Abstract
Aging in humans is associated with parallel changes in cognition, motivation, and motoric performance. Based on the human aging literature, we hypothesized that this constellation of age-related changes is mediated by the medial prefrontal cortex and that it would be observed in aging mice. Toward this end, we performed detailed assessments of cognition, motivation, and motoric behavior in aging mice. We assessed behavioral and cognitive performance in C57Bl/6 mice aged 6, 18, and 24 months, and followed this with microarray analysis of tissue from the medial prefrontal cortex and analysis of serum cytokine levels. Multivariate modeling of these data suggested that the age-related changes in cognition, motivation, motor performance, and prefrontal immune gene expression were highly correlated. Peripheral cytokine levels were also correlated with these variables, but less strongly than measures of prefrontal immune gene upregulation. To determine whether the observed immune gene expression changes were due to prefrontal microglial cells, we isolated CD11b-positive cells from the prefrontal cortex and subject them to next-generation RNA sequencing. Many of the immune changes present in whole medial prefrontal cortex were enriched in this cell population. These data suggest that, as in humans, cognition, motivation, and motoric performance in the mouse change together with age and are strongly associated with CNS immune gene upregulation.
Keywords: Aging, prefrontal cortex, motivation, locomotion, gene expression, microarray analysis, RNA sequencing, microglia, cognition
1 Introduction
Human aging, both normal and in age-related disorders, is associated with highly correlated changes in cognition, motivation, and motor performance. The frequent comorbidity of these changes has led to a growing interest in determining the biological mechanisms that may account for their association (Granholm and others 2008). Aging effects on the prefrontal cortex (PFC) are a particularly parsimonious explanation for age-related changes in cognition, motivation, and locomotor behavior. While aging does not solely affect the PFC (Dennis and others 2008; Raz and others 2005), this region exhibits age-dependent architectural changes (Peters and others 1998) and is particularly sensitive to age-related decline.
Normal aging is associated with declines in PFC performance (Mattay and others 2006; Rajah and D’Esposito 2005). For example, older individuals perform worse in tasks such as the Wisconsin Card Sort (MacPherson and others 2002) and the Stroop Task (Baena and others 2010). Other consequences of aging, such as reduced motivation (Brodaty and others 2010), often examined in the context of apathy, are also associated with PFC dysfunction. Apathy is more common in individuals with cognitive dysfunction, and may also serve as a predictor of cognitive decline (Onyike and others 2007). Although symptoms of apathy partially overlap with those of depression, which is also associated with PFC dysfunction (Drevets and others 2008), the two constructs can be distinguished based on the extent of strong negative emotions, depressed mood, and suicidal ideation which are unique to depression (Levy and others 1998). Apathy and depression also differ with regard to their association with cognition; apathy is more strongly correlated with cognitive dysfunction (Kuzis and others 1999; Levy and others 1998). Lastly, motoric behavior is also influenced by the PFC. In aging individuals, gait speed is correlated with executive function (de Bruin and Schmidt 2010) and gait slows with increasing cognitive demands (Cho and others 2008). Imaging studies have demonstrated significant correlations between PFC activation and walking speed (Harada and others 2009; Mihara and others 2010).
Although the rodent PFC is less sophisticated than that of the primate (Simen and others 2009; Uylings and others 2003), the mouse does serve as a suitable model organism for PFC-dependent tasks (Brown and Bowman 2002; Robbins 2002). As in higher organisms, the murine PFC can be defined by its reciprocal connections with the mediodorsal nucleus of the thalamus (Guldin and others 1981; Rios and Villalobos 2004). Similarly, certain subregions within the PFC have also been related to specific aspects of cognitive performance. The rodent medial PFC, for example, has been found to play a role in attention (Bissonette and others 2008) and motivation (Gourley and others 2010; Walton and others 2003). As discussed in detail below, PFC functioning also depends on structures with which it interconnects, which may also contribute to age-related decline. Given evidence that the mouse PFC is functionally and anatomically homologous to the PFC in higher mammals, and that motivation, cognition, and locomotor activity can easily be assessed using common laboratory paradigms, we sought to determine whether the aging mouse exhibits similar patterns to those seen in aging humans. Because peripheral inflammatory cytokines have also received attention in relation to normal and pathological aging (De Martinis and others 2006) we also sought to compare the impact of these factors on the same outcomes.
Using microarray analyses, previous work designed to investigate gene expression changes in aging have determined that immune genes account for many of those upregulated within the aging brain (Godbout and others 2005; Park and others 2009; Prolla 2002). Despite multiple studies being conducted on this subject, we are not currently aware of any systematic examination of age-related alterations in gene expression within the mouse PFC. We are also unaware of any previous attempts to relate cognitive performance, motivation, and locomotor behavior to whole genome expression data in the aging mouse. We hypothesized that, as in the human, parallel declines in cognition, motivation, and locomotion would be observed in aging mice. We further hypothesized that these changes would be highly correlated with age-related gene expression changes within the medial PFC, particularly those related to immune pathways.
Here, we describe age-related changes in cognition, motivation and motoric behavior in the mouse. These changes are highly correlated with one another, and with age-related upregulation of immune genes in the medial PFC. Peripheral cytokine levels were found to be less correlated with these age-dependent deficits. Furthermore, the observed immune changes in the medial PFC were strongly enriched in Cd11b-positive cells isolated from this region. Overall, our results indicate that the aging mouse is a suitable model organism for understanding the mechanisms responsible for parallel age-related changes in cognition, motivation, and motoric behavior, and that immune gene upregulation in the medial PFC is likely to contribute to these deficits.
2 Materials and methods
2.1 Subjects
Adult (6 months), Middle-aged (18 months) and Old-aged (24 months) male C57BL/6J mice were used as experimental subjects. With the exception of Cohort 1, in which mice were obtained from Jackson Laboratories and aged in-house, animals were obtained from the National Institute of Aging and housed 1-2 months prior to behavioral testing. Animals were group housed (2-4 animals/cage) whenever possible, and maintained in a temperature controlled environment (22°C), on a 12 hour light-dark cycle beginning at 0700 hr. In all cases, mice were food-restricted to 80-90% of their free-feeding body weight. Those that fell below this target weight were given 2hr ad libitum access to food in a separate cage (autoclaved Purina mouse chow; St. Louis, MO). Animals were maintained and treated in accordance with procedures approved by the Yale University Institutional Animal Care and Use Committee (protocol 2008-10975). Necropsies were performed on every animal immediately following sacrifice to rule out gross abnormalities and tumors; all animals used in this study were free of any identifiable pathology. A summary of the four cohorts of mice is shown in Table 1.
Table 1.
Summary of cohorts used in study.
| Cohort | N 6 months | N 18 months | N 24 months | Behavioral analyses |
Molecular analyses |
|---|---|---|---|---|---|
| 1 | 6 | 6 | 11 | CSRTT; PR; OF; NO |
Microarrays; Serum cytokines; Quantitative PCR |
| 2 | 8 | 0 | 9 | CSRTT | None |
| 3 | 10 | 10 | 10 | None | Microarrays; Serum cytokines |
| 4 | 6 | 0 | 6 | None | RNA sequencing of microglia |
2.2 Choice Serial Reaction Time Task (CSRTT)
In order to investigate the involvement of PFC dysfunction in age-related cognitive impairment, we used a modified version of the 5-Choice Serial Reaction Time Task (CSRTT), a well established test of attention and response inhibition in rodents (Bari and others 2008; Robbins 2002). This task was modified to include three, rather than five response apertures and consisted of several phases (i.e., Magazine training, Pre-CRSTT, CSRTT), all of which took place in a standard operant chamber (Med Associates) with a liquid dipper, house light, reward light, clicker and three apertures positioned on a curved wall, opposite the liquid dipper (Krueger and others 2006).
The first phase, magazine training, allowed for the association of auditory (clicker) and visual (reward light) cues with dipper presentation and ensuing reward availability. During this phase, all apertures were covered and, at random intervals, the house light would flash, the clicker would sound, the reward light would illuminate and the reward dipper would become available. The liquid reward consisted of roughly 30μl of commercially available chocolate whole-milk and was available for 5 seconds. Magazine training was conducted during the first three sessions and lasted 15 minutes.
For the subsequent phase (Pre-CSRTT), mice received daily sessions (30 minutes in duration) during which a stimulus (i.e., light) was randomly presented in one of the three apertures. An aperture was continuously illuminated until an animal made a correct response (i.e., nosepoke into the illuminated aperture) at which point the auditory and visual cues indicated reward availability. Following reward availability (5 seconds) a new trial was initiated and the stimulus light was randomly presented in one of the three apertures until the animal made another correct response. During the Pre-CSRTT phase, incorrect responses into non-illuminated apertures were not punished. The Pre-CSRTT was conducted until an individual animal exceeded 40 rewards with at least 40% of their responses being correct (note that chance performance is 33%) on two consecutive days, at which point the animal was graduated into the subsequent phase (CSRTT).
For the CSRTT phase, stimulus presentation times were initially established at 60 seconds and became progressively shorter in duration as mice met behavioral criterion. In order to progress through the task, mice were required to make more than ten successful responses on two consecutive days, with the total percentage of correct responses being greater than or equal to 80%. Once each animal had met criterion for advancement, the stimulus presentation time became shorter (6, 5, 4, 3, 2, 1 sec, 750, 500, 250, 125, 62, 31, 15ms), effectively increasing the difficulty of the task. Responses in the non-illuminated apertures (errors of commission), failure to respond during the stimulus presentation time (omission errors), and responses in one of the apertures prior to onset of the stimulus (premature errors) resulted in a 2 sec timeout, during which the house light and all other lights were extinguished.
2.3 Progressive Ratio
Following completion of the CSRTT, mice were given a test of progressive ratio responding in order to determine possible underlying differences in motivation to work for the reward. As previously described (Gourley and others 2008), response requirements (nose-pokes) began with a single nose-poke and increased by N+2 with each subsequent trial. Progressive ratio was assessed on three consecutive days and break point was deemed as the greatest number of response requirements met on any of the three days of testing.
2.4 Open Field
To assess potential age-related differences in locomotor speed, mice were placed in the center of a circular open field (diameter 55 cm) with an opaque floor and 30 cm opaque walls. In contrast to a square arena, the circular open field allows for the unambiguous assessment of distance from both the center and sides of the chamber (Lipkind and others 2004). The arena was placed in the center of a dimly lit room, devoid of any obvious visual cues, and was lit with a single lamp placed directly above the center. A video camera, suspended 3 ft above the middle of the arena, recorded each mouse during the exploration period at 30 frames per second. Testing was performed for 15 minutes and videos were scored offline using software written by the authors to determine position in the open field at each time point. Data were used to calculate speed, distance from the center, and distance traveled in 5 minute intervals over the course of testing.
2.5 Novel object responding
Exploration of a novel object (Carey and others 2008) was performed similarly to what has been reported elsewhere (Zueger and others 2005). Immediately following the completion of the open field assessment, mice were briefly removed while a novel object (black rubber stopper, roughly 3.5cm in diameter) was placed into the center of the arena. Mice were returned to the field for an additional 5 minutes, during which their behavior was video recorded. Total time in contact with the object and the number of contacts with the novel object were scored offline by a blind observer.
2.6 Visual Placing Test
We performed the Visual Placing Test (Pinto and Enroth-Cugell 2000), a measure of gross visual acuity, to determine whether age-related deficits in the CSRTT were an indication of a loss of visual function in old-aged mice. Mice were suspended by their tails and slowly lowered to a solid surface. Behavior was video recorded and the distance from the surface at which the animal extended its forelimbs was recorded. The average distance that elicited reaching on three separate tests of Visual Placing was analyzed for assessment of visual deficits.
2.7 Statistical analysis of behavioral data
All behavioral data were analyzed using SAS version 9.2 (SAS Institute, Cary NC). Repeated measures data (CSRTT, Open Field) were analyzed using mixed-effects repeated measures models (Brown and Prescott 1999; Pinheiro and Bates 2000) with the SAS MIXED procedure. The remaining data (Progressive Ratio, Visual Placing Test, and Novel Object interactions) were analyzed using analysis of variance models with the SAS GLM procedure. Mediation analysis was computed using a SAS macro (http://www.comm.ohio-state.edu/ahayes/sobel.htm). Principal components analysis was conducted using R software (http://cran.r-project.org). Statistical graphics were generated using SYTAT versions 12 and 13.
2.8 CD11b selection
For CD11b enrichment, animals were given an overdose of chloral hydrate (1500mg/kg; i.p.), decapitated, and brains were rapidly removed. A single coronal slice was made just anterior to the hypothalamus and both the frontal block and remaining tissue were placed in a tube containing 10% RNAlater (Qiagen). Pilot work determined that ice cold 10% RNAlater (1:10 dilution in water) maintains RNA integrity while still allowing for sufficient antibody recognition of CD11b using the Easy Sep kit (StemCell Technologies). Following a 24 hour incubation in 10% RNAlater (4°C), the PFC was microdissected, minced with a razor and placed in 500μl of PBS. A single-cell suspension was achieved by passing the tissue through a 1ml pipette tip followed by a 20g, and then 23g syringe needle. Microglial isolation was performed using the Easy Sep CD11b Positive Selection Kit (StemCell Technologies), according to the manufacturer’s protocol with slight modifications. Briefly, cells were incubated with CD11b PE labeling reagent (20 minutes) followed by PE selection cocktail (20 minutes) and finally with the magnetic beads (15 minutes). All incubations were performed on ice. Following the last incubation period, cells were placed on a magnet and all unbound cells (Unselected population) were aspirated off, transferred to a new 1.5ml tube and reserved for RNA/DNA/protein extraction. Bound (CD11b-positive) and unbound (CD11b-negative) cell populations were lysed in 350 and 600 μl RLT buffer (with 30μl/ml β-mercaptoethanol), respectively, and sonicated for 5 seconds at 10% power and again at 13% power (Branson Sonifier S-450). RNA, DNA and protein extractions were conducted immediately, using the Qiagen All Prep Kit, according to the manufacturer’s protocol (Qiagen).
2.9 Whole Genome Microarray
Tissue was obtained as follows: Mice were anesthetized with chloral hydrate (1500 mg/kg; i.p.), decapitated, and brains were immediately submerged in ice-cold RNAlater (Qiagen). Tissue was incubated overnight at +4°C, and subsequently stored at −20°C. At the time of dissection, brains were sectioned at 300 μm on a vibratory microtome (Vibratome), and sections were stored in 0.025% methylene blue in RNAlater at −20°C for 48 hours. Sections were examined microscopically to rule out intracranial tumors. Medial prefrontal cortex (PFC) was dissected under a stereomicroscope from two sections, placed in RNAlater at 4°C, and processed within 24 hours. Our “medial prefrontal cortex” dissections included anterior cingulate, pre-limbic, and infra-limbic prefrontal cortex as defined by comparison to a standard anatomical atlas, from two sections between approximately 2.1 and 1.5 mm from the Bregma (Paxinos and Franklin 2001).
At the time of processing, RNAlater was replaced with 600 μl of buffer RLT (Qiagen) containing β-mercaptoethanol, and tissue was sonicated for 5 seconds at 10% power. RNA and DNA were extracted using the Qiagen AllPrep mini kit, according to manufacturer’s instructions. RNA quality was first verified using Agilent 2100 Bioanalyzer RNA chips. An RNA integrity (RIN) cutoff of 8.0 was used for these analyses and all samples passed this quality control criterion. RNA was then hybridized to Illumina WG-6 microarrays which include 6 arrays per beadChip, at the Yale Keck Center. Data were normalized using the R/Bioconductor LUMI package (http://www.bioconductor.org/packages/2.2/bioc/html/lumi.html) and analyzed using one-way mixed-effects ANOVA models on a high performance computing cluster using R/Bioconductor and the Maanova package (http://www.bioconductor.org/packages/2.3/bioc/html/maanova.html). Models included beadChip and cohort as random effects, and age cohort as a fixed effect. P values were calculated using 1000 permutation tests and then adjusted to maintain a false discovery rate (Storey 2002) of 5% or 10%, as noted in the text.
Results were subjected to informatics analysis using MetaCore software (GeneGo Inc.) and PASTA software (http://trap.molgen.mpg.de/PASTAA.htm). PASTAA uses the TRAP method of defining the likely affinity of known transcription factors to a list of gene promoters associated with a set of dysregulated genes (Roider and others 2007).
2.10 RNA Sequencing
Independent pools of RNA (5-6 animals/pool) were analyzed using next generation RNA sequencing (RNA-seq) on the CD11b selected cells. RNA quality was first verified using Agilent 2100 Bioanalyzer RNA chips. An RNA integrity (RIN) cutoff of 8.0 was used for these analyses and all samples passed this quality control criterion. Quantity of RNA extracted from the CD11b selected cells was determined using Bioanalyzer readings as well as Nanodrop readings and Cd11b+ cellular fractions from N=5-6 mice were pooled and contained equivalent amounts of RNA from representative animals. RNA sequencing was performed by the Yale Genome Sequencing Center on an Illumina GA2 with 75 nt read lengths. Data were mapped to the mouse genome (mm9) using Cassava software (Illumina) and were subsequently analyzed using the DEGseq package (Wang and others 2010) in R/Bioconductor. P values were adjusted for multiple comparisons (Storey 2002).
2.11 Real Time PCR
cDNA synthesis was performed on 0.1 μg of RNA extracted from PFC lysates using SuperScript II reverse transcriptase (Invitrogen) and a blend of random hexamers and oligoDT primers, and included an RNAseH treatment step (Invitrogen). Quantitative PCR was performed on samples diluted 1:7 with RNAse free water and included 7.5μl of 2X QuantiTect SYBR Green Master Mix (Qiagen), 0.08μl of the forward primer (50 μM), 0.08μl of the reverse primer (50 μM), 4.59μl RNAse free water, 0.75μl DMSO, and 2μl of template cDNA. Following a 10 minute incubation period at 95° C, cycling consisted of 20 sec denaturation at 95° C, 20 sec annealing at 60° C, a 20 sec extension at 72° C, and a 10 second step at 80° C, for a total of 45 cycles. Fluorescence was determined during the step at 80° C for each cycle. PCR cycling was conducted using a Stratagene Mx3000p real-time PCR machine. Following amplification, a melt-curve beginning at 55° C and increasing to 95° C in 0.5° C increments was used to ensure that only a single PCR product resulted. All Ct values were normalized to the mean of Tbp and Rps27a. The following primers were used:
B2m (F),TGGCTCACACTGAATTCACCCCCA.
B2m (R),TCTCGATCCCAGTAGACGGTCTTGGGC.
C4 (F),TGAGCTCAGAGAGCCAGAGTCC
C4 (R),TGCCTTCAGAACAAATGTGCAA
Cd52 (F),GCCATTGGCTGTCAACTTTAGC
Cd52 (R),GCCACCATCAGTAGCGAGAGAC
H2-k1(F),TCTCCAACATGGCGACCGTTGCT.
H2-k1(R),AGGTCTGGGAGCCTGGAGCCAG.
Ifi27 (F),ATCAGCAGGGGTCCTTGGACTCTCC.
Ifi27 (R),GAGCAAGGCTCCAACAGCTGCCC.
Lgals3bp (F),GGTAGAAGGGGCGTATGACCAC
Lgals3bp (R),GACTCCCTCCCTCTTTCCCTGT
Psmb8 (F),AAGTGGTCATGGCGTTACTGGA
Psmb8 (R),CTTGCGCGGAGAAACTGTAGTG
Rps27a (F),GCTAGTGGCGCTACGCGTCGCT
Rps27a (R),GAGGGTTCAACCTCGAGCGTGATGGT
Tbp (F), AGCGGTGGCGGGTATCTGCTGGGC
Tbp (R), ATGGCGCCCTGTGGGGAGGCCAA
2.12 Serum cytokine analysis
Serum cytokine analyses were conducted as follows: Trunk blood was obtained after sacrifice and was subjected to coagulation on ice followed by centrifugation at 1000 × g. Serum was subsequently frozen at −80°C until the time of analysis. Samples were analyzed using a multiplexed ELISA method (Meso Scale Discovery) for IFN-gamma, IL-10, IL-12, IL-1, IL-6, TNF-alpha, and Cxcl1 (“KC antigen”). All data were calibrated using standard curves generated for each cytokine, and analyzed according to manufacturer’s directions.
2.13 Principal components analysis and regression analysis
Principal components analyses were conducted on the behavioral and molecular data using R version 2.11. All analyses were conducted on correlation matrices of the data after confirming that the data were reasonably linear by examining all possible bivariate scatter plots. Cytokine and microarray data were log transformed as this was found to cause the data to be approximately normally distributed. Eigenvalues were sorted and plotted (“scree plots”) to assess the adequacy of low-dimensional solutions. Factor scores were then computed for the retained factors and used in linear regression models as described in Results.
3. Results
3.1 Old age mice exhibit evidence of cognitive and motivational decline on the CSRTT
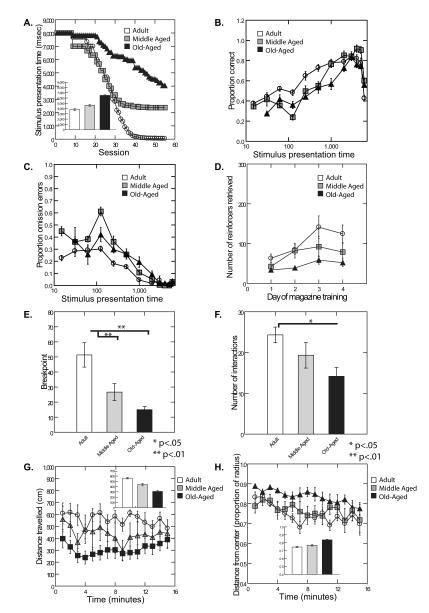
We used the Choice Serial Reaction Time Task (CSRTT) to determine whether aging mice exhibit impairments in spatial divided attention. Performance in this task is thought to be driven primarily by the medial PFC since selective lesions to areas such as the dorsal anterior cingulate lead to deficits in response accuracy (Chudasama and others 2003; Passetti and others 2002). A cohort of 23 C57Bl/6 mice (‘Cohort 1’; see Table 1) aged 6mo (n=6, ‘Adult’), 18mo (n=6, ‘Middle-aged’), and 24mo (n=11, ‘Old-aged’), were evaluated for the stimulus presentation time at which each animal could maintain a fixed level of response accuracy. Analysis of task acquisition revealed significant main effects of age, session, and a significant age × session interaction such that older animals required longer to acquire the task than younger mice (Fig. 1A). Age-related deficits remained evident during the last five days of testing (Fig. 1A), consistent with an age-associated decline in performance even after extended training.
FIGURE 1.
A Acquisition of the CSRTT was analyzed by analysis of the stimulus presentation time during each session as a function of test day, age group, and their interaction, using mixed effects analysis of variance. Significant main effects of age (F(2,20)=243.65, p<.0001), session (F(54,1080)=29.99, p<.0001), and a significant age × session interaction (F(108,1080)=2.56, p<.0001)) were noted. Age-related deficits remained evident during the last five days of testing (F(2, 20)=12.94, p=0.0002), consistent with an age-associated decline in performance even after extended training. Inset shows acquisition averaged across all sessions for the three age groups.
B Proportion of correct trials (number of correct trials/number of trials) as a function of age group and stimulus presentation time. Age cohort was significantly associated with overall performance on the CSRTT (F(2,19)=14.60, p=0.0001), stimulus presentation time (1,811=73.10, p<.0001), and the age cohort × stimulus presentation time interaction effect (F(2,811)=14.38) was significant. Younger animals performed better than older animals at the lower (more difficult) stimulus presentation times than older animals. Error bars represent the standard error of the mean.
C Proportion of omission errors (number of omission errors/number of trials) as a function of age group and stimulus presentation time. The effects of age cohort (F(2,19)=68.48, p<.0001), stimulus presentation time (F(1,811)=842.70, p<.0001), and the age cohort × stimulus presentation time interaction (F(2,811)=21.74), p<.0001) were all highly significant. Younger animals made fewer omission errors than older animals particularly at lower (more difficult) stimulus presentation times. Error bars represent the standard error of the mean.
D Number of rewards received during acquisition during the magazine training portion of the CSRTT is shown as a function of age group and day of training. Age group was associated with the number of rewards retrieved (F(2,20)=21.20, p<.0001), with Adult mice retrieving more rewards than Middle aged mice (F(1,20)=8.34, p=0.0091) and Middle aged mice retrieving more rewards than Old aged mice (F(1,20)=9.97, p=0.005). Error bars represent the standard error of the mean.
E Motivated responding was assessed using the progressive ratio test using an N+2 protocol. Adult animals extended a significantly greater number of nosepokes (higher breakpoint) than both Middle-aged and Old-aged mice [Cohort 1; F(2,20)=18.492, p= 0.000028]. Adult animals differed from Middle Aged animals (p=.008) and Adult animals differed from Old aged animals (p<.0001) by Tukey HSD comparisons. Error bars represent the standard error of the mean.
F Mice from Cohort 1 were subjected to novel object testing and the number of interactions with the novel object were recorded. Significant differences in the number of interactions with the object between the age groups were noted (F(2,20)=5.19, p=0.0153) such that interactions with the novel object declined with advancing age. Adult animals differed significantly from Old aged animals (p<.05) but not from Middle aged animals by Tukey HSD comparisons. Error bars represent the standard error of the mean.
G Motoric behavior was tested using the open field test. Analysis of total distance traveled in the open field during a 15 minutes test revealed significant main effects of age (Cohort 1; F(2,18)=60.24, p<0.0001) but no effect of time and no age group × time interaction effect.
H Anxiety-like behavior was also assessed from the open field, using distance from the center of the open field as an index of anxiety-like behavior. We noted a significant effect of age (Cohort 1; F(2,20)=55.12, p<.0001) and a significant effect of time (F(14,280)=4.21, p<.0001) but no age × time interaction. Error bars represent the standard error of the mean.
Because aging in humans is associated with PFC dysfunction, and the PFC is known to mediate effort and motivated responding (Rushworth 2008; Walton and others 2003; Walton and others 2002; Walton and others 2006; Walton and others 2007), we analyzed the CSRTT data for evidence of age-related motivational differences. Preliminary analyses of the CSRTT data indicated that age was inversely related to the total number of responses made on the task (F(2,22)=335.70, p<.0001; 38.47±1.57, 17.56±0.83, 7.55±0.35; mean±standard error for the adult, middle-aged and aged mice, respectively). We therefore divided the number of correct responses as well as the numbers of errors made by the number of responses made by the animal on each trial. In addition, because the difficulty of the task is governed by the stimulus presentation time, we controlled for this variable in our statistical analyses of the error data. After controlling for stimulus presentation time, age was still highly related to the number of responses made per trial (F(2,22)=414.06, p<.0001) with the younger animals making more responses than the older animals (30.18±0.68; 16.61±0.62; 11.92±0.51; marginal means and standard errors for adult, middle-aged and aged mice, respectively) further justifying the need for controlling for the number of responses made per trial.
Age cohort was significantly associated with overall performance (proportion of correct responses) on the CSRTT. As shown in Fig 1B, adult mice made a higher proportion of correct responses than the other groups at the lower (more difficult) presentation times. Analysis of the distinct error types (omission, premature, perseverative, and commission) revealed that the strongest effect of age was evident for omission errors where the effects of age cohort (F(2,19)=68.48, p<.0001), stimulus presentation time (F(1,811)=842.70, p<.0001), and the age cohort × stimulus presentation time interaction (F(2,811)=21.74), p<.0001) were all highly significant. As shown in Fig. 1C, younger animals made fewer omission errors than the middle aged and old aged mice especially at the shorter (more difficult) presentation times. The age effect on omission errors is consistent with an effect of age on attentional performance.
The results of the CSRTT were replicated in a second, independent cohort of C57Bl/6J mice (‘Cohort 2’; see Table 1) that included eight 6mo and nine 24mo old mice. Again, we observed more rapid acquisition in the younger animals (F(1,15)=158.49, p<.0001), a significant effect of trial (F(55,781)=13.63, p<.0001), and a small interaction effect that approached significance (F(55,781)=1.33, p=0.0574; data not shown). As we observed in Cohort 1, aged animals obtained fewer rewards than adult animals (F(1,15)=44.01, p<.0001) and a significant session × age group interaction was again noted (F(1,885)=20.29, p<.0001; data not shown).
Overall, results from CSRTT testing suggest that older mice acquired the task more slowly and received fewer rewards than younger animals. Older animals failed to obtain as many rewards as the younger animals per trial and failed to progress to more difficult levels of the task as quickly as the younger mice, and performed less well than younger animals after controlling for task difficulty. Analysis of the number of rewards retrieved also suggested the possibility that aging is associated with changes in motivation.
3.2 Old age mice exhibit evidence of decreased motivation on progressive ratio testing
The findings with respect to the number of responses made on the CSRTT led us to speculate that aging may affect motivated responding in addition to cognition. The dorsal-medial PFC has been shown to be involved in motivated behavior in rodents, non-human primates, and humans (Rushworth 2008; Walton and others 2003; Walton and others 2002; Walton and others 2006; Walton and others 2007). Age-related changes in motivation were initially suggested by the fact that older animals retrieved fewer rewards during magazine training on the CSRTT than older animals (Figure 1D). To confirm that aging is associated with changes in motivated responding, we used a progressive ratio operant task where mice were required to make increasing numbers of responses (nose pokes) in order to receive reward. The test was repeated over three consecutive days and the maximum level of responding (“breakpoint”) was analyzed. Adult animals extended a significantly greater number of nosepokes (higher breakpoint) than both Middle-aged and Old-aged mice (Cohort 1; Fig 1E). These results demonstrate that older mice show a reduction in motivated behavior compared to younger mice. We used the statistical method of mediation analysis (Preacher and Hayes 2008) to determine whether these motivational changes were responsible for the changes observed in CSRTT acquisition. Motivated responding as assessed by the progressive ratio task did not appear to mediate the effects of age on CSRTT acquisition (p=0.113 by mediation analysis).
3.3 Old age mice exhibit evidence of decreased interest on novel object testing
To obtain evidence for an age-dependent reduction in exploration, another apathy-like behavior, we examined interaction with a novel object (Carey and others 2008). While the novel-object recognition test is frequently used to assess differences in memory and recognition, exploration of a novel object has also served as an index of anhedonia (Bevins and Besheer 2005). Mice from Cohort 1 were subjected to a novel object exploration test in which the number of interactions with the object were recorded. Significant differences were noted between the age groups such that interactions with the object were smaller in number in the older animals (Fig 1F). These results are consistent with those observed in the CSRTT and progressive ratio tests, suggesting a decline in exploration and motivation with advancing age in the mouse.
We used the statistical method of mediation analysis to determine whether the observed effects on novel object responding where due to direct effects of age on object exploration or an indirect effect of age mediated by locomotor activity. This was motivated by the fact that distance traveled on the open field and novel object interactions were highly correlated (r=0.75, p<.0001). Mediation analysis demonstrated that motor speed does appear to underlie the relationship between age and novel object interactions (p=.0160). We also examined this using another statistical method. When locomotor speed was entered first in a hierarchical ANOVA model (F(1,17)=26.17, p<.0001), age cohort was no longer significant (F(1,17)=0.64, p=0.5414). However, when age cohort was entered first (F(1,17)=7.92, p=0.0037), locomotor speed remained significant (F(1,17)=11.60, p=0.0034). Taken together, these analyses are consistent with a model where locomotor speed mediates at least some of the relationship between age and novel object interactions.
3.4 Old-aged mice show evidence of motoric slowing and increased anxiety
Aging in humans is associated with changes in motoric behavior (i.e., gait speed) that parallel changes in cognition and motivation. We, therefore, sought to determine whether this is also true in the aging mouse. Open field activity, including total distance traveled and distance from the center of an open circular arena, was examined in order to determine whether aged mice show changes in locomotion and anxiety. Analysis of total distance traveled in an open field during a 15 minute test demonstrated that older animals traveled a shorter distance than younger animals (Cohort 1; Fig 1G).
Distance from the center of the open field is an index of unconditioned anxiety-like behavior and can also be determined from the open field test (Choleris and others 2001; Walsh and Cummins 1976). Older animals spent significantly more time further from the center of the open field than younger mice (Cohort 1; Fig 1H), consistent with increased avoidance of the center of the open field. Together, these results suggest both motoric slowing and heightened anxiety-like behavior in old aged mice.
3.5 Aging in C57BL/6 mice is not associated with gross visual deficits
Since behavior in both the CSRTT and the open field may have been affected by reductions in visual acuity, we used the Visual Placing Test (Pinto and Enroth-Cugell 2000) to determine whether Old-aged mice used in the current experiment were impaired in this regard. Analysis of the distance at which a mouse being lowered by its tail to a solid surface first extended its forepaws did not differ as a function of age (Cohort 1; F(2,19)=2.011, p=0.1614; data not shown). While not ruling out the possibility of small differences in acuity, these results suggest that there are no gross visual differences between Adult, Middle-aged and Old-aged in this cohort. Furthermore, the CSRTT task that we employed is dependent on stimulus duration, not intensity, and is therefore not likely to be confounded by differences in visual acuity.
3.6 Aging is related to immune gene expression changes in the PFC
Thus far, our behavioral data suggest that aging mice, like humans, show correlated cognitive, motivational, and locomotor changes. We hypothesized that these changes are attributable to age-related molecular changes in the medial PFC. Subsequent to the behavioral analyses, we extracted RNA from the PFC of the animals in Cohort 1 (see Table 1) to assess age-related changes in gene expression. To control for the possibility that lengthy behavioral testing affected gene expression, we also analyzed RNA extracted from the PFC of a separate group of 30 animals (‘Cohort 3’) consisting of 10 mice per age group (see Table 1).
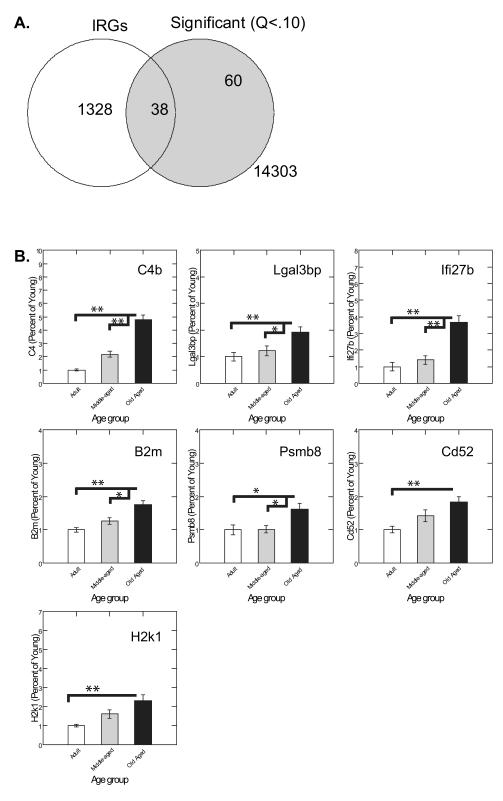
Microarray analysis of Cohorts 1 and 3, using a mixed effects ANOVA model that included beadChip (there were 6 arrays per beadChip) and Cohort as random effects and Age and Age × Cohort as fixed effects, revealed that the age effect yielded 85 and 112 significant probe sets representing 72 and 100 unique genes with a false discovery rate cutoff of Q<0.05 and Q<0.10, respectively. No genes were significant for the Age × Cohort interaction term. The 72 most significant genes identified (Q<.05) were subjected to Gene Ontology Analysis using GeneGo software (Metacore), revealing that “Immune gene upregulation, Interferon Signaling” (−log(P)=3.727), “Immune gene upregulation, Complement Signaling” (−log(P)=3.027) and “Immune gene upregulation, Innate inflammatory response” (−log(P)=2.896) were the top three GeneGo process networks affected by aging. Overall, aging was found to be associated with very strong upregulation of genes involved in immunity as well as other categories (Table 2; Supplementary table 1). A significant association was also found between age and genes known to be regulated by interferons (Fig. 2A), consistent with the results of our gene ontology analysis. Other major classes of dysregulated genes included genes involved in neuronal projection, motility, and/or migration, genes involved in cell cycle control and/or differentiation, and genes involved in transcriptional regulation, including transcription factors. We performed real-time PCR validation of selected immune-related genes from multiple classes (C4b, Lgal3bp, Ifi27b, B2m, Psmb8, Cd52, and H2k1) and all assays confirmed the upregulation observed in the array data (Fig. 2B).
Table 2.
Genes decreased and increased by age by microarray analysis.
| Genes decreased with aging |
|---|
| Cell cycle/growth/differentiation |
| Igfbpl1 |
| Wdr6 |
| Neuronal projection/motility/migration |
| Crmp1 |
| D0H4S114 |
| Dpysl3 |
| Ndn |
| Other genes |
| 1810018F18Rik |
| D0H4S114 |
| Mog |
| Pbk |
| Rem2 |
| Transcription factors/regulation |
| Cdca7 |
| Dlx1 |
| Sox4 |
| Sox11 |
| Tcfl5 |
FIGURE 2.
A. The significantly changed gene list was intersected with a list of known interferon regulated genes (IRGs) derived from the Interferome Project database (www.interferome.org). A significant association was noted between IRG status and age-related dysregulation on microarray by Pearson Chi-squared analysis (p<2.2·10−16).
B. PCR validations were conducted on RNA samples from Cohort 1. Data were normalized to the average of Tbp and Rps27a which were found not to be regulated by age based on microarray and real-time PCR analysis (p>0.9). C4 F(2,19)=46.45, p<.0001. Lgal3bp F(2,19)=7.31, p=0.0044. Ifi27b F(2,19)=20.40, p<.0001. B2m F(2,19)=13.73, p=0.0002. Psmb8 F(2,19)=5.60, p=0.0123. Cd52 F(2,19)=6.97, p=0.0054. H2k1 F(2,19)=7.06, p=0.0051. Error bars represent the standard error of the mean.
Furthermore, bioinformatic analysis of transcription factor binding sites present in the promoter region of upregulated genes (−400bp to +400bp of the transcription start site) using PASTAA (Roider and others 2007) found significant enrichment of immune related transcription factor binding sites. A highly significant overlap was observed between the upregulated genes and multiple interferon related transcription factors, namely Irf-1, Irf-2, Irf-7a, Irf-8, and Irf-10 (p<10−10) as well as Stat1alpha (p=0.000043). Significant enrichment was also observed for NFkappaB related transcription factor binding sites, including Rela (p<0.00001) and NFkappaB1/NFkappaB2 (p=0.00017). Thus, all significant associations detected between transcription factor binding sites and the genes upregulated with age were immune related.
To determine whether the increase in immune-gene expression was merely a reflection of a larger number of inflammatory cells in the aged brain, we examined whether there was an increase in markers for microglia. Importantly, the expression of Aif1 (Iba1), a marker of activated microglial cells, was not affected by age on any of the three microarray probes for this gene (Q values 0.10, 0.15, and 0.88). We also examined the expression of Itgam (CD11b) and Ptprc (CD45), both of which were expressed at levels that fell below the expression cutoff of our microarray analysis but we did not see any evidence of age-related dysregulation. Although indirect, these analyses suggest that the observed expression changes were not merely due to an increase in the number of microglial cells in the aged samples, or an increase in the number of cells with a macrophage phenotype (i.e., high Ptprc expression). To provide more direct evidence that the expression changes discussed above are not simply due to an increased number of microglial cells but due to gene expression changes in this cell type, we isolated Cd11b+ cells and analyzed expression within this cell type in isolation, as described below.
3.7 Aging is related to gene expression changes in purified Cd11b+ cells from PFC
The results of the microarray analysis suggest upregulation in immune related genes in the aging PFC. In order to determine whether these changes are occurring in Cd11b+ cells (a marker of microglia also expressed in macrophages and dendritic cells), we performed next-generation RNA sequencing (RNA-seq) on Cd11b+ cells immunopurified from the PFC of a fourth cohort (‘Cohort 4’; see Table 1) that included six 6mo and six 24mo old mice. Importantly, the Cd11b+ fraction was significantly enriched for genes characteristic of microglia relative to the non-selected fraction (e.g., Cd53 (3.4 fold change from CD11b-population by RNA-seq), Csf1r (2.4 fold), Emr1 (2.3 fold), Fcer1g (2.9 fold), Fcgr2b (2.6 fold), Aif1 (“Iba1”; 2.9 fold), and Itgam (“Cd11b”; 2.6 fold)), confirming the effectiveness of the immunoaffinity selection process. Analysis of the RNA-seq dataset demonstrated that a large number of genes were differentially expressed at 6mo as compared to 24mo (i.e., 4047 at Q<.05, 2542 at Q<.01, and 1591 at Q<.001). Interestingly, the Cd11b+ fraction was enriched for many of the same changes observed in whole PFC, in addition to other immune genes not previously identified. Aging was associated with upregulation of numerous CD antigens, chemokine related genes, complement genes, genes in the Jak-Stat and/or intereferon signaling pathway, cellular adhesion genes, MHC related genes, and Toll-like receptors in the Cd11b+ cells (see Table 3 and Supplementary table 2). Of note, there were no age-related changes in Ptprc (CD45), a marker that is highly expressed in macrophages and low in microglial cells, suggesting that the results are not due to a shift toward an increased proportion of infiltrating macrophages in the aged brain. Although we cannot exclude the possibility that other cell types also contribute to the gene expression changes observed in our microarray analysis of whole medial PFC, these results confirm the differential expression of immune-related genes in the aging PFC, and suggest that many of these changes are occurring within Cd11b+ cells, and likely in microglial cells.
Table 3. Genes found to be upregulated in purified CD11b+ cells by RNA sequencing.
Cd11b+ cells were isolated from the PFC of Adult and Old-aged mice as described in Methods and RNA was subjected to next-generation RNA sequencing. Aging was found to be associated with upregulation of numerous CD antigens, chemokine related genes, complement genes, genes in the Jak-Stat and/or intereferon signaling pathway, cellular adhesion genes, MHC related genes, and Toll-like receptors in microglial cells.
| CD antigens | Integrins | Other immune related genes |
|---|---|---|
| Cd3eap | Bcam | Atp1b3 |
| Cd52 | Itga3 | Bst2 |
| Cd59a | Itgax (“Cd11c”) | Csf2ra |
| Cd63 | Itgb4 | Ctsd |
| Cd74 | Ncam1 | Ddr1 |
| Cd81 | MHC related | F3 |
| Cd9 | B2m | Fgfr1 |
| Chemokines | H2-ab1 | Fgfr2 |
| Cxcl10 | H2-d1 | Fgfr3 |
| Cxcl13 | H2-k1 | Flt3 |
| Cxxc1 | Lag3 | Fth1 |
| Complement factors | Jak-Stat pathway | Igsf8 |
| C1qa | Cntfr | Il4ra |
| C1qb | Il11ra1 | Lamp1 |
| C3 | Irf2bp1 | Lpl |
| C4b | Irf3 | Ltbr |
| Osmr | Mcam | |
| Prlr | Plxnb2 | |
| Ptpn11 | Rps6kb2 | |
| Stat2 | Serping1 | |
| Stat3 | Sirpa | |
| Toll-like receptors | Slc44a1 | |
| Tlr2 | Trf |
 IRG
IRG
 Non-IRG
Non-IRG
3.8 Peripheral immune gene upregulation
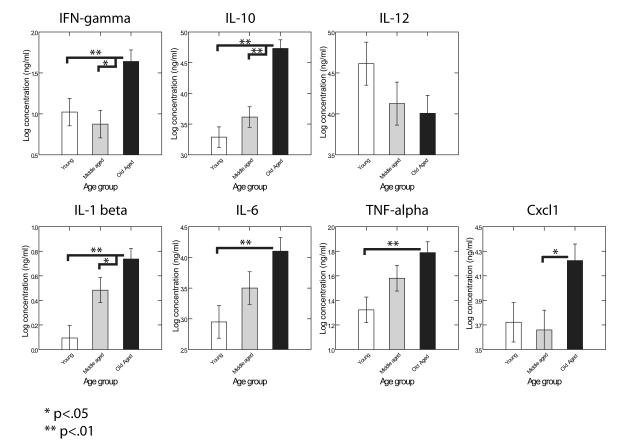
There is substantial evidence that aging is associated with increases in peripheral immune gene upregulation and that this is, in turn, associated with cognitive dysfunction (Gimeno and others 2008; Hilsabeck and others 2010; Rafnsson and others 2007; Reichenberg and others 2001; Rothenburg and others 2010; Tonelli and Postolache 2005). To address this possibility in the current experiment, we examined levels of pro-inflammatory cytokines in the serum of mice from Cohorts 1 and 3. Trunk blood was collected immediately after sacrifice and serum was extracted via coagulation and centrifugation. Samples were analyzed using a multiplexed ELISA method (Meso Scale Discovery) for IFN-gamma, IL-10, IL-12, IL-1, IL-6, TNF-alpha, and Cxcl1 (“KC antigen”). Significant age-related increases in IFN-gamma, IL-10, IL-1, IL-6, and TNF-alpha were observed (Fig. 3).
FIGURE 3.
Serum samples from Cohorts 1 and 3 were analyzed using a multiplexed ELISA method (Meso Scale Discovery) for IFN-gamma, IL-10, IL-12, IL-1, IL-6, TNF-alpha, and Cxcl1. Data were analyzed using mixed effects ANOVA with cohort as a random effect. Significant age-related increases in IFN-gamma (F(2,50)=7.29, p=0.0017), IL-10 (F(2,50)=25.24, p<.0001), IL-1 beta (F(2,50)=11.68, p<.0001), IL-6 (F(2,50)=5.60, p=0.0064), and TNF-alpha (F(2,50)= 5.87, p=0.0051) were noted.
3.9 Integrative analysis of behavioral, microarray, and cytokine data
Because we noted changes in multiple behavioral variables, genes, and cytokines with age, we sought to determine whether these changes were occurring in parallel. To address this issue, we utilized the statistical technique of Principal Components Analysis (PCA). In PCA, the variation in a group of variables is summarized by a smaller set of surrogate variables (“principal axes” or “factors”) that are linear functions of the original variables (Morrison 2005). Thus a set of highly correlated variables that are all functions of a single process should be well summarized by a single factor and should all show high correlations (“loadings”) with that factor.
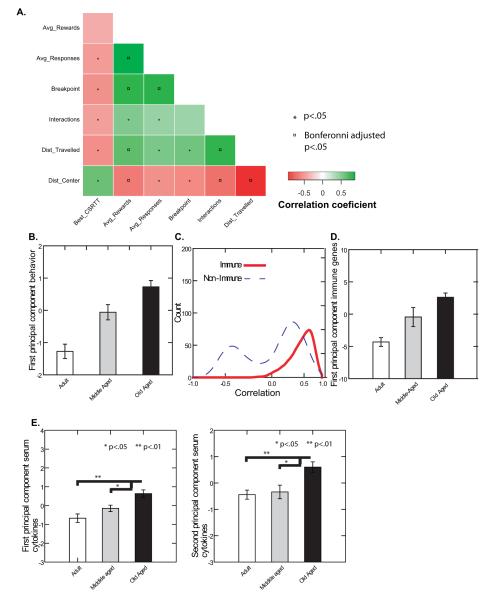
We computed linear correlations between the behavioral measures for animals in Cohort 1. As shown in Fig. 4A, CSRTT acquisition, average number of rewards obtained on the CSRTT, average number of responses made on the CSRTT, progressive ratio breakpoint, novel object interactions, and distance traveled in the open field were all highly correlated with one another. With the exception of two relationships (progressive ratio breakpoint with novel object interaction, and CSRTT acquisition with average rewards obtained on the CSRTT) all of the corresponding linear correlations were significant (p<.05) before multiple comparison correction, and 8 of these remained significant following Bonferroni correction (Fig. 4A). In addition, a principal components analysis of these data strongly suggested the presence of only a single factor; the first principal component accounted for 65.7% of the variance in the behavioral data. This first principal component was associated with better CSRTT performance, more rewards retrieved on the CSRTT, more responses on the CSRTT, a higher breakpoint in progressive ratio testing, more novel object interactions, a greater distance traveled on the open field, and more time spent toward the center of the open field (Table 4), and was significantly associated with age by ANOVA (Fig. 4B). These results suggest that, as in humans, cognition, motor performance, and motivation in the mouse are both highly correlated and age dependent (Granholm and others 2008) and also suggest an association with anxiety.
FIGURE 4.
A Heat map depicting Pearson correlation coefficients between CSRTT acquisition, average number of rewards obtained on the CSRTT, average number of responses made on the CSRTT, locomotor speed, distance from the open field center, and novel object interactions. With the exception of two relationships (progressive ratio breakpoint-novel object interaction and CSRTT acquisition-average rewards obtained on the CSRTT) all of the relationships were significant (p<.05) without multiple comparison correction (“*”) and seven relationships remained significant after Bonferonni correction (“¤”).
B The first principal component of the cognitive-behavioral data was significantly associated with age by analysis of variance (F(2,20)=48.79, p=0.000001). Error bars represent the standard error of the mean.
C Linear correlation coefficients between immune-related and non-immune-related genes (see Fig 2) were calculated and used to generate kernel density plots to illustrate the distributions of the correlation coefficients. The non-immune-related genes showed negative as well as positive correlations with one another, whereas the immune genes showed only positive inter-correlations, with a large proportion of strongly positive correlations, consistent with a high degree of internal consistency for this set of genes.
D The first principle component of the microarray data set (described above) was significantly associated with age for both cohorts (Cohort 1 F(2,20)=17.26, p=0.000044; Cohort 3 F(2,27)=15.56, p=0.000032). Error bars represent the standard error of the mean.
E The factor scores from the first two principal components of the cytokine data were related to age group by analysis of variance. Both factor 1 (left panel; F(2,53)=11.729, p<0.0001) and factor 2 (right panel; F(2,53)=8.410, p=0.001) were significantly related to age.
Table 4.
PCA analysis of behavioral measures. Principal components analysis of the cognitive-behavioral data shown in Figure 4A was performed and revealed only a single significant factor (4.6) accounting for 65.7% of the variance. Loadings on this first principal component are shown here.
| PCA loading | |
|---|---|
| Best CSRTT | −0.69 |
| Avg. Rewards | 0.85 |
| Avg. Responses | 0.81 |
| Breakpoint | 0.81 |
| Interactions | 0.74 |
| Dist. Traveled | 0.88 |
| Dist. Center | −0.88 |
Analysis of the microarray data also revealed significant correlations between genes dysregulated by aging, which was more pronounced for immune related genes than non-immune related genes (see Fig. 4C). PCA of the microarray data was also consistent with a single major factor that was strongly associated with age; the 85 array probes analyzed in this analysis yielded one eigenvalue that was much larger (48.01) than the next largest (6.63) and accounted for 56% of the variance in the data. We then calculated scores on the first principal component of the most highly correlated immune genes (Alox5ap, B2m, Ccl3, Cyba, H2-D1, H2-K1, Ifit3, Iigp2, Irf9, Lat2, Lgals3bp, Psmb8, Slc11a1, Tlr2, Tlr13) which differed significantly between the age groups (see Fig. 4D). PCA on peripheral cytokine levels obtained from individual mice, however, was consistent with two major sources of variation. IFN-gamma, IL-10, IL-1, IL-6, and TNF-alpha loaded heavily on the first factor, whereas Cxcl1 and IL-12 levels loaded heavily, and in opposite directions, on the second factor (Table 5). Both factors were significantly related to age (see Fig. 4E).
Table 5.
Principal components loadings for serum cytokines. Principal components analysis of the cytokine data was consistent with two factors. After removing the variance due to cohort, the first principal component accounted for approximately 49% of the variance in the data and the second component accounted for approximately 21% of the variance. Factor loadings are shown here.
| PCA1 | PCA2 | |
|---|---|---|
| IFN-gamma | 0.73 | −0.13 |
| IL-10 | 0.84 | 0.41 |
| IL-12 | 0.37 | −0.74 |
| IL-1 beta | 0.81 | 0.07 |
| IL-6 | 0.87 | 0.12 |
| TNF-alpha | 0.79 | −0.08 |
| Cxcl1 | −0.08 | 0.83 |
The results from PCA of the behavioral, microarray, and cytokine data were all consistent with aging acting on these outcome variables through one primary mechanism or a set of highly correlated mechanisms. We therefore determined how the microarray and cytokine data were related to the behavioral data. To determine whether the immune gene dysregulation observed in aging PFC is associated with behavior in the CSRTT, the first principal component from the microarray immune gene data was correlated with individual behavioral outcomes, and significant correlations were obtained for many of the behavioral measures (Table 6). The two serum cytokine factors described above were also related to these same behavioral measures (Table 6).
Table 6. Associations between behavioral measures and molecular analyses.
Associations between behavioral measures and molecules analyses. Correlation coefficients were calculated between central immune gene upregulation, as determined by the first microarray gene expression factor, and peripheral immune gene upregulation, as determined by the first and second cytokine factors, and the behavioral endpoints. We observed that “central immune gene upregulation”, as indexed by PFC immune gene expression, was a better predictor of behavior than “peripheral immune gene upregulation” as indexed by peripheral cytokine levels. Central immune gene upregulation was associated with significant associations with PR performance, average reward obtained on the CSRTT, average responses on the CSRTT, average distance traveled on the open field, average distance from the center of the open field, and the number of interactions with a novel object. All but PR performance survived Bonferonni correction for multiple comparisons. We also observed significant correlations between peripheral and central immune gene upregulation: r=0.495 for central immune gene upregulation and peripheral immune gene upregulation, factor 1, p=0.02; r=0.43, p=0.04 for central immune gene upregulation and peripheral immune gene upregulation, factor 2.
| Gene PCA1 | CYTO PCA1 | CTYO PCA2 | |
|---|---|---|---|
| Breakpoint | −0.55 b | −0.33 | −0.46 * |
| Best CSRTT | 0.22 | 0.19 | 0.39 |
| Avg. rewards | −0.77 b | −0.37 | −0.39 |
| Avg. responses | −0.64 b | −0.29 | −0.45 * |
| Dist. Traveled | −0.65 b | −0.49 * | −0.40 |
| Dist. Center | 0.67 b | 0.46 * | 0.42 * |
| Interactions | −0.69 b | −0.51 * | −0.43 * |
p<.05 (Bonferonni corrected)
p<.05
Central immune gene upregulation, as determined by the microarray based principal component, was significantly associated with progressive ratio performance, average reward obtained on the CSRTT, average responses on the CSRTT, average distance traveled on the open field, average distance from the center of the open field, and the number of interactions with a novel object. All but progressive ratio performance survived Bonferroni correction for multiple comparisons. Although significant associations were also noted for the two peripheral cytokine factors and some of the behavioral measures, none of these relationships survived Bonferroni correction. We also observed modest correlations between peripheral and central immune upregulation (see Table 6); although peripheral and central immune upregulation are correlated, this relationship was weak, accounting for no more than 25% of the variance in one another. Overall, central immune gene upregulation was more highly associated with the behavioral indices than were the peripheral cytokine levels.
4 Discussion
The current series of experiments were designed to test the hypotheses that aging in the mouse is associated with correlated changes in cognition, motivation, and locomotion, and that this aging phenotype is associated with gene expression changes within the medial PFC. We used several tests to examine these behavioral domains and confirmed that the aging mouse shows correlated alterations in cognition, motivation, and locomotion, paralleling what is observed with aging of humans. Microarray analyses of the medial PFC from two separate cohorts of mice revealed significant age-related gene expression changes, the majority of which were associated with immune gene upregulation. To investigate these immune related changes further, we examined isolated Cd11b+ from the PFC of Adult and Old-aged mice, and subjected RNA from these cells to RNA sequencing. As expected, there were a large number of age-related changes in the expression of immune genes within the Cd11b+ cells of the older animals.
Examination of behavior on the CSRTT, a well established test of attention in rodents (Bari and others 2008; Robbins 2002), revealed significant age-related impairments in task acquisition for the older mice. Analyses of task performance were complicated by the fact that younger animals made many more responses than older animals and progressed in the acquisition of the task more quickly than older animals. When CSRTT errors were normalized by the total number of responses made on the task and task difficulty was controlled for, older animals were found to make a lower proportion of correct responses and a greater fraction of omission errors than younger animals. A statistical test using mediation analysis was not consistent with motivation as determined from progressive ratio testing causing a spurious relationship between age and CSRTT acquisition, providing some evidence for a direct effect of age on CSRTT performance. Our results are similar to those of Harati et al. (Harati and others 2009) who found that aging in rats was associated with decreased performance on a five-choice version of the CSRTT.
Progressive ratio testing revealed a significant reduction in motivated responding in the older animals. Altered motivation to work for and consume an edible reinforcer could be driven by several factors, such as differences in caloric needs or taste. Indeed, it is possible that older animals were under a state of reduced caloric demand, therefore lessening their motivation to consume the edible reward. Age-related reductions in appetite have been described in humans, especially in the very old (Chapman and others 2002). This seems less likely in rodents, however, since age, body weight, and food intake have been found to be positively correlated with one another (Wolden-Hanson and others 2002). Although food intake was not directly assessed in the current experiment, body weights of animals used herein were greatest in the old mice (data not shown). Additional support for alterations in motivation in the absence of caloric confounds is provided by behavioral tests in which an edible reinforcer was not used. Importantly, in the case of both open field activity and novel object interaction, old mice exhibited comparable age-related deficits to those observed in the CSRTT. Similarly, there is evidence that aging affects olfaction (Cavallin and others 2010; Kondo and others 2010; Richard and others 2010), which also may have affected motivation to receive the reward. We cannot entirely rule out potential influences of age-related changes in appetite or olfaction with respect to the reinforcer on the basis of our data. Future studies should include assessments of these potential confounds.
We observed very significant age-related differences in the acquisition of the CSRTT task between the age groups. Numerous studies have demonstrated that aging humans also show deficits in the acquisition of a variety of skills. For example, when young and older subjects were compared on two probabilistic reward-based stimulus association learning tasks older subjects were found to show poorer acquisition of the task than younger subjects (Weiler and others 2008). Although procedural memory is not generally thought to be adversely affected by aging, there is evidence that older subjects show poorer skill consolidation than younger subjects based on performance at retest (Brown and others 2009). Similarly, there is evidence that older subjects acquire perceptual-motor skills more slowly than younger subjects (Rodrigue and others 2005). It has been demonstrated that older persons show slower acquisition and poorer asymptotic performance on a memory search task than younger subjects (Strayer and Kramer 1994). The neurobiological basis of these deficits in skill acquisition is not known with certainty. However, there is some evidence that both maximum performance and acquisition rate on PFC-dependent tasks are mediated by cellular architecture within the PFC. For example, a recent study examined the effects of aging on layer III pyramidal neurons in the dorsolateral PFC of rhesus monkeys (Dumitriu and others 2010). The authors determined that aging was associated with a loss of spines, especially small and thin spines, and a reduction in axospinous synapses. Synapse density and spine morphology were found to correlate with acquisition and performance on the delayed non-matching-to-sample test, a well established test of PFC performance.
As suggested by the principal components analysis, we observed parallel changes in performance on the CSRTT, open field, progressive ratio, and novel object tests with age. On the basis of our data, it is impossible to determine precisely which brain structures are contributing to these functional changes. The most likely model involves contributions from multiple brain regions. Here we will discuss some of the brain regions that are likely to contribute, in combination, to the observed cognitive and behavioral changes.
It is likely that age-related changes in the PFC are partially responsible for the observed cognitive and behavioral changes. The medial PFC in rodents has been found to mediate both motivation (Gourley and others 2010; Walton and others 2003) and performance on the CSRTT (Chudasama and others 2003; Passetti and others 2002). There is a growing literature on the mechanism of aging effects on gait speed in humans (Atkinson and others 2007; Holtzer and others 2006; Inzitari and others 2007). The PFC may also underlie age-related locomotor effects in the open field, as recent evidence from humans is consistent with an important role for PFC functioning in determining gait speed (Harada and others 2009; Mihara and others 2010). Although nearly all research conducted to date on gait in older persons has been conducted using laboratory-based tasks which typically involving walking in straight, predefined paths, some recent work has demonstrated that older persons take fewer steps than younger persons per unit time in their regular home environments (Harris and others 2009) and such measures of unstructured locomotion are analogous to the open field test (Choleris and others 2001; George and others 2010; Jahkel and others 2000). A recent study has implicated insufficient PFC activation in worry-related anxiety in the elderly (Andreescu and others 2011).
However, changes in the PFC are unlikely to be solely responsible. First, it is important to note that the functioning of the PFC depends heavily on other brain structures. For example, the PFC receives dopaminergic and GABAergic inputs from the ventral tegmental area (VTA) (Carr and Sesack 2000), and age-related changes in the VTA could therefore affect PFC function and could also affect motivation. Dopaminergic deficits, for example due to alterations in the substantia nigra which projects to the basal ganglia (Smith and Kieval 2000), could affect motor movements. In addition, the increased anxiety-like behavior that we observed in the aged animals on open field testing may be due in part to regions other than the PFC, including the locus coeruleus (Chiba and others 2009; Lacosta and others 1999) which provides significant noradrenergic inputs into the PFC. The infralimbic portion of the PFC plays an important role in suppressing amygdala activity (Quirk and others 2003; Vertes 2004) and therefore dysfunction of this circuit could cause an increase in anxiety-like behavior
Despite a likely role for the PFC in the observed cognitive and behavioral changes, we do not believe that age-related changes in this structure are solely responsible since aging affects numerous structures with a likely role in the tasks that we studied. Aging affects the hippocampus (Driscoll and Sutherland 2005; Lister and Barnes 2009), which also plays a role in CSRTT performance (Le Pen and others 2003). The hippocampus and other temporal lobe structures mediate at least some of the effects of age on object recognition (Baxter 2010; Burke and others 2010). Gait speed in humans is associated not only with PFC activity but also with activity of the supplementary motor area and medial sensorimotor cortex (Harada and others 2009), and the premotor cortex (Suzuki and others 2004). Age-related changes in the amygdala (Malykhin and others 2008; St Jacques and others 2010) could have played a role in the increased anxiety-like behavior we observed. Aging is also associated with a loss of neurons in the locus coeruleus (Lohr and Jeste 1988; Manaye and others 1995), a structure which sends noradrenergic projections to many brain regions, including the cortex and amygdala, which may have contributed to the observed behavioral and cognitive changes. Finally, aging is associated with changes in the striatum (Wang 2008), which would be expected to impact on the locomotor, motivational, and cognitive changes we observed.
The principal components analysis used here allowed us to determine the “dimensionality” of the behavioral and molecular data. Caution is required when inferring biological meaning from such statistical conclusions, and we cannot conclude that a single brain region, gene, or molecular pathway is responsible for the observed behavioral changes. However, the PCA analysis suggests that if multiple processes are involved, they are correlated. Therefore, a model positing that aging effects on the PFC cause these cognitive and behavioral changes is parsimonious and consistent with our data and the known function of the PFC, but will require further investigation to prove conclusively.
By examining changes in gene expression within the PFC of animals that were behaviorally characterized, we were able to identify age-dependent differences in gene expression, and test for association of these changes with performance on the behavioral tasks. Although causality cannot be established from these data, these analyses serve to synthesize a great deal of information and constrain models of brain aging to inform future studies. We report that immune-related genes within the PFC were significantly correlated with several behavioral measures, including days to criterion in the CSRTT, breakpoint in progressive ratio testing, average distance traveled in the open field, and average distance from the center of an open field. These results are consistent with a model in which age-related increases in immune signaling within the PFC are associated with reductions in both motivated responding and locomotion in Old-aged mice.
Immune gene upregulation, especially within the CNS, has been demonstrated to impair a variety of cognitive domains, including learning (Barrientos and others 2009; Barrientos and others 2006; Hein and others ; Terrando and others 2010), memory (Abraham and Johnson 2009; Frank and others ; Hirshler and others 2010; Wang and others 2009), and attention (Holden and others 2008). Administration of non-steroidal anti-inflammatory agents (e.g., celecoxib) has improved cognitive function in aging rodents (Trompet and others 2008) . While this study is the first, to our knowledge, to relate behavior with whole genome expression changes within the PFC of rodents, others have investigated PFC related behavior under inflammatory conditions. For example, rats given chronic ventricular administration of lipopolysaccharide (LPS), a potent activator of innate immunity, are significantly impaired in PFC dependent spatial working memory tasks (Rosi and others 2006). Similarly, there is evidence that age-related neuroimmune gene upregulation in humans is associated with cognitive decline (Simen and others 2011). The precise molecular mechanisms of such associations, however, have yet to be defined. Together with the existing literature, the data described here are consistent with a model where age-related neuroinflammatory changes lead to declines in prefrontal performance. One possible mechanism suggested by our datasets involves interferon-like signaling. An important function of interferons is to upregulate MHC Class I to enhance the presentation of peptides on the cell surface, effectively augmenting immune surveillance (Lampson and George 1986; Neumann and others 1997). Importantly, MHC Class I has recently been found to affect synaptic transmission (Shatz 2009) and could be, at least in part, responsible for some of the deficits in PFC function observed in older subjects. However, we cannot exclude an important role for other molecular mechanisms on the basis of our data as other classes of genes were also found to be dysregulated by aging.
With regard to our statistical association analyses, we observed a stronger relationship between measures of “central immune gene upregulation” and behavioral and cognitive indices than we did with measures of peripheral cytokine levels. There are a number of important caveats with regard to these analyses and any conclusions regarding which component is most important in relation to behavior and cognition. We employed different methods to assess cytokine levels in the periphery and gene expression in the CNS. Within the CNS, RNA expression was used to determine immune gene expression. In contrast, serum cytokine ELISAs were employed to assess peripheral cytokine expression. One would expect the protein-level quantification performed on peripheral tissue to be more biologically relevant than RNA expression. However, it is possible that temporal fluctuation in serum cytokine levels may have diminished the apparent impact of these factors on behavior and cognition (Lange and others 2010). Although there is evidence that peripheral inflammatory cytokines can induce inflammatory changes in the CNS, as reviewed above, CNS immune gene upregulation is likely to have additional determinants including age-dependent changes in microglial cells. Further research will be needed using alternative methods to assess our hypothesis that gene expression changes intrinsic to the CNS are the primary determinant of behavioral and cognitive changes with aging.
Given the evidence for dysregulation of immune processes within the PFC of the aging mouse, it is perhaps surprising that none of the prototypic proinflammatory cytokines (e.g., pro-IL-1b, IL-6, TNFa, INFg) were found to be differentially regulated by age. In the microarray analysis, RNAs for these factors fell below our expression cutoff, and focused analyses revealed no significant changes in the RNAs for these molecules. There is evidence to suggest that these cytokines can be elevated within areas such as the hippocampus (Blalock and others 2003) and cerebellum (Park and others 2009) of aging mice. However, cytokine expression in the brain is not uniform and may be differentially regulated in different brain regions (Simen and others 2011). Further research will be required to characterize the distribution of these factors in aged as compared to young brain.
Numerous publications have highlighted a possible neuroprotective role for microglia and a relative deficiency of microglial function in aging and neurodegenerative disease (Garden and Moller 2006; Lucin and Wyss-Coray 2009; Sawada and others 2008). We report an inverse relationship between immune-related gene expression in the PFC and various indices of behavior. Although we suspect that the increases in immune gene expression adversely affects behavior, it is possible that these changes in gene expression are the consequence of other gene expression changes that took place during the aging process, and that the immune-related changes are homeostatic responses or are in some other way secondary to other pathophysiological processes (Neumann and others 2009; Streit 2005; Streit and others 2008). Additional research will be needed to determine which of the upregulated immune genes that we observed have a neuroprotective role rather than a pathological role.
Despite the limitations of our study, as discussed above, this study has a number of unique strengths. This is the first detailed analysis of age-related gene expression changes in the medial prefrontal cortex of the rodent, including in Cd11b+ cells from this region, and the first attempt to relate such data to behavior and cognition that we are aware of. Given the importance of this brain region in age-related disability in humans such an analysis is of great importance. This is also the first demonstration that we are aware of that the aging rodent, like the aging human, shows highly correlated changes in cognition, motivation, and locomotor behavior, and highlights the suitability of the rodent as a model organism to study the mechanism of this phenomenon.
Overall, our results strongly suggest that age-related deficits in cognitive performance, motivation, and locomotion occur in healthy, aging mice, as they do in humans. These changes occur in parallel with dysregulation of immune gene expression within the medial prefrontal cortex of the aging brain. Further research will be required to establish additional brain regions involved in these changes. Understanding the biological mechanisms that link changes in cognition, motivation, and motor behavior during normal and pathological aging is important since these changes are associated with disability in humans (Granholm and others 2008). An adequate animal model is necessary for such progress to be made. Based on the data presented here, we believe that the aging mouse is a promising model for such mechanistic studies.
5 Conclusions
The series of experiments presented here are, to our knowledge, the first to show that aging in the mouse is associated with parallel declines in cognition, motivation, and motoric performance, and that such declines occur in conjunction with immune gene upregulation in the medial PFC. We believe that the aging mouse is a promising platform for future studies focused on the biological mechanisms responsible for the association in age-related changes in thinking, moving, and feeling in the elderly.
Supplementary Material
Supplementary table 1 Table of microarray results. The official gene symbol, Illumina probe ID, log2 transformed average expression estimates for Adult, Middle aged, and Old aged animals, and false discovery rate corrected P values (Q values) for the effects of Age, Cohort, and their interaction are shown, for each significant probe in our dataset (Q<0.1 for the effect of Age)
Supplementary table 2 Table of Cd11b+ RNA sequencing results. The official gene symbol, log2 fold change (corrected for library size), and false discovery rate corrected P values (Q values) for the effects of age, for each significant gene in our dataset (Q<.05 for the effect of Age).
Supplementary figure 1 - Cluster analysis of microarray results. Heat map showing results of hierarchical cluster analysis of array probes (rows) and animals (columns). Data were normalized to mean zero and standard deviation of one for each row and a Euclidian distance metric was used.
Research highlights.
Detailed cognitive, motor, and behavioral assessments as well as microarray analysis of tissue from the medial prefrontal cortex and serum cytokine analyses were performed.
Multivariate analysis of these data is consistent with association of the upregulation of inflammatory, and especially interferon regulated, genes in the prefrontal cortex and the behavioral and cognitive measures.
Next-generation RNA sequencing of Cd11+ cells shows enrichment of these genes in that specific cell population.
These results shed light on the mechanism by which aging causes parallel changes in cognition, motor performance, and affective behavior.
| Genes increased with aging | |||
|---|---|---|---|
| Cell cycle/growth/differentiation | Other genes | ||
| Anln | Ang | ||
| Ccnd1 | B230209E15Rik | ||
| Immune related genes | B230209E15Rik | ||
| Alox5ap | H2-K1 | Oasl2 | Cyp27a1 |
| B2m | H2-K2 | Psmb8 | ENSMUSG00074561 |
| C1qa | H2-Q8 | Serpina3n | Fbxo34 |
| C1qc | H2-T22 | Slamf9 | Gfap |
| C4a/b | Ifi27/Ifi27l2a | Slc11a1 | Gm4951 |
| Casp1 | Ifi27l1 | Slc11a1 | Hvcn1 |
| Ccl12 | Ifit3 | Stat1 | Isg15 |
| Ccl3 | Ifitm3 | Tlr13 | Ms4a6d |
| Ccl4 | Igtp | Tlr2 | Pisd-ps1 |
| Cd52 | Igtp | Tnfsf13b | Pisd-ps3 |
| Cd68 | Iigp2 | Trem2 | Plcg2 |
| Cd86 | Irf9 | Trim21 | Pmp22 |
| Cxcl16 | Lat2 | Pygl | |
| Cyba | Lgals3bp | Rab32 | |
| Fcer1g | Loh11cr2a | Rnf213 | |
| Fcgr4 | Ly6a | Samd9l | |
| Gbp3 | Lyz1 | Sat1 | |
| Gm10499 | Lyz2 | Skap2 | |
| H2-D1 | Oas1g | Slc15a3 | |
| Neuronal projection/motility/migration | Usp18 | ||
| Ntn5 | Xaf1 | ||
| Pcdhb14 | Transcription factors/regulation | ||
| Pcdhb3 | Srrt | ||
| Upk1b | Trim25 | ||
 IRG
IRG
 Non-IRG
Non-IRG
6 Acknowledgements
This work was supported by NIA grant P01 AG 030004-01A1, NIA/AFAR grant AG030970, a grant from the Yale Pepper Center (to A.S.), and NINDS grant 5U24NS051869 (to SM). KB and EG were supported by NIMH grant T32 MH014276. RK was supported by the UK EPSRC. The authors wish to acknowledge the support of the Yale Center for High Performance Computation in Biology and Biomedicine and NIH grant RR19895 which funded the computing instrumentation. The authors would also like to acknowledge the assistance of Kiran Patel, Yale University, for her excellent technical assistance, and Wendy VanScyoc, Ph.D., Meso-Scale Discovery, for her assistance with the cytokine assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12:445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Gross JJ, Lenze E, Edelman KD, Snyder S, Tanase C, Aizenstein H. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depress Anxiety. 2011;28:202–209. doi: 10.1002/da.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, Rapp SR, Cesari M, Newman AB, Harris TB, Rubin SM, Yaffe K, Satterfield S, Kritchevsky SB. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- Baena E, Allen PA, Kaut KP, Hall RJ. On age differences in prefrontal function: the importance of emotional/cognitive integration. Neuropsychologia. 2010;48:319–333. doi: 10.1016/j.neuropsychologia.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Baxter MG. “I’ve seen it all before”: explaining age-related impairments in object recognition. Theoretical comment on Burke et al. (2010) Behav Neurosci. 2010;124:706–709. doi: 10.1037/a0021029. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Novelty reward as a measure of anhedonia. Neurosci Biobehav Rev. 2005;29:707–714. doi: 10.1016/j.neubiorev.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodaty H, Altendorf A, Withall A, Sachdev P. Do people become more apathetic as they grow older? A longitudinal study in healthy individuals. Int Psychogeriatr. 2010;22:426–436. doi: 10.1017/S1041610209991335. [DOI] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied mixed models in medicine. J. Wiley & Sons; Chichester ; New York: 1999. [Google Scholar]
- Brown RM, Robertson EM, Press DZ. Sequence skill acquisition and off-line learning in normal aging. PLoS One. 2009;4:e6683. doi: 10.1371/journal.pone.0006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124:559–573. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Shanahan A, Damianopoulos EN, Muller CP, Huston JP. Effects on spontaneous and cocaine-induced behavior of pharmacological inhibition of noradrenergic and serotonergic systems. Pharmacol Biochem Behav. 2008;89:54–63. doi: 10.1016/j.pbb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Cavallin MA, Powell K, Biju KC, Fadool DA. State-dependent sculpting of olfactory sensory neurons is attributed to sensory enrichment, odor deprivation, and aging. Neurosci Lett. 2010;483:90–95. doi: 10.1016/j.neulet.2010.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman IM, MacIntosh CG, Morley JE, Horowitz M. The anorexia of ageing. Biogerontology. 2002;3:67–71. doi: 10.1023/a:1015211530695. [DOI] [PubMed] [Google Scholar]
- Chiba S, Matsuwaki T, Yamanouchi K, Nishihara M. Alteration in anxiety with relation to the volume of the locus ceruleus in progranulin-deficient mice. J Reprod Dev. 2009;55:518–522. doi: 10.1262/jrd.20239. [DOI] [PubMed] [Google Scholar]
- Cho CY, Gilchrist L, White S. A Comparison between young and old adults in their ability to rapidly sidestep during gait when attention is divided. Gerontology. 2008;54:120–127. doi: 10.1159/000118603. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- de Bruin ED, Schmidt A. Walking behaviour of healthy elderly: attention should be paid. Behav Brain Funct. 2010;6:59. doi: 10.1186/1744-9081-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Sutherland RJ. The aging hippocampus: navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci. 2010;11:123. doi: 10.1186/1471-2202-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008;33:1322–1334. doi: 10.1016/j.psyneuen.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol Psychiatry. 2008;64:884–890. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci. 2010;32:1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Boger H, Emborg ME. Mood, memory and movement: an age-related neurodegenerative complex? Curr Aging Sci. 2008;1:133–139. doi: 10.2174/1874609810801020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldin WO, Pritzel M, Markowitsch HJ. Prefrontal cortex of the mouse defined as cortical projection area of the thalamic mediodorsal nucleus. Brain Behav Evol. 1981;19:93–107. doi: 10.1159/000121636. [DOI] [PubMed] [Google Scholar]
- Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res. 2009;193:445–454. doi: 10.1007/s00221-008-1643-y. [DOI] [PubMed] [Google Scholar]
- Harati H, Majchrzak M, Cosquer B, Galani R, Kelche C, Cassel JC, Barbelivien A. Attention and memory in aged rats: Impact of lifelong environmental enrichment. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Owen CG, Victor CR, Adams R, Cook DG. What factors are associated with physical activity in older people, assessed objectively by accelerometry? Br J Sports Med. 2009;43:442–450. doi: 10.1136/bjsm.2008.048033. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O’Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsabeck RC, Anstead GM, Webb AL, Hoyumpa A, Ingmundson P, Holliday S, Zhang Q, Casas AM, Jovel M, Stern SL. Cognitive efficiency is associated with endogenous cytokine levels in patients with chronic hepatitis C. J Neuroimmunol. 2010;221:53–61. doi: 10.1016/j.jneuroim.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Hirshler YK, Polat U, Biegon A. Intracranial electrode implantation produces regional neuroinflammation and memory deficits in rats. Exp Neurol. 2010;222:42–50. doi: 10.1016/j.expneurol.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JM, Meyers-Manor JE, Overmier JB, Gahtan E, Sweeney W, Miller H. Lipopolysaccharide-induced immune activation impairs attention but has little effect on short-term working memory. Behav Brain Res. 2008;194:138–145. doi: 10.1016/j.bbr.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, Shorr R, Harris TB, Rosano C. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahkel M, Rilke O, Koch R, Oehler J. Open field locomotion and neurotransmission in mice evaluated by principal component factor analysis-effects of housing condition, individual activity disposition and psychotropic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:61–84. doi: 10.1016/s0278-5846(99)00081-0. [DOI] [PubMed] [Google Scholar]
- Kondo K, Suzukawa K, Sakamoto T, Watanabe K, Kanaya K, Ushio M, Yamaguchi T, Nibu K, Kaga K, Yamasoba T. Age-related changes in cell dynamics of the postnatal mouse olfactory neuroepithelium: cell proliferation, neuronal differentiation, and cell death. J Comp Neurol. 2010;518:1962–1975. doi: 10.1002/cne.22316. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Howell JL, Hebert BF, Olausson P, Taylor JR, Nairn AC. Assessment of cognitive function in the heterozygous reeler mouse. Psychopharmacology (Berl) 2006;189:95–104. doi: 10.1007/s00213-006-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzis G, Sabe L, Tiberti C, Dorrego F, Starkstein SE. Neuropsychological correlates of apathy and depression in patients with dementia. Neurology. 1999;52:1403–1407. doi: 10.1212/wnl.52.7.1403. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z.; Anisman, H. Behavioral and neurochemical consequences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res. 1999;818:291–303. doi: 10.1016/s0006-8993(98)01288-8. [DOI] [PubMed] [Google Scholar]
- Lampson LA, George DL. Interferon-mediated induction of class I MHC products in human neuronal cell lines: analysis of HLA and beta 2-m RNA, and HLA-A and HLA-B proteins and polymorphic specificities. J Interferon Res. 1986;6:257–265. doi: 10.1089/jir.1986.6.257. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Grottick AJ, Higgins GA, Moreau JL. Phencyclidine exacerbates attentional deficits in a neurodevelopmental rat model of schizophrenia. Neuropsychopharmacology. 2003;28:1799–1809. doi: 10.1038/sj.npp.1300208. [DOI] [PubMed] [Google Scholar]
- Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, Paulsen JS, Litvan I. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10:314–319. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- Lipkind D, Sakov A, Kafkafi N, Elmer GI, Benjamini Y, Golani I. New replicable anxiety-related measures of wall vs center behavior of mice in the open field. J Appl Physiol. 2004;97:347–359. doi: 10.1152/japplphysiol.00148.2004. [DOI] [PubMed] [Google Scholar]
- Lister JP, Barnes CA. Neurobiological changes in the hippocampus during normative aging. Arch Neurol. 2009;66:829–833. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- Lohr JB, Jeste DV. Locus ceruleus morphometry in aging and schizophrenia. Acta Psychiatr Scand. 1988;77:689–697. doi: 10.1111/j.1600-0447.1988.tb05189.x. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson SE, Phillips LH, Della Sala S. Age, executive function, and social decision making: a dorsolateral prefrontal theory of cognitive aging. Psychol Aging. 2002;17:598–609. [PubMed] [Google Scholar]
- Malykhin NV, Bouchard TP, Camicioli R, Coupland NJ. Aging hippocampus and amygdala. Neuroreport. 2008;19:543–547. doi: 10.1097/WNR.0b013e3282f8b18c. [DOI] [PubMed] [Google Scholar]
- Manaye KF, McIntire DD, Mann DM, German DC. Locus coeruleus cell loss in the aging human brain: a non-random process. J Comp Neurol. 1995;358:79–87. doi: 10.1002/cne.903580105. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Mihara M, Yagura H, Hatakenaka M, Hattori N, Miyai I. [Clinical application of functional near-infrared spectroscopy in rehabilitation medicine] Brain Nerve. 2010;62:125–132. [PubMed] [Google Scholar]
- Morrison DF. Multivariate statistical methods. Thomson/Brooks/Cole; Belmont, CA: 2005. [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyike CU, Sheppard JM, Tschanz JT, Norton MC, Green RC, Steinberg M, Welsh-Bohmer KA, Breitner JC, Lyketsos CG. Epidemiology of apathy in older adults: the Cache County Study. Am J Geriatr Psychiatry. 2007;15:365–375. doi: 10.1097/01.JGP.0000235689.42910.0d. [DOI] [PubMed] [Google Scholar]
- Park SK, Kim K, Page GP, Allison DB, Weindruch R, Prolla TA. Gene expression profiling of aging in multiple mouse strains: identification of aging biomarkers and impact of dietary antioxidants. Aging Cell. 2009;8:484–495. doi: 10.1111/j.1474-9726.2009.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. Springer; New York: 2000. [Google Scholar]
- Pinto LH, Enroth-Cugell C. Tests of the mouse visual system. Mamm Genome. 2000;11:531–536. doi: 10.1007/s003350010102. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Prolla TA. DNA microarray analysis of the aging brain. Chem Senses. 2002;27:299–306. doi: 10.1093/chemse/27.3.299. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Rumley A, Lowe GD, Fowkes FG. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J Am Geriatr Soc. 2007;55:700–707. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Richard MB, Taylor SR, Greer CA. Age-induced disruption of selective olfactory bulb synaptic circuits. Proc Natl Acad Sci U S A. 2010;107:15613–15618. doi: 10.1073/pnas.1007931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios O, Villalobos J. Postnatal development of the afferent projections from the dorsomedial thalamic nucleus to the frontal cortex in mice. Brain Res Dev Brain Res. 2004;150:47–50. doi: 10.1016/j.devbrainres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Raz N. Aging and longitudinal change in perceptual-motor skill acquisition in healthy adults. J Gerontol B Psychol Sci Soc Sci. 2005;60:P174–181. doi: 10.1093/geronb/60.4.p174. [DOI] [PubMed] [Google Scholar]
- Roider HG, Kanhere A, Manke T, Vingron M. Predicting transcription factor affinities to DNA from a biophysical model. Bioinformatics. 2007;23:134–141. doi: 10.1093/bioinformatics/btl565. [DOI] [PubMed] [Google Scholar]
- Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. 2006;142:1303–1315. doi: 10.1016/j.neuroscience.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Rothenburg LS, Herrmann N, Swardfager W, Black SE, Tennen G, Kiss A, Gladstone DJ, Ween J, Snaiderman A, Lanctot KL. The Relationship Between Inflammatory Markers and Post Stroke Cognitive Impairment. J Geriatr Psychiatry Neurol; 2010 doi: 10.1177/0891988710373598. [DOI] [PubMed] [Google Scholar]
- Rushworth MF. Intention, choice, and the medial frontal cortex. Ann N Y Acad Sci. 2008;1124:181–207. doi: 10.1196/annals.1440.014. [DOI] [PubMed] [Google Scholar]
- Sawada M, Sawada H, Nagatsu T. Effects of aging on neuroprotective and neurotoxic properties of microglia in neurodegenerative diseases. Neurodegener Dis. 2008;5:254–256. doi: 10.1159/000113717. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen AA, Bordner KA, Martin MP, Moy L, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Therapeutic advances in chronic disease. 2011 doi: 10.1177/2040622311399145. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen AA, DiLeone R, Arnsten AF. Primate models of schizophrenia: future possibilities. Prog Brain Res. 2009;179:117–125. doi: 10.1016/S0079-6123(09)17913-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Kieval JZ. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 2000;23:S28–33. doi: 10.1016/s1471-1931(00)00023-9. [DOI] [PubMed] [Google Scholar]
- St Jacques P, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: a network analysis of fMRI data. Neurobiol Aging. 2010;31:315–327. doi: 10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey J. A direct approach to false discovery rates. Journal of the Royal Statistical Society. 2002;64:479–498. [Google Scholar]
- Strayer DL, Kramer AF. Aging and skill acquisition: learning-performance distinctions. Psychol Aging. 1994;9:589–605. doi: 10.1037//0882-7974.9.4.589. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Brain Res Rev. 2005;48:234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Miller KR, Lopes KO, Njie E. Microglial degeneration in the aging brain--bad news for neurons? Front Biosci. 2008;13:3423–3438. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Miyai I, Ono T, Oda I, Konishi I, Kochiyama T, Kubota K. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. Neuroimage. 2004;23:1020–1026. doi: 10.1016/j.neuroimage.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Terrando N, Rei Fidalgo A, Vizcaychipi M, Cibelli M, Ma D, Monaco C, Feldmann M, Maze M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit Care. 2010;14:R88. doi: 10.1186/cc9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurol Res. 2005;27:679–684. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- Trompet S, de Craen AJ, Slagboom P, Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Ford I, Gaw A, Macfarlane PW, Packard CJ, Stott DJ, Jukema JW, Westendorp RG. Genetic variation in the interleukin-1 beta-converting enzyme associates with cognitive function. The PROSPER study. Brain. 2008;131:1069–1077. doi: 10.1093/brain/awn023. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–6479. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–11003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PE, Rushworth MF. Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Netw. 2006;19:1302–1314. doi: 10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Rudebeck PH, Bannerman DM, Rushworth MF. Calculating the cost of acting in frontal cortex. Ann N Y Acad Sci. 2007;1104:340–356. doi: 10.1196/annals.1390.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Feng Z, Wang X, Zhang X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- Wang WF, Wu SL, Liou YM, Wang AL, Pawlak CR, Ho YJ. MPTP lesion causes neuroinflammation and deficits in object recognition in Wistar rats. Behav Neurosci. 2009;123:1261–1270. doi: 10.1037/a0017401. [DOI] [PubMed] [Google Scholar]
- Wang Y. Differential effect of aging on synaptic plasticity in the ventral and dorsal striatum. Neurobiol Learn Mem. 2008;89:70–75. doi: 10.1016/j.nlm.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Bellebaum C, Daum I. Aging affects acquisition and reversal of reward-based associative learning. Learn Mem. 2008;15:190–197. doi: 10.1101/lm.890408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolden-Hanson T, Marck BT, Matsumoto AM. Troglitazone treatment of aging Brown Norway rats improves food intake and weight gain after fasting without increasing hypothalamic NPY gene expression. Exp Gerontol. 2002;37:679–691. doi: 10.1016/s0531-5565(01)00233-9. [DOI] [PubMed] [Google Scholar]
- Zueger M, Urani A, Chourbaji S, Zacher C, Roche M, Harkin A, Gass P. Olfactory bulbectomy in mice induces alterations in exploratory behavior. Neurosci Lett. 2005;374:142–146. doi: 10.1016/j.neulet.2004.10.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1 Table of microarray results. The official gene symbol, Illumina probe ID, log2 transformed average expression estimates for Adult, Middle aged, and Old aged animals, and false discovery rate corrected P values (Q values) for the effects of Age, Cohort, and their interaction are shown, for each significant probe in our dataset (Q<0.1 for the effect of Age)
Supplementary table 2 Table of Cd11b+ RNA sequencing results. The official gene symbol, log2 fold change (corrected for library size), and false discovery rate corrected P values (Q values) for the effects of age, for each significant gene in our dataset (Q<.05 for the effect of Age).
Supplementary figure 1 - Cluster analysis of microarray results. Heat map showing results of hierarchical cluster analysis of array probes (rows) and animals (columns). Data were normalized to mean zero and standard deviation of one for each row and a Euclidian distance metric was used.