Abstract
Sweet potato (Ipomoea batatas) root parenchyma and tobacco (Nicotiana tabacum) stem pith, both known to increase peroxidase activity after excision, differed from each other in their isoperoxidase patterns and in the isoperoxidase responses to injury and exogenous ethylene.
In potato root sections, the injury-dependent peroxidase increase was due to an induction of two isoenzymes, as well as to a promotion of some constitutive ones. In tobacco pith, this increase was entirely due to seven isoperoxidases not detectable, or detectable only in traces, immediately after excision. Actinomycin D did not inhibit the development of any isoperoxidases in the potato root sections and strongly repressed the development of all injury-induced isoenzymes in tobacco pith. Cycloheximide totally inhibited the development of all isoperoxidases in both species, with the exception of two injury-enhanced isoenzymes in root parenchyma.
In root sections, indoleacetic acid had a weak inhibitory effect on one injury-induced isoperoxidase only, whereas in tobacco pith it inhibited the development of the injury-induced, as well as the constitutive, isoperoxidases.
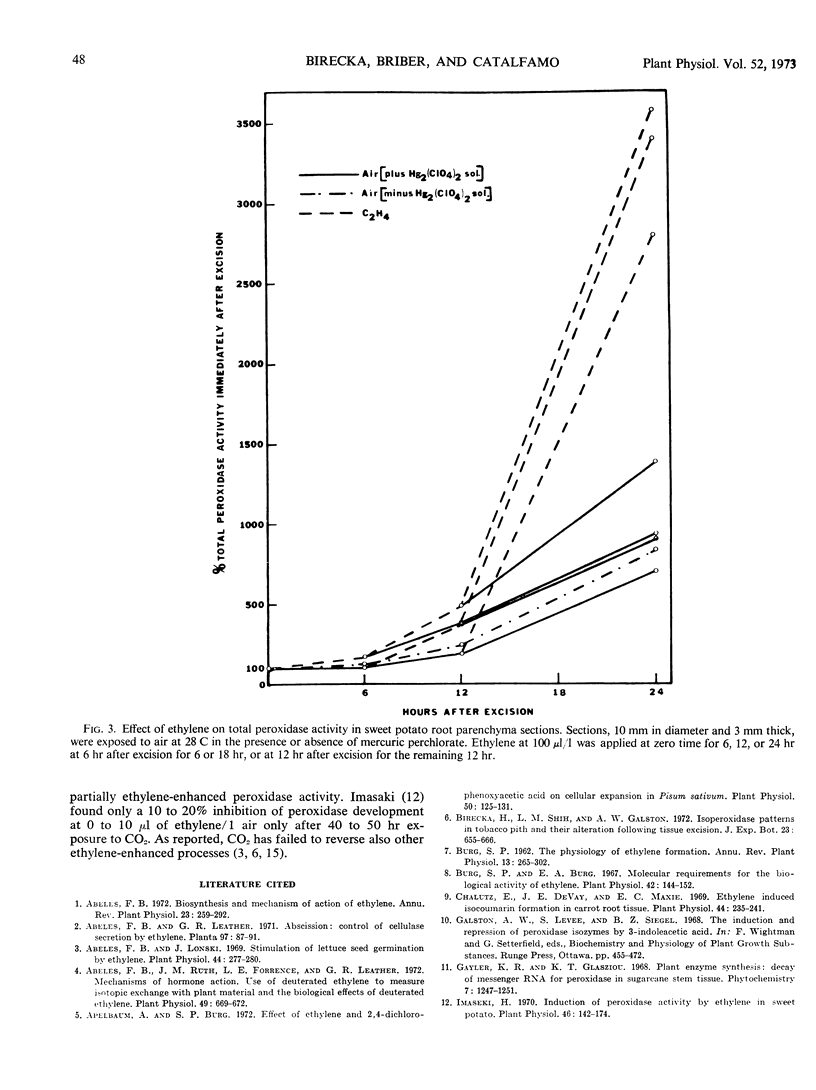
Exogenous ethylene did not induce, enhance, or significantly suppress any of the tobacco pith isoenzymes, whereas in potato root sections, it suppressed slightly the development of the injury-induced, had no effect on some of the injury-enhanced, and greatly promoted some of the injury-unaffected or-enhanced isoperoxidases. Removal of ethylene stopped the ethylene-dependent peroxidase increase without affecting the injury-induced increase. When applied to intact potato roots, ethylene did not induce any new isoperoxidases and promoted the same constitutive isoenzymes as it did in root sections.
Thus, the tissue peroxidase response to ethylene seems independent of its response to injury. Differences between tissue species in their response to ethylene may depend on the presence or absence of isoperoxidases sensitive to ethylene. The inhibition of injury-dependent peroxidase development by indoleacetic acid cannot be explained by an ethylene-induced inhibition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Lonski J. Stimulation of lettuce seed germination by ethylene. Plant Physiol. 1969 Feb;44(2):277–280. doi: 10.1104/pp.44.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B., Ruth J. M. Mechanisms of hormone action: use of deuterated ethylene to measure isotopic exchange with plant material and the biological effects of deuterated ethylene. Plant Physiol. 1972 May;49(5):669–671. doi: 10.1104/pp.49.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Burg S. P. Effects of Ethylene and 2,4-Dichlorophenoxyacetic Acid on Cellular Expansion in Pisum sativum. Plant Physiol. 1972 Jul;50(1):125–131. doi: 10.1104/pp.50.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalutz E., Devay J. E., Maxie E. C. Ethylene-induced Isocoumarin Formation in Carrot Root Tissue. Plant Physiol. 1969 Feb;44(2):235–241. doi: 10.1104/pp.44.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketring D. L., Morgan P. W. Physiology of Oil Seeds: IV. Role of Endogenous Ethylene and Inhibitory Regulators during Natural and Induced Afterripening of Dormant Virginia-type Peanut Seeds. Plant Physiol. 1972 Sep;50(3):382–387. doi: 10.1104/pp.50.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Uritani I. Effect of gamma radiation on peroxidase development in sweet potato disks. Radiat Res. 1970 Feb;41(2):342–351. [PubMed] [Google Scholar]
- Reid M. S., Pratt H. K. Effects of ethylene on potato tuber respiration. Plant Physiol. 1972 Feb;49(2):252–255. doi: 10.1104/pp.49.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon L. M., Uritani I., Imaseki H. De novo synthesis of peroxidase isozymes in sweet potato slices. Plant Physiol. 1971 Apr;47(4):493–498. doi: 10.1104/pp.47.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahmann M. A., Clare B. G., Woodbury W. Increased disease resistance and enzyme activity induced by ethylene and ethylene production of black rot infected sweet potato tissue. Plant Physiol. 1966 Nov;41(9):1505–1512. doi: 10.1104/pp.41.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]


