Abstract
Infection control for hospital pathogens such as methicillin-resistant Staphylococcus aureus (MRSA) often takes the form of a package of interventions, including the use of patient isolation and decolonization treatment. Such interventions, though widely used, have generated controversy because of their significant resource implications and the lack of robust evidence with regard to their effectiveness at reducing transmission. The aim of this study was to estimate the effectiveness of isolation and decolonization measures in reducing MRSA transmission in hospital general wards. Prospectively collected MRSA surveillance data from 10 general wards at Guy's and St. Thomas' hospitals, London, United Kingdom, in 2006–2007 were used, comprising 14,035 patient episodes. Data were analyzed with a Markov chain Monte Carlo algorithm to model transmission dynamics. The combined effect of isolation and decolonization was estimated to reduce transmission by 64% (95% confidence interval: 37, 79). Undetected MRSA-positive patients were estimated to be the source of 75% (95% confidence interval: 67, 86) of total transmission events. Isolation measures combined with decolonization treatment were strongly associated with a reduction in MRSA transmission in hospital general wards. These findings provide support for active methods of MRSA control, but further research is needed to determine the relative importance of isolation and decolonization in preventing transmission.
Keywords: Bayesian inference, data augmentation, infection control, Markov chain Monte Carlo, methicillin-resistant Staphylococcus aureus, patient isolation
Infections acquired by patients in hospitals are a major cause of illness and death worldwide, affecting 5%–10% of acute-care patients in developed countries and considerably higher numbers in developing countries (1). Despite a recent decline in infection rates in some European countries (2, 3), methicillin-resistant Staphylococcus aureus (MRSA) remains one of the most important nosocomial pathogens because of the wide dissemination of highly virulent pandemic lineages (4, 5) and multiple antibiotic resistance.
Although nonspecific interventions (principally hand hygiene and antibiotic stewardship) are widely considered to be essential components of strategies to prevent the nosocomial spread of antibiotic-resistant pathogens, there is debate about the additional value of interventions that target specific pathogens such as MRSA (6). Such targeted interventions screen patients to detect asymptomatic carriage and use patient isolation, often combined with decolonization therapy, in an attempt to reduce transmission.
Isolation measures typically range from contact precautions alone (wearing gloves and gowns for contact with known carriers), through patient cohorting (grouping carriers in one part of a ward), to single-room isolation. Isolation is controversial because it is resource intensive, and though studies have shown that such measures can be effective as part of a larger package of interventions, evidence to support the effectiveness of isolation and decolonization measures alone is lacking (6, 7). Moreover, concerns have been raised that isolation measures themselves could have a negative impact on patient welfare. This plausibly could occur as a result of reduced quality of care in an isolation setting (8–10). Despite this uncertainty, patient isolation remains a central component of many local control strategies and national guidelines (11–13).
In the present study, our primary aim was to estimate the combined effect of isolation and decolonization treatment in reducing the transmission rate of MRSA in hospital general wards. We achieved this by constructing a stochastic model to describe the transmission dynamics within each ward and by fitting this model to patient data. Although the impact of isolation in intensive care units (ICUs) has been assessed in some studies (14–16), to our knowledge, this is the first analysis of the effectiveness of isolation and decolonization treatment in a hospital general ward setting. General wards have a highly dynamic population, with frequent readmissions and ward transfers. Although patients in general wards typically have lower antibiotic consumption and are less susceptible to infection than those in ICUs, many more patient-days are spent in general wards, which makes them potentially important reservoirs for MRSA and locations for patient-to-patient MRSA transmission within the hospital.
MATERIALS AND METHODS
Data
We used data collected between January 2006 and April 2007 at Guy's and St. Thomas', a London teaching hospital (1,200 beds) on 2 sites, as part of a prospective cluster-randomized crossover trial to determine whether a policy of rapid screening with a polymerase chain reaction test for MRSA could reduce the rate of acquisition, as compared with a policy of conventional culture screening. Both screening policies were combined with isolation and decolonization treatment for positive patients. Ten hospital general wards were included in this study; ward specialties and characteristics are given in Web Table 1, available at http://aje.oxfordjournals.org/. Results of this study have been reported elsewhere (17).
All patients were screened by conventional culture within 48 hours of admission when possible, and most patients were also culture-screened on discharge. These routine screening swabs were taken at the nares, axillae, and groin (“screening sites”) and then pooled. In addition, swabs were taken from skin breaks and clinically indicated sites as appropriate, and positive results were recorded. Summary statistics are provided in Table 1. Full details of data collection, microbiological methods, and ethics approval were reported by Jeyaratnam et al. (17).
Table 1.
Summary Statistics for the MRSA Carriage Data Collected From Study Wards, Guy's and St. Thomas', London, United Kingdom, 2006–2007
| No. | Median (IQR) | % | |
|---|---|---|---|
| Study length, days | 452 | ||
| Unique patients | 10,845 | ||
| Patient episodes | 14,035 | ||
| Patient-days | 94,747 | ||
| Length of stay, days | 3.3 (2–7.4) | ||
| Patients not swabbed on admission | 1,019 | 7.3a | |
| Patients positive on admission | 847 | 6.5b | |
| Patients positive on admission by surveillance screening of nares, axillae, and groin | 640 | 5.3c | |
| Patients with missing or invalid discharge swab | 5,358 | 41.1b | |
| Valid admission-discharge swab pairs | 7,665 | 54.6a | |
| Observed MRSA acquisitions | 238 | 3.1d |
Abbreviations: IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus.
a Based on total admissions.
b Based on total admission swabs.
c Based on total “valid” swabs, where a swab is considered valid if it is taken in a correct manner and within a defined time scale (14).
d Based on total valid admission-discharge swab pairs.
Newly admitted patients considered to be at high risk for MRSA carriage were isolated, where possible, before the admission swab result. A decision to implement this “preemptive isolation” was based on a previous MRSA-positive swab or the presence of 1 or more risk factors for MRSA carriage (i.e., living in a nursing or residential home; an inpatient stay during the previous year; or a direct transfer from another hospital, from abroad, or from a high-risk area within the hospital). Patients found to be MRSA positive by the admission screen (by culture or polymerase chain reaction) also were isolated, and decolonization treatment was started. Single-bedded side rooms were used if available and appropriate; otherwise, the MRSA-positive patients were nursed on the open ward with standard contact precautions (staff donned disposable gowns and gloves before patient contact). Isolation policies and practices were strictly enforced. When more than 1 MRSA-positive patient had to be on the open ward, they were placed together in a separate bay where possible (patient cohorting). Decolonization treatment with chlorhexidine for the skin, povidone iodine or silver sulphadiazine for colonized wounds, and mupirocin nasal ointment (neomycin for mupirocin-resistant strains) were initiated for all patients found to be MRSA positive.
Although screening data were collected uniformly for all patients and from the same screening sites, clinical isolates were taken only from sites of suspected infection, and these data therefore cannot be treated in the same manner. Moreover, only positive clinical isolates were recorded, precluding attempts to measure sensitivity. Here we adopt 2 approaches: In the primary analysis, only the screening data are used. The second approach makes use of both screening and clinical isolate data, but patients are assumed positive after a positive clinical result, and subsequent screening results are ignored.
Transmission model
We considered all patients to be either colonized (MRSA positive) or susceptible to colonization (MRSA negative). We supposed that a susceptible patient's risk of colonization depended on the number of MRSA-positive patients in the ward at the same time. Furthermore, we supposed that the risk of transmission from a colonized and isolated patient might differ from that of a colonized and unisolated patient. We defined the rate of MRSA transmission acting on each susceptible patient at time t to be q(t) = a0 + a1CN(t) + a2CI(t), where CN(t) is the number of colonized patients under no isolation precautions, and CI(t) is the number of colonized and isolated patients at time t. Background transmission is represented by a0 and accounts for transmission from other sources, such as long-term staff carriers, persistent environmental contamination, and the introduction of the pathogen from elsewhere in the hospital. We worked in discrete time, assuming events occur in daily intervals. Under the assumption that susceptible persons experience effective contacts at a rate q(t) on day t, the probability of any given susceptible person avoiding colonization on day t is exp(−q(t)) (18). Estimating the transmission parameters a0, a1, and a2 allowed us to estimate the effectiveness of isolation and decolonization in reducing transmission. We expressed this as a relative risk, defined as the ratio of the risk of transmission from an isolated colonized patient to the risk of transmission from an unisolated colonized patient. This can be achieved by considering the daily probability of acquiring MRSA, given exposure to a colonized patient in isolation versus exposure to an unisolated positive patient, and by calculating the associated relative risk:
 |
A value below 1 would indicate that isolation had a positive effect in reducing transmission. Because decolonization treatment usually was initiated at the same time as isolation in this study, it was not possible to consider the effects of these 2 interventions separately, so we calculated the combined effect. In addition, we estimated the prevalence on admission (p) and the swab sensitivity (z).
If transmission dynamics were perfectly observed, it would be straightforward to calculate the likelihood of the data (comprising admission, isolation, and discharge times, as well as screening dates and results) given parameters θ = {p, z, a0, a1, a2}. However, transmission typically is unobserved; uncertainty exists about patients' true colonization times and therefore about the true prevalence at any given time. This means the likelihood is not tractable. We accounted for this by using a data-augmented Markov chain Monte Carlo algorithm to fit a stochastic transmission model to the observed data (19–21). This allowed us to estimate posterior distributions of model parameters θ while sampling over colonization times for all patients. This accounts for imperfect and infrequent screening, as well as for missing admission and discharge swabs. At each iteration, we sampled values for the colonization times and model parameters θ and also kept track of other quantities of interest, such as the proportion of colonized days out of isolation.
This process allowed us to estimate the true, rather than the observed, prevalence on admission and the transmission rate, as well as swab sensitivity. Precise details of the analysis can be found in the Web Appendix.
We estimated parameters on a ward-by-ward basis. To obtain overall estimates, a random-effects meta-analysis was used to pool the individual ward estimates (22). Parameter estimates for each ward were weighted by inverse variance.
Assumptions
Several assumptions were made when we ran our analysis:
Patients who became colonized with MRSA remained so for the remainder of their stay on a study ward, because of the typically lengthy carriage times of MRSA (23, 24) and the relatively short length of a typical ward episode (refer to Table 1).
For the purpose of calculating the daily population count, we assumed that admission and isolation entry times occurred at the start of each day, whereas discharge and isolation exit times occurred at the end of the day.
We did not explicitly model contact patterns between patients and health-care workers or direct patient-to-patient interactions, instead assuming that all susceptible patients were exposed to the same colonization pressure on a given day and faced the same risk of acquisition. Similarly, we assumed that compliance with barrier precautions and the application of decolonization treatment were the same for all patients within a ward, regardless of isolation type.
We assumed no difference in infectiousness for patients with positive screening results and patients with positive clinical isolates.
We assumed test specificity to be 100%.
We did not differentiate between strains of MRSA.
RESULTS
Screening data
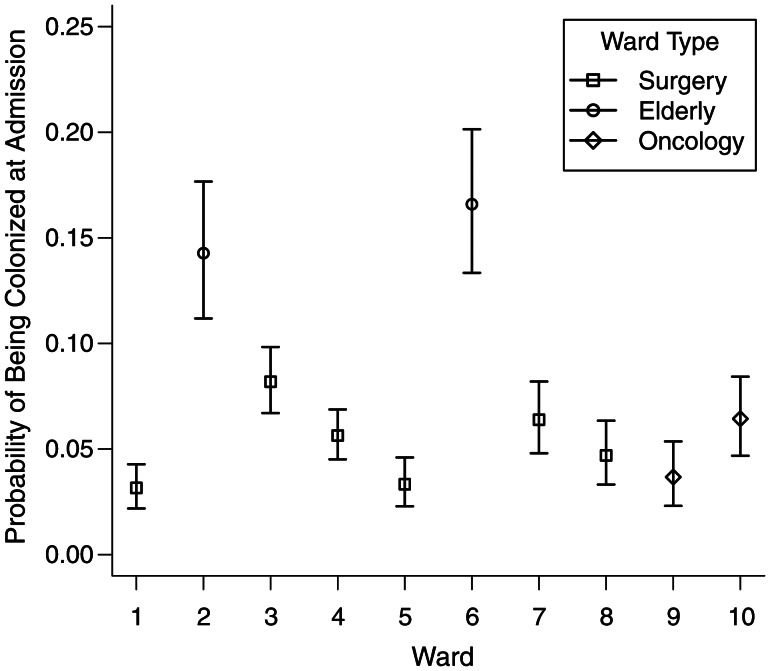
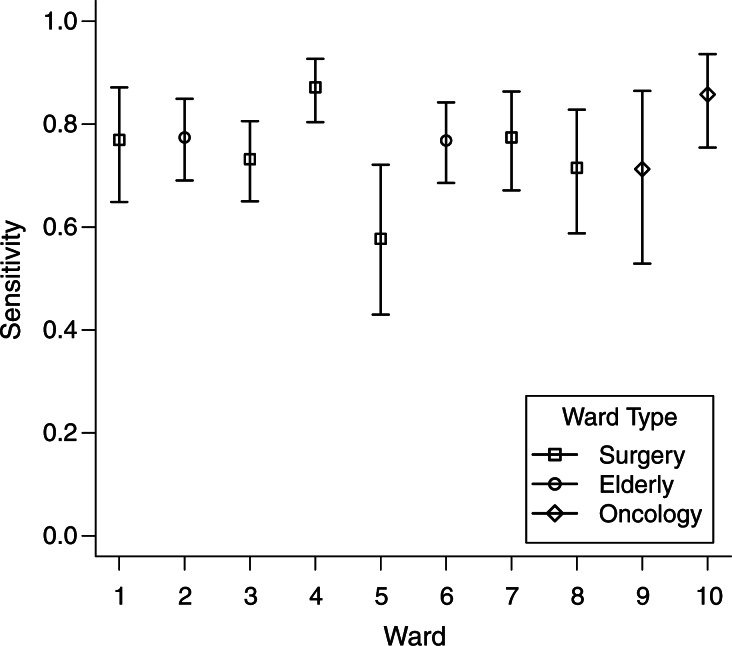
Posterior estimates of the model parameters for each study ward are reported in Table 2. MRSA carriage prevalence on admission varied considerably by ward (Figure 1). Estimates ranged from 3% to 16% and were highest for the 2 elderly care wards 2 and 6. Estimates for the screening test sensitivity ranged from 58% to 86% on individual wards (Figure 2). The pooled ward estimate was 77% (95% confidence interval (CI): 72, 82). We estimated that the proportion of colonized patient-days spent out of isolation ranged by ward from 40% to 65%, with a pooled estimate of 54% (95% CI: 47, 60). We found that the average transmission rate ranged from 1.3 to 4.7 acquisitions per 1,000 patient-days across the wards (Table 3).
Table 2.
Transmission Parameter Estimates for Study Wards, Guy's and St. Thomas', London, United Kingdom, 2006–2007
| Ward | Prevalence on Admission |
Screening Sensitivity |
Background Effect (a0 × 105) |
Transmission Effect From Unisolated Patients (a1 × 105) |
Transmission Effect From Isolated Patients (a2 × 105) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CrI | Mean | 95% CrI | Median | 95% CrI | Median | 95% CrI | Median | 95% CrI | |
| 1 | 0.03 | 0.02–0.04 | 0.77 | 0.65–0.87 | 15 | 0.4–55.9 | 299.7 | 101–555 | 27.9 | 0.8–97.3 |
| 2 | 0.14 | 0.11–0.18 | 0.78 | 0.69–0.85 | 30.9 | 0.8–107.9 | 222 | 116.3–341.6 | 16.1 | 0.4–57.7 |
| 3 | 0.08 | 0.07–0.1 | 0.74 | 0.66–0.81 | 46.7 | 1.4–145.3 | 62.4 | 5.8–142.5 | 45.3 | 1.6–137.9 |
| 4 | 0.06 | 0.05–0.07 | 0.87 | 0.81–0.93 | 24.3 | 0.6–81.2 | 311.4 | 168.4–488 | 21.7 | 0.6–76.7 |
| 5 | 0.03 | 0.02–0.05 | 0.58 | 0.43–0.72 | 18.9 | 0.5–67.9 | 221.3 | 77.6–399.4 | 69.4 | 2.2–222.4 |
| 6 | 0.17 | 0.13–0.2 | 0.77 | 0.68–0.84 | 53.2 | 1.2–218.6 | 83.3 | 23.4–146.1 | 20.3 | 0.7–70.7 |
| 7 | 0.06 | 0.05–0.08 | 0.78 | 0.68–0.86 | 40.1 | 1–137.2 | 38.3 | 1.6–97 | 59.3 | 2.8–153.5 |
| 8 | 0.05 | 0.03–0.06 | 0.72 | 0.59–0.83 | 21.9 | 0.6–78.1 | 132.2 | 58.7–213.8 | 33.3 | 1–110.2 |
| 9 | 0.04 | 0.02–0.05 | 0.71 | 0.53–0.86 | 21.9 | 0.6–78.2 | 81.9 | 4.4–220.1 | 94.6 | 6–236.6 |
| 10 | 0.06 | 0.05–0.08 | 0.86 | 0.75–0.94 | 25.1 | 0.6–90.1 | 99.2 | 10.1–226.2 | 40.1 | 1.2–132.6 |
Abbreviation: CrI, credible interval.
Figure 1.
Ward estimates of probability of colonization on admission, Guy's and St. Thomas', London, United Kingdom, 2006–2007. Mean estimates for the parameter p, probability of colonization on admission, together with 95% equitailed credible intervals calculated from Markov chain Monte Carlo samples, for study wards are shown.
Figure 2.
Ward estimates of sensitivity, Guy's and St. Thomas', London, United Kingdom, 2006–2007. Mean estimates for the parameter z, test sensitivity, together with 95% equitailed credible intervals calculated from Markov chain Monte Carlo samples, for study wards are shown.
Table 3.
Estimated Colonization Rates for Study Wards at Guy's and St. Thomas', London, United Kingdom, 2006–2007
| Ward | Type | Estimated Acquisitions Per 1,000 Patient-Daysa |
|
|---|---|---|---|
| Posterior Mean | 95% CrI | ||
| 1 | Surgery (plastics) | 1.7 | 0.7–3.0 |
| 2 | Elderly care | 4.7 | 2.5–7.0 |
| 3 | Surgery (urology) | 2.4 | 0.8–4.5 |
| 4 | Surgery (ear, nose, and throat) | 3.5 | 2.1–5.1 |
| 5 | Surgery (cardiothoracic) | 2.0 | 0.9–3.6 |
| 6 | Elderly care | 3.8 | 2.0–5.8 |
| 7 | Surgery (vascular) | 2.3 | 1.3–3.6 |
| 8 | Surgery (gastrointestinal) | 2.7 | 1.6–4.2 |
| 9 | Oncology | 1.3 | 0.5–2.5 |
| 10 | Oncology | 1.5 | 0.6–2.6 |
Abbreviation: CrI, credible interval.
a Estimates derived by using an average colonized population.
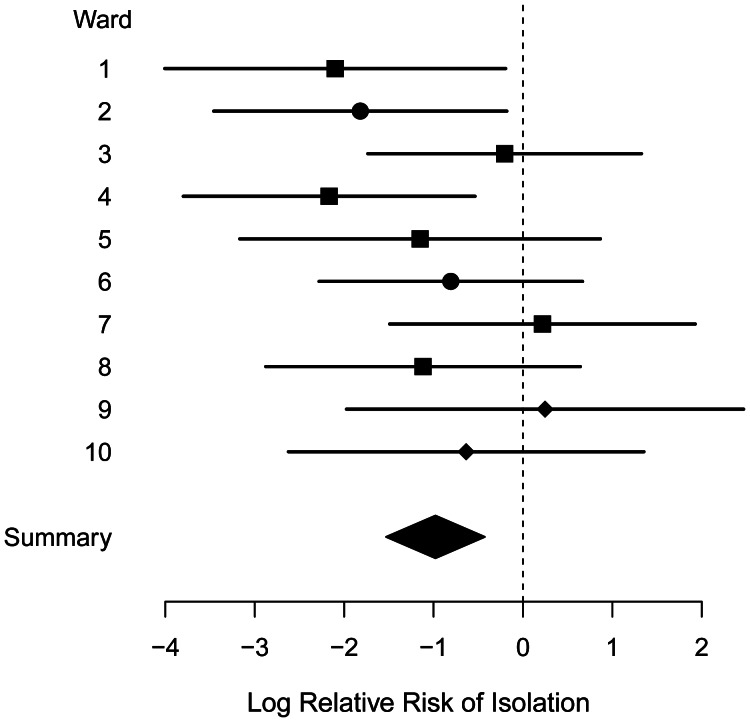
Estimates for the effectiveness of isolation and decolonization in reducing transmission in each ward are shown in Table 4. A forest plot for the isolation relative risk is shown in Figure 3. The pooled estimate for the relative risk is 0.36 (95% CI: 0.21, 0.63), indicating that isolation and decolonization treatment were associated with a reduction in transmission of approximately 64%. In 2 wards (ward 7: surgery; ward 9: oncology), the point estimates indicate a negative isolation effect (increased transmission associated with isolation and decolonization), though in both cases the 95% credible intervals are wide and include the pooled estimate. We found our estimates to be robust to the choice of prior for transmission parameters (Web Table 2).
Table 4.
Isolation Use and Estimated Effectiveness in Study Wards, Guy's and St. Thomas', London, United Kingdom, 2006–2007
| Ward | Total Recorded Patient-Days | Estimated Colonized Daysa | Recorded Isolation Days | Estimated Colonized and Isolated Daysa | Estimated Relative Risk of MRSA Transmission Associated With Isolation Measures, Eiso |
|
|---|---|---|---|---|---|---|
| Posterior Median | 95% CrI | |||||
| 1 | 9,975 | 1,225 | 1,692 | 800 | 0.12 | 0.02–0.66 |
| 2 | 10,734 | 3,786 | 2,371 | 1,762 | 0.16 | 0.02–0.54 |
| 3 | 7,907 | 1,267 | 1,263 | 726 | 0.80 | 0.15–3.65 |
| 4 | 11,020 | 1,938 | 2,169 | 1,228 | 0.12 | 0.02–0.45 |
| 5 | 8,685 | 462 | 1,067 | 302 | 0.31 | 0.04–1.72 |
| 6 | 10,539 | 3,691 | 2,549 | 1,750 | 0.48 | 0.08–1.29 |
| 7 | 10,264 | 2,567 | 1,771 | 1,334 | 1.23 | 0.22–9.21 |
| 8 | 8,959 | 1,686 | 1,672 | 831 | 0.31 | 0.05–1.33 |
| 9 | 8,797 | 1,201 | 4,039 | 655 | 1.14 | 0.12–9.71 |
| 10 | 7,867 | 959 | 1,666 | 580 | 0.48 | 0.06–3.45 |
| All | 94,747 | 18,782 | 20,259 | 9,967 | 0.36 | 0.21–0.63 |
Abbreviations: CrI, credible interval; MRSA, methicillin-resistant Staphylococcus aureus.
a Posterior mean estimates.
Figure 3.
Isolation effectiveness, Guy's and St. Thomas', London, United Kingdom, 2006–2007. Median estimates and 95% credible intervals for the log relative risk of isolation, log(Eiso), for each study ward are shown. Values below zero indicate effectiveness of isolation and decolonization measures. Ward types are indicated by squares (surgery), circles (elderly care), and diamonds (oncology).
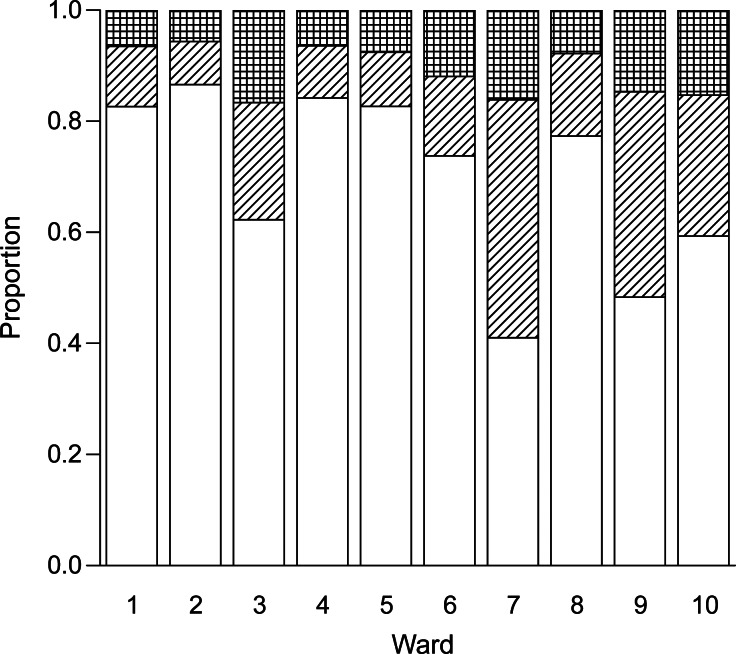
The expected proportions of transmission events due to 3 sources (background, isolated MRSA-positive patients within the same ward, unisolated MRSA-positive patients within the same ward) and rate of transmission for each ward are shown in Figure 4. An estimated 75% (95% CI: 67, 86) of the transmission in this setting was attributable to MRSA-positive patients who were not under isolation precautions or receiving decolonization therapy. We estimated that background transmission (i.e., transmission not linked to patient-to-patient spread within the ward) accounted for 9% (95% CI: 3, 14) of within-ward transmission, with 0.23 new colonization events per 1,000 patient-days caused by a background effect.
Figure 4.
Each column represents the transmission within a particular study ward at Guy's and St. Thomas', London, United Kingdom, in 2006–2007. Transmission is split into the proportions expected (top to bottom): background transmission (hatched), colonized patients in isolation (striped), and colonized patients not receiving isolation precautions (white). Mean rates of transmission for each ward are given in Table 3.
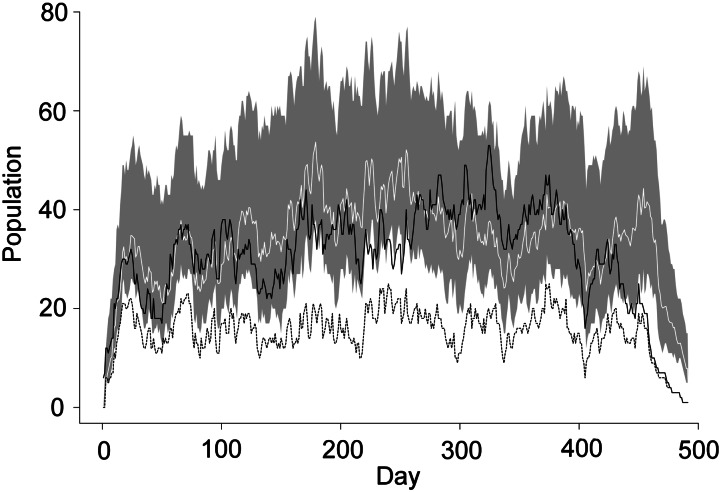
Plotting the inferred MRSA-colonized population based on the surveillance screens alongside the number of observed positive patients based on all isolates (Figure 5) shows that our estimated colonized population is a reasonable approximation to the total number of patients observed to be positive by clinical isolate or surveillance swab.
Figure 5.
Estimated and observed colonized population, Guy's and St. Thomas', London, United Kingdom, 2006–2007. Population count (across all study wards) of patients observed to be positive via screening only (dashed) and via either a screening test or a clinical isolate (black) is shown. The colonized population predicted by the model from the surveillance screens is shown in white (95% credible interval shaded in gray).
We checked the model fit by using posterior predictive analysis and found no evidence to suggest a lack of fit. We found little evidence of parameter correlation (Web Figure 1). In addition, we ran our analysis on various simulated data sets, which confirmed the ability of the model to identify parameters adequately (an example is shown in Web Figure 2). Details of model assessment procedures can be found in the Web Appendix.
Clinical isolate data
For comparison, we ran analyses incorporating the positive clinical isolates by overriding surveillance swabs with clinical swab results where applicable. As expected, this resulted in a higher prevalence on admission; a pooled estimate was 0.09 (95% CI: 0.07, 0.11), compared with 0.07 in the initial analysis. We found that almost 30% of available positive surveillance swabs were overridden by other positive results in this process, which greatly reduced the information available to inform the estimate of swab sensitivity. This resulted in increased uncertainty, and the pooled estimate of sensitivity dropped to 0.57 (95% CI: 0.42, 0.71). We considered using an informative prior for sensitivity and found this had little effect on our other estimates (Web Table 3).
We found that the transmission parameters differed from the initial estimates; the estimated transmission rate from unisolated colonized patients (a1) increased, whereas the estimated transmission rate from isolated and colonized patients (a2) decreased slightly. As a consequence, our estimate of isolation effectiveness was higher; we estimated Eiso to be 0.23 (95% CI: 0.13, 0.42), which corresponds to a reduction in transmission of 77% (95% CI: 58, 87), compared with 64% estimated previously. Ward-by-ward estimates are given in Web Table 4. Furthermore, we estimated that 44% (95% credible interval: 40, 48) of MRSA-positive patient-days were not in isolation, compared with 54% in our previous estimate.
DISCUSSION
To our knowledge, no previous study has estimated the effect of isolation and decolonization measures on MRSA transmission rates in general wards. Our analysis provides strong evidence that isolation precautions in combination with decolonization treatment were associated with a reduction in transmission in these settings and suggests that most transmission within these wards was due to patient-to-patient spread from unisolated MRSA-positive patients. Our results, however, do not tell us about the relative importance of isolation and decolonization or about the causal mechanisms responsible for the association.
In this paper, our main focus has been on the analysis of surveillance screening alone. However, the addition of clinical data supported our main conclusions and increased our estimate of the effect size associated with isolation and decolonization. Figure 5 shows that the colonized population across all wards inferred by using this method provides a reasonable approximation to the known prevalence based on all culture results. Use of surveillance screening results alone leads to underestimation of the prevalence on admission and therefore to underestimation of the colonization pressure. This does not influence the estimated isolation effectiveness greatly, providing only a slightly more conservative estimate. Repeating this analysis with additional clinical isolate data accounts for additional colonization pressure by including those with MRSA at clinical culture sites. The majority of “extra” patients are likely to have been in isolation because, according to hospital policy, patients with a positive clinical specimen were isolated when possible. Therefore, the MRSA-positive patients missed by routine screening swabs but detected by clinical cultures were those most likely to contribute to the colonized and isolated population count (CI(t)). With lower estimates of colonized and isolated patients and a similar transmission rate, results in which surveillance screens are used alone are likely to provide a lower estimate of a2 and so in turn a more conservative estimate of Eiso.
In 1 surgical ward and 1 oncology ward, the point estimates suggested that isolation had a detrimental effect on MRSA transmission, and in 1 further surgical ward, only a negligible beneficial effect was seen (Figure 3). These results are most parsimoniously explained by chance variation because in all cases associated credible intervals were wide. Nevertheless, other explanations are possible; there are case reports of superspreading events (25), and recent modeling work has highlighted the potential for a peripatetic health-care worker with poor hand hygiene compliance to influence the transmission dynamics greatly (26). This could reduce the effectiveness of isolation.
We assumed specificity of the screening test to detect MRSA to be 100%, and independent estimates have confirmed that specificity closely approaches this value. Two culture tests were used in the clinical trial: CHROMagar MRSA (Oxoid, Basingstoke, United Kingdom) and a MRSA-selective broth, which were estimated to have specificities of 99.3% and 92.8%, respectively, after 22–24 hours (27). Sensitivity estimates varied slightly across the wards. Sensitivity can depend on swabbing techniques and the time between taking the swab and testing, which might account for the differences between ward estimates.
Table 4 shows the number of patient-days spent under isolation precautions for each ward, with estimates derived from clinical isolates and screening results. We estimated that almost half of all colonized patient-days were spent out of isolation. False negative swab results and delays in screening (and the processing of results) contributed to this. We also estimated the number of colonized patient-days spent in each form of isolation (cohort, side room, open ward with precautions) and found that side-room isolation accounted for more than three quarters of these. Our assumption that all forms of isolation have the same effectiveness is therefore unlikely to greatly affect our conclusions, because in the majority of cases, isolation procedures were the same.
We are not aware of a similar study estimating the effectiveness of isolation and decolonization treatment in hospital general wards, so directly comparable results are not available. A systematic review of literature on isolation of MRSA patients conducted in 2004 (7) concluded that no well-designed studies had assessed the effect of isolation alone. However, some evidence supported a package of intervention measures, which included isolation. Since that review, Cepeda et al. (14) reported a prospective trial during which positive patients were not moved to isolation areas (single rooms or cohorts) for a period of 6 months in 2 ICUs in a London hospital. This period was then compared with a control phase in which isolation in a side room or cohort was used. Standard precautions were maintained throughout. Acquisition rates were found to be similar in both periods, and there was no evidence to suggest increased transmission during the period with no isolation. More recently, Huskins et al. (28) showed that active surveillance and increased use of barrier precautions had no significant effect on the transmission of MRSA or vancomycin-resistant enterococcus, again in an ICU setting, compared with standard hospital policy. Jain et al. (29), however, recently reported the results of introducing a bundle of MRSA control measures in acute Veterans Affairs hospitals in the United States. The measures included universal nasal admission screening, contact precautions for patients known to have MRSA, hand hygiene promotion, and culture change. This combined intervention was associated with a reported reduction of transmission in ICUs of 17% and a reduction in other wards of 21%.
A model-based analysis by Kypraios et al. (15) was designed to assess the impact of isolation on MRSA transmission by using data collected from 8 ICUs in a Boston hospital. All ICU beds in this setting were in single rooms, and isolation was considered to be the use of barrier precautions. Bayesian inference was used to measure isolation effectiveness, assessed with the measure a1/a2 (which approximates our measure Eiso), and this was estimated to be 0.75 (95% CI: 0.25, 2.22), pooled across each of the ICUs.
The effect of decolonization therapy on MRSA transmission rates is unclear. In a systematic review of the effect of mupirocin nasal ointment on S. aureus infection rates in nasal carriers, no evidence was found to support its effectiveness; however, reduction in transmission was not investigated in any of the included studies, and in only 1 study were methicillin-resistant strains considered separately (30). To consider the effect of decolonization treatment separately, it would be necessary to conduct a study in which isolation and decolonization were used nonconcurrently.
Our analysis indicates that isolation in combination with decolonization treatment is associated with a reduction in MRSA transmission of around 64% in hospital general wards and that approximately three quarters of ward transmission is due to unisolated colonized patients. We estimated that more than 40% of colonized patient-days were spent out of isolation. Therefore, attempting to minimize this figure could be key to reducing MRSA acquisitions in the general ward setting. Further research into the separate effects of decolonization and isolation, as well as an estimate of the difference between open-ward and side-room isolation, would be required to provide statistical evidence supporting the precise components of a package of interventions for a newly discovered MRSA-positive patient. With appropriate data from a purpose-designed trial, our model might be extended to consider these factors.
ACKNOWLEDGMENTS
Author affiliations: School of Mathematical Sciences, University of Nottingham, Nottingham, United Kingdom (Colin J. Worby, Theodore Kypraios, Philip D. O'Neill); Statistics, Modelling and Bioinformatics Department, Health Protection Agency, London, United Kingdom (Colin J. Worby, Julie V. Robotham); King's College Hospital National Health Service Foundation Trust, London, United Kingdom (Dakshika Jeyaratnam); Medical Research Council Biostatistics Unit, University of Cambridge, Cambridge, United Kingdom (Daniela De Angelis); Guy's and St. Thomas’ National Health Service Foundation Trust, London, United Kingdom (Gary French); Centre for Clinical Vaccinology and Tropical Medicine, Nuffield Department of Clinical Medicine, University of Oxford, Oxford, United Kingdom (Ben S. Cooper); and Mahidol-Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand (Ben S. Cooper).
This work was supported by funding from the European Community (Mastering Hospital Antimicrobial Resistance (MOSAR) network contract LSHP-CT-2007-037941) and was conducted on behalf of the MOSAR (Work Package 7) Study Team. B.S.C. acknowledges support from the Oak Foundation. The Mahidol-Oxford Tropical Medicine Research Unit is supported by the Wellcome Trust of Great Britain (Major Overseas Programme, Thailand Unit Core Grant). The original clinical trial was funded by the United Kingdom Department of Health.
Conflict of interest: none declared.
REFERENCES
- 1.Pittet D, Allegranzi B, Storr J, et al. Infection control as a major World Health Organization priority for developing countries. J Hosp Infect. 2008;68(4):285–292. doi: 10.1016/j.jhin.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Pearson A, Chronias A, Murray M. Voluntary and mandatory surveillance for methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus bacteraemia in England. J Antimicrob Chemother. 2009;64(suppl 1):i11–i17. doi: 10.1093/jac/dkp260. [DOI] [PubMed] [Google Scholar]
- 3.Navarro MB, Huttner B, Harbarth S. Methicillin-resistant Staphylococcus aureus control in the 21st century: beyond the acute care hospital. Curr Opin Infect Dis. 2008;21(4):372–379. doi: 10.1097/QCO.0b013e3283013add. [DOI] [PubMed] [Google Scholar]
- 4.Harris SR, Feil EJ, Holden MTG, et al. Evolution of MRSA during hospital transmission and intercontinental spread. Science. 2010;327(5964):469–474. doi: 10.1126/science.1182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb GF, Horn MA, D'Agata EMC, et al. Competition of hospital-acquired and community-acquired methicillin-resistant Staphylococcus aureus strains in hospitals. J Biol Dyn. 2010;4(1):115–129. doi: 10.1080/17513750903026411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farr BM, Bellingan G. Pro/con clinical debate: isolation precautions for all intensive care unit patients with methicillin-resistant Staphylococcus aureus colonization are essential. Crit Care. 2004;8(3):153–156. doi: 10.1186/cc2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper BS, Stone SP, Kibbler CC, et al. Isolation measures in the hospital management of methicillin resistant Staphylococcus aureus (MRSA): systematic review of the literature. BMJ. 2004;329(7465):533. doi: 10.1136/bmj.329.7465.533. doi:10.1136/bmj.329.7465.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354(9185):1177–1178. doi: 10.1016/S0140-6736(99)04196-3. [DOI] [PubMed] [Google Scholar]
- 9.Evans HL, Schaffer MM, Hughes MG, et al. Contact isolation in surgical patients: a barrier to care? Surgery. 2003;134(2):180–188. doi: 10.1067/msy.2003.222. [DOI] [PubMed] [Google Scholar]
- 10.Saint S, Higgins LA, Nallamothu BK, et al. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354–356. doi: 10.1016/s0196-6553(02)48250-8. [DOI] [PubMed] [Google Scholar]
- 11.Nicolle LE. Infection Control Programmes to Control Antimicrobial Resistance. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 12.Werkgroep Infectiepreventie. Policy for methicillin-resistant Staphylococcus aureus in hospitals. Leiden, Netherlands: Dutch Working Party on Infection Prevention; 2012. http://www.wip.nl/UK/free_content/Richtlijnen/110530%20MRSA%20hospital%20def.pdf. (Accessed August 28, 2012) [Google Scholar]
- 13.Department of Health. Screening for meticillin-resistant Staphylococcus aureus (MRSA) colonisation—a strategy for NHS trusts: a summary of best practice. London, UK: Department of Health; 2006. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_063188. (Accessed August 28, 2012) [Google Scholar]
- 14.Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet. 2005;365(9456):295–304. doi: 10.1016/S0140-6736(05)17783-6. [DOI] [PubMed] [Google Scholar]
- 15.Kypraios T, O'Neill PD, Huang SS, et al. Assessing the role of undetected colonization and isolation precautions in reducing methicillin-resistant Staphylococcus aureus transmission in intensive care units. BMC Infect Dis. 2010;10:29. doi: 10.1186/1471-2334-10-29. doi:10.1186/1471-2334-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall IM, Barrass I, Leach S, et al. Transmission dynamics of methicillin-resistant Staphylococcus aureus in a medical intensive care unit. J R Soc Interface. 2012;9(75):2639–2652. doi: 10.1098/rsif.2012.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeyaratnam D, Whitty CJM, Phillips K, et al. Impact of rapid screening tests on acquisition of meticillin resistant Staphylococcus aureus: cluster randomised crossover trial. BMJ. 2008;336:927–930. doi: 10.1136/bmj.39525.579063.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson H, Britton T. Stochastic Epidemic Models and Their Statistical Analysis. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 19.Gilks WR, Richardson S, Spiegelhalter DJ. Markov Chain Monte Carlo in Practice. London, UK: Chapman & Hall/CRC; 1996. [Google Scholar]
- 20.Cooper BS, Medley GF, Bradley SJ, et al. An augmented data method for the analysis of nosocomial infection data. Am J Epidemiol. 2008;168(5):548–557. doi: 10.1093/aje/kwn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrester M, Pettitt A, Gibson G. Bayesian inference of hospital-acquired infectious diseases and control measures given imperfect surveillance data. Biostatistics. 2007;8(2):383–401. doi: 10.1093/biostatistics/kxl017. [DOI] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-analysis. Chichester, UK: John Wiley & Sons, Ltd.; 2009. [Google Scholar]
- 23.Scanvic A, Denic L, Gaillon S, et al. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis. 2001;32(10):1393–1398. doi: 10.1086/320151. [DOI] [PubMed] [Google Scholar]
- 24.Robicsek A, Beaumont J, Peterson L. Duration of colonisation with methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2009;48(7):910–913. doi: 10.1086/597296. [DOI] [PubMed] [Google Scholar]
- 25.Sherertz RJ, Reagan DR, Hampton KD, et al. A cloud adult: the Staphylococcus aureus-virus interaction revisited. Ann Intern Med. 1996;124(6):539–547. doi: 10.7326/0003-4819-124-6-199603150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Temime L, Opatowski L, Pannet Y, et al. Peripatetic health-care workers as potential superspreaders. Proc Natl Acad Sci U S A. 2009;106(43):18420–18425. doi: 10.1073/pnas.0900974106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry JD, Davies A, Butterworth LA, et al. Development and evaluation of a chromogenic agar medium for methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2004;42(10):4519–4523. doi: 10.1128/JCM.42.10.4519-4523.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huskins WC, Huckabee CM, O'Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407–1418. doi: 10.1056/NEJMoa1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 30.van Rijen M, Bonten M, Wenzel R, et al. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers (review) Cochrane Database Syst Rev. 2008;(4):CD006216. doi: 10.1002/14651858.CD006216.pub2. doi:10.1002/14651858.CD006216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]