Abstract
Myocardial infarction (MI) is one of the leading causes of morbidity and mortality world-wide. Whether endogenous repair and regenerative ability could be augmented by drug administration is an important issue for generation of novel therapeutic approach. Recently it was reported that in mice pretreated with thymosin beta 4 (TB4) and subsequently subjected to experimental MI, a subset of epicardial cells differentiated into cardiomyocytes. In clinical settings, epicardial priming with TB4 prior to MI is impractical. Here we tested if TB4 treatment after MI could reprogram epicardium into cardiomyocytes and augment the epicardium’s injury response. Using epicardium genetic lineage trace line Wt1CreERT2/+ and double reporter line Rosa26mTmG/+, we found post-MI TB4 treatment significantly increased the thickness of epicardium and coronary capillary density. However, epicardium-derived cells did not adopt cardiomyocyte fate, nor did they migrate into myocardium to become coronary endothelial cells. Our result thus indicates that TB4 treatment after MI does not alter epicardial cell fate to include the cardiomyocyte lineage, providing both cautions and insights for the full exploration of the potential benefits of TB4 in the clinical settings. This article is part of a Special Issue entitled ‘Possible Editorial’.
Keywords: Epicardium, Thymosin beta 4, Cardiomyocytes, Myocardial infarction, Reprogram
1. Introduction
Myocardial infarction (MI) is a one of the leading cause of mortality and morbidity. As a result of inadequate myocardial perfusion, billions of cardiomyocytes are lost in human hearts after an acute myocardial infarction [1,2]. Recent work showed that extracardiac or intracardiac progenitor cells have beneficial effects after MI, suggesting a potential therapeutic role for regenerative approaches to heart failure [3]. However, stem cell transplantation approaches still face numerous hurdles, including graft survival, effective functional integration with existing myocardium, immune responses to non-autologous donor cells, and uncertain long term benefits [4]. An attractive alternative approach is to augment the regenerative capacity of resident cardiac progenitor cells. Success hinges on identification of suitable cardiac progenitors resident in the adult heart, and uncovering factors and pathways that increase the regenerative ability of these progenitors.
One potential progenitor population is located within the epicardium. This single layer of epithelial cells is pivotal for normal heart development, as it provides both mesenchymal cells (derived through an epithelial to mesenchymal transition) and paracrine factors necessary for growth of the myocardium and the coronary vasculature [5]. Using genetic fate mapping approaches, we and others found that epicardium cells contribute to fibroblast, vascular smooth muscle, endothelial, and cardiomyocyte lineages during heart development [6,7]. However, the epicardial origin of cardiomyocytes during heart development remains controversial [8,9].
While the role of epicardium in heart development has been intensely studied, relatively little is known about epicardial function in the normal and injured adult heart. In adult zebrafish, amputation of the myocardial apex reactivated fetal epicardial genes. The epicardium appeared to be intimately involved in the regenerative response [10,11], although there was no direct epicardial contribution to the cardiomyocyte lineage in zebrafish hearts [12]. Recently, we showed that MI markedly expanded the adult murine epicardium and reactivated expression of fetal epicardial genes [13]. Augmenting the epicardial response to myocardial injury could be an attractive approach for promoting cardiac repair and regeneration.
Thymosin beta 4 (TB4), a 43 amino acid actin monomer-binding peptide, plays an important role in normal heart development, promoting myocardial growth, coronary vasculogenesis, and epicardial integrity. Administration of TB4 promoted cardiac repair in a murine MI model [14], and TB4 has been undergoing clinical trials as a treatment for MI (www.clinicaltrials.gov, NCT00743769). In the murine model, TB4 reduced cardiomyocyte apoptosis and promote cardiac function recovery after MI [14]. While TB4 appears to have beneficial activity in promoting myocardial repair, the mechanisms of action remain uncertain. At least one site of TB4 action is the epicardium, as TB4-stimulated epicardium expanded markedly in vitro and in vivo [15,16]. In explant culture, TB4 also reactivated fetal epicardial properties, including restoring the capacity of adult epicardial explants to generate mesenchymal cells that differentiate into fibroblast, endothelial cell, and smooth muscle cell fates [16]. However whether TB4 administered after MI influences epicardial differentiation programs has not been directly investigated in vivo. Here we addressed this question using genetic lineage tracing in a murine model.
2. Methods
An expanded Methods section is available in the data supplement and includes detailed information regarding the following experimental procedures: myocardium infarction, epicardial cell isolation and culture, quantitative RT-PCR, immunostaining and immunohistochemistry, and statistical analysis.
3. Results
To begin to better understand potential roles of TB4 in myocardial repair, we measured TB4 expression in the postnatal heart by qRTPCR and immunohistochemistry. MI significantly upregulated TB4 expression in the heart, with expression peaking 5 days following infarction (Supplementary Fig. 1A). At 1 week post MI, immunostaining indicated that the epicardium strongly expressed TB4 (Supplementary Fig. 1B). Enrichment of TB4 in epicardium and its derivatives was confirmed in both fetal and post-MI heart by qRTPCR of FACS-purified cells isolated from Wt1CreERT2/±;Rosa26mTmG/± mice, in which GFP indelibly labels epicardial cells and their descendants (Supplementary Figs. 1C–E) [13]. For simplicity, in this report we refer to these GFP-labeled cells, composed of epicardium and its derivatives, as epicardium-derived cells (EPDCs), and we use the term ‘epicardial layer’ to refer to the region of the adult heart overlying the myocardium that contains these EPDCs. In conjunction with the beneficial effects of exogenous TB4 in the MI model [14], these data suggest that native, epicardium-secreted TB4 might also protect and promote repair and vascularization of the underlying myocardium.
Next, we investigated the effect of exogenous TB4 administration on the myocardial injury response, using a genetic lineage tracing approach to evaluate the effect of TB4 treatment on epicardial cell fate in vivo. Previous work indicated that TB4 administered after MI influenced epicardial activity [15], but the effect of TB4 on epicardial cell fate after MI has not been investigated using direct genetic lineage tracing approaches. We addressed this point using the Wt1CreERT2/+; Rosa26mTmG/+ system to follow the fate of EPDCs through their indelible GFP label. We induced MI in these mice, then treated with TB4 each day after MI for 1 week, and analyzed the fate of GFP-expressing EPDCs at 2 weeks post-MI. We confirmed that TB4 was effective in our hands by reproducing the previously reported beneficial effects of TB4 on myocardium after infarction [14,17]. Specifically, as previously reported TB4 significantly reduced infarct size, cardiac fibrosis, and cardiomyocyte apoptosis, and increased vessel density (Supplementary Figs. 2A–C). Interestingly, TB4 treatment also augmented thickening of the epicardial layer that occurs following MI, as determined by immunohistochemistry for GFP-labeled EPDCs in Wt1CreERT2/+;Rosa26mTmG/+ mice (Figs. 1A, B; Supplementary Fig. 3A). Our recent work suggested that thickening of the epicardial layer, comprised of EPDCs and epicardial cells, after MI contributes importantly to its secretion of paracrine factors that mitigate myocardial injury and adverse cardiac remodeling [13]. Thus, TB4-induced epicardial thickening likely participates in the salutary effects of TB4. The above result showed that the TB4 in our models (post-MI) was effective and beneficial in protection for injured heart.
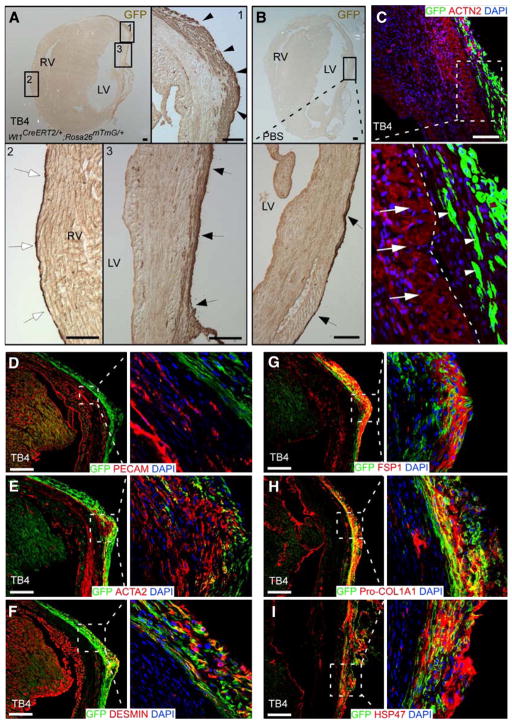
Fig. 1.
Reactivated epicardial layer and cell fate after myocardial infarction followed by thymosin β4 (TB4) treatment. After myocardial infarction followed by TB4 or PBS treatment, Wt1CreERT2/+;Rosa26mTmG/+ hearts were analyzed by immunochemistry. (A, B) Immunostaining for GFP in TB4-treated (A) or PBS-treated (B) heart. GFP marks EPDCs. The GFP+ epicardial layer covering the heart was thicker in the peri-infarct (arrowheads, inset 1) and infarct (arrows, inset 3) regions with TB4 treatment. The epicardial layer remained thin in the remote (right ventricular) region (inset 2). LV, left ventricle; RV, right ventricle; bar=100 μm. (C) EPDCs (white arrowheads) did not migrate into myocardium or express cardiomyocyte markers in Wt1CreERT2/+;Rosa26mTmG/+ hearts that underwent experimental infarction followed by TB4 treatment. White arrows indicate cardiomyocytes and white arrowheads indicate EPDCs. White dotted line indicates the border between myocardium and the epicardial layer. Bar=100 μm. (D–I) In infarcted, TB4-treated heart, EPDCs (GFP+) did not differentiate into coronary endothelial cells, marked by PECAM. A subset of cells expressed smooth muscle actin (ACTA2) or DESMIN, markers of myofibroblast and smooth muscle cells. Many GFP+ cells also co-expressed fibroblast markers FSP1, Pro-Collagen I and HSP47. Bar=200 μm.
Given the putative, albeit controversial, capacity of fetal epicardium to differentiate into cardiomyocytes, we asked if post-MI TB4 treatment induces cardiomyocyte differentiation from adult epicardium. Using the GFP lineage marker, we dissected the fate of epicardium cells by immunostaining TB4-treated infarcted hearts for coexpression of cardiomyocyte, fibroblast, smooth muscle, and endothelial cell markers. Like control (PBS-treated) MI hearts at 2 week post MI, GFP+ EPDCs in TB4-treated MI hearts did not migrate into myocardium nor did they express cardiomyocyte or endothelial cell markers (Figs. 1C, D; Supplementary Figs. 3B, C; data not shown). A subset of EPDCs expressed myofibroblast/fibroblast or smooth muscle markers smooth muscle actin alpha (ACTA2), desmin, fibroblast-specific protein (FSP1), procollagen alpha1 type I (Pro-Col1a1), heat shock protein 47 (HSP47), collagen III (ColIII) and discoidin domain receptor 2 (DDR2) (Figs. 1E–I and Supplementary Figs. 2D, E). To confirm these results, we analyzed FACS-purified EPDCs from Wt1CreERT2/+;Rosa26mTmG/+ MI hearts treated with TB4 (Fig. 2A). Strong enrichment of epicardial genes Wt1 and Tbx18 in the GFP+ population validated the purification procedure (Fig. 2B). Immunostaining of the isolated cells permitted analysis of lineage markers in isolated cells and thereby avoided potential artifacts from staining of tissue sections. The isolated GFP+ cells expressed epicardial markers WT1 and TBX18, but did not co-express cardiomyocyte markers cardiac troponin T (TNNT2) or cardiac actinin alpha (ACTN2, Fig. 2C). In control experiments, TNNT and ACTN2 were readily stained cells in the GFP− population (data not shown), excluding a technical staining failure. In accordance with results from tissue section staining, a subset of EPDCs differentiated into myofibroblast and smooth muscle cells (Fig. 2C). EPDCs isolated from PBS-treated mice adopted a similar set of cell fates, which did not include cardiomyocytes (Fig. 2D).
Fig. 2.
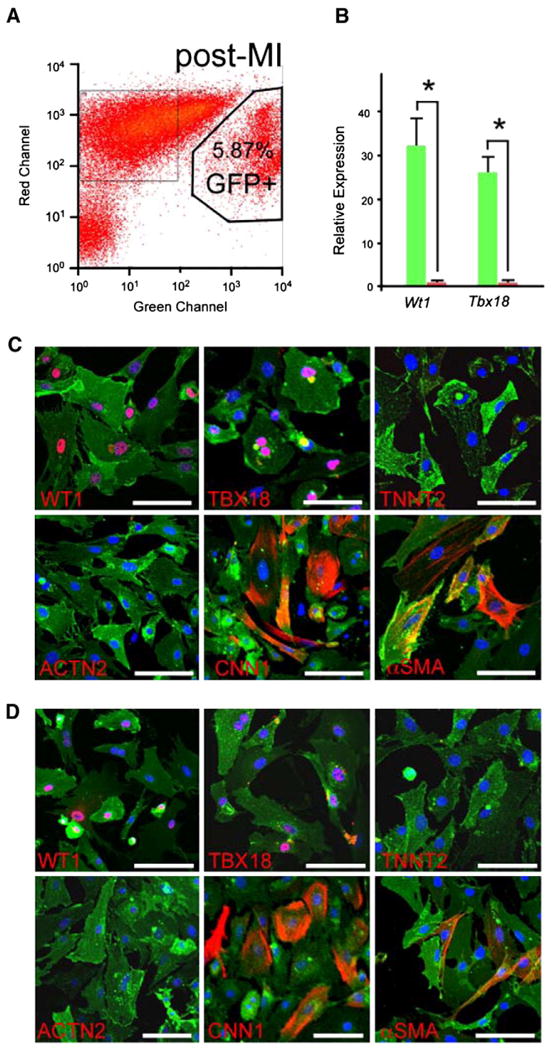
Non-cardiomyocyte fate of epicardial cells after TB4 injection. (A) FACS isolation of GFP+ EPDCs from Wt1CreERT2/+;Rosa26mTmG/+ hearts treated by LAD ligation followed by TB4 injection. (B) Quantitative RT-PCR showed highly purified EPDCs after FACS isolation (n=3). (C) Immunostaining of primary EPDCs isolated from infarcted, TB4-treated hearts showed co-expression of markers of epicardial cells (WT1, TBX18) and smooth muscle or myofibroblast cells (CNN1, αSMA), but not cardiomyocytes (TNNT2, ACTN2). (D) Immunostaining of primary isolated EPDCs from infarcted, PBS-treated hearts. White bar=50 μm.
4. Discussion
Our present work used mouse genetic models to directly show that TB4 administered after myocardial infarction increased expansion of epicardium and reduced infarct size. Endogenous TB4 was upregulated in epicardium after MI, suggesting that native TB4 participates in the myocardial injury response by promoting epicardial expansion, reducing myocardial apoptosis, and improving myocardial vascularization. The epicardial actions of TB4 are likely linked to its beneficial effects in MI, as thickening of the epicardium layer likely enhances its production of beneficial paracrine factors [10,13] and contributes to production of smooth muscle cells and fibroblasts that may provide mechanical protection as the myocardium thins due to loss of myocytes after MI.
Our data indicate that TB4 administered post-MI does not mobilize EPDCs so that they cannot migrate into the myocardium like fetal EPDCs [6,7]. Furthermore, post-MI TB4 did not direct EPDC differentiation into cardiomyocytes. This result contrasts with the effect of TB4 administered prior to MI, where “priming” of epicardium by TB4 reprogrammed EPDCs so that a subset differentiated into cardiomyocytes after subsequent MI [18]. Both experiments used clinical grade TB4 from the same source. Importantly, we also observed the beneficial effect of TB4 for injury repair after MI, supporting biological activity of TB4 in our system. Both experiments also employed the same epicardium specific inducible Cre line (Wt1CreERT2/±) [4] for genetic fate mapping [6]. Thus the lack of cardiomyocyte fate in our experiment is unlikely due to labeling of different epicardial cell subpopulations.
The principal difference between the experiments was the timing of TB4 injection, as we administered TB4 following MI, while Smart and colleagues primed mice by TB4 pretreatment. Myocardial injury substantially alters epicardial cell properties and gene expression [13], and it is possible that these changes impair the ability of TB4 to augment epicardial cell plasticity. Another possibility is that myocardial injury disrupts the architecture of an epicardial niche that is required for nurturing epicardial cells to be reprogrammed. In clinical practice, it will not be feasible to administer TB4 prior to MI. Thus, effective translation of TB4-primed EPDC differentiation into cardiomyocytes will require understanding why TB4 fails to induce EPDC plasticity when administered following an MI, and the molecular mechanisms underlying the difference.
Supplementary Material
Acknowledgments
We appreciated the help from Elizabeth Boush in FACS procedures. This work was supported by funding from NIH (RO1 HL094683 and U01 HL100401 to WTP), an American Heart Association Postdoctoral Fellowship (BZ), a Career Development Award from the Translational Research Program at Children’s Hospital Boston (WTP), and charitable support from James Smith and Gail Federici-Smith (WTP) and the Simeon Burt Wolbach Research Fund (BZ). TB4 was generously provided by RegeneRx Biopharmaceuticals Inc, Rockville, MD.
Appendix A. Supplementary data
Supplementary data to this article can be found online at doi:10. 1016/j.yjmcc.2011.08.020.
Footnotes
Disclosures
None.
Contributor Information
Bin Zhou, Email: houbin@sibs.ac.cn.
William T. Pu, Email: wpu@enders.tch.harvard.edu.
References
- 1.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344(23):1750–7. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 2.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5(4):364–77. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, et al. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006;20(12):1651–66. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454(7200):104–8. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grieskamp T, Rudat C, Ludtke TH, Norden J, Kispert A. Notch signaling regulates smooth muscle differentiation of epicardium-derived cells. Circ Res. 2011;108(7):813–23. doi: 10.1161/CIRCRESAHA.110.228809. [DOI] [PubMed] [Google Scholar]
- 9.Christoffels VM, Grieskamp T, Norden J, Mommersteeg MT, Rudat C, Kispert A. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458:7240 E8–9. doi: 10.1038/nature07916. discussion E9–10. [DOI] [PubMed] [Google Scholar]
- 10.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127(3):607–19. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–5. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y, et al. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development. 2011;138(14):2895–902. doi: 10.1242/dev.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121(5):1894–904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432(7016):466–72. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 15.Bock-Marquette I, Shrivastava S, Pipes GC, Thatcher JE, Blystone A, Shelton JM, et al. Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. J Mol Cell Cardiol. 2009;46(5):728–38. doi: 10.1016/j.yjmcc.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445(7124):177–82. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 17.Riley PR, Smart N. Thymosin beta4 induces epicardium-derived neovascularization in the adult heart. Biochem Soc Trans. 2009;37(Pt 6):1218–20. doi: 10.1042/BST0371218. [DOI] [PubMed] [Google Scholar]
- 18.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474(7353):640–4. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.