Abstract
Studying empirically derived dietary patterns is useful in understanding dietary practice. We classified women by their dietary patterns using latent class analysis of 66 foods and studied the association of these patterns with neural tube defects (NTDs) and congenital heart defects (CHDs) in the US National Birth Defects Prevention Study (1997–2005). Logistic regression models used data from 1,047 with an NTD, 6,641 with a CHD, and 6,123 controls that were adjusted for maternal characteristics and tested the effect modification of multivitamin supplement use. Four latent dietary patterns were identified: prudent, Western, low-calorie Western, and Mexican. Among participants who did not use supplements, those in the Mexican, Western, and low-calorie Western classes were significantly more likely (odds ratios of 1.6, 1.5, and 1.4, respectively) to have offspring born with NTDs than were those in the prudent class after adjustment of for dietary folic acid intake. In contrast, among supplement users, there was no difference in the incidence of NTDs between classes. Associations between dietary class and CHD subgroups were not modified by supplement use except for tetralogy of Fallot; among supplement users, those in the Western class were twice as likely (95% confidence interval: 1.4, 2.8) as the prudent class to have offspring with tetralogy of Fallot. Women who adhered to a Western diet were 1.2 (95% confidence interval: 1.03, 1.35) times more likely to have an infant with septal heart defect than were women who adhered to a prudent diet. A prudent dietary pattern, even with folate fortification, may decrease the risk of NTDs and some heart defects.
Keywords: congenital anomalies, diet, eating patterns, folic acid, latent class analysis
The infant mortality rate in the United States remains at a troublesome 6.7 per 1,000 babies (1). Approximately half of all infant deaths can be explained by disorders related to shortened gestation, congenital anomalies, respiratory distress syndrome, and sudden infant death syndrome. Major birth defects are the leading cause of infant death and account for more than 20% of all infant deaths (1), with congenital heart defects (CHDs) being one of the most deadly (2). On the other hand, neural tube defects (NTDs) are among the most common birth defects, and maternal nutrition plays a key role in their complex causes (3, 4). Understanding the etiology of CHDs and NTDs may lead to a decrease in associated morbidity and mortality and is a global priority. Modifiable factors that may be associated with NTDs and CHDs include pregravid weight status, maternal diet, and use of medications, alcohol, tobacco, and vitamin/mineral supplements. The most successful intervention that has evolved from this field of study has been supplementation with folic acid to prevent NTDs (5). However, recent studies have implicated that other B vitamins also contribute to the methylation pathway (6, 7). Although it is important to tease out which nutrients, if any, contribute to the development of these birth defects, humans eat foods that contain many nutrients and nonnutrients, and thus identifying dietary patterns may also aid in prevention.
The dominating epidemiologic approach of examining the impact of single nutrients or foods on health does not account for the complexity of the relation between dietary intake and health outcomes (8). The high degree of intercorrelation among nutrients or foods makes it difficult to attribute effects to single independent components. Separation of effects using statistical adjustment is hard to accomplish because high correlations between individual nutrients and among groups of nutrients result in unstable models. Creating dietary patterns that inherently account for interactions between single micronutrients and estimate overall dietary effects may provide a more robust method for determining associations. Diet quality indexes, which also capture the complexity of the diet, use prevailing hypotheses and guidance from current dietary recommendations (9), whereas empirically derived dietary patterns are data-driven (10).
To date, there have been 5 studies in which investigators analyzed the association between dietary patterns and birth defects, specifically on CHDs (11), spina bifida (12, 13), cleft lip (13, 14), and hypospadias (15). In each of these studies, healthy dietary patterns were associated with reductions in the risk of birth defects. Whereas Carmichael et al. (13) used a diet quality index to a priori derive the dietary patterns, the rest used statistical methods to empirically derive the patterns (3 used principal component analysis and 1 used cluster analysis). Like cluster analysis, latent class analysis (LCA) (16) can be used to classify individuals into mutually exclusive dietary patterns such that variation in diet is maximized across different dietary patterns while individuals within dietary patterns have similar food intakes (17–19). Unlike other methods, LCA allows adjustment for covariates, quantification of the uncertainty of class membership, and assessment of goodness of fit.
The objective of our analysis was to derive dietary patterns in the National Birth Defects Prevention Study (NBDPS) by conducting a LCA of 64 foods and drinks and then to examine the association between these dietary classes and NTDs and CHDs.
MATERIALS AND METHODS
The NBDPS is an ongoing multistate population-based case-control study of birth defects in the United States (20). Briefly, study populations from 7 of 10 states participating in the NBDPS included live-born, stillborn, prenatally diagnosed, and electively terminated cases (Arkansas, California, Georgia, Iowa, North Carolina, Texas, and Utah), 1 state included only live-born and stillborn cases (Massachusetts), and 2 states included only live-born cases (New Jersey and New York). Diagnostic case information was obtained from hospital and medical records and entered into a standardized database for clinician review and classification. Control infants were not matched with cases; they were live-born infants without birth defects who were randomly selected from either birth certificates or hospital records, depending on the study center. Mothers were interviewed by telephone in either English or Spanish using a computer-based questionnaire 6 weeks to 24 months after the estimated date of delivery to obtain information on maternal demographic characteristics and many different exposures, such as nutritional and behavioral characteristic, both before and during pregnancy. The NBDPS was approved by the institutional review boards of the Centers of Disease Control and Prevention and the participating study centers.
We used the NBDPS analytic data file version 7.04, which included women with expected due dates from 1997 to 2005. Included in this population were 1,223 cases with nonsyndromic, isolated, or multiple NTDs, 8,091 CHD cases, and 6,807 nonmalformed controls. We excluded mothers who had multiple births (646 cases and 213 controls), had preexisting diabetes (type I or II; 321 cases and 57 controls), used folate antagonist medications (phenytoin, valproic acid, valproate sodium, carbamazepine, methotrexate, trimethoprim hydrocholoride, trimethoprim sulfate, trimethoprim, aminopterin sodium, phenobarbital, phenobarbital sodium, primidone, divalproex, and sodium) from the time period 3 months before pregnancy to the end of pregnancy (357 cases and 197 controls), reported extreme values in total calorie intake (<500 or >5,000 kcal), missed more than 1 item in the food frequency questionnaire (206 cases and 128 controls), or lacked data on folic acid/multivitamin supplement use (210 cases and 134 controls). After exclusions, analyses include 1,047 cases with nonsyndromic, isolated, or multiple NTDs, 6,641 CHD cases, and 6,123 nonmalformed controls. In particular, we studied 4 common types of CHD (21): septal defects, left ventricular outflow tract obstructive defects, right ventricular outflow tract obstructive defects, and conotruncal defects (dextrotransposition of the great arteries and tetralogy of Fallot, separately). Folic acid/multivitamin supplement use was defined as multivitamin, prenatal vitamin, or single-component vitamin use for 30 days or more from 3 months before pregnancy to the first month of pregnancy.
In NBDPS, dietary intake was assessed using a modified 58-item Willet food frequency questionnaire (22) administered during the interview that refers to the year before pregnancy. In total, 16 response categories for frequency of consumption were possible, ranging from never or less than once a month to 6 or more times per day. In addition, intakes of cereals, soft drinks, coffee, and tea were assessed using separate detailed questions. We classified 166 cereals as being highly folic acid–fortified cereals (100% of the daily recommended value per serving) or not and 32 soft drinks as being low-calorie or not. Grams per day of foods consumed were calculated by multiplying the frequency of consumption by the standard portion size listed on the questionnaire for each food item. Nutrient estimates were determined using the US Department of Agriculture National Nutrient Database, version 20 (23). Neither folic acid/multivitamin supplements nor food supplements were included in the calculated nutrient variables.
LCA (16, 18) for categorical outcomes and covariates was used to estimate 2 sets of parameters: regression coefficients predicting class membership (e.g., energy intake and dietary class) and conditional probabilities of the observed indicators given the class (e.g., the probability of consumption of fortified cereal among women adhering to the prudent diet). These 2 sets of probabilities were then used, via Bayes' theorem, to predict for each subject the probability of belonging to each class given her dietary data and covariates. Finally, subjects were classified to the class for which they had the highest predicted probability of class membership.
We used LCA to derive dietary patterns of 64 food items (each relative to total consumption), adjusting for energy intake (kcal/day) and using dietary data only from controls. In particular, we determined the number of classes and estimated both the regression coefficient of energy intake that predicted class membership and the conditional probabilities of food intake given the dietary pattern. Then, using these estimates, we predicted the posterior probabilities of class membership for not only controls but also cases. Intake of each food (g/day) was divided by the total grams per day of food consumed to quantify the amount consumed relative to the total. Hence, for a fixed energy intake of 2,000 kcal, we were able to differentiate between 2 subjects who consumed 250 g/day of French fries but with different total overall food intakes per day, for instance, 1,000 and 2,000 g/day. Because this derived variable had the typical spike at 0 for nonconsumers and was constrained between 0 and 1, we categorized the relative food item consumption into 4 levels: no consumption and tertiles of nonzero consumption (calculated among control consumers). Foods that were rarely consumed (chicken liver and beef tongue) or were consumed by less than 20% of the women (low-calorie soft drinks and cereals fortified with high levels of folic acid) were treated as binary variables (consumed or not). Foods that were consumed almost ubiquitously such as cheese, chicken, potatoes, and spaghetti did not have a nonconsumption category. We interpreted and named the dietary classes based on the conditional food intake probabilities. The number of classes was determined based on the Lo-Mendel-Rubin likelihood ratio test and the Bayesian information criteria.
Statistical analysis
We compared the nutrient intake geometric means among classes by adjusting for energy intake and sociodemographic and health behavior characteristics. We measured the association between maternal dietary class and birth defects using adjusted odds ratios estimated from logistic regression models that included dietary class as a nominal predictor and were adjusted for folic acid intake, maternal age, race/ethnicity, educational level, smoking status, and pregravid body mass index (BMI). We tested the effect measure modification of folic acid/multivitamin supplement use. We conducted a sensitivity analysis restricted to live-born children because 3 states did not ascertain information on elective terminations and there could be selection bias for terminated cases with regard to dietary intake measured retrospectively and administered after termination. Finally, to assess the impact of classification quality, we excluded women with low predicted probabilities of class membership (<(K − 1)/K in LCA with K classes) for their assigned dietary class. We classified women into the following categories of BMI (measured as weight in kilograms divided by height in meters squared) using the National Institutes of Health definitions: underweight (<18.5), normal (18.5–24.9), overweight (25–29.9), and obese (≥30). Statistical analyses were performed using SAS/STAT software, version 9.2, of the SAS System for Windows (24) and the procedure PROC LCA, version 1.2.7 alpha (25).
RESULTS
The maternal race/ethnicity distribution was very similar for CHD cases and controls; 60% were white, 11% were black, and 23% were Hispanic (Table 1). In contrast, among mothers of infants with an NTD, 50% were white and 34% were Hispanic. Maternal age distribution, periconceptional smoking, and drinking were not different between mothers of cases and controls. Mothers of infants with NTDs were significantly more likely to be less educated (P < 0.001) and to be more obese than controls. One third of women reported folic acid/multivitamin use, with a significant (P = 0.033) but modest lower prevalence for mothers of CHD cases compared with controls.
Table 1.
Maternal Characteristics of Cases and Controls, National Birth Defects Prevention Study, 1997–2005
| Participants With Offspring With an NTD (n = 1,047a) |
Participants With Offspring With a CHD (n = 6,641a) |
Controls (n = 6,123) |
||||
|---|---|---|---|---|---|---|
| Variable | No.b | % | No.b | % | No.b | % |
| Race/ethnicity | ||||||
| Non-Hispanic white | 522 | 50.00 | 3,905 | 58.96 | 3,658 | 60.00 |
| Non-Hispanic black | 98 | 9.39 | 735 | 11.10 | 687 | 11.27 |
| Hispanic | 358 | 34.29 | 1,565 | 23.63 | 1,372 | 22.50 |
| Other | 66 | 6.32 | 418 | 6.31 | 380 | 6.23 |
| Age, years | ||||||
| <25 | 362 | 34.57 | 2,167 | 32.64 | 2,048 | 33.45 |
| 25–35 | 548 | 52.34 | 3,475 | 52.33 | 3,239 | 52.90 |
| >35 | 137 | 13.09 | 998 | 15.03 | 836 | 13.65 |
| Years of education | ||||||
| 0–12 | 518 | 49.57 | 2,913 | 43.99 | 2,520 | 41.28 |
| >12 | 527 | 50.43 | 3,709 | 56.01 | 3,584 | 58.72 |
| Body mass indexc category | ||||||
| Underweight (<18.5) | 44 | 4.47 | 349 | 5.49 | 325 | 5.53 |
| Normal (18.5– 24.9) | 497 | 50.51 | 3,280 | 51.56 | 3,305 | 56.21 |
| Overweight (25– 29.9) | 222 | 22.56 | 1,521 | 23.91 | 1,313 | 22.33 |
| Obese (≥30) | 221 | 22.46 | 1,212 | 19.05 | 937 | 15.94 |
| Smokerd | 171 | 16.36 | 1,327 | 20.01 | 1,146 | 18.73 |
| Periconceptional drinkingd | 335 | 32.18 | 2,332 | 35.28 | 2,251 | 36.92 |
| Folic acid/ multivitamin supplement usee | 321 | 30.66 | 2,033 | 30.61 | 2,023 | 33.04 |
Abbreviations: CHD, congenital heart defect; NTD, neural tube defect.
a Twenty-eight cases had a child with both a neural tube defect and a congenital heart defect.
b Some frequencies do not add to the total sample size because of missing values.
c Weight (kg)/height (m)2.
d Assessed during the period of 1 month before pregnancy to the third month of pregnancy.
e Assessed during the period of 3 months before pregnancy to the first month of pregnancy.
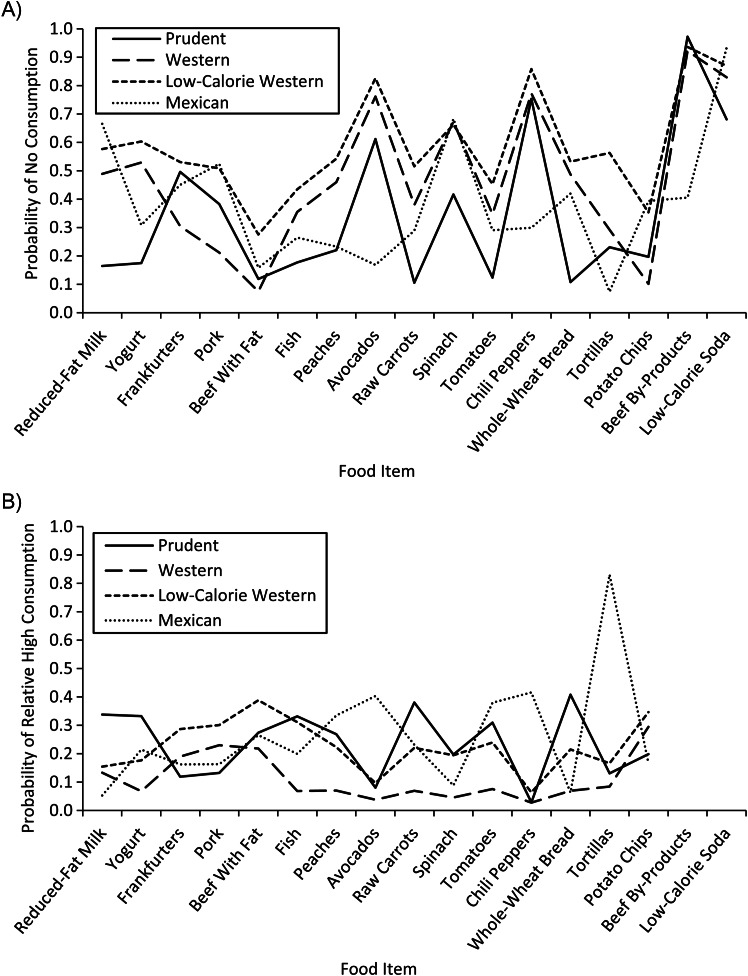
We fitted latent class models with 2–5 classes and chose 4 dietary pattern classes based on the Bayesian information criteria and the Lo-Mendel-Rubin likelihood ratio test. We named the classes prudent, Western, low-calorie Western, and Mexican. Women in the prudent class had the lowest probabilities of not consuming fruits and vegetables and had higher probabilities of having high intakes of healthy foods such as yogurt, reduced-fat milk, whole-wheat bread, fortified cereal, and fish (Figure 1; data for all foods are shown in Web Figure 1, available online at http://aje.oxfordjournals.org/). Women who were in the Mexican dietary class were more likely to consume salsa, chili peppers, avocados, refried beans, tortillas, and chicken or beef by-products (liver, tongue). Similar to women in the prudent class, women in the Mexican class were also more likely to consume fruits and vegetables. There were 2 classes of Western diet, and both were characterized by high rates of consumption of frankfurters, bacon, French fries, white bread, potato chips, and regular soda and low rates of consumption of fruits and vegetables. The difference between these 2 Western classes was the amount of energy (kilocalories) and food (grams) consumed. On average, women in the low-calorie Western class consumed 273 fewer calories than did women in the Western class, and their average intakes of energy and food were similar (1,409 kcal and 1,544 g/day, respectively) (Table 2). In contrast, women in the Western class consumed 50% more grams per day of food than kilocalories of energy (1,682 kcal and 2,484 g/day). Further, women in the low-calorie Western class had higher extreme probabilities (i.e., higher levels of no consumption and higher amounts consumed relative to the total consumption) compared with women in the other Western class for most foods. For example, the percent of women with no fish consumption was 43.3% for women in the low-calorie Western group compared with 35.6% in the Western class, but the percentages of women with relatively high fish consumption were 31.2% and 6.8%, respectively. Also, women in the low-calorie Western class had much higher proportions (relative to their total food intake) of chicken, potato (baked, boiled, or mashed), and rice/pasta intakes. Class membership probability depended on energy intake (P < 0.0001); the higher the energy intake, the higher the probability of belonging to the Mexican class and the lower the probability of belonging to the prudent or low-calorie Western class. Overall, 32% of control women were in the prudent class, 26% were in each Western class, and 15% were in the Mexican class.
Figure 1.
Food-item consumption level probabilities by dietary pattern class, National Birth Defects Prevention Study, 1997–2005. A) Probability of no consumption conditional on dietary pattern class. B) Probability of relative high (third tertile) consumption conditional on dietary pattern class. Beef by-products and low-calorie soda are not displayed in B because there was no category for relative high consumption; because there were few participants who consumed these products, they were treated as binary variables (consumed vs. not).
Table 2.
Mean Daily Nutrient Intakea by Dietary Pattern Class for Controls, National Birth Defects Prevention Study, 1997–2005
| Intake by Dietary Pattern Class |
||||
|---|---|---|---|---|
| Nutrient | Prudent (n = 1,989) | Western (n = 1,617) | Low-Calorie Western (n = 1,585) | Mexican (n = 932) |
| Energyb, kcal | 1,462.2 (494) | 1,682.0 (730) | 1,409.5 (622) | 2,043.9 (786) |
| Grams of food per dayb | 1,768.2 (652) | 2,484.7 (1,061) | 1,544.1 (778) | 2,470.7 (998) |
| Calcium, mg | 860.71 | 633.83 | 623.01 | 678.18 |
| Caffeine, g | 33.63 | 109.08 | 14.19 | 29.59 |
| α-carotene, μg RE | 581.39 | 180.34 | 200.08 | 468.66 |
| β-carotene, μg RE | 2,871.27 | 1,112.76 | 1,487.02 | 2,443.99 |
| Carbohydrate (by difference), g | 202.01 | 209.16 | 194.61 | 214.56 |
| Cholesterol, mg | 218.15 | 203.11 | 220.43 | 218.51 |
| Total choline, g | 272.61 | 224.67 | 248.90 | 288.68 |
| Total monounsaturated fatty acids, g | 17.17 | 16.53 | 16.69 | 16.36 |
| Total polyunsaturated fatty acids, g | 5.67 | 4.74 | 5.22 | 5.01 |
| Total saturated fatty acids, g | 19.99 | 19.01 | 18.56 | 16.41 |
| Total lipids (fat), g | 47.16 | 44.74 | 45.28 | 42.56 |
| Total trans fats, g | 0.05 | 0.12 | 0.04 | 0.07 |
| Iron, mg | 12.86 | 11.59 | 12.12 | 13.28 |
| Total dietary fiber, g | 17.24 | 11.35 | 14.48 | 24.67 |
| Folic acid, μg | 154.62 | 130.76 | 138.34 | 108.08 |
| Natural folate, μg | 240.05 | 170.92 | 201.27 | 261.63 |
| Folate, μg DFE | 543.14 | 430.70 | 489.14 | 493.82 |
| Protein, g | 70.74 | 58.39 | 63.49 | 65.16 |
| Retinol, μg | 386.46 | 297.52 | 301.45 | 352.24 |
| Vitamin E, mg α-TE | 4.97 | 3.26 | 3.87 | 5.11 |
| Vitamin A, IU | 7,181.84 | 3,608.13 | 4,547.25 | 6,426.68 |
| Vitamin A, μg RAE | 712.65 | 450.42 | 515.44 | 655.13 |
| Vitamin B12, μg | 5.37 | 4.66 | 4.78 | 5.62 |
| Vitamin B6, mg | 2.06 | 1.66 | 1.88 | 2.16 |
| Vitamin C (ascorbic acid), mg | 110.09 | 69.66 | 91.89 | 132.83 |
| Vitamin D, IU | 123.13 | 97.70 | 83.98 | 91.36 |
| Vitamin K (phylloquinone), μg | 80.14 | 36.36 | 51.46 | 63.86 |
| Zinc, mg | 11.44 | 10.19 | 10.45 | 10.73 |
Abbreviations: DFE, dietary folate equivalents; IU, international units; RAE, retinol activity equivalents; RE, retinol equivalent; TE, tocopherol equivalent.
a Values for energy and grams of food per day are presented as arithmetic means, and values for all nutrients are presented as geometric means adjusted for energy intake.
b Values are expressed as mean (standard deviation).
The average energy intake of women in the Mexican class was 582 kcal, 360 kcal, and 634 kcal higher than that of women in the prudent, Western, and low-calorie Western classes, respectively (Table 2). Women in the prudent class had the highest means for almost all micronutrients except choline, iron, and vitamins E, B-6, and B-12, for which women in the Mexican class had the highest intake. In contrast, the women in the Western class had the lowest mean intakes of all micronutrients and the highest mean intakes of trans fat and caffeine. Women in the Mexican dietary class had the highest mean intakes of carbohydrates and fiber and the lowest intakes of saturated fat, total fat, and folic acid.
Associations between maternal characteristics and dietary class were very similar for cases and controls (data not shown). Among controls, most women in the prudent and Western classes were white (81.1% and 71.9%, respectively), whereas in the Mexican class, 87.5% were Hispanic (Table 3). The low-calorie Western class was the most diverse in terms of race/ethnicity, with 50% being white, 22.1% being black, and 15.7% being Hispanic. Eighty-six percent of the women in the prudent class had 12 or more years of education, compared with 21.5% of the women in the Mexican class and half in the Western classes. The percentage of obese women was almost 2-fold higher in the Western and Mexican classes than in the prudent class, which had an obesity rate of 11.8%. The percentage of gestational diabetes in the Mexican class was 6.9%, which was twice the percentage in the other dietary classes. The Western class had the highest percentage of smokers (from 1 month before pregnancy to 3 months into pregnancy) at 34.8%, and the Mexican class had the lowest percentage at 6.7%. In total, 81% of the women in the Mexican class did not report drinking alcohol, and 6.4% reported no binge drinking. In contrast, 11% of women in the prudent and low-calorie Western classes reported binge drinking and 18.5% of women in the Western class reported binge drinking. In total, 72% of women in the prudent class became pregnant intentionally, opposed to 50% of the women in all other dietary classes.
Table 3.
Association of Maternal Characteristics and Dietary Pattern Class for Controls, National Birth Defects Prevention Study, 1997–2005
| Dietary Pattern Class |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prudent (n = 1,989) |
Western (n = 1,617) |
Low-Calorie Western (n = 1,585) |
Mexican (n = 932) |
Overall (n = 6,123) |
||||||
| Variable | No.a | % | No.a | % | No.a | % | No.a | % | No.a | % |
| Race/ethnicity | ||||||||||
| Non-Hispanic white | 1,627 | 82.13 | 1,160 | 71.96 | 830 | 52.66 | 41 | 4.42 | 3,658 | 60.00 |
| Non-Hispanic black | 124 | 6.26 | 209 | 12.97 | 345 | 21.89 | 9 | 0.97 | 687 | 11.27 |
| Hispanic | 111 | 5.60 | 173 | 10.73 | 276 | 17.51 | 812 | 87.50 | 1,372 | 22.50 |
| Other | 119 | 6.01 | 70 | 4.34 | 125 | 7.93 | 66 | 7.11 | 380 | 6.23 |
| Age, years | ||||||||||
| <25 | 281 | 14.13 | 700 | 43.29 | 643 | 40.57 | 424 | 45.49 | 2,048 | 33.45 |
| 25–35 | 1,282 | 64.45 | 783 | 48.42 | 735 | 46.37 | 439 | 47.10 | 3,239 | 52.90 |
| >35 | 426 | 21.42 | 134 | 8.29 | 207 | 13.06 | 69 | 7.40 | 836 | 13.65 |
| Years of education | ||||||||||
| 0–12 | 283 | 14.24 | 770 | 47.71 | 741 | 46.96 | 726 | 78.49 | 2,520 | 41.28 |
| >12 | 1,704 | 85.76 | 844 | 52.29 | 837 | 53.04 | 199 | 21.51 | 3,584 | 58.72 |
| Body mass indexb category | ||||||||||
| Underweight (<18.5) | 72 | 3.63 | 102 | 6.37 | 111 | 7.18 | 40 | 5.33 | 325 | 5.53 |
| Normal (18.5–24.9) | 1,261 | 63.59 | 810 | 50.56 | 863 | 55.86 | 371 | 49.47 | 3,305 | 56.21 |
| Overweight (25–29.9) | 417 | 21.03 | 355 | 22.16 | 338 | 21.88 | 203 | 27.07 | 1,313 | 22.33 |
| Obese (≥30) | 233 | 11.75 | 335 | 20.91 | 233 | 15.08 | 136 | 18.13 | 937 | 15.94 |
| Smokingc | 224 | 11.26 | 563 | 34.84 | 297 | 18.75 | 62 | 6.66 | 1,146 | 18.73 |
| Binge drinkingc | ||||||||||
| No drinking | 1,092 | 55.29 | 910 | 56.84 | 1,096 | 69.81 | 748 | 80.86 | 3,846 | 63.35 |
| Binge drinking (≥4 drinks/occasion) | 220 | 11.14 | 296 | 18.49 | 164 | 10.45 | 59 | 6.38 | 739 | 12.17 |
| Drinking but not binge drinking | 663 | 33.57 | 395 | 24.67 | 310 | 19.75 | 118 | 12.76 | 1,486 | 24.48 |
| Folic acid/multivitamin supplement used | 1,072 | 53.90 | 405 | 25.05 | 443 | 27.95 | 103 | 11.05 | 2,023 | 33.04 |
| Intended to get pregnant | 1,443 | 72.77 | 848 | 52.61 | 835 | 52.81 | 529 | 56.94 | 3,655 | 59.87 |
| Gestational diabetes | 73 | 3.67 | 61 | 3.77 | 55 | 3.47 | 64 | 6.87 | 253 | 4.13 |
| Gestational age category | ||||||||||
| Very preterm (<32 weeks) | 17 | 0.85 | 23 | 1.42 | 18 | 1.14 | 5 | 0.54 | 63 | 1.03 |
| Preterm (32–36 weeks) | 117 | 5.88 | 134 | 8.29 | 131 | 8.26 | 53 | 5.69 | 435 | 7.10 |
| Full-term (≥37) | 1,855 | 93.26 | 1,460 | 90.29 | 1,436 | 90.60 | 874 | 93.78 | 5,625 | 91.87 |
| Birth weight category | ||||||||||
| Very low (<1,500 g) | 12 | 0.60 | 14 | 0.87 | 11 | 0.70 | 0 | 0.00 | 37 | 0.61 |
| Low (1,500–2,499 g) | 61 | 3.07 | 74 | 4.59 | 81 | 5.12 | 28 | 3.03 | 244 | 4.00 |
| Normal (2,500–3,999 g) | 1,661 | 83.64 | 1,352 | 83.92 | 1,340 | 84.76 | 828 | 89.61 | 5,181 | 84.91 |
| Macrosomic (≥4,000 g) | 252 | 12.69 | 171 | 10.61 | 149 | 9.42 | 68 | 7.36 | 640 | 10.49 |
a Some frequencies do not add to the total sample size because of missing values.
b Weight (kg)/height (m)2.
c Assessed during the period of 1 month before pregnancy to the third month of pregnancy.
d Assessed during the period of 3 months before pregnancy to the first month of pregnancy.
The association between dietary class and having a child with an NTD was modified by folic acid/multivitamin use (P = 0.0105). Among women who did not use folic acid supplements or multivitamins, those in the Mexican class (adjusted odds ratio (AOR) = 1.58, 95% confidence interval (CI): 1.15, 2.19), the Western class (AOR = 1.45, 95% CI: 1.10, 1.90), and the low-calorie Western class (AOR = 1.38, 95% CI: 1.05, 1.83) were significantly more likely to have infants with NTDs than were women in the prudent class (Table 4). In contrast, among folic acid/multivitamin supplement users, there was no association between the rate of NTDs and dietary class. The associations between dietary class and CHD subgroups (Table 4) were not modified by folic acid/multivitamin use, except tetralogy of Fallot (P = 0.03). Among folic acid/multivitamin users, women in the Western class were twice as likely to have an infant with tetralogy of Fallot (AOR = 1.96, 95% CI: 1.37, 2.80) than were women in the prudent class. Women in both the Western and low-calorie Western classes were more likely (AOR = 1.18, 95% CI: 1.03, 1.36 and AOR = 1.16, 95% CI: 1.09, 1.52, respectively) to have infants with septal heart defects than were women in the prudent class, and women in the Western class were more likely (AOR = 1.22, 95% CI: 1.03, 1.46) to have infants with conotruncal CHDs than were women in the prudent class. The associations between dietary class and dextrotransposition of the great arteries, left ventricular outflow tract obstructive defects, and right ventricular outflow tract obstructive defects were not significant.
Table 4.
Association Between Dietary Pattern Class and Having a Child With a Neural Tube Defect or Congenital Heart Defect, National Birth Defects Prevention Study, 1997–2005
| Total Cases | Dietary Pattern Class |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Western |
Low-Calorie Western |
Mexican |
||||||||
| Birth Defect | No. | AORa | 95% CI | No. | AORa | 95% CI | No. | AORa | 95% CI | |
| Neural tube defectb | ||||||||||
| No folic acid/multivitamin usec | 726 | 211 | 1.45 | 1.10, 1.90 | 184 | 1.38 | 1.05, 1.83 | 234 | 1.58 | 1.15, 2.19 |
| Folic acid/multivitamin supplement usec | 321 | 76 | 1.08 | 0.80, 1.47 | 58 | 0.80 | 0.58, 1.12 | 21 | 0.66 | 0.37, 1.17 |
| Congenital heart defect | ||||||||||
| Septal | 2,972 | 883 | 1.18 | 1.03, 1.35 | 803 | 1.16 | 1.01, 1.32 | 509 | 1.11 | 0.91, 1.35 |
| LVOTO | 1,018 | 248 | 0.93 | 0.77, 1.14 | 260 | 1.12 | 0.92, 1.35 | 139 | 0.93 | 0.68, 1.28 |
| RVOTO | 1,006 | 285 | 1.03 | 0.85, 1.26 | 281 | 1.12 | 0.92, 1.36 | 136 | 1.18 | 0.85, 1.63 |
| Conotruncal | 1,288 | 370 | 1.22 | 1.03, 1.46 | 334 | 1.06 | 0.89, 1.27 | 181 | 0.93 | 0.71, 1.23 |
| DTGA | 406 | 114 | 1.02 | 0.77, 1.35 | 95 | 0.84 | 0.63, 1.12 | 47 | 0.71 | 0.45, 1.14 |
| Tetralogy of Fallotb | ||||||||||
| No folic acid/multivitamin usec | 384 | 111 | 1.17 | 0.85, 1.62 | 112 | 1.16 | 0.84, 1.61 | 82 | 1.21 | 0.79, 1.85 |
| Folic acid/multivitamin supplement usec | 206 | 60 | 1.96 | 1.37, 2.80 | 48 | 1.26 | 0.86, 1.83 | 7 | 0.59 | 0.23, 1.54 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; DTGA, dextrotransposition of the great arteries; LVOTO, left ventricular outflow tract obstructive; RVOTO, right ventricular outflow tract obstructive.
a Odds ratios were adjusted for folic acid/multivitamin use, folic acid intake, race, age, body mass index category, educational level, smoking, and site using logistic regression. Prudent class was the referent.
b Significant interaction between dietary pattern and folic acid/multivitamin (P < 0.05).
c Assessed during the period of 3 months before pregnancy to the first month of pregnancy.
There were no changes in our findings when we restricted the analyses to live-born children (6,111 controls, 6,577 with CHDs, and 740 with NTDs; data not shown). For NTDs, the interaction between dietary class and folic acid/multivitamin supplement use was significant (P = 0.0240), and the adjusted odds ratios were very similar except that for Western class, which was attenuated from 1.45 (95% CI: 1.10, 1.90) to 1.32 (95% CI: 0.96, 1.81). Finally, we assessed the impact of the quality of dietary classification by excluding women without a high predicted probability (>0.75) of class membership (8.8% of controls, 9.7% of participants who had children with CHDs, and 8.4% of participants who had children with NTDs). None of the associations between dietary class and NTDs or CHDs changed substantially.
DISCUSSION
Maternal diet plays an important role in the etiology of birth defects. Findings from our study confirm the importance of maternal nutrition and dietary choices. Specifically, we derived dietary patterns empirically using LCA and classified women into the 1 of 4 dietary classes (prudent, Western, low-calorie Western, and Mexican) to which they were more likely to belong given their relative food intake and energy intake. The protective effect of prudent class on having an infant with an NTD was among nonusers of folic acid/multivitamin supplements, whereas the protective effect of prudent class on having an infant with Tetralogy of Fallot defect was among users of folic acid/multivitamin supplements. In particular, we found that, among nonusers of folic acid/multivitamin supplements, women in the prudent class were significantly less likely to have infants with NTDs than were women in the Western and Mexican classes, even after adjustment for folic acid intake. Hence, the protective association of prudent class with NTDs among folic acid/multivitamin nonusers was not driven by the higher levels of folic acid intake in this healthier dietary class. Further, similar to Mosley et al. (26), we found that NTDs were 1.4 times more likely (95% CI: 1.2, 1.7) in the offspring of women with folic acid intakes in the 11th–30th percentiles compared with those with intakes in the 50th percentile or higher, independent of folic acid/multivitamin supplement use. The lack of association between dietary class and having offspring with NTDs among folic acid/multivitamin users supports the well-known protective effect of folic acid/multivitamin supplement use.
Although a prudent dietary pattern had a protective effect with regard to NTDs among folic acid/multivitamin nonusers, it was among users of supplements that the prudent dietary pattern had a protective effect with regard to tetralogy of Fallot. This latter finding could be due to residual confounding in which an unexplained behavior related to the use of folic acid/multivitamin supplements is associated with tetralogy of Fallot defects, or it could be just due to chance; replication studies could help us understand. In any case, our results suggest that the prudent dietary pattern has a protective effect for some of the studied birth defects beyond that resulting from the use of folic acid/multivitamin supplements and dietary folic acid intake. Similarly, in the same study, Carmichael et al. (13) found that a healthier maternal dietary pattern, as measured by the diet quality index, was associated with reduced risks of NTDs and clefts after adjustment for confounders, including use of folic acid supplements. The 4 studies that analyzed the impact of empirically derived dietary patterns on birth defects also found that dietary patterns associated with healthy diets reduced the risk of birth defects even after adjusting for periconception folic acid/multivitamin supplement use. Vujkovic et al. (14) found that being in the highest tertile of participants who adhered to the Western diet was associated with a higher risk of having a child with a cleft lip or cleft palate (AOR = 1.9, 95% CI: 1.2, 3.1) than was being in the first tertile of the Western diet. In the same Dutch study, the maternal Mediterranean dietary factor was associated with a reduced risk of having a child with spina bifida (AOR = 2.7, 95% CI: 1.2, 6.1) (12). Similarly, the one-carbon-rich dietary pattern (11) characterized by a high intake of fish and seafood was associated with a reduced risk of having children with CHDs.
The NBDPS has a population-based design with a rich racial/ethnic, geographic, and socioeconomic diversity, and cases were ascertained in a rigorous and standardized way from multiple sources. Methodologically, strengths include the use of LCA to classify women into mutually exclusive dietary patterns, which allowed us to estimate the risk of birth defects for one dietary pattern compared with a referent one. Further, cases' dietary classes were predicted from parameters estimated using only data from controls, which provides a more standardized estimation of the dietary patterns. Finally, associations between dietary class and birth defects were adjusted for important covariates, including race/ethnicity, which was highly associated with dietary class. One drawback is the use of a reduced food frequency questionnaire to derive dietary patterns, which may increase misclassification. However, less than 10% of the participants were difficult to classify into a single dietary class, and the association between class and birth defects did not change after excluding these women. Another drawback is the reduced number of food items that are indeed aggregated food groups, which limits the possibility of identifying specific foods appropriately. However, the benefit of this approach is that it gives us the ability to assess the impact of groups of nutrients not in isolation but in patterns or groups, as they are commonly consumed.
Our findings provide more evidence of the benefits of a healthy diet on birth outcomes. Promoting a healthy diet is particularly important because we showed that folic acid/multivitamin nonusers were less likely to have infants with an NTD. The behavior of taking a multivitamin regularly is hard to maintain, and presently only 40% of women of reproductive age report engaging in such a behavior (27).Our findings highlight the importance of stressing the need to make healthy dietary choices in preconceptional counseling to optimize not only reproductive outcomes but also general maternal health. Overall, a healthy dietary pattern may prevent NTDs and selected CHDs even in the era of fortification.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Daniela Sotres-Alvarez, Amy H. Herring); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Anna Maria Siega-Riz, Andrew F. Olshan); Neonatal and Developmental Medicine, Department of Pediatrics, School of Medicine, Stanford University, Palo Alto, California (Suzan L. Carmichael); Division of Medical Genetics, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, Utah (Marcia L. Feldkamp); Utah Birth Defect Network, Utah Department of Health, Salt Lake City, Utah (Marcia L. Feldkamp); and Department of Pediatrics, Birth Defects Research Section, University of Arkansas for Medical Sciences and Arkansas Children's Hospital Research Institute, Little Rock, Arkansas (Charlotte A. Hobbs).
This work was supported in part through cooperative agreements under Program Announcement 02081 from the Centers for Disease Control and Prevention to the centers participating in the National Birth Defects Prevention Study, and by the National Institute of Environmental Health Sciences (grant P30ES10126).
We thank Jing Zhou for her assistance in replicating the statistical analysis to ensure reproducibility. We also thank the California Department of Public Health Maternal Child and Adolescent Health Division for providing data.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the California Department of Public Health.
Conflict of interest: None declared.
REFERENCES
- 1.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2007 period linked birth/infant death data set. Natl Vital Stat Rep. 2011;59(6):1–30. [PubMed] [Google Scholar]
- 2.Yang Q, Chen H, Correa A, et al. Racial differences in infant mortality attributable to birth defects in the United States, 1989–2002. Birth Defects Res A Clin Mol Teratol. 2006;76(10):706–713. doi: 10.1002/bdra.20308. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael SL, Shaw GM, Schaffer DM, et al. Dieting behaviors and risk of neural tube defects. Am J Epidemiol. 2003;158(12):1127–1131. doi: 10.1093/aje/kwg286. [DOI] [PubMed] [Google Scholar]
- 4.Shaw GM, Carmichael SL, Yang W, et al. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160(2):102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 5.Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150(9):626–631. doi: 10.7326/0003-4819-150-9-200905050-00009. [DOI] [PubMed] [Google Scholar]
- 6.Stover PJ. One-carbon metabolism–genome interactions in folate-associated pathologies. J Nutr. 2009;139(12):2402–2405. doi: 10.3945/jn.109.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benevenga NJ. Consideration of betaine and one-carbon sources of N5-methyltetrahydrofolate for use in homocystinuria and neural tube defects. Am J Clin Nutr. 2007;85(4):946–949. doi: 10.1093/ajcn/85.4.946. [DOI] [PubMed] [Google Scholar]
- 8.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Waijers PM, Feskens EJ, Ocké MC. A critical review of predefined diet quality scores. Br J Nutr. 2007;97(2):219–231. doi: 10.1017/S0007114507250421. [DOI] [PubMed] [Google Scholar]
- 10.Kant AK. Dietary patterns and health outcomes. J Am Diet Assoc. 2004;104(4):615–635. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Obermann-Borst S, Vujkovic M, de Vries J, et al. A maternal dietary pattern characterised by fish and seafood in association with the risk of congenital heart defects in the offspring. BJOG. 2011;118(10):1205–1215. doi: 10.1111/j.1471-0528.2011.02984.x. [DOI] [PubMed] [Google Scholar]
- 12.Vujkovic M, Steegers E, Looman C, et al. The maternal Mediterranean dietary pattern is associated with a reduced risk of spina bifida in the offspring. BJOG. 2009;116(3):408–415. doi: 10.1111/j.1471-0528.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 13.Carmichael SL, Yang W, Feldkamp ML, et al. Reduced risks of neural tube defects and orofacial clefts with higher diet quality. Arch Pediatr Adolesc Med. 2012;166(2):121–126. doi: 10.1001/archpediatrics.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vujkovic M, Ocke MC, van der Spek PJ, et al. Maternal Western dietary patterns and the risk of developing a cleft lip with or without a cleft palate. Obstet Gynecol. 2007;110(2):378. doi: 10.1097/01.AOG.0000268799.37044.c3. [DOI] [PubMed] [Google Scholar]
- 15.de Kort CA, Nieuwenhuijsen MJ, Mendez MA. Relationship between maternal dietary patterns and hypospadias. Paediatr Perinat Epidemiol. 2011;25(3):255–264. doi: 10.1111/j.1365-3016.2011.01194.x. [DOI] [PubMed] [Google Scholar]
- 16.Collins LM, Lanza ST. Latent Class and Latent Transition Analysis : With Applications in the Social, Behavioral, and Health Sciences. Hoboken, NJ: John Wiley & Sons; 2010. [Google Scholar]
- 17.Padmadas SS, Dias JG, Willekens FJ. Disentangling women's responses on complex dietary intake patterns from an Indian cross-sectional survey: a latent class analysis. Public Health Nutr. 2006;9(2):204–211. doi: 10.1079/phn2005842. [DOI] [PubMed] [Google Scholar]
- 18.Fahey MT, Thane CW, Bramwell GD, et al. Conditional Gaussian mixture modelling for dietary pattern analysis. J R Stat Soc Ser A Stat Soc. 2007;170(1):149–166. [Google Scholar]
- 19.Sotres-Alvarez D, Herring AH, Siega-Riz AM. Latent class analysis is useful to classify pregnant women into dietary patterns. J Nutr. 2010;140(12):2253–2259. doi: 10.3945/jn.110.124909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon PW, Rasmussen SA, Lynberg M, et al. The National Birth Defects Prevention Study. Public Health Rep. 2001;116(suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botto LD, Lin AE, Riehle-Colarusso T, et al. Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79(10):714–727. doi: 10.1002/bdra.20403. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Reynolds RD, Cottrell-Hoehner S, et al. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87(1):43–47. [PubMed] [Google Scholar]
- 23.Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 20. Washington, DC: Agricultural Research Service; 2007. http://www.ars.usda.gov/ba/bhnrc/ndl . [Google Scholar]
- 24.SAS Institute, Inc. SAS System for Windows. Cary, NC: 2002. [Google Scholar]
- 25.Lanza ST, Collins LM, Lemmon DR, et al. PROC LCA: A SAS procedure for latent class analysis. Struct Equ Modeling. 2007;14(4):671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosley BS, Cleves MA, Siega-Riz AM, et al. Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am J Epidemiol. 2009;169(1):9–17. doi: 10.1093/aje/kwn331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsey LLM, Petrini JR, Carter H, et al. Use of supplements containing folic acid among women of childbearing age—United States. MMWR Morb Mortal Wkly Rep. 2007;57(1):5–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.