Abstract
An increase in circulating catecholamines constitutes one of the mechanisms whereby human body responds to stress. In response to chronic stressful situations, the adrenal medullary tissue exhibits crucial morphological and functional changes that are consistent with an improvement of chromaffin cell stimulus-secretion coupling efficiency. Stimulus-secretion coupling encompasses multiple intracellular (chromaffin cell excitability, Ca2+ signaling, exocytosis, endocytosis) and intercellular pathways (splanchnic nerve-mediated synaptic transmission, paracrine and endocrine communication, gap junctional coupling), each of them being potentially subjected to functional remodeling upon stress. This review focuses on three chromaffin cell incontrovertible actors, the cholinergic nicotinic receptors and the voltage-dependent T-type Ca2+ channels that are directly involved in Ca2+-dependent events controlling catecholamine secretion and electrical activity, and the gap junctional communication involved in the modulation of catecholamine secretion. We show here that these three actors react differently to various stressors, sometimes independently, sometimes in concert or in opposition.
Keywords: adrenal stimulus-secretion coupling, voltage-gated calcium channels, gap junctions, nicotinic receptors, chromaffin cells, stress
Introduction
In mammals, catecholamine secretion from adrenal chromaffin cells represents an ubiquitous mechanism helping the organism to cope with stressful situations. In response to stress, catecholamines are among the first mediators to be released. Once delivered into the blood circulation, these stress hormones exert multiple actions in particular on the cardiovascular system, leading to appropriate adjustments of blood pressure and cardiac rhythm, and of the energy metabolism, enabling the organism to respond with a threat for survival. To ensure appropriate catecholamine secretion, whose needs strongly differ from basal to stressful conditions, stimulus-secretion coupling of adrenal chromaffin cells undergoes continuous adaptation. This is achieved by transient or sustained remodeling of pivotal molecular, cellular and tissular adrenal medullary determinants. Among them, synaptic cholinergic neurotransmission between splanchnic nerve endings and chromaffin cells, gap junction-mediated intercellular communication between chromaffin cells and voltage-gated calcium channels at the membrane of chromaffin cells, appear to play substantial roles. For each of these three stimulus-secretion coupling components, this review will describe their contribution to catecholamine exocytosis in basal resting conditions when the hormonal need is low, and how they remodel in response to stressors when the catecholamine demand is high. We will show that the initial stress experienced at birth occurs in the absence of effective acetylcholine neurotransmission and that response of the adrenal medulla involves a non-neurogenic catecholamine secretion in which both gap junctions and low-voltage-activated T-type channels are key actors. Subsequently, the development of the neurogenic control down-regulates the contribution of gap junctions and T-type channels and devotes the predominant control of catecholamine secretion to acetylcholine receptors and high-voltage activated calcium channels (L, N, P/Q, R). In response to chronic stress in adulthood, concerted remodeling of cholinergic synaptic transmission, gap junctions and calcium channels occurs, so as to optimize the excitation-secretion coupling to the new body conditions.
1. Nicotinic acetylcholine receptor channels: the primary target of stimulus-secretion coupling
The primary stimulus of catecholamine secretion from the adrenal medulla comes from the activation of the splanchnic nerve, which releases acetylcholine from its axon terminals and stimulates postsynaptic cholinergic receptors located on chromaffin cells (Douglas, 1968; Kidokoro and Ritchie, 1980; Wakade, 1981). Although cholinergic muscarinic receptors can also participate in chromaffin cell stimulus-secretion coupling (Barbara et al., 1998; Olivos and Artalejo, 2008), we focus here exclusively on nicotinic receptors (nAChRs). Indeed, while nAChRs ensure a rapid transmission of preganglionic impulses, muscarinic receptors are considered to play a more subsidiary role mostly by facilitating the nicotinic responses. In particular, they are proposed to contribute to long-lasting excitation of chromaffin cells by regulating the catecholamine secretory response upon graded preganglionic stimulation and prolonged periods of time (Olivos and Artalejo, 2008).
1.1. Chromaffin cell nicotinic acetylcholine receptors under resting conditions
The classical view is that activation of nAChRs evokes a membrane depolarization, which in turn triggers electrical activity and calcium influx from voltage-dependant calcium channels (Kilpatrick et al., 1982). This massive calcium influx, potentiated by a calcium-induced calcium release from the endoplasmic reticulum via the ryanodine receptors (Alonso et al., 1999; Inoue et al., 2003; Wu et al., 2010), would then be responsible for triggering exocytosis of catecholamine-containing granules. Additionally, calcium influx through the nAChR channel itself may directly contribute to intracellular calcium elevation and to catecholamine release. Although challenged recently (Arnaiz-Cot et al., 2008), this hypothesis is strongly supported by the fact that activation of nAChRs triggers exocytosis from bovine chromaffin cells in the absence of membrane depolarization (Mollard et al., 1995).
Chromaffin cell nAChRs belong to the neuronal nAChR family (Gotti et al., 2006; Sala et al., 2008). They are composed of five principal transmembrane subunits of the a and β families. Since calcium permeability and duration of pore opening largely depend on the subunit composition (Albuquerque et al., 2009), it is worth studying this composition and its putative regulation during physiological (development, aging,…) and/or physiopathological (stress, hypertension,…) situations. Heteromeric α3β4 receptors are considered the main nAChRs expressed in bovine (Criado et al., 1992; Campos-Caro et al., 1997) or rat (Di Angelantonio et al., 2003; Colomer et al., 2010) chromaffin cells, although α5 subunit transcript has also been detected in bovine chromaffin cells (Campos-Caro et al., 1999). α3β4 nAChRs belong to the α-bungarotoxin insensitive receptor class and they are believed to account for most of the acetylcholine-triggered catecholamine release in control conditions, which is mainly insensitive to this toxin (Kilpatrick et al., 1981; Sala et al., 2008). In addition to this dominant form, α-bungarotoxin sensitive homomeric α7- and α9/α10-built nAChRs are also expressed in bovine (Garcia-Guzman et al., 1995) and rat (Martin et al., 2003; Colomer et al., 2010) chromaffin cells, but their role in catecholamine secretion is less clear. Using specific toxins, Lopez and collaborators (Lopez et al., 1998) have demonstrated that α7 nAChRs do contribute to acetylcholine-induced inward current and to catecholamine secretion from bovine chromaffin cells. However, it remains that catecholamine release is insensitive to α-bungarotoxin in many preparations (Sala et al., 2008), shading doubts on the ubiquitous contribution of this receptor to catecholamine exocytosis. Regarding α9-containing nAChRs, they contribute to spontaneous excitatory postsynaptic and acetylcholine-evoked inward currents (Colomer et al., 2010), but their contribution to catecholamine exocytosis remains to be investigated. Human chromaffin cells express both homomeric and heteromeric nAChRs, as evidenced by the identification of α7 (Perez-Alvarez et al., 2011c) and α6β4-containing channels (Perez-Alvarez et al., 2011a). From a functional point of view, these nAChRs differentially contribute to catecholamine exocytosis. Ca2+ entry through α7 nAChRs is unable to evoke exocytosis by itself, but these receptors contribute to fast-onset membrane depolarization and subsequently to hormone secretion (Perez-Alvarez et al., 2011c). By contrast, α6β4-containing nAChRs are directly involved in the exocytotic process (Perez-Alvarez et al., 2011a).
1.2. Modulation in response to stressful situations
Direct sensitivity to hypoxia is the mean by which chromaffin cells release catecholamines during fetus expulsion, when the transition from the intra-uterine cocoon to the airborne outside induce a lack of oxygen and threatens all organs. The neonate copes with this stressful situation by triggering a catecholamine surge. By stimulating the cardiovascular and respiratory systems, catecholamines contribute to the ability of the organism to withstand acute oxygen deprivation and to protect organs from severe damages. The striking features of catecholamine secretion occurring in neonates are that i) it occurs before the complete maturation of splanchnic nerve synapses, adrenal medullary innervation being non-functional until the end of the first postnatal week (Slotkin, 1986; Parker et al., 1988) and ii) it is triggered directly by the propensity of some chromaffin cells to sense oxygen decrease and to respond by catecholamine release (Seidler and Slotkin, 1985; Thompson et al., 1997; Garcia-Fernandez et al., 2007) and reviewed in (Colomer et al., 2011). Maturation of splanchnic nerve synapses during the first postnatal week allows the neurogenic control of catecholamine release to take place and sets a tonic inhibition on the direct sensitivity of chromaffin cells to hypoxia (Thompson et al., 1997; Garcia-Fernandez et al., 2007; Nurse et al., 2009). This negative control is indeed mediated by acetylcholine and by the contribution of the α7-built nAChRs (Buttigieg et al., 2009). Further support for this contribution comes from the observation that long-term hypoxia, supposed to stimulate the direct sensitivity of chromaffin cells to hypoxia, decreases the expression of α7 subunit in ovine fetus adrenal gland (Ducsay et al., 2007). Recently, it has been reported that the expression of α9-containing nAChRs (measured both at the messenger and protein levels) is specifically upregulated by cold exposure-evoked stress (Colomer et al., 2010). Although their contribution to catecholamine release has not been evaluated yet, in view of their high calcium permeability as compared to α3-containing nAChRs (Jagger et al., 2000; Fucile, 2004; Fucile et al., 2006), it is highly probable that α9-containing nAChRs significantly contribute to the high catecholamine secretion needed during chronic stressful situations. All these findings strengthen an important contribution of nAChRs in adaptive response to stress.
As an attempt to schematize the respective role of nAChRs in the adrenal medullary tissue, we can state that the α3β4-built receptor is the pillar on which acetylcholine-evoked catecholamine release lays primarily. Homomeric α7- and α9-containing receptors, although probably contributing to this process, seem mainly involved in adjoining but crucial long-term regulatory processes. Indeed, cholinergic innervation of the adrenal medulla does not only trigger secretion, it also maintains a negative control on two specific properties of chromaffin cells: their specific activation by hypoxia (α7-built nAChRs, see above) and their degree of gap junction-mediated intercellular communication (α9-built nAChRs, see chapter 3).
In summary, a global view would be that, in control conditions, heteromeric α3β4 receptors carry out the main part of catecholamine secretion while other cholinergic functions, involving long-term regulations of chromaffin cells, would be taken in charge by homomeric α7 and/or α9/α10 nAChRs. In response to the threat of chronic stress (long-term hypoxia, cold exposure, …), up- or down-regulation of these homomeric channels would allow chromaffin cells to recover some of their inhibited functions and to be better armed to respond and protect the individual. Consistent with this hypothesis, α7- but not α4β2-built nAChRs have been described as the main nAChR subtype responsible for the attenuation of lipopolysaccharide-induced neuroinflammation in the brain (Tyagi et al., 2010). A further support for the important role of homomeric nAChRs in the stress response comes from the finding that α7 nAChRs are strongly regulated by chronic stress and corticosteroids/glucocorticoids (Carrasco-Serrano and Criado, 2004), especially in hippocampus (Hunter et al., 2010), a brain region involved in stress reactivity. It is noteworthy that beside the adrenal medullary tissue, stress also induces remodeling of nAChRs in other sympathetic ganglia, as recently reported in sympathetic neurons in response to hyperglycemia (Campanucci et al., 2010). Altogether and in addition to numerous studies performed in brain of stressed animals, neuronal nAChRs appear to be crucial targets in the response to stress.
2. Gap junction channels: a positive modulator of catecholamine secretion
As mentioned above, adrenal catecholamine secretion in under a dual regulation involving an incoming initial cholinergic command from the splanchnic nerve terminals synapsing onto chromaffin cells and a local modulation through gap junctions expressed by chromaffin cells (Colomer et al., 2009, 2011 for two recent reviews). The cholinergic control of catecholamine secretion is known for several decades (Douglas et al., 1967; Douglas, 1968; Axelrod, 1971; Douglas, 1975). By contrast, the involvement of gap junctional coupling between chromaffin cells in catecholamine release has been recently reported (Martin et al., 2001), although it was suspected for a long time (Cena et al., 1983).
Gap junction channels are built of the apposition of two hemichannels in adjacent cells. Each hemichannel is composed of six protein subunits called connexins in vertebrates. Gap junction channels connect the cytoplasm of two contiguous cells, therefore providing a direct route for electrical and metabolic signaling between coupled cells. By regulating the passage of ions and small molecules, gap junction channels are critically important in many biological activities, including hormone secretion from endocrine/neuroendocrine cells (Caton et al., 2002; Colomer et al., 2009; Jain and Lammert, 2009; Stojilkovic et al.; Bosco et al., 2011).
2.1. Connexin expression in the adrenal medullary tissue
The presence of intercellular junctions in the adrenal medullary tissue was initially uncovered by freeze-fracture (Grynszpan-Wynograd and Nicolas, 1980). Gap junction plaques were identified in various species, including mouse, guinea-pig, hamster and rabbit, but not in rat (Grynszpan-Wynograd and Nicolas, 1980). Regarding protein subunit expression, six connexins (Cx26, Cx29, Cx32, Cx36, Cx43 and Cx50) have been unambiguously found in the adrenal medullary tissue to date (recently reviewed in (Colomer et al., 2009; Colomer et al., 2011). Table 1 summarises the main connexins expressed in the mammalian adrenal medulla, both in normal and tumoral tissue. The presence of connexin-built gap junctions has been described in several adrenal medullary cell types, including both secreting and non-secreting cells.
Table 1. Summary of the main connexins expressed in the normal and tumoral adrenal medullary tissue from various species.
Data collected from the combination of western blot, immunostaining, single-cell PCR, real-time PCR and β-galactosidase assay techniques. (+/−, weakly expressed; +, robustly expressed)
| MOUSE | RAT | GUINEA-PIG | HUMAN | ||||
|---|---|---|---|---|---|---|---|
| normal tissue | PC-12 cells | normal tissue | benign pheochromocytoma | malignant pheochromocytoma | |||
| Cx26 | + (Willenberg et al., 2006) | + (Willenberg et al., 2006) | |||||
| Cx29 | + (Eiberger et al., 2006) | ||||||
| Cx32 | +/− (Willenberg et al., 2006) | +/− (Willenberg et al., 2006) | |||||
| Cx36 | + (Degen et al., 2004 ; Li et al., 2004) | + (Martin et al., 2001 ; Colomer et al., 2008b) | + (Lu et al., 2007) | ||||
| Cx43 | +/− (Murray and Pharrams, 1997) | + (Meda et al., 1993 ; Martin et al., 2001 ; Colomer et al., 2008b) | +/− (Murray and Pharrams, 1997) | +/− (Willenberg et al., 2006) | +/− (Willenberg et al., 2006) | ||
| Cx50 | + (Willenberg et al., 2006) | + (Willenberg et al., 2006) | +/− (Willenberg et al., 2006) | ||||
To date, three connexins (Cx36, Cx43 and Cx50) have been identified in chromaffin cells (figure 1), with a differential expression between species. While rat neuroendocrine chromaffin cells exhibit both Cx36 and Cx43 (Meda et al., 1993; Martin et al., 2001; Colomer et al., 2008b), mouse chromaffin cells appear to be dominantly coupled by Cx36-built gap junctions, Cx43 being weakly expressed (Murray and Pharrams, 1997; Degen et al., 2004; Li et al., 2004), personal observations). In the human adrenal medulla, the main connexin expressed in chromaffin cells is Cx50 (Willenberg et al., 2006). In addition, the expression of connexins also depends on the normal or tumoral state of the tissue, as reported in human. Indeed, in pheochromocytomas, tumors arising from catecholamine-secreting cells, expression of Cx26 and Cx32, which are not present in the normal tissue, has been detected (Willenberg et al., 2006; Colomer et al., 2011 for review) (table 1).
Figure 1. Cellular distribution of connexins within the mammalian adrenal medullary tissue.
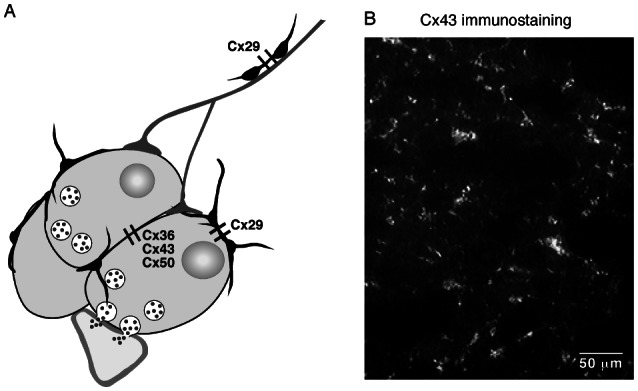
A. Neuroendocrine chromaffin cells are coupled by three connexins (Cx36, Cx43 and Cx50), while non-secreting sustentacular cells express a unique connexin (Cx29). In addition, note that Cx29 is also present in cells surrounding preganglionic sympathetic nerve fibers innervating the medulla. B. Representative example of Cx43 immunolabeling in the rat adrenal medulla (polyclonal rabbit anti-Cx43 from Zymed Laboratories).
Regarding non-secreting cells in the adrenal medullary tissue, Cx29 is expressed in S100-positive cells (Eiberger et al., 2006), targeting likely the glial-like sustentacular cell population (Cocchia and Michetti, 1981; Lloyd et al., 1985) (figure 1A). This is consistent with previous studies illustrating the presence and functionality of gap junction channels between glial-like non-endocrine pituitary folliculostellate cells (Morand et al., 1996; Fauquier et al., 2001). Cx29 is also expressed in cells surrounding the preganglionic sympathetic nerve fibers that innervate the medulla (Eiberger et al., 2006).
2.2. Possible functions of gap junction channels in the adrenal medulla
2.2.1. Under resting conditions: a moderate contribution to catecholamine release
The involvement of intercellular junction-mediated electrocoupling process in adrenal catecholamine release has been initially proposed by Ceña and colleagues (Cena et al., 1983). Consistent with this assumption, a junctional communication mediated by a low-conductance electrical coupling between chromaffin cells has been recorded in mouse acute slices (Moser, 1998), thus opening the possibility that depolarization of one cell may trigger action potentials in adjacent cells and that electrical coupling may ensure activation of several, if not all cells within a cluster. The contribution of gap junctions to the propagation of electrical (and ensuing Ca2+) signals between chromaffin cells has been unambiguously demonstrated in rat adrenal slices in the beginning of the 2000s, by the work of Martin and collaborators (Martin et al., 2001). It is noteworthy that under basal conditions, most of the coupled cells exhibit a weak electrical coupling, both in females (Martin et al., 2001) and males (Colomer et al., 2008b). This results in the propagation of a small depolarizing waveform in response to action potentials evoked in the stimulated cells (figure 2A). However, when the membrane potential of the coupled cell is close to action potential threshold, a firing activity can be evoked (figure 2B). This gap junction-mediated electrical propagation is physiologically relevant, as evidenced by the detection of exocytotic signals in coupled cells in response to stimulation (depolarization-evoked action potentials or iontophoretic application of nicotine) of a single chromaffin cell (Martin et al., 2001). The current hypothesis is that, under basal conditions corresponding to low frequency splanchnic nerve discharge, few synaptic boutons are stimulated and the propagation of secretory events (electrical and Ca2+ signals) through gap junctions represents an efficient complement to synaptic transmission to amplify catecholamine release without mobilizing a large number of synapses.
Figure 2. Weak electrical coupling between a chromaffin cell pair in a control rat.
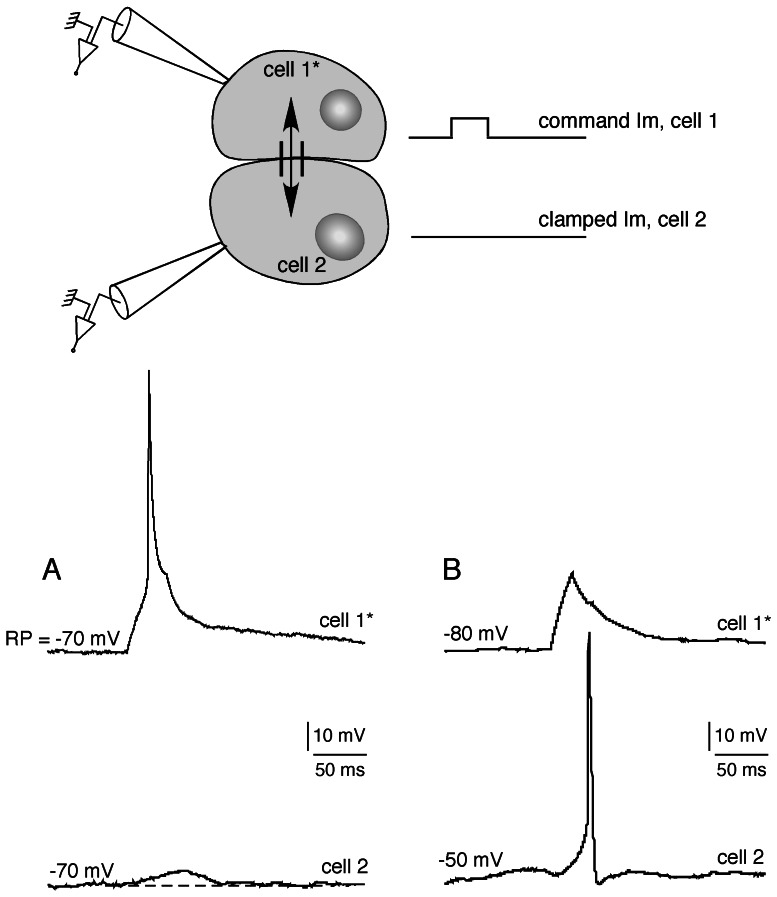
Two adjacent cells were recorded in the current-clamp mode using the dual patch-clamp technique. A depolarizing current was applied in cell 1 (cell 1*) and the subsequent membrane potential changes simultaneously monitored in both cell 1* and cell 2. A. Cells 1* and 2 were maintained at a membrane potential of −70 mV. Chart recording illustrating that emission of an action potential in cell 1* results in a small membrane depolarization in cell 2. B. Cell 1* was current-clamped at −80 mV and cell 2 was slightly depolarized to −50 mV, a membrane potential close to action potential threshold. The amplitude of the depolarizing wave transmitted to cell 2 in response to a weak depolarization of cell 1* (not robust enough to generate action potentials in the stimulated cell), is sufficient to trigger an action potential. These findings show that, even if weak, an electrical coupling between chromaffin cells can have physiologically relevant consequences.
2.2.2. In response to stressful situations: a significant contribution to catecholamine release
Stressful situations are associated with a massive and robust release of catecholamines, and as proposed by Ceña and collaborators (Cena et al., 1983), an amplifying signal is likely required. Because gap junctions enable propagation of secretory signals between adjacent chromaffin cells, we previously hypothesized that they can be plausible candidates involved in the amplification process. Several findings support the proposal of an up-regulated gap junctional coupling between chromaffin cells in stressed animals (cold exposure): i) Cx36 and Cx43 expression increases in stressed rats (Colomer et al., 2008b) (figure 3A), ii) the connexin-permeant fluorescent tracer Lucifer yellow more widely spreads within the medullary tissue of stressed rats (Colomer et al., 2008a) (figure 3B) and iii) half of coupled chromaffin cell pairs in stressed rats exhibit a robust electrical coupling that allows the transmission of unattenuated action potentials between cells (Colomer et al., 2008b). Consistently, the cytosolic Ca2+ concentration rise triggered by iontophoretic application of nicotine in a single chromaffin cell simultaneously occurs in a larger number of cells when recorded in adrenal slices from stressed rats (Colomer et al., 2008b) (figure 4). Although the direct contribution of increased gap junctional coupling to increased catecholamine secretion has not been evidenced in stressed rats yet, all these findings are consistent with. Altogether, this indicates that gap junction-driven intercellular communication between chromaffin cells is an important determinant of the regulation of catecholamine secretion from the adrenal medullary tissue.
Figure 3. Up-regulation of gap junctional communication in stressed rat.
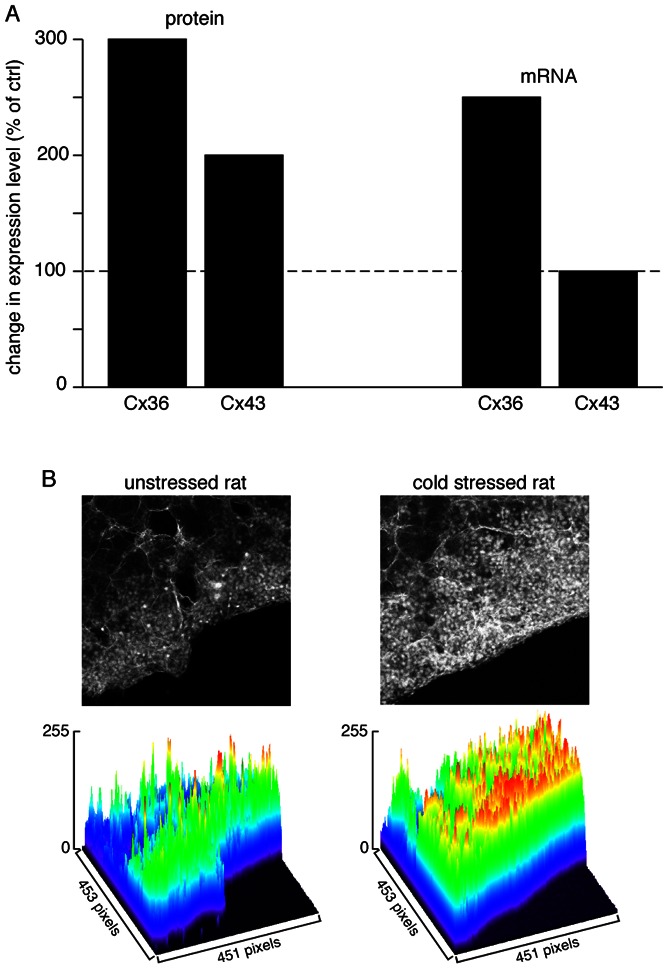
Histogram showing that protein expression of both Cx36 and Cx43 is enhanced in cold stressed rats (3- and 2-fold, respectively). This parallels an increased expression of Cx36 mRNA (2.5-fold) but not Cx43 mRNA. B. As a functional consequence, an increased diffusion of Lucifer yellow within the adrenal medulla is observed. Lucifer yellow was introduced into the adrenal gland by scrape-loading. Pictures illustrate raw data (focus on the medullary tissue) and the surface plot analysis (pseudo-colored wireframe plot) shows an extended Lucifer yellow diffusion in the medulla of a cold stressed rat as compared to an unstressed rat (modified from (Colomer et al., 2008a, figure 3).
Figure 4. Extended nicotine-induced simultaneous [Ca2+]i rises in cold stressed rat chromaffin cells.
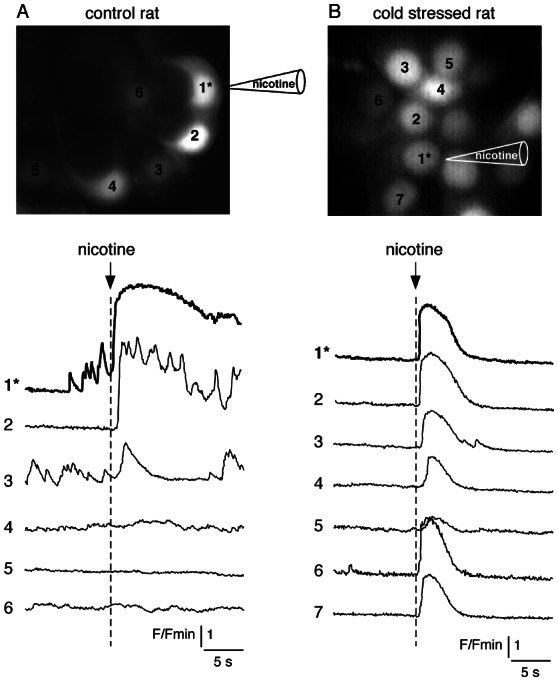
Adrenal slices were loaded with the Ca2+-sensitive fluorescent probe Oregon Green 488 BAPTA-1. Nicotine (200 mM) was iontophoretically applied on cell 1* through a sharp microelectrode leading to focal stimulation of a single cell (the onset of the nicotinic stimulation is indicated by an arrow) and [Ca2+]i changes were imaged using real-time confocal microscopy. In a control rat (panel A), the [Ca2+]i increase originating in the stimulated cell was simultaneously detected in only one adjacent cell while in a stressed rat (panel B), a simultaneous [Ca2+]i increase was recorded into up to 6 adjacent cells.
2.3. Interaction between synaptic transmission and gap junctional coupling
2.3.1. Under resting conditions: a negative regulatory control
As mentioned above, both cholinergic synaptic neurotransmission and gap junctional coupling between chromaffin cells are involved in adrenal catecholamine secretion. Interestingly, these two intercellular communication pathways do not function independently but are tightly co-regulated and continuously interact with each other. Indeed, cholinergic innervation enters the play during early postnatal life when synapses mature and begin to exert a negative control on gap junctions (Martin et al., 2005). This tonic inhibitory control persists in adults, as evidenced by the increased gap junctional communication between chromaffin cells in response to the pharmacological blockade of postsynaptic nAChRs (Martin et al., 2003; Colomer et al., 2010). In addition, the junctional coupling between chromaffin cells can also be re-activated by sectioning the splanchnic nerve (Martin et al., 2003). Conversely, it is inhibited by a prolonged stimulation of nAChRs (Colomer et al., 2011). In summary, in all cases in which the cholinergic synaptic transmission is not competent, junctional coupling between chromaffin cells is significantly enhanced and relays neurotransmission in the control of catecholamine secretion. To date, the cellular mechanisms targeted by nAChR signaling and involved in the inhibitory control of synaptic transmission on gap junctions are largely unknown. The trafficking intracellular machinery is the only identified mediator (Martin et al., 2003) but most of the characterization remains to be performed.
2.3.2. In response to stressful situations: a synergistic action
In response to chronic stress, gap junctional and synaptic communications undergo profound anatomical and functional remodeling, resulting in increased synaptic inputs on highly coupled chromaffin cells (Colomer et al., 2008a; Colomer et al., 2008b). One remarkable feature of this plasticity is the abolition of the tonic inhibitory control exerted by synaptic transmission on junctional coupling. By acting in synergy, the two pathways likely allow the adrenal medulla to respond to an increased catecholamine demand.
Thus, it is clear that, in the adrenal medullary tissue, gap junctional communication between chromaffin cells and synaptic cholinergic neurotransmission undergo parallel or opposite regulations. In control conditions, cholinergic innervation down-regulates gap junctional coupling but, in stressful situations, this inhibition is abolished and an increase of both communication pathways is observed. To date, the mechanisms shutting down the inhibitory control are unknown. In a finely tuned coordination with synaptic transmission, gap junctional channels contribute to the adrenal chromaffin cell function, not only at rest when the hormonal need is weak but also in response to a huge catecholamine demand. Our proposal is that in basal conditions, the firing nerve discharge is low and chromaffin cells exhibit an action potential firing matching the sympathetic tone and release catecholamines at a modest rate (Fulop and Smith, 2007). Under these conditions, gap junction-mediated coupling may help chromaffin cells to coordinate their electrical and ensuing calcium activities and may therefore represent an efficient complement to synaptic transmission to amplify catecholamine release. In response to an acute stress occurring with a sudden increase in splanchnic nerve discharge frequency, gap junctional intercellular communication would also facilitate catecholamine release. By contrast, when the firing discharge of the splanchnic nerve is high and sustained, as observed in response to a chronic stress, increased strength of gap junctional coupling could either have a synergistic effect with synaptic transmission, or exert a shunting effect preventing over-secretion of catecholamines as recently proposed (Colomer et al., 2009).
3. Voltage-gated Ca2+ channels: crucial players of chromaffin cell excitability and catecholamine secretion
Voltage-gated Ca2+ channels are protein complexes which encompass a main pore-forming α1-subunit of 190–250 kDa in association with auxiliary β-, α2δ- and γ-subunits. The α1-subunit is composed of four membrane-spanning domains (I–IV) linked together in a single polypeptide chain. Each domain contains six putative transmembrane segments (S1–S6) plus a “P” loop that dips partially into the membrane to form the pore lining (Catterall, 2011). The cytoplasmic loops linking the four domains are the sites of interactions with β-subunits, second messengers, membrane-binding proteins and channel gating. Presently, ten α1-subunits have been cloned with specialized functions and distributions. Four members belong to the Cav1 group (L-type), three to the Cav2 group (N, P/Q and R-types) and three to the Cav3 group (T-type). Based on the activation threshold, the Cav1 and Cav2 channels are also indicated as “high-voltage-activated” (HVA) channels, while the Cav3 are “low-voltage-activated” (LVA) channels. This classification, however, is only indicative since L-type Cav1 subunits (Cav1.3) open at voltages very close to the negative values of Cav3 (T-type) channel activation (Lipscombe et al., 2004; Singh et al., 2008; Marcantoni et al., 2010; Mahapatra et al., 2011).
3.1. Chromaffin cell Ca2+ channels under resting conditions
The adrenal chromaffin cells of mammalian species express all types of voltage-gated Ca2+ channels described above (Carbone et al., 2001; Carbone et al., 2006; Garcia et al., 2006; Marcantoni et al., 2008; Vandael et al., 2010). Their expression density changes remarkably among animal species although their function remains surprisingly identical. Ca2+ channels shape action potential waveforms, control catecholamine secretion and vesicle retrieval and regulate Ca2+-dependent events that originate near the membrane. An obvious question is: why chromaffin cells, which are round-shaped with no synaptic contacts and neuronal-like morphology, need so many Ca2+ channel types (L, N, P/Q, R, T) to perform few simple basal functions? There is no clear answer to this question, but increasing evidence indicates that Ca2+ channels in chromaffin cells play roles similar to those in other tissues. Before focusing on the more relevant aspects of Ca2+ channel remodeling during stress, we will briefly summarize here the function that each Ca2+ channel group (Cav1, Cav2 and Cav3) plays in the physiology of chromaffin cells during normal functioning.
3.1.1. The Cav1 (L-type) channels
L-type Ca2+ channels (LTCCs) are widely expressed in many tissues and control a number of Ca2+-dependent responses in excitable cells. Of the four identified α1-subunits (Cav1.1, Cav1.2, Cav1.3 and Cav1.4), chromaffin cells express only the Cav1.2 and Cav1.3 isoforms (Garcia-Palomero et al., 2000; Baldelli et al., 2004; Benavides et al., 2004; Marcantoni et al., 2010), which are both highly sensitive to 1,4-dihydropyridines blockers (Ca2+ antagonists) or activators (Ca2+ agonists). Cav1.2 and Cav1.3 are highly expressed in rat (RCCs), mouse (MCCs) and human chromaffin cells (HCCs) and they control a large fraction of catecholamine release induced by different stimuli such as: sustained splanchnic nerve stimulation (Lopez et al., 1992), KCl- and ACh-induced depolarization (Cena et al., 1983; Lopez et al., 1994) and voltage-clamp pulses (Horrigan and Bookman, 1994; Carabelli et al., 2003; Garcia et al., 2006; Marcantoni et al., 2008).
Since chromaffin cells constantly release catecholamines, during low and sustained sympathetic stimulation (Chan and Smith, 2001), LTCCs and the other Ca2+ channels experience a basal and a stimulus-induced autocrine modulation, which alters their activation/inactivation time course and the amount of Ca2+ entering the cell during activity (Carbone et al., 2001; Marcantoni et al., 2007; Marcantoni et al., 2008). This effect is mediated by adrenergic, opioidergic and purinergic G protein-coupled receptors (GPCRs) and occurs in both isolated cells (Hernandez-Guijo et al., 1999; Cesetti et al., 2003) and adrenal gland slices (Hernandez et al., 2011). In the case of Cav1.2 and Cav1.3, the modulation is dual: it can either down- or up-regulate LTCC currents. The down-modulation is fast, mediated by PTX-sensitive G proteins (Hernandez-Guijo et al., 1999; Hernandez et al., 2011) and limited to membrane micro-domains (Carabelli et al., 2001), while the up-regulation is slow, remote and mediated by the cAMP/PKA phosphorylation pathway (Carabelli et al., 2001; Cesetti et al., 2003; Marcantoni et al., 2009; Hernandez et al., 2011). Given that LTCCs can also be down-regulated by the NO/cGMP/PKG phosphorylation pathway (Carabelli et al., 2002), all this suggests that, depending on cell conditions (high cAMP/low cGMP versus low cAMP/high cGMP), chromaffin cell LTCCs can undergo extreme up- or down-modulations which may change Ca2+ currents and catecholamine release by one order of magnitude (Mahapatra, Marcantoni, Carabelli and Carbone, unpublished results).
LTCCs also possess two further key properties that make them even more strategic for chromaffin cell physiology at rest and during stressful stimulations. One is related to the role that Cav1.3 (and Cav1.2) plays in pacemaking mouse chromaffin cells near resting conditions (Marcantoni et al., 2010; Vandael et al., 2010) and another relates to the strict control that LTCCs exert on endocytosis following secretion (Rosa et al., 2007; Rosa et al., 2010; Rosa et al., 2011). The long form of Cav1.3 (Cav1.349) is highly expressed in mouse chromaffin cells (Marcantoni et al., 2010) and activates at about 9 and 24 mV more negative voltages than Cav1.2 and Nav1.7 channels (half activation at −27 mV in 2 mM Ca2+ (Mahapatra et al., 2011). Furthermore, Cav1.349 inactivates slowly and only partially during pulses of 0.5 to 1 s. Thus, this channel is suitable for pacemaking chromaffin cells with spontaneous firing frequencies of 0.5–2 Hz and interpulse potential of −50 mV. Indeed, removal of Cav1.3 in Cav1.3−/− KO mice causes a dramatic decrease of L-type pacemaking currents and a drastic reduction in the number of spontaneously firing cells in an external medium containing 4 mM KCl (Marcantoni et al., 2010). This estimate can be overlooked if MCCs are kept at more depolarized resting potentials using higher KCl concentrations (Mahapatra et al., 2011; Perez-Alvarez et al., 2011b).
LTCCs do also participate to the control of vesicle endocytosis in bovine chromaffin cells (Rosa et al., 2007). Block of LTCCs by dihydropyridines has little effects on the fast exocytosis but largely prevents both compensatory and excess endocytosis, thus causing an increased slow exocytosis during prolonged Ca2+ entries that stimulate vesicle retrieval. There is not yet a clear explanation to this phenomenon but it seems evident that endocytosis is favored by Ca2+ channels, like the L-type, that are able to maintain prolonged Ca2+ entries during sustained depolarizations (Rosa et al., 2011) and that sphingosine plays a permissive role in the regulation of Ca2+-dependent endocytosis (Rosa et al., 2010).
3.1.2. The Cav2 (N, P/Q, R-type) channels
N-, P/Q- and R-type channels are highly expressed in the nervous system, where they conduct the presynaptic Ca2+ currents that initiate synaptic transmission. The efficiency of neurotransmitter release is steeply dependent on the 3rd to 4th power of Ca2+ entry through these voltage-gated channels making them an important target of synaptic regulation. Cav2.1 channels carrying P/Q-type Ca2+ currents and Cav2.2 channels carrying N-type Ca2+ currents are the predominant pathways through which Ca2+ initiates the rapid release of neurotransmitters (glutamate, acetylcholine, GABA). Extensive studies indicate that Cav2.1 and Cav2.2 functioning is critically regulated by many different protein interactions (SNARE complex and G protein subunits) with their intracellular domains, which form the basis of Ca2+- and voltage-dependent signal transduction at the synaptic terminal (Catterall, 2011).
In chromaffin cells, Cav2.1 and Cav2.2 are expressed at different densities in all animal species (Garcia et al., 2006) while Cav2.3 seems to be preferentially expressed in MCCs and RCCs (Albillos et al., 2000; Marcantoni et al., 2010). Cav2.1 and Cav2.2 are effectively coupled to secretion but the Ca2+-dependence is nearly linear and comparable to the Ca2+-dependence experienced by the other calcium channels expressed in chromaffin cells (Horrigan and Bookman, 1994; Kim et al., 1995; Carabelli et al., 2003; Thiagarajan et al., 2004; Giancippoli et al., 2006; Carabelli et al., 2007a; Rosa et al., 2011). This indicates functional “loose coupling” of Cav2 channels to the secretory vesicles ready for release, which is consistent with the idea that Ca2+ channels and release sites are not tightly co-localized (Chow et al., 1992), but distributed within an average distance of 200–300 nm and assembled in specialized regions of the surface membrane with dimensions of several micrometers (Klingauf and Neher, 1997; Neher, 2006). In this way, the dominant Ca2+ signal regulating vesicle release derives from the activation of multiple channels operating over distances of many micrometers rather than from Ca2+ channel clusters localized in microdomains (Wu et al., 2009).
At variance with LTCCs, which control the subthreshold current regulating action potential firing in RCCs and MCCs, the Cav2 channels contribute mostly to the upstroke and falling phase of action potentials. They are also differently modulated in a voltage-dependent manner by G protein-coupled pathways (Ikeda and Dunlap, 1999). In chromaffin cells, Cav2.1 and Cav2.2 are autocrinally inhibited by ATP and opioids that are released during cell activity (Gandia et al., 1993; Albillos et al., 1996a; Albillos et al., 1996b; Currie and Fox, 1996). The inhibition occurs in membrane microdomains, without the involvement of diffusible second messengers, and is manifested by a long delay of channel openings (slow activation) at negative potentials (Carabelli et al., 1996; Carabelli et al., 1998). This effect, however, can be reversed by depolarization. The normal fast activation is recovered during strong depolarizations (Marchetti et al., 1986; Bean, 1989) or when a strong pre-pulse anticipates a weak depolarization (Elmslie et al., 1990). This phenomenon is called “voltage-dependent facilitation” and is attributed to a protein-protein interaction between the receptor activated Gβγ subunit and the II-III loop of Cav2 channels (Ikeda, 1996; Herlitze et al., 1997; Zamponi et al., 1997). Interestingly, this Cav2.1 and Cav2.2 down-modulation can be partially reverted during short depolarizations repeated at high frequency (Currie and Fox, 2002) mimicking high frequency action potential trains that occur under stress conditions. The final result is that the attenuated Ca2+ influx through Cav2.1 and Cav2.2 at rest, due to the down-regulation induced by the released ATP and opioids, can be partially reverted during sustained cell activity. This phenomenon is particularly evident in clusters of bovine chromaffin cells (Hernandez-Guijo et al., 1998b) and slices of mouse adrenal glands (Hernandez et al., 2011) where the packed organization of the cells is well preserved and the released neurotransmitters (ATP and opioids) can accumulate in the extracellular space and exert a basal tonic inhibition on cell firing and catecholamine secretion.
3.1.3. The Cav3 (T-type) channels
T-type Ca2+ channels (TTCCs) are transient, low-voltage activated Ca2+ channels that control Ca2+ entry during mild depolarizations near resting potential. Studies in the past 25 years have clarified their functional role in controlling: low-threshold spikes, oscillatory cell activity, muscle contraction, hormone release, cell growth, differentiation and proliferation (Perez-Reyes, 2003; Carbone et al., 2006). Due to their widespread localization and function, TTCCs are proposed as therapeutic targets for a variety of diseases like: hypertension, angina pectoris, heart failure, atrial fibrillation, neuropathic pain, epilepsy, sleep disorders, obesity and cancer (Giordanetto et al., 2011).
TTCCs possess unique properties, which facilitate their biophysical identification: (1) activation is sharply voltage-dependent and channels open at hyperpolarized potentials (−50 mV in 5 mM Ca2+); (2) inactivation is voltage-dependent, independent of Ca2+ and complete within tens of milliseconds at positive potentials; (3) deactivation is slow at potentials near rest; (4) Ca2+ and Ba2+ are carried with equal efficacy and single channel conductance is a factor 3- to 4-fold lower than that of Cav1 and Cav2; (5) activation and steady-state inactivation (channel availability) overlap near resting potential, providing a 3–20 pA inward ‘window current’. Molecular cloning of TTCCs has provided evidence for the existence of three different pore forming α1 subunits (Cav3.1, Cav3.2 and Cav3.3), which possess only 25% amino acid homology but similar pore structure organization to Cav1 and Cav2 (Perez-Reyes, 2003).
Despite their widespread distribution in most tissues (neuronal, muscular, endocrine), TTCCs are either absent or weakly expressed in adult chromaffin cells. In adult bovine chromaffin cells, the mRNA encoding Cav3.1 and Cav3.2 is expressed (Garcia-Palomero et al., 2001), but functional TTCCs have not been detected (Cena et al., 1983; Artalejo et al., 1991; Albillos et al., 1993; Carabelli et al., 1998), except in one case (Diverse-Pierluissi et al., 1991). On the contrary, TTCCs are functionally expressed in embryonic and neonatal RCCs (Bournaud et al., 2001; Levitsky and Lopez-Barneo, 2009; Souvannakitti et al., 2010) and are available in a small percentage of adult RCCs (Hollins and Ikeda, 1996; Novara et al., 2004; Carabelli et al., 2007b). TTCCs channels are also not expressed in adult MCCs (Hernandez-Guijo et al., 1998a; Marcantoni et al., 2010), but these cells sometimes display the typical “low-threshold shoulder” on the I/V relationship of ramp commands, which is the fingerprint of TTCCs (see Fig. 4 in (Gosso et al., 2011). These low-threshold currents are also present in Cav1.3 KO MCCs (Navarro-Tableros, Carabelli and Carbone, unpublished observation), excluding the possibility that they derive from the C-terminus “short” splice variant of Cav1.3 α1-subunit (Cav1.342a), which activates at very negative potentials and inactivates quickly in a Ca2+ dependent manner (Singh et al., 2008). It is thus very likely that availability of TTCCs may depend critically on chromaffin cell conditions or stimulations, as in the case of applied chronic stressors (Novara et al., 2004; Giancippoli et al., 2006; Carabelli et al., 2007b) or acute sympatho-adrenal stress stimuli (Hill et al., 2011), which up-regulate Cav3.2 channels in RCCs and MCCs (figures 5A and 5B).
Figure 5. cAMP-pre-treatment induces de novo expression of Cav3.2 (T-type) channels in rat chromaffin cells.
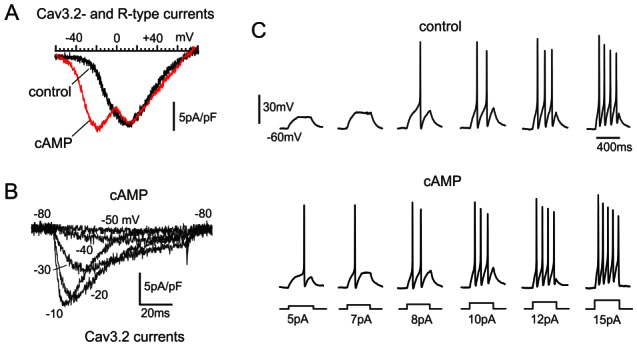
A. I-V curves of R- and T-type channels expressed in control and cAMP-treated cells after exposure to 200 μM pCPT-cAMP. Both cells were pretreated with ω-toxins and bathed in solutions containing 1 μM nifedipine to minimize N-, P/Q and L-type Ca2+ currents. Cav3.2 channels are responsible for the low-threshold early peak of current at about −22 mV in cAMP-treated cells. The high-threshold peak at about +12 mV is mainly due to R-type channels. B. T-type currents recorded in isolation from a rat chromaffin cell incubated with 3.2 μM ω-CTx-GVIA, 2 μM ω-Aga-IVA, 1 μM SNX-482 (10 min) and bathed with 1 μM nifedipine to nearly block all HVA channels. C. cAMP-recruited T-type channels lower the threshold of action potential firing in rat chromaffin cells. On the upper and lower panels are shown action potentials recorded from a control (upper traces) and a cAMP-treated rat chromaffin cell (lower traces) during current-clamp stimulations of increasing amplitude (5–15 pA). Cells were maintained at −60 mV by injecting −2 to −3 pA. Note that in the cAMP-treated cell, 5 pA is sufficient to elicit a spike, while in control the minimal current is significantly higher (8 pA) (adapted from Novara et al., 2004).
When expressed in chromaffin cells, TTCCs are shown to control a sizeable “low-threshold” catecholamine release that is absent in cells possessing only HVA channels (Giancippoli et al., 2006; Carabelli et al., 2007a; Carabelli et al., 2007b; Marcantoni et al., 2008). Of relevance is the effective coupling existing between TTCCs and the pool of readily releasable vesicles. The Ca2+-dependence of TTCCs-controlled secretion is linear and strikingly similar to that of LTCCs (Carabelli et al., 2007a), which in turn is comparable to that of P/Q and N-type channels (Carabelli et al., 2003). Thus, although preserving a low-voltage range of activation, TTCCs appear similarly distributed around the docked vesicles as the HVA channels. In conclusion, TTCCs lower the threshold of action potential generation and ensure a broader interval of voltage control on catecholamine secretion, which may be significant when chromaffin cells depolarize steadily or fire at high frequencies (15–20 Hz) during stress conditions.
3.2. Chromaffin cell Ca2+ channel remodeling in response to stressful situations
As for the nicotinic receptors and gap junction channels (Guerineau and Desarmenien, 2010), voltage-gated Ca2+ channels undergo functional remodeling during stress (Carbone et al., 2006). This is particularly evident in chromaffin cells of adult animals as an adaptation response to stressful situations including chronic and intermittent hypoxia (Carabelli et al., 2007b; Souvannakitti et al., 2010), β-adrenergic receptor (AR) and high-frequency sympathetic stimulation (Novara et al., 2004; Hill et al., 2011) and hypoxia occurring during the transition from intrauterine to air-breathing life (Levitsky and Lopez-Barneo, 2009).
A common molecular mechanism in response to the indicated stressors is a marked recruitment of functioning Cav3.2 channels that: i) lowers the threshold of action potential firing (Novara et al., 2004) (figure 5C), ii) increases the amount of secreted catecholamines at low potentials (−30, −40 mV) (Giancippoli et al., 2006; Carabelli et al., 2007b) (figures 6A and 6B) and iii) mobilizes a pool of readily releasable vesicles equivalent to that recruited by L-type channels (Carabelli et al., 2007a). Figures 5C and figures 6A and 6B summarize these properties associated with Cav3.2 channels specifically up-regulated by stressors. Most likely, an increased density of T-type channels is also the cause of depolarized resting potential and increased firing frequency occurring in spontaneously firing RCCs in the adrenal gland slices of stressed animals (Colomer et al., 2008a). An increased density of functioning T-type channels during stressful stimuli, appears also to be a more general remodeling mechanism that many cell types and tissues experience in response to hormones (ACTH, aldosterone), cell proliferation and cell conditions associated to stressors. Table 2 lists a number of stressors and stress-induced conditions which lead to an up-regulation or recruitment of newly available Cav3.2 T-type channels in a variety of cells. Notice how neuronal, cardiac and endocrine tissues all respond to stressors with an increased density of Cav3.2 (and Ca3.1) channels, justifying the informal terminology that T-type channels are “stress-induced channels” (Carbone et al., 2006; Hill et al., 2011).
Figure 6. Cav3.2 channels control “low-threshold” exocytosis in rat chromaffin cells exposed to hypoxic conditions or cAMP.
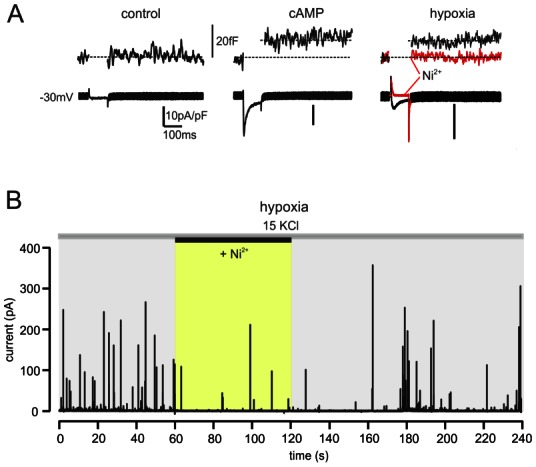
A. Ca2+ currents and membrane capacitance changes at −30 mV (10 mM external Ca2+) in control, cAMP-treated and hypoxic cells. Notice the potent block of Ca2+ currents and secretion induced by 50 μM Ni2+ in an hypoxic cell (red trace) in which N-, P/Q- and L-type channels were blocked by ω-toxins and nifedipine. B. Amperometric spikes evoked by small membrane depolarizations using 15 mM KCl, recorded from a hypoxic cell expressing T-type channels. Addition of 50 μM Ni2+ caused a marked depression of amperometric spikes frequency. The cell was pre-incubated with ω-toxins and perfused with solutions containing 1 μM nifedipine (adapted from (Carabelli et al., 2007b).
Table 2.
Type of stressor or stressor-like stimuli that induce recruitment or up-regulation of Cav3 (T-type) channels in different cells and tissues
| Stressors | Cell type, tissue | Cav3 type | Reference |
|---|---|---|---|
| Chronic hypoxia | PC12 | Cav3.2 | Del Toro et al., 2003 |
| Long-term β-AR exposure | Adult RCCs | Cav3.2 | Novara et al., 2004 |
| Chronic hypoxia | Adult RCCs | Cav3.2 | Carabelli et al., 2007b |
| Adrenal gland splanchnic denervation | Neonatal RCCs | Cav3.2 | Levitsky & Lopez-Barneo, 2009 |
| Intermittent hypoxia | Neonatal RCCs | Cav3.1, Cav3.2 | Souvannakitti et al., 2010 |
| Acute high-frequency sympathetic stimulation | Adult MCCs (adrenal gland slices) | Cav3.2 | Hill et al., 2011 |
| Acute PACAP-mediated stimulation | Adult MCCs (adrenal gland slices) | Cav3.2 | Kuri et al., 2009 |
| Long-term ACTH exposure | Bovine zona fasciculata cells | Cav3.2 |
Barbara & Takeda, 1995 Liu et al., 2010 |
| Long-term VIP exposure | Bovine zona fasciculata cells | Cav3 (?) | Barbara & Takeda, 1995 |
| Chronic hypoxia | Neonatal rat ventricular myocytes | Cav3.2 | Pluteanu & Cribbs, 2009 |
| Pulmonary hypertension | Rat ventricular myocytes | Cav3 (?) | Takebayashi et al., 2006 |
| Cardiomyopathic heart | Hamster ventricular myocytes | Cav3 (?) | Sen & Smith, 1994 |
| Hypertrophic heart | Feline ventricular myocytes | Cav3 (?) | Nuss & Hauser, 1993 |
| Long-term aldosterone exposure | Neonatal rat ventricular myocytes | Cav3.2 | Lalevée et al., 2005 |
| Long-term aldosterone exposure | Human adrenocarcinoma | Cav3.2 | Lesouhaitier et al., 2001 |
| Cell proliferation | Human cancer cells | Cav3.1, Cav3.2 |
Mariot et al., 2002 Panner & Wurster, 2006 |
| Diabetic neuropathic pain | Rat hindpaws sensory neurons | Cav3.2 | Jagodic et al., 2007 |
| Chronic visceral pain | Rat colonic sensory neurons | Cav3.2 | Marger et al., 2011 |
3.2.1. The signaling pathways underlying T-type channels up-regulation in chromaffin cells
Table 2 illustrates the multiple pathways through which Cav3.2 channels are up-regulated by stressors. Some act acutely within minutes (PACAP and high-frequency sympathetic stimulation) while the majority act more slowly, requiring days for full development (hypoxia, β1-AR stimulation, VIP, aldosterone and ACTH exposure). In addition, the action of stressors is mediated by different intracellular messengers and transcription factors, suggesting the existence of multiple pathways converging on a common target: the activation, up-regulation or recruitment of Cav3.2 channels.
Regarding the stress response of chromaffin cells, there is now consisting evidence in favor of two well distinct pathways in which Cav3.2 are either activated or up-regulated by stressors. One pathway develops rapidly (minutes) and is most evident in mouse adrenal gland slices. The cascade of events is activated by PACAP (released during high-frequency splanchnic stimulation) and mediated by cAMP-activated exchange proteins (Epac) that stimulates PLC and PKC through a non-canonical cAMP-dependent pathway (Hill et al., 2011). The PKC target is the phosphorylation of the Na+/Ca2+ exchanger which causes a small depolarization near resting potential, sufficient to activate a robust low-threshold current carried by Cav3.2 channels, which are also up-regulated by the stimulation (Kuri et al., 2009) for a schematic model). Significantly different, although involving similar molecular components, are the long-lasting mechanisms by which hypoxia and cell exposure to β1-AR agonists (isoprenaline) act on Cav3.2 channels. Both systems require hours or days to fully develop. They cause increased levels of CACNA1H mRNA (Cav3.2 gene) and their action is prevented by specific inhibitors of protein synthesis (Novara et al., 2004; Giancippoli et al., 2006; Carabelli et al., 2007a; Carabelli et al., 2007b; Souvannakitti et al., 2010), suggesting a net recruitment of newly available Cav3.2 channels (figure 7). For the case of β1-AR stimulation, Cav3.2 recruitment is mediated by a cAMP-dependent Epac pathway which leads to the activation of transcription factors (Novara et al., 2004), possibly through the phosphorylation of ERK, as reported for other Epac-regulated cell signaling pathways (Borland et al., 2009).
Figure 7. Schematic drawings of cell signaling pathways leading to CACNA1H gene expression and Cav3.2 channels incorporation during stressor stimulation.
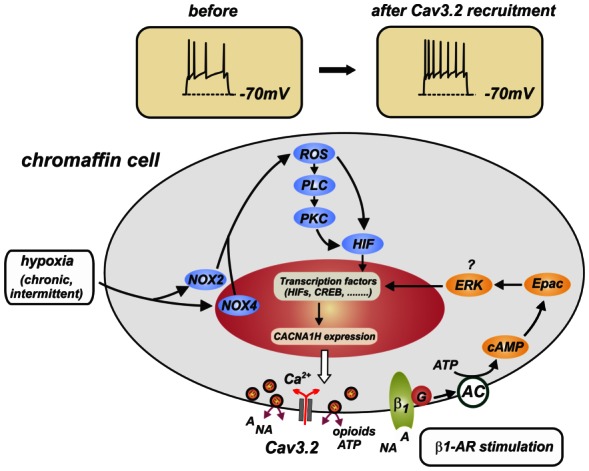
The final result of β1-adrenergic receptor stimulation and chronic/intermittent hypoxia is the activation of transcription factors (HIF, CREB, …) and CACNA1H gene expression that lead to Cav3.2 channel incorporation into the plasma membrane. The pathway driven by β1-adrenergic receptor stimulation (isoprenaline, forskolin, pCPT-cAMP) is derived from Novara et al. (2004). The pathway induced by chronic/intermittent hypoxia (NOX, ROS, PLC, PKG, HIF) is taken from Yuan et al. (2008).
For the case of hypoxia (figure 7), the present findings indicate the involvement of hypoxia-inducible factors (HIF-1α, HIF-2α), although a direct proof of their participation is still missing. HIF-2α appears to be involved in the up-regulation of Cav3.2 in PC12 cells exposed to chronic hypoxia (Del Toro et al., 2003) and overnight incubation with the unspecific HIF activator desferrioxamine (DFX) mimics the effects of hypoxia in PC12 cells (Del Toro et al., 2003) and RCCs (Carabelli et al., 2007b). In addition to HIFs, NADHP-oxidases (NOX2, NOX4) and reactive-oxygen species (ROS) appear to play a main role in the recruitment of Cav3.2 in neonatal RCCs subjected to intermittent hypoxia. A hypothetical scheme in which NOX, ROS and HIF are linked together is reported in figure 7. The scheme is derived from the work of Prabakhar’s group in which ROS are shown to activate PLC, DAG and PKC, favoring the stabilization and translocation of HIF-1α to the nucleus to start transcription and gene expression (Yuan et al., 2008).
In conclusion, several issues remain to be clarified about the molecular pathways regulating the stress-induced response of chromaffin cells, but it seems very likely that with some adjustments, the two pathways illustrated in figure 7 could cover a large number of stress-induced effects observed in different cells and tissues (see for instance (Liu et al., 2010). Additional pathways cannot be excluded and further work will possibly highlight alternative ones.
3.2.2. T-type channel remodeling in chromaffin cells during intrauterine-neonate transition and cholinergic splanchnic denervation
A unique property of chromaffin cells is their high chemosensitivity to hypoxia during fetal life, when these cells respond to acute lowering of O2 levels with a marked catecholamine release, mostly controlled by Ca2+ influx through Cav3.2 channels (Levitsky and Lopez-Barneo, 2009). As discussed previously, in neonatal animals (<7 days), the control of hormone release from the adrenal medulla is mostly non-neurogenic (Slotkin, 1986) and strongly linked to the robust gap junction coupling existing between neighboring cells (Martin et al., 2003; Guerineau and Desarmenien, 2010). Tight electrical coupling and high density of T-type channels facilitate action potential generation and propagation, as well as synchronous release of catecholamines from extended cell populations of the adrenal medulla, resulting in an increased amount of hormone release (Colomer et al., 2009; Guerineau and Desarmenien, 2010). It is however interesting that the T-type channels expressed in the chromaffin cells of newborn rats, not only are responsible for the catecholamine release during acute hypoxia, but are also up-regulated during intermittent hypoxia (Souvannakitti et al., 2010). As intermittent hypoxia mimics the physiological conditions experienced by the fetus during delivery, the up-regulation of T-type channels underscores the specific role that these channels play in sustaining the O2-sensitivity of the adrenal medulla under stressful conditions (hypoxia and hypoglycemia), during adaptation to the extra-uterine life.
Of relevance is also the observation that cholinergic innervation of the adrenal gland, which follows postnatal development (Seidler and Slotkin, 1985), causes a loss of functional Cav3.2 channels (Levitsky and Lopez-Barneo, 2009) paralleled by an increased sensitivity to nicotine (Nurse et al., 2009) and an increased density of available α7-built nAChRs (Buttigieg et al., 2009). The close correlation between chromaffin cell innervation and O2 sensitivity is further strengthened by the finding that both O2 sensitivity of chromaffin cells and expression of Cav3.2 channels re-appear after adrenal gland denervation. This suggests that: i) Cav3.2 are the Ca2+ channels mainly responsible for chromaffin cell response to hypoxia and ii) cholinergic splanchnic innervation of the adrenal medulla down-regulates the expression of Cav3.2 channels through an unknown mechanism (Levitsky and Lopez-Barneo, 2009).
An interesting question is whether there is a link between the lowering of functional Cav3.2 channels and the increased expression of nAChRs. In sensory neurons, T-type channels can undergo down-regulations following intracellular Ca2+ overloading (Carbone and Lux, 1987). If this is a possible mechanism, a way for Ca2+ to massively enter the cells is through the activation of ion channel receptors which are permeable to Na+ and Ca2+, like TRPV1 and nAChRs (Chung et al., 2008; Albuquerque et al., 2009). Recent studies in rat sensory neurons have shown that Cav3.2 channels are effectively down-regulated if large Ca2+ fluxes are passed through capsaicin-activated TRPV1 channels located close to T-type channels (Comunanza et al., 2011). The same may occur in developing chromaffin cells if, for instance, increasing densities of nAChRs are repeatedly activated and large Ca2+ inward currents flow through the activated nAChRs. Explaining whether and how up-regulation of nAChRs and down-regulation of T-type channels are linked together in developing chromaffin cells is an intriguing issue that future work will certainly help to clarify.
Concluding remarks
The continuous and coherent remodeling of the adrenal stimulus-secretion coupling is essential to the achievement of appropriate catecholamine secretion in response to the body demand. It is now established that gap junctional communication, nAChRs and voltage-gated T-type channels play major roles in chromaffin cell plasticity. Noteworthy, the up-regulation of one partner is associated with the up-, or down-regulation of the other two. Much remains to be done and future studies will address the molecular mechanisms regulating the crosstalks among these membrane channels and the identification of other molecular determinants that undergo an effective remodeling during stress. Concerning this latter, the C-terminus slowly inactivating “long form” of Cav1.3 (L-type) channels is shown to be down-regulated and replaced by the fast-inactivating Cav1.3 “short variant” in chromaffin cells of spontaneously hypertensive rats (Segura-Chama et al., 2011). Since similar remodeling may also occur for other components regulating chromaffin cells excitability (K+ channels, G proteins-coupled receptors, ion pumps and ion exchangers), it is obvious that a complete picture of chromaffin cells plasticity during stress might include many more actors, whose identification would be beneficial for a full understanding of the chromaffin cell role in regulating stress response.
Acknowledgments
The authors thank the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Ministère de l’Enseignement Supérieur et de la Recherche, Fondation pour la Recherche Médicale, Association pour la Recherche sur le Cancer, Région Languedoc-Roussillon, Région Pays de la Loire, Conseil Général de Maine et Loire, Angers-Loire Métropole, Marie Curie Research Training Network “CavNET”, Italian M.I.U.R., Regione Piemonte, Università di Torino, Compagnia di San Paolo di Torino.
References
- Albillos A, Garcia AG, Gandia L. omega-Agatoxin-IVA-sensitive calcium channels in bovine chromaffin cells. FEBS Lett. 1993;336:259–262. doi: 10.1016/0014-5793(93)80815-c. [DOI] [PubMed] [Google Scholar]
- Albillos A, Carbone E, Gandia L, Garcia AG, Pollo A. Opioid inhibition of Ca2+ channel subtypes in bovine chromaffin cells: selectivity of action and voltage-dependence. Eur J Neurosci. 1996a;8:1561–1570. doi: 10.1111/j.1460-9568.1996.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Albillos A, Gandia L, Michelena P, et al. The mechanism of calcium channel facilitation in bovine chromaffin cells. J Physiol. 1996b;494 ( Pt 3):687–695. doi: 10.1113/jphysiol.1996.sp021524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albillos A, Neher E, Moser T. R-Type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J Neurosci. 2000;20:8323–8330. doi: 10.1523/JNEUROSCI.20-22-08323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MT, Barrero MJ, Michelena P, et al. Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J Cell Biol. 1999;144:241–254. doi: 10.1083/jcb.144.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaiz-Cot JJ, de Diego AM, Hernandez-Guijo JM, Gandia L, Garcia AG. A two-step model for acetylcholine control of exocytosis via nicotinic receptors. Biochem Biophys Res Commun. 2008;365:413–419. doi: 10.1016/j.bbrc.2007.10.151. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Dahmer MK, Perlman RL, Fox AP. Two types of Ca2+ currents are found in bovine chromaffin cells: facilitation is due to the recruitment of one type. J Physiol. 1991;432:681–707. doi: 10.1113/jphysiol.1991.sp018406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J. Noradrenaline: fate and control of its biosynthesis. Science. 1971;173:598–606. doi: 10.1126/science.173.3997.598. [DOI] [PubMed] [Google Scholar]
- Baldelli P, Hernandez-Guijo JM, Carabelli V, et al. Direct and remote modulation of L-channels in chromaffin cells: distinct actions on alpha1C and alpha1D subunits? Mol Neurobiol. 2004;29:73–96. doi: 10.1385/MN:29:1:73. [DOI] [PubMed] [Google Scholar]
- Barbara JG, Takeda K. Voltage-dependent currents and modulation of calcium channel expression in zona fasciculata cells from rat adrenal gland. J Physiol. 1995;488:609–622. doi: 10.1113/jphysiol.1995.sp020994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara JG, Lemos VS, Takeda K. Pre- and post-synaptic muscarinic receptors in thin slices of rat adrenal gland. Eur J Neurosci. 1998;10:3535–3545. doi: 10.1046/j.1460-9568.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Benavides A, Calvo S, Tornero D, Gonzalez-Garcia C, Cena V. Adrenal medulla calcium channel population is not conserved in bovine chromaffin cells in culture. Neuroscience. 2004;128:99–109. doi: 10.1016/j.neuroscience.2004.06.042. [DOI] [PubMed] [Google Scholar]
- Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009;158:70–86. doi: 10.1111/j.1476-5381.2008.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco D, Haefliger JA, Meda P. Connexins: key mediators of endocrine function. Physiol Rev. 2011;91:1393–1445. doi: 10.1152/physrev.00027.2010. [DOI] [PubMed] [Google Scholar]
- Bournaud R, Hidalgo J, Yu H, Jaimovich E, Shimahara T. Low threshold T-type calcium current in rat embryonic chromaffin cells. J Physiol. 2001;537:35–44. doi: 10.1111/j.1469-7793.2001.0035k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttigieg J, Brown S, Holloway AC, Nurse CA. Chronic nicotine blunts hypoxic sensitivity in perinatal rat adrenal chromaffin cells via upregulation of KATP channels: role of alpha7 nicotinic acetylcholine receptor and hypoxia-inducible factor-2alpha. J Neurosci. 2009;29:7137–7147. doi: 10.1523/JNEUROSCI.0544-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanucci V, Krishnaswamy A, Cooper E. Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron. 2010;66:827–834. doi: 10.1016/j.neuron.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Campos-Caro A, Smillie FI, Dominguez del Toro E, et al. Neuronal nicotinic acetylcholine receptors on bovine chromaffin cells: cloning, expression, and genomic organization of receptor subunits. J Neurochem. 1997;68:488–497. doi: 10.1046/j.1471-4159.1997.68020488.x. [DOI] [PubMed] [Google Scholar]
- Campos-Caro A, Carrasco-Serrano C, Valor LM, Viniegra S, Ballesta JJ, Criado M. Multiple functional Sp1 domains in the minimal promoter region of the neuronal nicotinic receptor alpha5 subunit gene. J Biol Chem. 1999;274:4693–4701. doi: 10.1074/jbc.274.8.4693. [DOI] [PubMed] [Google Scholar]
- Carabelli V, Lovallo M, Magnelli V, Zucker H, Carbone E. Voltage-dependent modulation of single N-Type Ca2+ channel kinetics by receptor agonists in IMR32 cells. Biophys J. 1996;70:2144–2154. doi: 10.1016/S0006-3495(96)79780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli V, Carra I, Carbone E. Localized secretion of ATP and opioids revealed through single Ca2+ channel modulation in bovine chromaffin cells. Neuron. 1998;20:1255–1268. doi: 10.1016/s0896-6273(00)80505-x. [DOI] [PubMed] [Google Scholar]
- Carabelli V, Hernandez-Guijo JM, Baldelli P, Carbone E. Direct autocrine inhibition and cAMP-dependent potentiation of single L-type Ca2+ channels in bovine chromaffin cells. J Physiol. 2001;532:73–90. doi: 10.1111/j.1469-7793.2001.0073g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli V, D’Ascenzo M, Carbone E, Grassi C. Nitric oxide inhibits neuroendocrine Ca(V)1 L-channel gating via cGMP-dependent protein kinase in cell-attached patches of bovine chromaffin cells. J Physiol. 2002;541:351–366. doi: 10.1113/jphysiol.2002.017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli V, Giancippoli A, Baldelli P, Carbone E, Artalejo AR. Distinct potentiation of L-type currents and secretion by cAMP in rat chromaffin cells. Biophys J. 2003;85:1326–1337. doi: 10.1016/S0006-3495(03)74567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli V, Marcantoni A, Comunanza V, Carbone E. Fast exocytosis mediated by T- and L-type channels in chromaffin cells: distinct voltage-dependence but similar Ca2+-dependence. Eur Biophys J. 2007a;36:753–762. doi: 10.1007/s00249-007-0138-2. [DOI] [PubMed] [Google Scholar]
- Carabelli V, Marcantoni A, Comunanza V, et al. Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol. 2007b;584:149–165. doi: 10.1113/jphysiol.2007.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Lux HD. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Carabelli V, Cesetti T, Baldelli P, Hernandez-Guijo JM, Giusta L. G-protein- and cAMP-dependent L-channel gating modulation: a manyfold system to control calcium entry in neurosecretory cells. Pflugers Arch. 2001;442:801–813. doi: 10.1007/s004240100607. [DOI] [PubMed] [Google Scholar]
- Carbone E, Marcantoni A, Giancippoli A, Guido D, Carabelli V. T-type channels-secretion coupling: evidence for a fast low-threshold exocytosis. Pflugers Arch. 2006;453:373–383. doi: 10.1007/s00424-006-0100-7. [DOI] [PubMed] [Google Scholar]
- Carrasco-Serrano C, Criado M. Glucocorticoid activation of the neuronal nicotinic acetylcholine receptor alpha7 subunit gene: involvement of transcription factor Egr-1. FEBS Lett. 2004;566:247–250. doi: 10.1016/j.febslet.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Caton D, Calabrese A, Mas C, Serre-Beinier V, Wonkam A, Meda P. Beta-cell crosstalk: a further dimension in the stimulus-secretion coupling of glucose-induced insulin release. Diabetes Metab. 2002;28:3S45–53. discussion 43S108–112. [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cena V, Nicolas GP, Sanchez-Garcia P, Kirpekar SM, Garcia AG. Pharmacological dissection of receptor-associated and voltage-sensitive ionic channels involved in catecholamine release. Neuroscience. 1983;10:1455–1462. doi: 10.1016/0306-4522(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Cesetti T, Hernandez-Guijo JM, Baldelli P, Carabelli V, Carbone E. Opposite action of beta1- and beta2-adrenergic receptors on Ca(V)1 L-channel current in rat adrenal chromaffin cells. J Neurosci. 2003;23:73–83. doi: 10.1523/JNEUROSCI.23-01-00073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SA, Smith C. Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells. J Physiol. 2001;537:871–885. doi: 10.1111/j.1469-7793.2001.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- Cocchia D, Michetti F. S-100 antigen in satellite cells of the adrenal medulla and the superior cervical ganglion of the rat. An immunochemical and immunocytochemical study. Cell Tissue Res. 1981;215:103–112. doi: 10.1007/BF00236252. [DOI] [PubMed] [Google Scholar]
- Colomer C, Lafont C, Guerineau NC. Stress-induced intercellular communication remodeling in the rat adrenal medulla. Ann N Y Acad Sci. 2008a;1148:106–111. doi: 10.1196/annals.1410.040. [DOI] [PubMed] [Google Scholar]
- Colomer C, Olivos Ore LA, Coutry N, et al. Functional remodeling of gap junction-mediated electrical communication between adrenal chromaffin cells in stressed rats. J Neurosci. 2008b;28:6616–6626. doi: 10.1523/JNEUROSCI.5597-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer C, Desarmenien MG, Guerineau NC. Revisiting the stimulus-secretion coupling in the adrenal medulla: role of gap junction-mediated intercellular communication. Mol Neurobiol. 2009;40:87–100. doi: 10.1007/s12035-009-8073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer C, Olivos-Ore LA, Vincent A, McIntosh JM, Artalejo AR, Guerineau NC. Functional characterization of alpha9-containing cholinergic nicotinic receptors in the rat adrenal medulla: implication in stress-induced functional plasticity. J Neurosci. 2010;30:6732–6742. doi: 10.1523/JNEUROSCI.4997-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer C, Martin AO, Desarmenien MG, Guerineau NC. Gap junction-mediated intercellular communication in the adrenal medulla: An additional ingredient of stimulus-secretion coupling regulation. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamem.2011.07.034. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Comunanza V, Carbone E, Marcantoni A, Sher E, Ursu D. Calcium-dependent inhibition of T-type calcium channels by TRPV1 activation in rat sensory neurons. Pflugers Arch. 2011;462:709–722. doi: 10.1007/s00424-011-1023-5. [DOI] [PubMed] [Google Scholar]
- Criado M, Alamo L, Navarro A. Primary structure of an agonist binding subunit of the nicotinic acetylcholine receptor from bovine adrenal chromaffin cells. Neurochem Res. 1992;17:281–287. doi: 10.1007/BF00966671. [DOI] [PubMed] [Google Scholar]
- Currie KP, Fox AP. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron. 1996;16:1027–1036. doi: 10.1016/s0896-6273(00)80126-9. [DOI] [PubMed] [Google Scholar]
- Currie KP, Fox AP. Differential facilitation of N- and P/Q-type calcium channels during trains of action potential-like waveforms. J Physiol. 2002;539:419–431. doi: 10.1113/jphysiol.2001.013206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen J, Meier C, Van Der Giessen RS, et al. Expression pattern of lacZ reporter gene representing connexin36 in transgenic mice. J Comp Neurol. 2004;473:511–525. doi: 10.1002/cne.20085. [DOI] [PubMed] [Google Scholar]
- Del Toro R, Levitsky KL, Lopez-Barneo J, Chiara MD. Induction of T-type calcium channel gene expression by chronic hypoxia. J Biol Chem. 2003;278:22316–22324. doi: 10.1074/jbc.M212576200. [DOI] [PubMed] [Google Scholar]
- Di Angelantonio S, Matteoni C, Fabbretti E, Nistri A. Molecular biology and electrophysiology of neuronal nicotinic receptors of rat chromaffin cells. Eur J Neurosci. 2003;17:2313–2322. doi: 10.1046/j.1460-9568.2003.02669.x. [DOI] [PubMed] [Google Scholar]
- Diverse-Pierluissi M, Dunlap K, Westhead EW. Multiple actions of extracellular ATP on calcium currents in cultured bovine chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:1261–1265. doi: 10.1073/pnas.88.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas WW, Kanno T, Sampson SR. Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J Physiol. 1967;188:107–120. doi: 10.1113/jphysiol.1967.sp008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas WW. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968;34:451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas WW. Secretomotor control of adrenal medullary secretion: synaptic, membrane and ionic events in stimulus-secretion coupling. In: Blaschko H, Sayers G, Smith AD, editors. Handbook of Physiology. Am. Physiol. Soc; Baltimore, MA: 1975. pp. 367–388. [Google Scholar]
- Ducsay CA, Hyatt K, Mlynarczyk M, Root BK, Kaushal KM, Myers DA. Long-term hypoxia modulates expression of key genes regulating adrenomedullary function in the late gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1997–2005. doi: 10.1152/ajpregu.00313.2007. [DOI] [PubMed] [Google Scholar]
- Eiberger J, Kibschull M, Strenzke N, et al. Expression pattern and functional characterization of connexin29 in transgenic mice. Glia. 2006;53:601–611. doi: 10.1002/glia.20315. [DOI] [PubMed] [Google Scholar]
- Elmslie KS, Zhou W, Jones SW. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990;5:75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- Fauquier T, Guerineau NC, McKinney RA, Bauer K, Mollard P. Folliculostellate cell network: a route for long-distance communication in the anterior pituitary. Proc Natl Acad Sci U S A. 2001;98:8891–8896. doi: 10.1073/pnas.151339598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Fucile S, Sucapane A, Eusebi F. Ca2+ permeability through rat cloned alpha9-containing nicotinic acetylcholine receptors. Cell Calcium. 2006;39:349–355. doi: 10.1016/j.ceca.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Fulop T, Smith C. Matching native electrical stimulation by graded chemical stimulation in isolated mouse adrenal chromaffin cells. J Neurosci Methods. 2007;166:195–202. doi: 10.1016/j.jneumeth.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandia L, Garcia AG, Morad M. ATP modulation of calcium channels in chromaffin cells. J Physiol. 1993;470:55–72. doi: 10.1113/jphysiol.1993.sp019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AG, Garcia-De-Diego AM, Gandia L, Borges R, Garcia-Sancho J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev. 2006;86:1093–1131. doi: 10.1152/physrev.00039.2005. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez M, Mejias R, Lopez-Barneo J. Developmental changes of chromaffin cell secretory response to hypoxia studied in thin adrenal slices. Pflugers Arch. 2007;454:93–100. doi: 10.1007/s00424-006-0186-y. [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M, Sala F, Sala S, et al. alpha-Bungarotoxin-sensitive nicotinic receptors on bovine chromaffin cells: molecular cloning, functional expression and alternative splicing of the alpha 7 subunit. Eur J Neurosci. 1995;7:647–655. doi: 10.1111/j.1460-9568.1995.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Palomero E, Cuchillo-Ibanez I, Garcia AG, Renart J, Albillos A, Montiel C. Greater diversity than previously thought of chromaffin cell Ca2+ channels, derived from mRNA identification studies. FEBS Lett. 2000;481:235–239. doi: 10.1016/s0014-5793(00)01984-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Palomero E, Renart J, Andres-Mateos E, et al. Differential expression of calcium channel subtypes in the bovine adrenal medulla. Neuroendocrinology. 2001;74:251–261. doi: 10.1159/000054692. [DOI] [PubMed] [Google Scholar]
- Giancippoli A, Novara M, de Luca A, et al. Low-threshold exocytosis induced by cAMP-recruited CaV3.2 (alpha1H) channels in rat chromaffin cells. Biophys J. 2006;90:1830–1841. doi: 10.1529/biophysj.105.071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordanetto F, Knerr L, Wallberg A. T-type calcium channels inhibitors: a patent review. Expert Opin Ther Pat. 2011;21:85–101. doi: 10.1517/13543776.2011.536532. [DOI] [PubMed] [Google Scholar]
- Gosso S, Gavello D, Giachello CN, Franchino C, Carbone E, Carabelli V. The effect of CdSe-ZnS quantum dots on calcium currents and catecholamine secretion in mouse chromaffin cells. Biomaterials. 2011;32:9040–9050. doi: 10.1016/j.biomaterials.2011.08.031. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Grynszpan-Wynograd O, Nicolas G. Intercellular junctions in the adrenal medulla: a comparative freeze-fracture study. Tissue Cell. 1980;12:661–672. doi: 10.1016/0040-8166(80)90020-8. [DOI] [PubMed] [Google Scholar]
- Guerineau NC, Desarmenien MG. Developmental and stress-induced remodeling of cell-cell communication in the adrenal medullary tissue. Cell Mol Neurobiol. 2010;30:1425–1431. doi: 10.1007/s10571-010-9583-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Hockerman GH, Scheuer T, Catterall WA. Molecular determinants of inactivation and G protein modulation in the intracellular loop connecting domains I and II of the calcium channel alpha1A subunit. Proc Natl Acad Sci U S A. 1997;94:1512–1516. doi: 10.1073/pnas.94.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Segura-Chama P, Jimenez N, Garcia AG, Hernandez-Guijo JM, Hernandez-Cruz A. Modulation by endogenously released ATP and opioids of chromaffin cell calcium channels in mouse adrenal slices. Am J Physiol Cell Physiol. 2011;300:C610–623. doi: 10.1152/ajpcell.00380.2010. [DOI] [PubMed] [Google Scholar]
- Hernandez-Guijo JM, de Pascual R, Garcia AG, Gandia L. Separation of calcium channel current components in mouse chromaffin cells superfused with low- and high-barium solutions. Pflugers Arch. 1998a;436:75–82. doi: 10.1007/s004240050606. [DOI] [PubMed] [Google Scholar]
- Hernandez-Guijo JM, Gandia L, Lara B, Garcia AG. Autocrine/paracrine modulation of calcium channels in bovine chromaffin cells. Pflugers Arch. 1998b;437:104–113. doi: 10.1007/s004240050754. [DOI] [PubMed] [Google Scholar]
- Hernandez-Guijo JM, Carabelli V, Gandia L, Garcia AG, Carbone E. Voltage-independent autocrine modulation of L-type channels mediated by ATP, opioids and catecholamines in rat chromaffin cells. Eur J Neurosci. 1999;11:3574–3584. doi: 10.1046/j.1460-9568.1999.00775.x. [DOI] [PubMed] [Google Scholar]
- Hill J, Chan SA, Kuri B, Smith C. PACAP recruits a low voltage activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells. J Biol Chem. 2011;286:42459–42469. doi: 10.1074/jbc.M111.289389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollins B, Ikeda SR. Inward currents underlying action potentials in rat adrenal chromaffin cells. J Neurophysiol. 1996;76:1195–1211. doi: 10.1152/jn.1996.76.2.1195. [DOI] [PubMed] [Google Scholar]
- Horrigan FT, Bookman RJ. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994;13:1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Bloss EB, McCarthy KJ, McEwen BS. Regulation of the nicotinic receptor alpha7 subunit by chronic stress and corticosteroids. Brain Res. 2010;1325:141–146. doi: 10.1016/j.brainres.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Dunlap K. Voltage-dependent modulation of N-type calcium channels: role of G protein subunits. Adv Second Messenger Phosphoprotein Res. 1999;33:131–151. doi: 10.1016/s1040-7952(99)80008-1. [DOI] [PubMed] [Google Scholar]
- Inoue M, Sakamoto Y, Fujishiro N, et al. Homogeneous Ca2+ stores in rat adrenal chromaffin cells. Cell Calcium. 2003;33:19–26. doi: 10.1016/s0143-4160(02)00178-1. [DOI] [PubMed] [Google Scholar]
- Jagger DJ, Griesinger CB, Rivolta MN, Holley MC, Ashmore JF. Calcium signalling mediated by the 9 acetylcholine receptor in a cochlear cell line from the Immortomouse. J Physiol. 2000;527:49–54. doi: 10.1111/j.1469-7793.2000.t01-1-00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodic MM, Pathirathna S, Nelson MT, et al. Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J Neurosci. 2007;27:3305–3316. doi: 10.1523/JNEUROSCI.4866-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Lammert E. Cell-cell interactions in the endocrine pancreas. Diabetes Obes Metab. 2009;11(Suppl 4):159–167. doi: 10.1111/j.1463-1326.2009.01102.x. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y, Ritchie AK. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 1980;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DL, Slepetis R, Kirshner N. Ion channels and membrane potential in stimulus-secretion coupling in adrenal medulla cells. J Neurochem. 1981;36:1245–1255. doi: 10.1111/j.1471-4159.1981.tb01724.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DL, Slepetis RJ, Corcoran JJ, Kirshner N. Calcium uptake and catecholamine secretion by cultured bovine adrenal medulla cells. J Neurochem. 1982;38:427–435. doi: 10.1111/j.1471-4159.1982.tb08647.x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lim W, Kim J. Contribution of L- and N-type calcium currents to exocytosis in rat adrenal medullary chromaffin cells. Brain Res. 1995;675:289–296. doi: 10.1016/0006-8993(95)00085-5. [DOI] [PubMed] [Google Scholar]
- Klingauf J, Neher E. Modeling buffered Ca2+ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri BA, Chan SA, Smith CB. PACAP regulates immediate catecholamine release from adrenal chromaffin cells in an activity-dependent manner through a protein kinase C-dependent pathway. J Neurochem. 2009;110:1214–1225. doi: 10.1111/j.1471-4159.2009.06206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalevee N, Rebsamen MC, Barrere-Lemaire S, et al. Aldosterone increases T-type calcium channel expression and in vitro beating frequency in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;67:216–224. doi: 10.1016/j.cardiores.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Lesouhaitier O, Chiappe A, Rossier MF. Aldosterone increases T-type calcium currents in human adrenocarcinoma (H295R) cells by inducing channel expression. Endocrinology. 2001;142:4320–4330. doi: 10.1210/endo.142.10.8435. [DOI] [PubMed] [Google Scholar]
- Levitsky KL, Lopez-Barneo J. Developmental change of T-type Ca2+ channel expression and its role in rat chromaffin cell responsiveness to acute hypoxia. J Physiol. 2009;587:1917–1929. doi: 10.1113/jphysiol.2009.168989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Olson C, Lu S, Nagy JI. Association of connexin36 with zonula occludens-1 in HeLa cells, betaTC-3 cells, pancreas, and adrenal gland. Histochem Cell Biol. 2004;122:485–498. doi: 10.1007/s00418-004-0718-5. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Liu H, Enyeart JA, Enyeart JJ. ACTH induces Cav3.2 current and mRNA by cAMP-dependent and cAMP-independent mechanisms. J Biol Chem. 2010;285:20040–20050. doi: 10.1074/jbc.M110.104190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd RV, Blaivas M, Wilson BS. Distribution of chromogranin and S100 protein in normal and abnormal adrenal medullary tissues. Arch Pathol Lab Med. 1985;109:633–635. [PubMed] [Google Scholar]
- Lopez MG, Shukla R, Garcia AG, Wakade AR. A dihydropyridine-resistant component in the rat adrenal secretory response to splanchnic nerve stimulation. J Neurochem. 1992;58:2139–2144. doi: 10.1111/j.1471-4159.1992.tb10956.x. [DOI] [PubMed] [Google Scholar]
- Lopez MG, Albillos A, de la Fuente MT, et al. Localized L-type calcium channels control exocytosis in cat chromaffin cells. Pflugers Arch. 1994;427:348–354. doi: 10.1007/BF00374544. [DOI] [PubMed] [Google Scholar]
- Lopez MG, Montiel C, Herrero CJ, et al. Unmasking the functions of the chromaffin cell alpha7 nicotinic receptor by using short pulses of acetylcholine and selective blockers. Proc Natl Acad Sci U S A. 1998;95:14184–14189. doi: 10.1073/pnas.95.24.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SJ, Li H, Zhou FH, et al. Connexin 36 is expressed and associated with zonula occludens-1 protein in PC-12 cells. Gen Physiol Biophys. 2007;26:33–39. [PubMed] [Google Scholar]
- Mahapatra S, Marcantoni A, Vandael DH, Striessnig J, Carbone E. Are Ca(v)1.3 pacemaker channels in chromaffin cells? Possible bias from resting cell conditions and DHP blockers usage. Channels (Austin) 2011;5:219–224. doi: 10.4161/chan.5.3.15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantoni A, Baldelli P, Hernandez-Guijo JM, Comunanza V, Carabelli V, Carbone E. L-type calcium channels in adrenal chromaffin cells: role in pace-making and secretion. Cell Calcium. 2007;42:397–408. doi: 10.1016/j.ceca.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Marcantoni A, Carabelli V, Comunanza V, Hoddah H, Carbone E. Calcium channels in chromaffin cells: focus on L and T types. Acta Physiol (Oxf) 2008;192:233–246. doi: 10.1111/j.1748-1716.2007.01815.x. [DOI] [PubMed] [Google Scholar]
- Marcantoni A, Carabelli V, Vandael DH, Comunanza V, Carbone E. PDE type-4 inhibition increases L-type Ca(2+) currents, action potential firing, and quantal size of exocytosis in mouse chromaffin cells. Pflugers Arch. 2009;457:1093–1110. doi: 10.1007/s00424-008-0584-4. [DOI] [PubMed] [Google Scholar]
- Marcantoni A, Vandael DH, Mahapatra S, et al. Loss of Cav1.3 channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J Neurosci. 2010;30:491–504. doi: 10.1523/JNEUROSCI.4961-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Carbone E, Lux HD. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986;406:104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Marger F, Gelot A, Alloui A, et al. T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci U S A. 2011;108:11268–11273. doi: 10.1073/pnas.1100869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariot P, Vanoverberghe K, Lalevee N, Rossier MF, Prevarskaya N. Overexpression of an alpha 1H (Cav3.2) T-type calcium channel during neuroendocrine differentiation of human prostate cancer cells. J Biol Chem. 2002;277:10824–10833. doi: 10.1074/jbc.M108754200. [DOI] [PubMed] [Google Scholar]
- Martin AO, Mathieu MN, Chevillard C, Guerineau NC. Gap junctions mediate electrical signaling and ensuing cytosolic Ca2+ increases between chromaffin cells in adrenal slices: A role in catecholamine release. J Neurosci. 2001;21:5397–5405. doi: 10.1523/JNEUROSCI.21-15-05397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AO, Mathieu MN, Guerineau NC. Evidence for long-lasting cholinergic control of gap junctional communication between adrenal chromaffin cells. J Neurosci. 2003;23:3669–3678. doi: 10.1523/JNEUROSCI.23-09-03669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AO, Alonso G, Guerineau NC. Agrin mediates a rapid switch from electrical coupling to chemical neurotransmission during synaptogenesis. J Cell Biol. 2005;169:503–514. doi: 10.1083/jcb.200411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda P, Pepper MS, Traub O, et al. Differential expression of gap junction connexins in endocrine and exocrine glands. Endocrinology. 1993;133:2371–2378. doi: 10.1210/endo.133.5.8404689. [DOI] [PubMed] [Google Scholar]
- Mollard P, Seward EP, Nowycky MC. Activation of nicotinic receptors triggers exocytosis from bovine chromaffin cells in the absence of membrane depolarization. Proc Natl Acad Sci U S A. 1995;92:3065–3069. doi: 10.1073/pnas.92.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand I, Fonlupt P, Guerrier A, et al. Cell-to-cell communication in the anterior pituitary: evidence for gap junction-mediated exchanges between endocrine cells and folliculostellate cells. Endocrinology. 1996;137:3356–3367. doi: 10.1210/endo.137.8.8754762. [DOI] [PubMed] [Google Scholar]
- Moser T. Low-conductance intercellular coupling between mouse chromaffin cells in situ. J Physiol. 1998;506 (Pt 1):195–205. doi: 10.1111/j.1469-7793.1998.195bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SA, Pharrams SY. Comparison of gap junction expression in the adrenal gland. Microsc Res Tech. 1997;36:510–519. doi: 10.1002/(SICI)1097-0029(19970315)36:6<510::AID-JEMT8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Neher E. A comparison between exocytic control mechanisms in adrenal chromaffin cells and a glutamatergic synapse. Pflugers Arch. 2006;453:261–268. doi: 10.1007/s00424-006-0143-9. [DOI] [PubMed] [Google Scholar]
- Novara M, Baldelli P, Cavallari D, Carabelli V, Giancippoli A, Carbone E. Exposure to cAMP and beta-adrenergic stimulation recruits Ca(V)3 T-type channels in rat chromaffin cells through Epac cAMP-receptor proteins. J Physiol. 2004;558:433–449. doi: 10.1113/jphysiol.2004.061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse CA, Buttigieg J, Brown S, Holloway AC. Regulation of oxygen sensitivity in adrenal chromaffin cells. Ann N Y Acad Sci. 2009;1177:132–139. doi: 10.1111/j.1749-6632.2009.05031.x. [DOI] [PubMed] [Google Scholar]
- Nuss HB, Houser SR. T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res. 1993;73:777–782. doi: 10.1161/01.res.73.4.777. [DOI] [PubMed] [Google Scholar]
- Olivos L, Artalejo AR. Muscarinic excitation-secretion coupling in chromaffin cells. Acta Physiol (Oxf) 2008;192:213–220. doi: 10.1111/j.1748-1716.2007.01816.x. [DOI] [PubMed] [Google Scholar]
- Panner A, Wurster RD. T-type calcium channels and tumor proliferation. Cell Calcium. 2006;40:253–259. doi: 10.1016/j.ceca.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Parker TL, Kesse WK, Tomlinson A, Coupland RE. Ontogenesis of preganglionic sympathetic innervation of rat adrenal chromaffin cells. In: Dahlström A, Belmaker RH, Sandler M, editors. Progress in Catecholamine Research, part A: Basic aspects and peripheral mechanisms. Liss A. R; New York: 1988. pp. 227–232. [Google Scholar]
- Perez-Alvarez A, Hernandez-Vivanco A, Caba-Gonzalez JC, Albillos A. Different roles attributed to Cav1 channel subtypes in spontaneous action potential firing and fine tuning of exocytosis in mouse chromaffin cells. J Neurochem. 2011a;116:105–121. doi: 10.1111/j.1471-4159.2010.07089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvarez A, Hernandez-Vivanco A, McIntosh JM, Albillos A. Native {alpha}6{beta}4* nicotinic receptors control exocytosis in human chromaffin cells of the adrenal gland. FASEB J. 2011b doi: 10.1096/fj.11-190223. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvarez A, Hernandez-Vivanco A, Gregorio SA, Tabernero A, McIntosh JM, Albillos A. Pharmacological characterization of native alpha7 nAChRs and their contribution to depolarization-elicited exocytosis in human chromaffin cells. Br J Pharmacol. 2011c doi: 10.1111/j.1476-5381.2011.01596.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Pluteanu F, Cribbs LL. T-type calcium channels are regulated by hypoxia/reoxygenation in ventricular myocytes. Am J Physiol Heart Circ Physiol. 2009;297:H1304–1313. doi: 10.1152/ajpheart.00528.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa JM, de Diego AM, Gandia L, Garcia AG. L-type calcium channels are preferentially coupled to endocytosis in bovine chromaffin cells. Biochem Biophys Res Commun. 2007;357:834–839. doi: 10.1016/j.bbrc.2007.03.207. [DOI] [PubMed] [Google Scholar]
- Rosa JM, Gandia L, Garcia AG. Permissive role of sphingosine on calcium-dependent endocytosis in chromaffin cells. Pflugers Arch. 2010;460:901–914. doi: 10.1007/s00424-010-0861-x. [DOI] [PubMed] [Google Scholar]
- Rosa JM, Torregrosa-Hetland CJ, Colmena I, Gutierrez LM, Garcia AG, Gandia L. Calcium entry through slow-inactivating L-type calcium channels preferentially triggers endocytosis rather than exocytosis in bovine chromaffin cells. Am J Physiol Cell Physiol. 2011;301:C86–98. doi: 10.1152/ajpcell.00440.2010. [DOI] [PubMed] [Google Scholar]
- Sala F, Nistri A, Criado M. Nicotinic acetylcholine receptors of adrenal chromaffin cells. Acta Physiol (Oxf) 2008;192:203–212. doi: 10.1111/j.1748-1716.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- Segura-Chama P, Rivera-Cerecedo CV, Gonzalez-Ramirez R, Felix R, Hernandez-Guijo JM, Hernandez-Cruz A. Atypical Ca2+ currents in chromaffin cells from SHR and WKY rat strains result from the deficient expression of a splice variant of alpha1D Ca2+ channel. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00849. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Adrenomedullary function in the neonatal rat: responses to acute hypoxia. J Physiol. 1985;358:1–16. doi: 10.1113/jphysiol.1985.sp015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen L, Smith TW. T-type Ca2+ channels are abnormal in genetically determined cardiomyopathic hamster hearts. Circ Res. 1994;75:149–155. doi: 10.1161/01.res.75.1.149. [DOI] [PubMed] [Google Scholar]
- Singh A, Gebhart M, Fritsch R, et al. Modulation of voltage- and Ca2+-dependent gating of CaV1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J Biol Chem. 2008;283:20733–20744. doi: 10.1074/jbc.M802254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Development of the sympathoadrenal axis. In: Gootman PM, editor. Developmental Neurobiology of the Autonomic Nervous System. Humana; Totowa, NJ: 1986. pp. 69–96. [Google Scholar]
- Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci. 2010;30:10763–10772. doi: 10.1523/JNEUROSCI.2307-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev. 2010;31:845–915. doi: 10.1210/er.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi S, Li Y, Kaku T, et al. Remodeling excitation-contraction coupling of hypertrophied ventricular myocytes is dependent on T-type calcium channels expression. Biochem Biophys Res Commun. 2006;345:766–773. doi: 10.1016/j.bbrc.2006.04.146. [DOI] [PubMed] [Google Scholar]
- Thiagarajan R, Tewolde T, Li Y, Becker PL, Rich MM, Engisch KL. Rab3A negatively regulates activity-dependent modulation of exocytosis in bovine adrenal chromaffin cells. J Physiol. 2004;555:439–457. doi: 10.1113/jphysiol.2003.056333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Jackson A, Nurse CA. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J Physiol. 1997;498 ( Pt 2):503–510. doi: 10.1113/jphysiol.1997.sp021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi E, Agrawal R, Nath C, Shukla R. Inhibitory role of cholinergic system mediated via alpha7 nicotinic acetylcholine receptor in LPS-induced neuro-inflammation. Innate Immun. 2010;16:3–13. doi: 10.1177/1753425909104680. [DOI] [PubMed] [Google Scholar]
- Vandael DH, Marcantoni A, Mahapatra S, et al. Ca(v)1.3 and BK channels for timing and regulating cell firing. Mol Neurobiol. 2010;42:185–198. doi: 10.1007/s12035-010-8151-3. [DOI] [PubMed] [Google Scholar]
- Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenberg HS, Schott M, Saeger W, Tries A, Scherbaum WA, Bornstein SR. Expression of connexins in chromaffin cells of normal human adrenals and in benign and malignant pheochromocytomas. Ann N Y Acad Sci. 2006;1073:578–583. doi: 10.1196/annals.1353.060. [DOI] [PubMed] [Google Scholar]
- Wu MM, Llobet A, Lagnado L. Loose coupling between calcium channels and sites of exocytosis in chromaffin cells. J Physiol. 2009;587:5377–5391. doi: 10.1113/jphysiol.2009.176065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PC, Fann MJ, Kao LS. Characterization of Ca2+ signaling pathways in mouse adrenal medullary chromaffin cells. J Neurochem. 2010;112:1210–1222. doi: 10.1111/j.1471-4159.2009.06533.x. [DOI] [PubMed] [Google Scholar]
- Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel alpha1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]


