Abstract
Many adverse pregnancy outcomes differ by race. We examined the association between self-reported race and miscarriage (pregnancy loss at <20 weeks) in a community-based pregnancy cohort. Women from the southeastern United States (North Carolina, Texas, and Tennessee) were enrolled in “Right from the Start” from 2000 to 2009. They were recruited while trying to conceive or during early pregnancy. Participants completed study ultrasound examinations, interviews, and consent forms for review of medical records. We used proportional hazard models to examine miscarriage risk among black women compared with white women, adjusted for confounders. There were 537 observed miscarriages among 4,070 women, 23% of whom self-identified as black (n = 932). The life table–adjusted cumulative risk of loss after gestational week 5 was 21.3%. With adjustment for age and alcohol use, blacks had increased risk of miscarriage compared with whites (adjusted hazard ratio = 1.57, 95% confidence interval: 1.27, 1.93). When risk of loss before gestational week 10 was dichotomized at the median gestational age, there was little difference, but black women had a greater risk thereafter compared with white women (adjusted hazard ratio = 1.93, 95% confidence interval: 1.48, 2.51). Early pregnancy ultrasound examinations did not differ by race. In summary, self-reported race is independently associated with risk of miscarriage, and the higher risk for black women is concentrated in gestational weeks 10–20.
Keywords: disparity, miscarriage, pregnancy, prospective cohort, race, reproductive epidemiology
Approximately 10%–15% of clinically identified pregnancies end in recognized miscarriage, a pregnancy loss before completion of 20 weeks’ gestation (1–4). More than a third of all conceptions that can be identified hormonally may end in loss when taking into account unrecognized pregnancies (1). Miscarriage is challenging to study because risk varies markedly by gestational age; thus, the gestational age at study entry must be considered when estimating risk of loss. Few biological, behavioral, or socioeconomic factors have been definitively associated with risk of miscarriage (1–3, 5, 6). Increasing maternal age and history of miscarriage are the strongest predictors of miscarriage (1–3).
Studies incorporating race into multivariable models of miscarriage risk have not specifically focused on evaluating the presence or magnitude of disparity. One study reported race as a confounder, suggesting that blacks have twice the risk of miscarriage compared with other racial groups (7), and others report no association between race and miscarriage risk (8–10). Understanding whether race has an independent influence on miscarriage risk has not been the goal of prior analyses.
Although there is limited research on disparities in risk of miscarriage, other adverse pregnancy outcomes, including spontaneous preterm birth and fetal growth restriction, have been shown to differ significantly by race (11–15). For example, in the United States, the risk of spontaneous preterm birth for non-Hispanic black women is approximately 1.5 times greater than the risk observed among non-Hispanic white women (16, 17). Studies have shown that the overrepresentation of preterm births in non-Hispanic black women is observed independently of conventional maternal medical and socioeconomic factors captured in epidemiologic research and that complex causal pathways may link the social construct of race to the biological outcome of preterm birth (13, 17, 18).
Given significant racial and ethnic disparities observed in other adverse pregnancy outcomes that may have origins in early pregnancy, such as placentation (19–22), we examined the association between self-reported race and miscarriage risk in a community-recruited prospective pregnancy cohort to determine if racial disparities in reproductive outcomes include increased miscarriage risk.
MATERIALS AND METHODS
Study population and data collection
“Right from the Start” (RFTS) is an ongoing prospective community-based pregnancy cohort study that began enrollment in 2000. Over time, the study has included 3 phases designated “RFTS 1,” “RFTS 2,” and “RFTS 3.” Women, either pregnant or planning a pregnancy, enrolled from 9 areas in 3 states (North Carolina, Texas, and Tennessee). Participants were between 18 and 45 years of age, spoke English or Spanish, intended to carry the pregnancy to term, and had not used assisted reproductive technologies to conceive (5, 23, 24). The study was designed to recruit women from a variety of clinic- and community-based settings and has been described in detail (24).
Briefly, women who were not yet pregnant but trying to conceive were followed until a positive pregnancy test and enrolled at that time. To avoid overenrollment of subfertile women, nonpregnant participants in the study must have been attempting to get pregnant for fewer than 6 months (RFTS 1 and RFTS 2) or fewer than 3 months (RFTS 3). Women planning pregnancies were eligible to be followed for up to 12 months. Pregnant women formally enrolled in the study before 12 completed weeks of gestation (RFTS 1), before 9 completed weeks of gestation (RFTS 2), or at the time of a positive pregnancy test after preenrollment prior to pregnancy (RFTS 3) (23). Informed, written consent was obtained from each study participant in compliance with institutional review board procedures and approvals.
Women who had their last menstrual period before November 12, 2009, were included in this data set (n = 4,887). Participants had an early pregnancy ultrasound examination for assessment of embryological viability, documentation of stage of development, and confirmation of gestational dating. The accuracy of self-reported last menstrual period dating in this cohort is excellent and has been described (25). Research ultrasound examinations were conducted at a time in gestation (>54/7 weeks from the last menstrual period) in which normal pregnancies would be expected to have a fetal pole and heart rate. Participants completed a baseline interview at the time of enrollment and a comprehensive computer-assisted telephone interview in the first trimester. In the interview, information collected included reproductive and medical history, sociodemographic characteristics, and health behaviors around the time of conception or during pregnancy. Participants who experienced pregnancy loss before the scheduled interview were interviewed as soon as possible after the loss.
Pregnancy outcomes were self-reported by study participants and verified by medical records. Exclusions from the analysis include women who enrolled during more than one pregnancy (n = 240, only the first pregnancy was included), women who had induced abortions (n = 17), women who had a missing pregnancy status at the time of analysis (n = 10), and women who had ectopic/molar pregnancies (n = 7).
Variable definitions
The primary exposure of interest in this analysis is maternal race. Race is a self-reported measure acquired during the baseline interview. For this analysis, race was grouped into the following categories: non-Hispanic white (referred to throughout as “white”) and non-Hispanic black (referred to throughout as “black”). Women with missing information for race (n = 7) or who decline to self-identify their race (n = 2) were excluded from this analysis. Women who self-identified as Hispanic regardless of white or black racial self-identification or as other races, which include Native Americans and Asians, were also excluded from this analysis (n = 327 and n = 207, respectively). A total of 4,070 women contributed to this analysis.
Miscarriage was defined as loss of a recognized pregnancy prior to 20 completed weeks of gestation from the last menstrual period. We documented miscarriage in 537 women (13.2%) during the study period. Pregnancy was verified by ultrasound examination or repeat pregnancy tests. Participants who had birth outcomes at a gestational age later than 20 weeks served as a comparison group. The comparison group (n = 3,533) consisted of women who had livebirths (n = 3,510) or stillbirths (n = 23).
Statistical analysis
Potential confounders examined from baseline and the first-trimester interviews included factors known to be associated with both miscarriage and race. Candidate confounders related to sociodemographic factors included age (years); body mass index calculated as weight (kg)/height (m)2 (<18.5, 18.5–24.9 (referent), 25.0–29.9, ≥30); household income (≤$40,000, $40,001–$80,000, >$80,000 (referent)); maternal education (high school or less, some college, 4 or more years of college (referent)); marital status (married/living as married (referent), other); and insurance coverage (none, private (referent), public, other). In addition, we assessed potential confounders related to maternal reproductive history and health behaviors during pregnancy, namely, parity (yes/no), previous induced abortion (yes/no), diabetes status (yes/no), prenatal vitamin use (yes/no), alcohol use (never, current, former, within 4 months prior to the interview (meaning exposures in the pregnancy and/or periconception window) or ≥4 months from interview), smoking status (never, current, former), and study site (Galveston, Texas; Raleigh and Research Triangle Park, North Carolina (referent); Nashville, Tennessee; Memphis, Tennessee). We did not consider prior history of miscarriage as a confounder in our data because we would be potentially overadjusting when a factor that caused a previous miscarriage may also be a causal factor in the current pregnancy (26).
Gestational age at the time of loss was calculated from the first day of the last menstrual period for the index pregnancy to the end of that gestation. Four women did not have complete last menstrual period information and were not included. Of the 537 women with miscarriage, 18 did not complete the first-trimester interview (3.4%), and 325 completed the interview after their loss (60.5%). Among women who experienced a loss prior to their interview, the mean interval between loss and first-trimester interview was less than 3.5 weeks (24.4 (standard deviation, 18.3) days). To investigate timing of loss, we dichotomized loss at 10 weeks, the median gestational age at time of loss for our cohort. We grouped losses into early loss (prior to 10 weeks’ gestation) and late loss (≥10 weeks’ gestation). In addition, we used ultrasound examination findings by grouping losses into developmental stage documented on ultrasound examination prior to pregnancy loss.
Cox regression was used to estimate hazard ratios for the association between race and risk of miscarriage. Participants were followed from the time of enrollment in the study and contributed to analysis until an outcome or loss to follow-up occurred. Cox models accounted for variable gestational age at study entry and were used to screen candidate confounders. Confounding was defined as a greater than 10% change from the crude hazard ratio for miscarriage risk for black women compared with white women (referent). If a 10% change was observed from the crude hazard ratio, the variable was retained in the final models. Cox models were used to compare overall risk for miscarriage as well as early versus late miscarriage. We restricted the analysis to those with complete covariate information, excluding 118 pregnancies. Final hazard models included adjustment for maternal age and alcohol use. Analyses were conducted by using Stata IC/11.1 software (StataCorp LP, College Station, Texas).
RESULTS
Nearly 23% of participants self-identified as black (n = 932) (Table 1). Compared with white women, black women were more likely to be younger, to have a higher body mass index (≥30 kg/m2), to have income <$80,000, not to have a college degree, to be unmarried, to be parous (>1), and to abstain from alcohol and tobacco products. Additionally, blacks were more likely than whites to have Medicaid coverage rather than private insurance and to have had a prior induced abortion. Mean gestational age at the time of enrollment was later for blacks (7.9 weeks) than for whites (6.8 weeks) (Table 1). There were 537 miscarriages. Among those women who experienced a loss prior to their interview, the mean number of days between loss and interview was similar for whites and blacks (25.5 (standard deviation, 18.0) vs. 20.2 (standard deviation, 17.0) days, respectively). Twenty-three percent of the women in our cohort were recruited prior to pregnancy (n = 943).
Table 1.
Characteristics by Race of “Right From the Start” Participants, 2000–2009
| Baseline Characteristics | RFTS Study Participantsa |
|||||
|---|---|---|---|---|---|---|
| Whites |
Blacks |
|||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | |
| Maternal age, years | 29.8 (4.7) | 26.5 (5.7) | ||||
| Maternal age, years | ||||||
| <20 | 51 | 1.6 | 86 | 9.2 | ||
| 20–24 | 322 | 10.3 | 317 | 34.0 | ||
| 25–29 | 1,121 | 35.7 | 250 | 26.8 | ||
| 30–34 | 1,123 | 35.8 | 187 | 20.1 | ||
| ≥35 | 520 | 16.6 | 92 | 9.9 | ||
| Missing | 1 | 0 | ||||
| Body mass indexb | 24.9 (5.5) | 29.3 (7.9) | ||||
| Body mass index | ||||||
| Underweight (<18.5) | 86 | 2.8 | 18 | 2.0 | ||
| Normal weight (18.5–24.9) | 1,874 | 60.3 | 283 | 30.8 | ||
| Overweight (25.0–29.9) | 678 | 21.8 | 257 | 28.0 | ||
| Obese (≥30.0) | 472 | 15.2 | 360 | 39.2 | ||
| Missing | 28 | 14 | ||||
| Household income | ||||||
| ≤$40,000 | 581 | 19.4 | 528 | 62.9 | ||
| $40,001–$80,000 | 1,250 | 41.7 | 208 | 24.8 | ||
| >$80,000 | 1,169 | 39.0 | 103 | 12.3 | ||
| Missing | 138 | 93 | ||||
| Maternal education | ||||||
| High school or less | 320 | 10.2 | 373 | 40.0 | ||
| Some college | 455 | 14.5 | 263 | 28.2 | ||
| College (≥4 years) | 2,362 | 75.3 | 296 | 31.8 | ||
| Missing | 1 | 0 | ||||
| Marital status | ||||||
| Married, living as married, single | 2,994 | 95.4 | 599 | 64.3 | ||
| Other | 144 | 4.6 | 333 | 35.7 | ||
| Missing | 0 | 0 | ||||
| Insurance coverage | ||||||
| None | 64 | 3.6 | 26 | 10.2 | ||
| Private | 1,583 | 89.8 | 142 | 55.5 | ||
| Public | 104 | 5.9 | 85 | 33.2 | ||
| Other | 11 | 0.6 | 3 | 1.2 | ||
| Missing | 1,376 | 676 | ||||
| Current birth outcome miscarriage | ||||||
| No | 2,732 | 87.1 | 801 | 85.9 | ||
| Yes | 406 | 12.9 | 131 | 14.1 | ||
| Gestational age at enrollment, weeks | ||||||
| Overall | 6.8 (1.8) | 6.6c | 7.9 (2.0) | 7.9c | ||
| If miscarriage occurred | 6.4 (1.7) | 6.0c | 7.4 (1.8) | 7.4c | ||
| Parity | ||||||
| Nulliparous | 1,467 | 48.3 | 390 | 44.8 | ||
| 1 | 1,099 | 36.2 | 264 | 30.3 | ||
| ≥2 | 473 | 15.6 | 217 | 24.9 | ||
| Missing | 99 | 61 | ||||
| Previous miscarriage | ||||||
| No | 2,388 | 78.6 | 651 | 74.7 | ||
| Yes | 651 | 21.4 | 220 | 25.3 | ||
| Missing | 99 | 61 | ||||
| Previous induced abortion | ||||||
| No | 2,657 | 87.4 | 621 | 71.3 | ||
| Yes | 382 | 12.6 | 250 | 28.7 | ||
| Missing | 99 | 61 | ||||
| Pregnant at time of recruitment | ||||||
| No | 845 | 26.9 | 98 | 10.5 | ||
| Yes | 2,293 | 73.1 | 834 | 89.5 | ||
| Missing | 0 | 0 | ||||
| Diabetes | ||||||
| No | 2,973 | 97.5 | 869 | 96.4 | ||
| Type 1 | 9 | 0.3 | 5 | 0.6 | ||
| Type 2 | 2 | 0.1 | 9 | 1.0 | ||
| Gestational diabetes | ||||||
| Nulliparous | 1 | 0.0 | 2 | 0.2 | ||
| 1 | 22 | 0.7 | 1 | 0.1 | ||
| ≥2 | 43 | 1.4 | 15 | 1.7 | ||
| Missing | 88 | 31 | ||||
| Prenatal vitamin use | ||||||
| No | 297 | 10.7 | 177 | 20.7 | ||
| Yes | 2,471 | 89.3 | 680 | 79.3 | ||
| Missing | 370 | 75 | ||||
| Alcohol use | ||||||
| Never | 273 | 8.9 | 195 | 21.6 | ||
| Current | 197 | 6.5 | 10 | 1.1 | ||
| Former | 2,581 | 84.6 | 697 | 77.3 | ||
| Missing | 87 | 30 | ||||
| Smoking | ||||||
| Never | 2,172 | 69.2 | 714 | 76.6 | ||
| Current | 113 | 3.6 | 41 | 4.4 | ||
| Former | 767 | 24.4 | 148 | 15.9 | ||
| Missing | 86 | 2.7 | 29 | 3.1 | ||
Abbreviations: RFTS, “Right from the Start”; SD, standard deviation.
a RFTS participants included 3,138 (77.1%) whites and 932 (22.9%) blacks.
b Body mass index: weight (kg)/height (m)2.
c Median for gestational age at enrollment.
The life table–adjusted cumulative incidence of miscarriage after gestational week 5 was 21.3%. Risk of loss was greater for women who were older, had a higher body mass index, were nulliparous, did not use prenatal vitamins, and had a previous history of miscarriage. Maternal age per year (hazard ratio (HR) = 1.06, 95% confidence interval (CI): 1.04, 1.08), alcohol intake during pregnancy (HR = 4.84, 95% CI: 3.53, 6.63) comparing current drinkers with never drinkers, and unmarried status (HR = 1.46, 95% CI: 1.15, 1.84) compared with married/living as married were associated with increased miscarriage risk in crude analyses. With each recruitment phase, there were increasing proportions of women recruited prior to pregnancy.
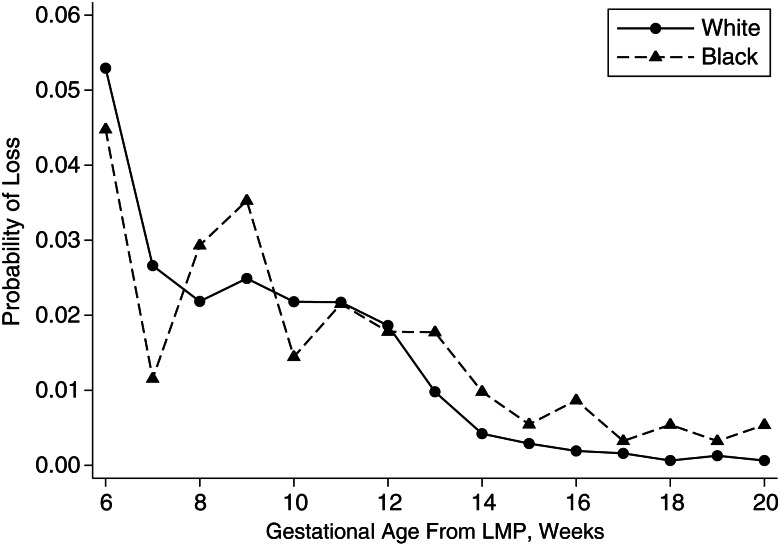
Week-specific risk of miscarriage started at about 5% during gestational week 6 and declined over gestation for both blacks and whites (Figure 1). The risk was higher for blacks than whites for all weeks after gestational week 12. We calculated life table–adjusted cumulative risk of loss for 3 time periods during gestation that may have different pathologies. The cumulative risk of embryonic loss (gestational weeks 6–9) was 15% for whites and 14% for blacks. The cumulative risk for early fetal loss (weeks 10–15) was 6% and 8% and, for late fetal loss (weeks 16–19), was <1% and 2% for whites and blacks, respectively. Black women had a greater hazard for miscarriage compared with white women in unadjusted comparisons (unadjusted HR = 1.32, 95% CI: 1.09, 1.62) (Table 2). With adjustment for age and alcohol consumption, black women had a 57% greater hazard for miscarriage compared with white women (blacks: adjusted hazard ratio (aHR) = 1.57, 95% CI: 1.27, 1.93) (Table 2). When we dichotomized losses at the median gestational age of loss, black women had a 93% greater hazard for later miscarriage (≥10 weeks) compared with white women (aHR = 1.93, 95% CI: 1.48, 2.51) (Table 2). There was no significant association between race and risk of early miscarriage for blacks compared with whites (aHR = 1.15, 95% CI: 0.82, 1.62) (Table 2).
Figure 1.
Week-specific probability of pregnancy loss by race among “Right From the Start” participants, 2000–2009. The x-axis is gestational age at loss from last menstrual period (LMP); the y-axis is probability of pregnancies ending in miscarriage.
Table 2.
Risk of Miscarriage by Race Among “Right From the Start” Participants, 2000–2009
| No. of Losses | Unadjusted |
Adjusted |
|||
|---|---|---|---|---|---|
| HR | 95% CI | aHRa | 95% CI | ||
| Total | |||||
| Whites | 406 | 1.00 | Referent | 1.0 | Referent |
| Blacks | 131 | 1.32 | 1.09, 1.62 | 1.57 | 1.27, 1.93 |
| Early loss (<10 weeks) | |||||
| Whites | 214 | 1.00 | Referent | 1.0 | Referent |
| Blacks | 44 | 1.00 | 0.72, 1.40 | 1.15 | 0.82, 1.62 |
| Late loss (≥10 weeks) | |||||
| Whites | 192 | 1.00 | Referent | 1.00 | Referent |
| Blacks | 87 | 1.59 | 1.24, 2.05 | 1.93 | 1.48, 2.51 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio.
a Adjusted for age and alcohol use.
Data from ultrasound examinations were available for the majority of women who experienced a miscarriage (n = 384, 71.5%) (Table 3). Ultrasound examinations were conducted at a time in which normal pregnancies are clinically expected to have a fetal pole and heart rate. Among those women who experienced a loss and had ultrasound imaging data available, key categories of early embryonic arrest (anembryonic gestations and fetal pole without heart rate or with abnormal heart rate) did not differ significantly by race (Table 3).
Table 3.
Mean Gestational Age at Loss and Ultrasound Characteristics by Race of “Right From the Start” Participants Who Experienced Miscarriage, 2000–2009
| Ultrasound Characteristics | RFTS Study Participants |
|||||
|---|---|---|---|---|---|---|
| Whites (n = 406) |
Blacks (n = 131) |
|||||
| Gestational Age at Loss, Weeks (Mean (SD)) | No. of Losses | % of Loss | Gestational Age at Loss, Weeks (Mean (SD)) | No. of Losses | % of Loss | |
| Loss before ultrasound examination | 122 | 31 | ||||
| Fetal pole | ||||||
| With normal heart rate | 11.73 (3.04) | 101 | 35.6 | 14.88 (3.95) | 36 | 36.0 |
| With abnormal or no heart rate | 10.35 (1.60) | 74 | 26.1 | 11.24 (1.85) | 28 | 28.0 |
| Anembryonic gestationa | 9.51 (2.40) | 109 | 38.4 | 10.53 (3.13) | 36 | 36.0 |
Abbreviations: RFTS, “Right from the Start”; SD, standard deviation.
a Anembryonic gestation includes only gestational sac present (n = 44 (whites), n = 12 (blacks)); gestational and yolk sac present (n = 49 (whites), n = 13 (blacks)); and empty uterus (n = 16 (whites), n = 11 (blacks)).
DISCUSSION
Black women in our cohort were more likely than white women to experience a pregnancy loss. The increased risk was greater in the period from 10 to 20 weeks’ gestation. Previous studies have built multivariable models considering race as a confounder in assessing miscarriage risk (7, 27–29), but they have not examined race as a main effect. As a multistate prospective community-based cohort, with 23% black participants, “Right from the Start” (24) is well-suited to examine race as an independent factor in assessing miscarriage risk.
The study of miscarriage requires careful assessment of gestational time at study entry because women who enter a study later will have less opportunity for a miscarriage to be observed than women who enter very early in pregnancy. A recent review found only 4 prior studies that collected adequate data to estimate miscarriage risk by week of gestation from early in pregnancy (30). Three of the 4 prior studies showed the highest risk of loss early in gestation. All 4 prior studies showed declining risk from gestational weeks 12–20, as in our study. None of those prior studies examined differences in miscarriage risk by race. Our study supports the earlier studies that show higher risk of loss very early in pregnancy that declines with increasing gestational age but does not support the prior study (10) that found the highest risk during gestational weeks 8–10. Estimates of miscarriage risk very early in pregnancy are in the time period most vulnerable to bias, so further studies with early ascertainment of pregnancy and careful longitudinal follow-up are needed. The finding of increased risk for blacks during weeks 10–20 is less vulnerable to such biases because most women have recognized their pregnancy by week 10. Only the first phase of RFTS enrollment allowed women to enter the study later than week 9.
Our primary finding was that black women have a nearly 2-fold higher risk of miscarriage compared with white women during gestational weeks 10–20, while there was no apparent difference in the risk of earlier miscarriage. We conducted several analyses to evaluate possible bias in our findings. Because previous history of miscarriage is an established predictor for future miscarriage (1, 2, 9), we did a simple sensitivity analysis, removing women who had ever reported having a prior miscarriage (n = 871) from the analysis. Estimates for risk of miscarriage did not change appreciably when these women were removed, and risk among black women remained elevated (adjusted HR = 1.51, 95% CI: 1.18, 1.95). We were also concerned about possible bias due to differences among the 3 separate phases of our study, but we found that study site, a good surrogate for study phase, was not a confounder for the association between race and miscarriage risk. Another concern with all prospective studies of miscarriage is that women who exhibit symptoms suggestive of pregnancy problems will enroll in order to seek help. This is rare in our study, because all women had to identify a prenatal care plan to enroll. However, 36 women had losses within 3 days of enrollment (6.7%). When we remove these women, our results are robust; the risk of miscarriage remains elevated for blacks compared with whites (aHR = 1.61, 95% CI: 1.31, 1.99). Finally, for 60% of women with miscarriages, ascertainment of behavioral factors during pregnancy, including alcohol consumption and smoking, was collected after the loss, raising the potential for recall bias. However, we interviewed women as soon as possible following their loss, on average less than 3.5 weeks, and the questions were clearly asking about behaviors during the pregnancy. When we simply adjusted for age, our findings remained.
The primary strength of our study is our ability to follow a large sample of women recruited from the community prospectively through their pregnancies, many of whom were enrolled prior to pregnancy (24). In addition, we were able to evaluate numerous potential confounders (9, 28, 31) and analyze the data with hazard models that account for variation in gestational age at study entry. “Right from the Start” avoids overselection of women who may be subtly symptomatic or at high risk by advertising as a study about pregnancy health. Women do not alter prenatal care choices to enroll, and therefore it is unlikely that enrollment procedures and study activities influenced behaviors or outcomes within this population.
Generalizability of our findings may be limited by enrollment of volunteers. Volunteers tend to be better educated, to be more health conscious with lifestyle factors related to pregnancy planning, and to have access to care before pregnancy (5). However, even in our cohort, not all pregnancies were intended; 39% of women reported getting pregnant despite using a method to prevent pregnancy. Furthermore, the risk of miscarriage remained elevated for blacks compared with whites regardless of stated pregnancy intention. Given the volunteer nature of our sample with nearly a third of black women having 4 years of college, our sample of black women might be expected to be at lower risk of miscarriage than blacks in the general population. Despite this possibility which would attenuate effects, we observed a clear elevation in risk of late miscarriage for blacks.
Biological mechanisms to explain miscarriage are not well-understood (1, 31, 32). Chromosomal abnormalities have been recognized in at least half of all pregnancy losses occurring in the first trimester, but they are less common in later losses (4, 31). Postimplantation events of pregnancy prior to week 10 that put a conceptus at risk include failure of trophoblast tissue to block the spiral arteries resulting in failure to maintain the low oxygen environment needed for organogenesis and failure of a timely luteal-placental shift resulting in failure to maintain adequate progesterone support (30). Remodeling of the spiral arteries to allow adequate maternal blood flow for fetal development occurs toward the end of the first trimester, and this would be one candidate for further study to explain the elevated risk we observed in blacks. Alternatively, intrauterine hematomas at 13–14 weeks’ gestation increase the risk for preeclampsia, which may be associated with chronic inflammatory reactions resulting in preterm rupture and delivery that may explain the disparities seen in adverse pregnancy outcomes (20, 33).
Silver et al. (34) argue that one approach to coping with etiologic heterogeneity in miscarriage is to consider miscarriages of different gestational ages as different outcomes. They note that losses occurring after the 16th week may share a pathophysiology similar to that of early preterm birth or stillbirth (34), and combining those outcomes might be useful. We do not have adequate data on such factors as infection that are associated with early preterm birth to investigate late fetal loss in detail, but our findings of elevated fetal loss in blacks are consistent with such shared etiologies and suggest that they may also be shared by early fetal losses (loss during weeks 10–15). We used ultrasound examination data to evaluate fetal viability among study participants and to assess developmental stage prior to pregnancy loss. Ultrasound information was available for the majority of women who experienced a loss in our cohort, and we observed similar patterns of early embryological arrest in blacks and whites. This also suggests that the increased risk of later loss among black women may reflect events during fetal development after initial organogenesis is complete (rather than early embryologic insults).
The elevated risk of fetal loss in blacks could involve several plausible causal pathways including differences in the following: 1) environmental or product exposures that accrue over weeks across pregnancy; 2) risk of insult from existing health vulnerabilities such as anemia or insulin resistance that vary by race and exert greater influence on fetal than embryological viability; or 3) genetic mechanisms such as those in inflammatory or immunological pathways that may vary by race and influence fetal well-being. Further investigation is warranted to examine biologically plausible explanations of the disparity in risk and pattern of loss between black women and white women. Such research has potential to advance overall knowledge about causes of pregnancy loss and to identify risks that may be preventable.
ACKNOWLEDGMENTS
Author affiliations: Vanderbilt Epidemiology Center, Institute for Medicine and Public Health, Vanderbilt University, Nashville, Tennessee (Sudeshna Mukherjee, Digna R. Velez Edwards, Katherine E. Hartmann); Department of Obstetrics and Gynecology, Vanderbilt University Medical Center, Nashville, Tennessee (Digna R. Velez Edwards, Katherine E. Hartmann); National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Donna D. Baird); Department of Epidemiology, Brown University, Providence, Rhode Island (David A. Savitz); and Department of Obstetrics and Gynecology, Brown University, Providence, Rhode Island (David A. Savitz).
The field research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grants R01HD043883 and R01HD049675) and the American Water Works Association Research Foundation (grant 2579). Additional funds were provided by the Building Interdisciplinary Research Careers in Women's Health career development program (grant K12HD4383), the Vanderbilt Clinical and Translational Science Award UL1 RR024975-01, and the Intramural Research Program of the National Institutes of Health (National Institute of Environmental Health Sciences).
Conflict of interest: none declared.
REFERENCES
- 1.Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 2.Maconochie N, Doyle P, Prior S, et al. Risk factors for first trimester miscarriage—results from a UK-population-based case-control study. BJOG. 2007;114(2):170–186. doi: 10.1111/j.1471-0528.2006.01193.x. [DOI] [PubMed] [Google Scholar]
- 3.Kline J, Stein Z, Susser M. Conception to Birth: Epidemiology of Prenatal Development. New York, NY: Oxford University Press; 1989. [Google Scholar]
- 4.Michels TC, Tiu AY. Second trimester pregnancy loss. Am Fam Physician. 2007;76(9):1341–1346. [PubMed] [Google Scholar]
- 5.Hasan R, Olshan AF, Herring AH, et al. Self-reported vitamin supplementation in early pregnancy and risk of miscarriage. Am J Epidemiol. 2009;169(11):1312–1318. doi: 10.1093/aje/kwp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavanaugh K, Hershberger P. Perinatal loss in low-income African American parents. J Obstet Gynecol Neonatal Nurs. 2005;34(5):595–605. doi: 10.1177/0884217505280000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang HP, Bracken MB. Tree-based, two-stage risk factor analysis for spontaneous abortion. Am J Epidemiol. 1996;144(10):989–996. doi: 10.1093/oxfordjournals.aje.a008869. [DOI] [PubMed] [Google Scholar]
- 8.Goldhaber MK, Fireman BH. The fetal life table revisited: spontaneous abortion rates in three Kaiser Permanente cohorts. Epidemiology. 1991;2(1):33–39. [PubMed] [Google Scholar]
- 9.Risch HA, Weiss NS, Clarke EA, et al. Risk factors for spontaneous abortion and its recurrence. Am J Epidemiol. 1988;128(2):420–430. doi: 10.1093/oxfordjournals.aje.a114982. [DOI] [PubMed] [Google Scholar]
- 10.Wen W, Shu XO, Jacobs DR, Jr, et al. The associations of maternal caffeine consumption and nausea with spontaneous abortion. Epidemiology. 2001;12(1):38–42. doi: 10.1097/00001648-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Janevic T, Stein CR, Savitz DA, et al. Neighborhood deprivation and adverse birth outcomes among diverse ethnic groups. Ann Epidemiol. 2010;20(6):445–451. doi: 10.1016/j.annepidem.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiely M, El-Mohandes AAE, El-Khorazaty MN, et al. An integrated intervention to reduce intimate partner violence in pregnancy: a randomized controlled trial. Obstet Gynecol. 2010;115(2):273–283. doi: 10.1097/AOG.0b013e3181cbd482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Love C, David RJ, Rankin KM, et al. Exploring weathering: effects of lifelong economic environment and maternal age on low birth weight, small for gestational age, and preterm birth in African-American and white women. Am J Epidemiol. 2010;172(2):127–134. doi: 10.1093/aje/kwq109. [DOI] [PubMed] [Google Scholar]
- 14.Miranda ML, Swamy GK, Edwards S, et al. Disparities in maternal hypertension and pregnancy outcomes: evidence from North Carolina, 1994–2003. Public Health Rep. 2010;125(4):579–587. doi: 10.1177/003335491012500413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise LA, Palmer JR, Heffner LJ, et al. Prepregnancy body size, gestational weight gain, and risk of preterm birth in African-American women. Epidemiology. 2010;21(2):243–252. doi: 10.1097/EDE.0b013e3181cb61a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahern J, Pickett KE, Selvin S, et al. Preterm birth among African American and white women: a multilevel analysis of socioeconomic characteristics and cigarette smoking. J Epidemiol Community Health. 2003;57(8):606–611. doi: 10.1136/jech.57.8.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kistka Z, Palomar L, Lee K, et al. Racial disparity in the frequency of recurrence of preterm birth. Am J Obstet Gynecol. 2007;196(2):131.e1–131.e6. doi: 10.1016/j.ajog.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 18.Kramer MR, Hogue CR. What causes racial disparities in very preterm birth? A biosocial perspective. Epidemiol Rev. 2009;31:84–98. doi: 10.1093/ajerev/mxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baptiste-Roberts K, Salafia CM, Nicholson WK, et al. Maternal risk factors for abnormal placental growth: the National Collaborative Perinatal Project. BMC Pregnancy Childbirth. 2008;8:44. doi: 10.1186/1471-2393-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12(6):747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasdaglis T, Aberdeen G, Turan O, et al. Placental growth factor in the first trimester: relationship with maternal factors and placental Doppler studies. Ultrasound Obstet Gynecol. 2010;35(3):280–285. doi: 10.1002/uog.7548. [DOI] [PubMed] [Google Scholar]
- 22.Papaioannou GI, Syngelaki A, Maiz N, et al. Ultrasonographic prediction of early miscarriage. Hum Reprod. 2011;26(7):1685–1692. doi: 10.1093/humrep/der130. [DOI] [PubMed] [Google Scholar]
- 23.Hasan R, Baird DD, Herring AH, et al. Association between first-trimester vaginal bleeding and miscarriage. Obstet Gynecol. 2009;114(4):860–867. doi: 10.1097/AOG.0b013e3181b79796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Promislow JH, Makarushka CM, Gorman JR, et al. Recruitment for a community-based study of early pregnancy: the Right From The Start study. Paediatr Perinat Epidemiol. 2004;18(2):143–152. doi: 10.1111/j.1365-3016.2003.00546.x. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman CS, Messer LC, Mendola P, et al. Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatr Perinat Epidemiol. 2008;22(6):587–596. doi: 10.1111/j.1365-3016.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 26.Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol. 1993;137(1):1–8. doi: 10.1093/oxfordjournals.aje.a116591. [DOI] [PubMed] [Google Scholar]
- 27.Guendelman S, Gould JB, Hudes M, et al. Generational differences in perinatal health among the Mexican American population: findings from HHANES 1982–84. Am J Public Health. 1990;80(suppl):61–65. doi: 10.2105/ajph.80.suppl.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li DK, Liu LY, Douh R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. BMJ. 2003;327(7411):368–371. doi: 10.1136/bmj.327.7411.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver RM, Zhao Y, Spong CY, et al. Prothrombin gene G20210A mutation and obstetric complications. Obstet Gynecol. 2010;115(1):14–20. doi: 10.1097/AOG.0b013e3181c88918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol. 2012;94(6):417–423. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- 31.Wilcox AJ, Weinberg CR, Baird DD. Risk factors for early pregnancy loss. Epidemiology. 1990;1(5):382–385. doi: 10.1097/00001648-199009000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340(23):1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 33.Picklesimer AH, Jared HL, Moss K, et al. Racial differences in C-reactive protein levels during normal pregnancy. Am J Obstet Gynecol. 2008;199(5):523 e1–523 e6. doi: 10.1016/j.ajog.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver RM, Branch DW, Goldenberg R, et al. Nomenclature for pregnancy outcomes: time for a change. Obstet Gynecol. 2011;118(6):1402–1408. doi: 10.1097/AOG.0b013e3182392977. [DOI] [PubMed] [Google Scholar]