonstrating that green tea effectively inhibits functional vessel formation (Fig. 2c).
Fig. 2.
Poly E inhibits neovascularization in vivo. A matrigel plug assay was performed in C57Bl6 mice treated with Poly E (0.75, 1.5, or 2.5 mg) five times weekly for a duration of 2 weeks. a Cell migration was significantly reduced by all doses of Poly E treatment as indicated by H&E staining of matrigel plug sections. b Poly E treatment significantly inhibited MVD as indicated by reduced CD31 staining of plug sections. Images reflect representative staining of all sections. c The hemoglobin content of matrigel plugs, assayed by Drabkin’s reagent, was reduced by all doses of Poly E. Data points indicate an average of five samples with bars representing SD. *P < 0.05 by Student’s t test compared to the control group
Green tea modulates vascular endothelial growth factor expression
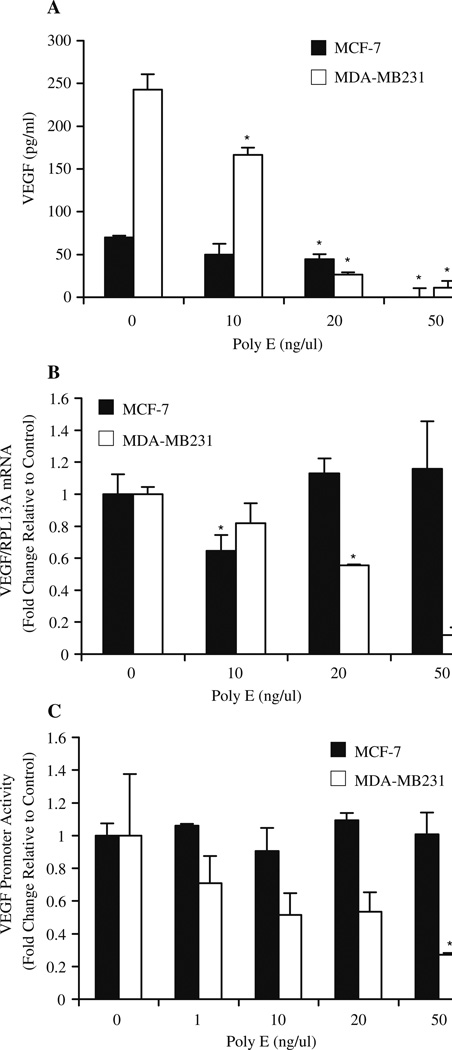
The impact of green tea on VEGF expression levels was determined as a measure of green tea’s effect on angiogenic growth factors. Conditioned media from MCF-7 and MDA-MB231 cells was concentrated after 48 h administration of the indicated concentrations of Poly E and assessed for VEGF secretion by ELISA analysis. Poly E (10–50 ng/µl) substantially inhibited VEGF levels in a concentration-dependent manner from 70 pg/ml to 242 pg/ml in MCF-7 and MDA-MB231 cells to 2 pg/ml and 11 pg/ml, respectively (Fig. 3a).
Fig. 3.
Poly E modulates VEGF expression and secretion. MCF-7 and MDA-MB231 cells were treated with Poly E for 24 h. a The release of VEGF into cell culture media, as determined by ELISA assay, was significantly inhibited by Poly E in MCF-7 (10–50 ng/µl) and MDA-MB231 cells (50 ng/µl). b Poly E significantly inhibited VEGF mRNA expression by quantitative RTPCR analysis in a concentration-dependent manner in MDA-MB231 but not in MCF-7 cells. c A similar decrease in VEGF promoter activity was observed by luciferase assay. Bars represent SD. *P < 0.05 by Student’s t test compared to the respective control group.
To assess whether the observed effect occurred at the transcriptional level, VEGF mRNA expression was measured by quantitative real-time PCR. VEGF mRNA expression in MDA-MB231 cells was reduced by Poly E treatment for 24 h in a concentration-dependent manner (10–50 ng/µl) by up to almost 90% (Fig. 3b). The effect of Poly E in MCF-7 cells was inconsistent, showing only a 35% decrease in mRNA expression at 10 ng/µl. As an alternate method, we tested the effect of green tea on VEGF promoter activity. Cells were transfected with pGL13-VEGF and treated for 24 h with the indicated concentrations of Poly E. Similar to the gene expression studies, Poly E inhibited promoter activity by up to 60% compared to controls in MDA-MB231 cells while Poly E showed no effect in MCF-7 cells (Fig. 3c). These combined results suggest that the effect of green tea on VEGF expression is mediated at the transcriptional level in MDA-MB231 cells and at the translational level in MCF-7 cells.
Effects of green tea on MMP-9 activity and expression
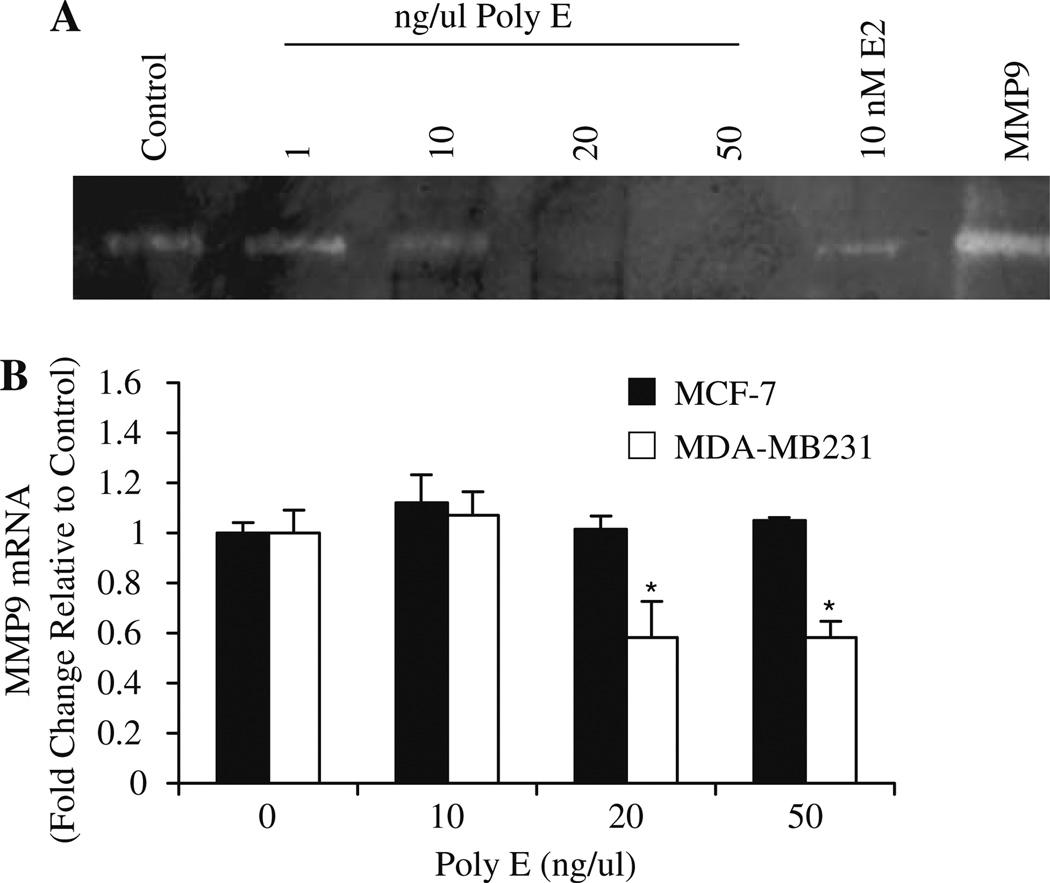
Metalloproteases, such as MMP-9, are involved in both angiogenic and tumorigenic processes. To evaluate the effect of green tea on MMP-9 activity, Poly E was administered to MDA-MB231 cells for 48 h, and conditioned media was collected and analyzed by gelatin zymography. Although MDA-MB231 cells secrete low levels of MMP-9, results clearly indicate that treatment with Poly E caused a concentration-dependent suppression of MMP-9 activity with complete inhibition reached by 20 ng/µl Poly E (Fig. 4a). Basal secretion of MMP-9 from MCF-7 cells was below detectable limits (data not shown). Further examination of MMP-9 expression by quantitative RTPCR indicated that treatment with 20 ng/µl or greater concentrations of Poly E for 48 h inhibited MMP-9 mRNA by greater than 40% compared to controls in MDA-MB231 cells while having no effect in MCF-7 cells (Fig. 4b). The observed results indicate that similar to VEGF, green tea also modulates MMP-9 through transcriptional mechanisms in MDA-MB231 cells.
Fig. 4.
Poly E downregulates the expression and secretion of MMP9. MDA-MB231 and MCF-7 cells were treated with Poly E for 48 h. a MMP9 activity was significantly decreased by Poly E (20–50 ng/µl) as determined by zymography analysis of conditioned media of MDA-MB231-treated cells. MMP9 was loaded in the last lane as an internal positive control. b Poly E inhibited MMP9 mRNA expression by quantitative RTPCR analysis. Bars represent SD. *P < 0.05 by Student‘s t test compared control.
Green tea interferes with STAT1 and STAT3 activation
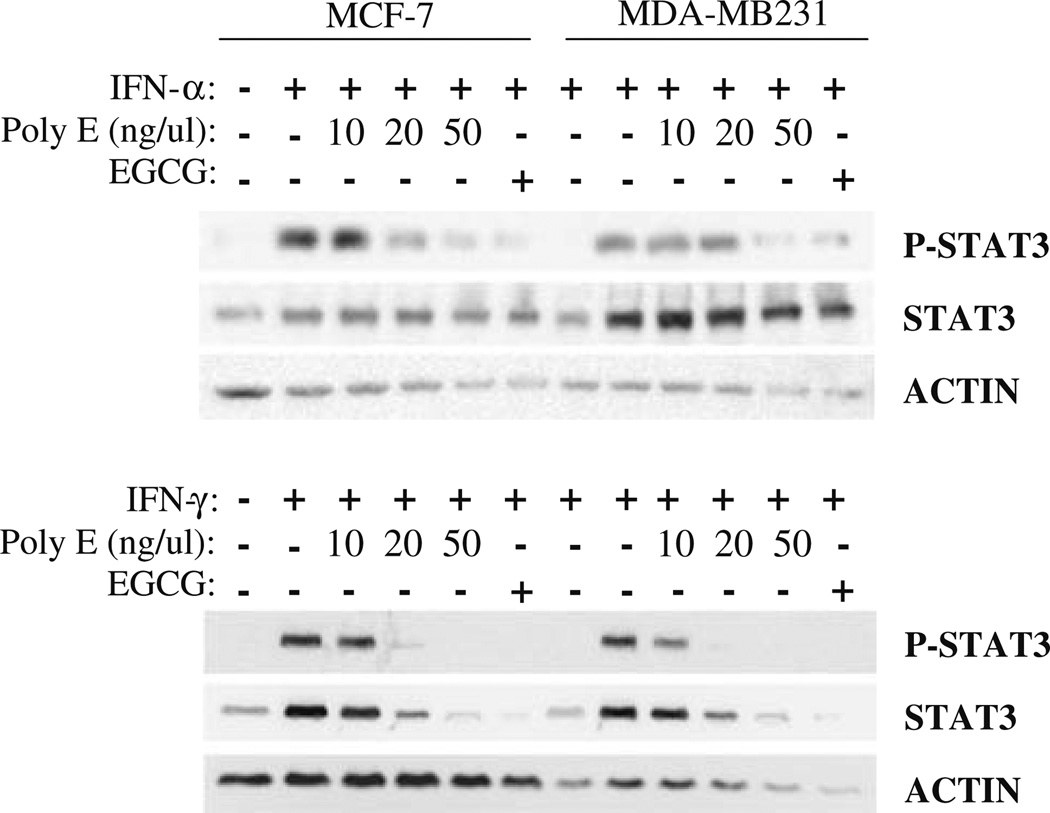
Several members of the signal transducers and activators of transcription (STAT) family play a role in tumorigenesis [29]. Studies demonstrate that STAT3 activity is commonly upregulated in breast cancer, and that STAT3 regulates the expression of angiogenic genes including VEGF and MMP9 [30]. Accordingly, we examined the effect of green tea on modulation of STAT3 activity by western analyses. MCF-7 and MDA-MB231 cells were treated with Poly E or EGCG for 24 h in the presence of 1,000 U/ml interferon-alpha (IFN-α) or interferon-gamma (IFN-γ), cytokines which are known activators of STAT1 and STAT3. Immunoblotting for phosphorylated STAT3 indicated that EGCG and Poly E inhibited interferon-induced activation of STAT3 in a concentration-dependent manner (Fig. 5a, b). Poly E administration almost completely inhibited interferon-activated STAT forms in both MCF-7 and MDA-MB231 cells.
Fig. 5.
Inhibition of STAT3 activation by Poly E. MDA-MB231 and MCF-7 cells were treated with Poly E or EGCG in the presence or absence of 1,000 U/ml IFN-α or IFN-γ. Immunoblotting for total and phosphorylated forms of STAT3 were performed after 24 h of treatment. Actin was detected as an internal loading control.
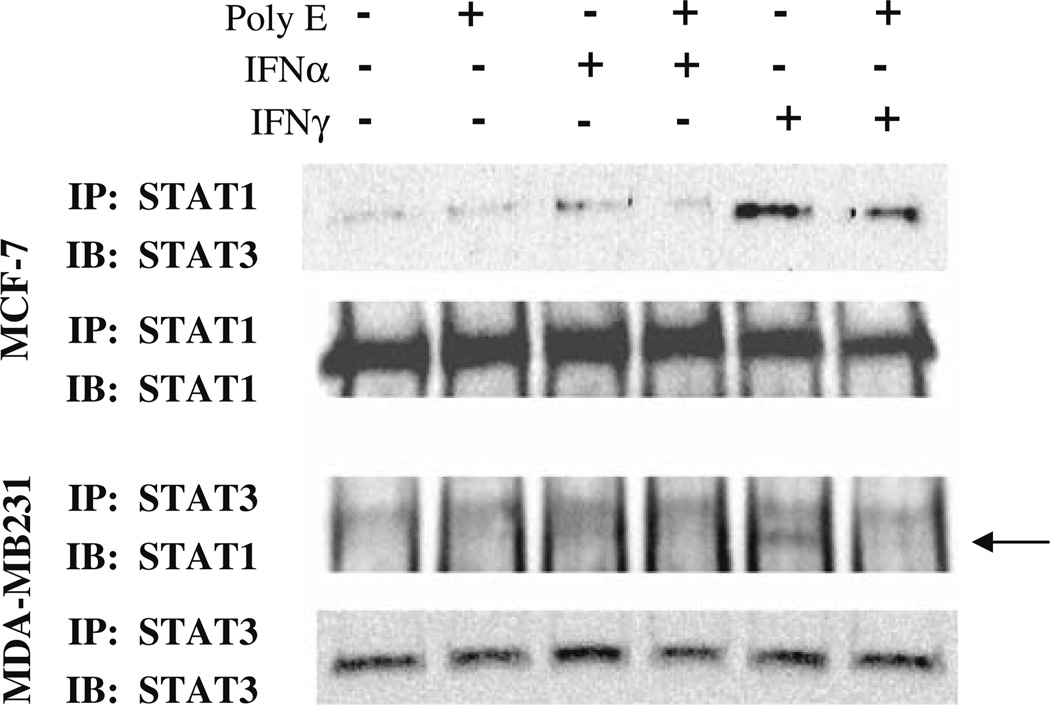
We also examined the effect of Poly E on the association of STAT3 with STAT1, as activation of STAT-regulated gene transcription involves the phosphorylation and formation of hetero- and homodimers of STAT complexes, which then associate with and increase the transcription of specific gene promoters [31]. Following 24 h administration of Poly E (20 ng/µl) in the presence or absence of 1,000 U/ ml IFN-α or IFN-γ, MCF-7, and MDA-MB231 cell extracts were coimmunoprecipitated with STAT1 or STAT3 antibody and analyzed for STAT1 and STAT3 association by western analysis. While IFN-α produced a weaker effect, both IFN-α and IFN-γ induced the association of STAT1 with STAT3 in MCF-7 and MDA-MB231 cells (Fig. 6). Poly E treatment reduced the formation of all interferoninduced STAT1-STAT3 complexes, indicating that green tea inhibits STAT dimerization.
Fig. 6.
Poly E inhibits interferon-induced STAT1/STAT3. MDA-MB231 and MCF-7 cells were treated for 24 h with Poly E (20 ng/µl) in the presence or absence of IFN-α or IFN-γ. Cell extracts were coimmunoprecipitated with STAT1 or STAT3 antibody and analyzed for STAT1 and STAT3 association by western analysis.
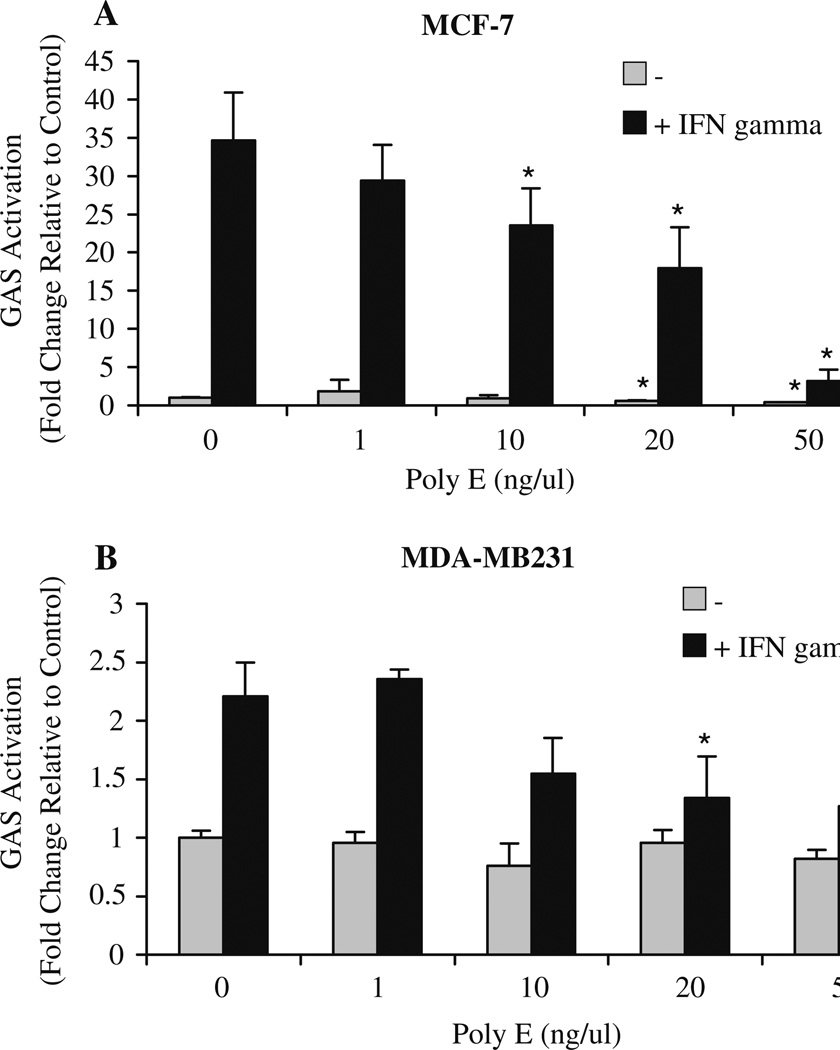
As a functional test of STAT inhibition, we examined the effect of green tea on interferon-regulated gene transcription using a luciferase reporter under the regulatory control of IFN-γ-activating sequence (GAS) elements. Treatment of MCF-7 cells with Poly E resulted in a significant concentration-dependent suppression of IFN-γ-induced reporter activity by greater than 85% (Fig. 7a). Poly E also reduced IFN-γ-induced GAS activation in MDA-MB231 cells by ~45% (Fig. 7b). Altogether, these results suggest that Poly E reduces the ability of STAT1 and STAT3 to form active transcriptional complexes through the inhibition of STAT nuclear localization and dimerization.
Fig. 7.
Poly E suppresses interferon-induced GAS activation. a MCF-7, and b MDA-MB231, cells were transfected with pGAS-Luc and control β-galactosidase expression reporter plasmids. Cells were treated with vehicle or Poly E in the presence or absence of 1,000 U/ml IFN-α or IFN-γ. Luciferase activity was assayed and reported as the fold induction relative to vehicle-treated control. Bars indicate SD. *P < 0.05 by Student’s t test compared to the respective control group.
STAT3 mediates the antiangiogenic effects of green tea
To determine whether the disruption of STAT3 signaling is required for the observed antiangiogenic effects of green tea, we examined the effect of Poly E on angiogenic events in cells transiently expressing constitutively active STAT3 (STAT3C). STAT3C activity was verified by western analysis for phospho-STAT3 under basal conditions (data not shown).
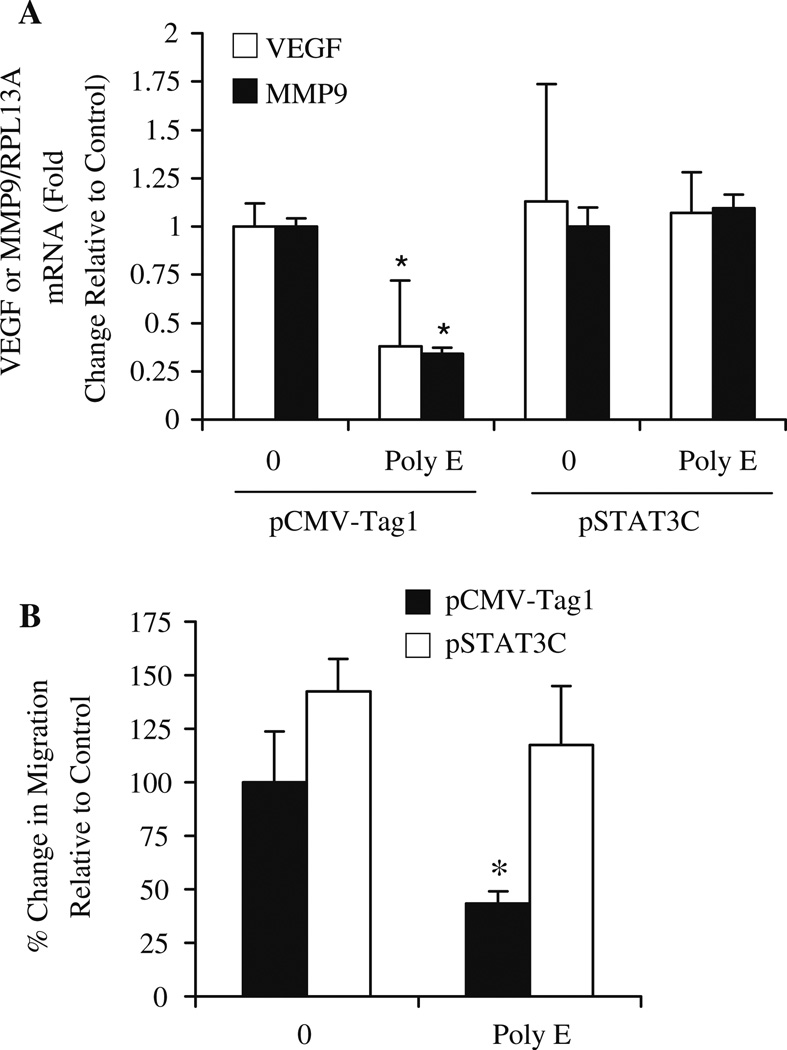
To determine the role of STAT3 in mediating the effects of green tea on VEGF and MMP9, we investigated the influence of STAT3C transient expression on transcriptional regulation of these genes in MDA-MB231 cells by quantitative RTPCR analysis. In cells expressing control vector, 20 ng/µl Poly E treatment for 24 h inhibited both VEGF and MMP9 gene expression by over 50% (Fig. 8a). However, Poly E did not downregulate gene expression in cells expressing STAT3C.
Fig. 8.
Poly E mediates antiangiogenic effects through STAT-3 dependent mechanisms. a MDA-MB231 cells transiently transfected with pSTAT3C or control pCMV-Tag1 expression vector were treated with Poly E (20 ng/µl) for 24 h. Quantitative RTPCR analysis demonstrated that constitutive activation of STAT3 blocked the inhibitory effects of Poly E on VEGF and MMP9 gene expression. Results are expressed as fold induction relative to control cells transfected with pCMV-Tag1 in the absence of Poly E treatment. Bars represent SD. *P < 0.05 by Student’s t test compared to the respective control group. b MDA-MB231 cells were plated in Boyden chambers following transient transfection with pSTAT3C or control pCMV-Tag1 expression vector. The suppressive effect of 24 h Poly E (20 ng/µl) treatment on cell migration was inhibited by constitutive STAT3 activation. Data points indicate the percent change in migration relative to control cells transfected with pCMV-Tag1. Bars represent SD. *P < 0.05 by Student’s t test compared to the respective control group.
Invasion assays were performed as a functional test to confirm the requirement of STAT3 inhibition for the antiangiogenic effects of green tea. Using MDA-MB231 cells, we investigated the effect of STAT3C expression on invasive behavior. Following transfection of cells with pSTAT3C or pCMV-Tag1 control vector, cells were plated in Boyden chambers using 10% FBS as a chemoattractant and treated with 20 ng/µl Poly E for 24 h in the presence or absence of IFN-γ. Poly E administration inhibited migration under basal conditions by greater than 50% (Fig. 8b). In cells expressing STAT3C, expression completely blocked the anti-invasive effects of Poly E. Altogether, these combined results clearly indicate that the antiangiogenic actions of Poly E are partially mediated by the inhibition of STAT3 activity.
Discussion
In this study, we demonstrate a novel mechanism by which green tea inhibits angiogenic signaling in breast cancer cells. We show that green tea inhibits neovascularization in vivo and inhibits the expression of the proangiogenic factors VEGF and MMP9 in breast cancer cells. Green tea regulates such factors in a cell-specific manner by inhibiting expression through translational and/or transcriptional mechanisms in MDA-MB231 and MCF-7 cells. We demonstrate that both mechanisms require the inhibition of STAT3 activation, as constitutive activation of STAT3 blocks the antiangiogenic effects of green tea. To the best of our knowledge, this is the first report to establish that green tea suppression of angiogenesis is in part mediated through STAT3. Previous mechanistic reports have focused mainly on the disruption of VEGF signaling pathways [25]. For example, others have reported the suppression of VEGF and MMP9 expression by EGCG as well as the inhibition of VEGFR-2 expression, activity, and binding to VEGF [12, 20, 22, 32, 33]. The downregulation of VEGF-induced angiogenesis by EGCG has been attributed to the inhibition of downstream target activation including Akt, PI3K, ERK1/2, and NFκB in endothelial cells [23, 25, 34]. However, few studies have described the exact mechanisms by which green tea modulates upstream signaling pathways that regulate VEGF or MMP9 expression. Reports have suggested that EGCG may suppress VEGF expression in breast cancer and endothelial cells by inhibiting the activity of PKC and NFκB or by downregulating the expression of c-fos and c-jun, which regulate VEGF promoter activity [21, 32]. Thus, our study provides further insight into the molecular mechanisms by which green tea inhibits angiogenesis.
STAT proteins are nuclear transcription factors that regulate the expression of diverse genes in combination with other transcriptional factors. STAT activation occurs by ligand binding of specific cell surface receptors and is controlled by several mechanisms including the phosphorylation state of the receptor, interaction of STATs with a family of inhibitory factors known as protein inhibitors of activated STAT (PIAS), and feedback inhibition by suppressor of cytokine signaling (SOCS) proteins [21, 35, 36]. In the current study, we demonstrate that the antiangiogenic effects of green tea are at least partially dependent on STAT3 inhibition. Inhibiting STAT3 is especially significant in tumor cells as STAT3 is constitutively active in several tumor types compared to normal cells [37]. Human cancers expressing active STAT3 include carcinomas of the lung, pancreas, head and neck, ovarian, prostate, and breast [37, 38]. Persistent STAT3 activation contributes to tumorigenesis by deregulating the expression of genes involved in cell proliferation, immune response, angiogenesis, and metastasis [39]. Specifically, activated STAT3 may enhance tumor survival by increasing the expression of genes including MCL-1 and BCL-XL, increase proliferation by enhancing cyclin D1 and myc expression, and promote angiogenesis by inducing the expression of angiogenic genes including VEGF and MMP-2 and enhancing the proliferation, migration, and differentiation of endothelial cells [39].
STAT3 clearly plays a critical role in regulating tumor angiogenesis. Thus, we are not surprised to find that the antiangiogenic effects of green tea are mediated by the inhibition of STAT3 in breast cancer cells in the present study. To our knowledge, only one research group has reported the inhibitory effect of EGCG on STAT3 phosphorylation in head and neck and breast cancer cells [14, 21]. However, the role of STAT3 inhibition in the suppression of angiogenic events by EGCG was not established. Here, we demonstrate that green tea interferes with STAT3 activation at multiple levels including phosphorylation and heterodimerization with STAT1. The observed STAT3 dysregulation is essential for the inhibition of VEGF and MMP-9 gene expression and invasive activity of MDA-MB231 cells. Previous studies demonstrate that STAT3 activation upregulates VEGF expression and correlates with increased MMP-9 activity while expression of dominant negative STAT3 leads to decreased angiogenesis and suppression of cell growth including breast cancer cells [40–43]. Due to the nearly complete abrogation of green tea’s effect on angiogenic events by the expression of constitutively active STAT3, we suggest that STAT3 inhibition is a primary mechanism by which green tea exerts antiangiogenic effects in MDA-MB231 cells. Although green tea inhibited STAT3 activation in MCF-7 cells, only translational effects on VEGF expression were noted. These results suggest that green tea may influence the transcriptional control of other STAT3-regulated genes involved in angiogenesis that were not tested here. Due to the observed cell-specific effects, further studies are required to identity whether angiogenesis-related STAT3-regulated genes are directly or indirectly inhibited by green tea.
Elevated STAT3 activity has been associated with breast cancer progression and inversely correlated with response to chemotherapy [44]. Thus, small molecule inhibitors of STAT3, such as EGCG found in green tea, could be used in combination with chemotherapy to improve the therapeutic response in breast cancer patients. Previous studies have already demonstrated that blocking STAT3 signaling in tumor cells expressing constitutively active STAT3 results in tumor growth inhibition and increased tumor apoptosis [37]. Green tea has already been found to block breast tumorigenesis and angiogenesis through multiple mechanisms, for example, by altering p21 and p27 expression levels, c-fos promoter activity, and plasma estrone levels [45]. The ability of green tea to suppress STAT3, in which a wide variety of oncogenic signaling pathways converge, further highlights the potent antitumor effects green tea. In summary, our findings further support the use of green tea in breast cancer treatment and prevention as an antiangiogenic agent.
Acknowledgments
This work was supported by the generous donation of Mr. and Mrs. Gordon I. Segal (H. Leong, P. S. Mathur, G. L. Greene), Susan G. Komen Breast Cancer Foundation Postdoctoral Fellowship PDF0503835 (H. Leong.), and the Ludwig Fund for Cancer Research (G. L. Greene). We also thank Dr. Yukihiko Hara from Mitsui Norin Co. Ltd. for the supply of Poly E.
Footnotes
Author contributions: H. Leong and G. L. Greene designed research, H. Leong and P. S. Mathur performed research, and H. Leong analyzed data and wrote the paper.
Contributor Information
Hoyee Leong, The Ben May Department for Cancer Research, The University of Chicago, GCIS W325D, 929 East 57th Street, Chicago, IL 60637, USA.
Priya S. Mathur, The Ben May Department for Cancer Research, The University of Chicago, GCIS W325D, 929 East 57th Street, Chicago, IL 60637, USA, pmathur@surgery.bsd.uchicago.edu
Geoffrey L. Greene, The Ben May Department for Cancer Research, The University of Chicago, GCIS W330, 929 East 57th Street, Chicago, IL 60637, USA, ggreene@uchicago.edu
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Atiqur Rahman M, Toi M. Anti-angiogenic therapy in breast cancer. Biomed Pharmacother. 2003;57:463–470. doi: 10.1016/j.biopha.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Rosen LS. VEGF-targeted therapy: therapeutic potential and recent advances. Oncologist. 2005;10:382–391. doi: 10.1634/theoncologist.10-6-382. [DOI] [PubMed] [Google Scholar]
- 4.Guidi AJ, Schnitt SJ, Fischer L, Tognazzi K, Harris JR, Dvorak HF, Brown LF. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in patients with ductal carcinoma in situ of the breast. Cancer. 1997;80:1945–1953. doi: 10.1002/(sici)1097-0142(19971115)80:10<1945::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Foekens JA, Peters HA, Grebenchtchikov N, Look MP, Meijervan Gelder ME, Geurts-Moespot A, van der Kwast TH, Sweep CG, Klijn JG. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61:5407–5414. [PubMed] [Google Scholar]
- 6.Thomas AL, Morgan B, Drevs J, Unger C, Wiedenmann B, Vanhoefer U, Laurent D, Dugan M, Steward WP. Vascular endothelial growth factor receptor tyrosine kinase inhibitors: PTK787/ZK 222584. Semin Oncol. 2003;30:32–38. doi: 10.1016/s0093-7754(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 7.Singh RP, Sharma G, Dhanalakshmi S, Agarwal C, Agarwal R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Biomarkers Prev. 2003;12:933–939. [PubMed] [Google Scholar]
- 8.Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Flynn E, Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- 9.Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, Kim YM, Kwon YG. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335:300–308. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 10.Park JS, Kim MH, Chang HJ, Kim KM, Kim SM, Shin BA, Ahn BW, Jung YD. Epigallocatechin-3-gallate inhibits the PDGF-induced VEGF expression in human vascular smooth muscle cells via blocking PDGF receptor and Erk-1/2. Int J Oncol. 2006;29:1247–1252. [PubMed] [Google Scholar]
- 11.Rosen LS. Clinical experience with angiogenesis signaling inhibitors: focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control. 2002;9:36–44. doi: 10.1177/107327480200902S05. [DOI] [PubMed] [Google Scholar]
- 12.Fassina G, Vene R, Morini M, Minghelli S, Benelli R, Noonan DM, Albini A. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin Cancer Res. 2004;10:4865–4873. doi: 10.1158/1078-0432.CCR-03-0672. [DOI] [PubMed] [Google Scholar]
- 13.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 14.Masuda M, Suzui M, Lim JT, Weinstein IB. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin Cancer Res. 2003;9:3486–3491. [PubMed] [Google Scholar]
- 15.Fujiki H, Suganuma M, Kurusu M, Okabe S, Imayoshi Y, Taniguchi S, Yoshida T. New TNF-alpha releasing inhibitors as cancer preventive agents from traditional herbal medicine and combination cancer prevention study with EGCG and sulindac or tamoxifen. Mutat Res. 2003;523-524:119–125. doi: 10.1016/s0027-5107(02)00327-5. [DOI] [PubMed] [Google Scholar]
- 16.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 17.Clark J, You M. Chemoprevention of lung cancer by tea. Mol Nutr Food Res. 2006;50:144–151. doi: 10.1002/mnfr.200500135. [DOI] [PubMed] [Google Scholar]
- 18.Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and breast cancer risk: a meta-analysis of epidemiological studies. Carcinogenesis. 2006;27:1310–1315. doi: 10.1093/carcin/bgi276. [DOI] [PubMed] [Google Scholar]
- 19.Tang FY, Meydani M. Green tea catechins and vitamin E inhibit angiogenesis of human microvascular endothelial cells through suppression of IL-8 production. Nutr Cancer. 2001;41:119–125. doi: 10.1080/01635581.2001.9680622. [DOI] [PubMed] [Google Scholar]
- 20.Lamy S, Gingras D, Beliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62:381–385. [PubMed] [Google Scholar]
- 21.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 22.Kojima-Yuasa A, Hua JJ, Kennedy DO, Matsui-Yuasa I. Green tea extract inhibits angiogenesis of human umbilical vein endothelial cells through reduction of expression of VEGF receptors. Life Sci. 2003;73:1299–1313. doi: 10.1016/s0024-3205(03)00424-7. [DOI] [PubMed] [Google Scholar]
- 23.Tang FY, Nguyen N, Meydani M. Green tea catechins inhibit VEGF-induced angiogenesis in vitro through suppression of VE-cadherin phosphorylation and inactivation of Akt molecule. Int J Cancer. 2003;106:871–878. doi: 10.1002/ijc.11325. [DOI] [PubMed] [Google Scholar]
- 24.Garbisa S, Sartor L, Biggin S, Salvato B, Benelli R, Albini A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer. 2001;91:822–832. doi: 10.1002/1097-0142(20010215)91:4<822::aid-cncr1070>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez SK, Guo W, Liu L, Band MA, Paulson EK, Meydani M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int J Cancer. 2006;118:1635–1644. doi: 10.1002/ijc.21545. [DOI] [PubMed] [Google Scholar]
- 26.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 27.Leong H, Sloan JR, Nash PD, Greene GL. Recruitment of histone deacetylase 4 to the N-terminal region of estrogen receptor alpha. Mol Endocrinol. 2005;19:2930–2942. doi: 10.1210/me.2005-0178. [DOI] [PubMed] [Google Scholar]
- 28.Leong H, Mathur P, Greene G. Inhibition of mammary tumorigenesis in the C3(1)/SV40 mouse model by green tea. Breast Cancer Res Treat. 2007;107(3):359–369. doi: 10.1007/s10549-007-9568-x. [DOI] [PubMed] [Google Scholar]
- 29.Clevenger CV. Roles and regulation of stat family transcription factors in human breast cancer. Am J Pathol. 2004;165:1449–1460. doi: 10.1016/S0002-9440(10)63403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haura EB, Turkson J, Jove R. Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 31.Brierley MM, Fish EN. Stats: multifaceted regulators of transcription. J Interferon Cytokine Res. 2005;25:733–744. doi: 10.1089/jir.2005.25.733. [DOI] [PubMed] [Google Scholar]
- 32.Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132:2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- 33.Kondo T, Ohta T, Igura K, Hara Y, Kaji K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002;180:139–144. doi: 10.1016/s0304-3835(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 34.Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 38.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 39.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–327. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez JV, Febbo PG, Ramaswamy S, Loda M, Richardson A, Frank DA. Identification of a genetic signature of activated signal transducer and activator of transcription 3 in human tumors. Cancer Res. 2005;65:5054–5062. doi: 10.1158/0008-5472.CAN-04-4281. [DOI] [PubMed] [Google Scholar]
- 41.Dechow TN, Pedranzini L, Leitch A, Leslie K, Gerald WL, Linkov I, Bromberg JF. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci USA. 2004;101:10602–10607. doi: 10.1073/pnas.0404100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh FC, Cheng G, Lin J. Evaluation of potential Stat3-regulated genes in human breast cancer. Biochem Biophys Res Commun. 2005;335:292–299. doi: 10.1016/j.bbrc.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 44.Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, Cantor A, Lee JH, Beam CA, Sullivan D, Jove R, Muro-Cacho CA. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res. 2006;12:20–28. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- 45.Stuart EC, Scandlyn MJ, Rosengren RJ. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sci. 2006;79:2329–2336. doi: 10.1016/j.lfs.2006.07.036. [DOI] [PubMed] [Google Scholar]