Summary
The mechanisms underlying the silencing of alternative fate potentials in very early B cell precursors remain unclear. Using gain- and loss-of-function approaches together with a synthetic Zinc-finger polypeptide (6ZFP) engineered to prevent transcription factor binding to a defined cis element, we show that the transcription factor EBF1 promotes B cell lineage commitment by directly repressing expression of the T-cell-lineage-requisite Gata3 gene. Ebf1-deficient lymphoid progenitors exhibited increased T cell lineage potential and elevated Gata3 transcript expression, whereas enforced EBF1 expression inhibited T cell differentiation and caused rapid loss of Gata3 mRNA. Notably, 6ZFP-mediated perturbation of EBF1 binding to a Gata3 regulatory region restored Gata3 expression, abrogated EBF1-driven suppression of T cell differentiation, and prevented B cell differentiation via a GATA3-dependent mechanism. Furthermore, EBF1 binding to Gata3 regulatory sites induced repressive histone modifications across this region. These data identify a transcriptional circuit critical for B cell lineage commitment.
Highlights
-
•
The essential T cell gene Gata3 is a direct repressive target of EBF1
-
•
Inhibiting repression of Gata3 by EBF1 enhances T cell differentiation
-
•
Inhibiting repression of Gata3 by EBF1 prevents B cell differentiation
-
•
Binding of EBF1 to Gata3 induces repressive histone modifications
Introduction
The development of multicellular systems requires that multipotent progenitors differentiate into specialized lineage-restricted daughter cells. The adoption of a particular cell fate by multipotent cells is orchestrated by networks of transcription factors, which act to coordinate changes in gene expression commensurate with the ultimate function of the cell fate in question. Commitment of multipotent cells to a particular lineage often requires the silencing of gene products that are incompatible with the function of end-product cells. For instance, during hematopoiesis, erythroid and myeloid lineage genes are silenced during the generation of lymphocyte-biased progenitors (Miyamoto et al., 2002) and B cell and myeloid-affiliated genes are actively repressed in early T lineage cells (Yang et al., 2010; Zhang et al., 2012). Understanding the regulation of cell fate decisions in hematopoiesis should provide insights into the development of a wide array of multicellular systems and lead to strategies to enhance or limit the generation of particular cell types.
Early B cell development is regulated by several transcription factors. These include Ikaros and PU.1, which promote the generation of lymphoid-biased precursors, and early B cell factor-1 (EBF1), Pax5, and the E2a isoforms E12 and E47 (encoded by the Tcf3 locus), which coordinate the differentiation of lymphoid progenitors into lineage-restricted pro-B cells (reviewed in Mandel and Grosschedl, 2010). Given that each of these factors is essential for generating B cell lineage precursors, much work has been devoted to identifying regulated and coregulated target genes. Ebf1, Pax5, and Tcf3 gene products synergize to activate the expression of the pre-BCR components λ5 and VpreB and the B cell signaling protein Ig-α (encoded by Igll1, Vpreb1, and Cd79a, respectively) (reviewed in Busslinger, 2004; Hagman and Lukin, 2006). Notably, Pax5, Ebf1, and Tcf3 gene products are each proposed to suppress differentiation of alternative fates (Ikawa et al., 2004; Nutt et al., 1999; Pongubala et al., 2008). In this regard, Pax5 is regarded as the dominant determinant of B cell commitment, because deletion of Pax5 in pro-B cells or mature peripheral B cells allows these cells to adopt alternative fates (Cobaleda et al., 2007; Mikkola et al., 2002). A key but unresolved question is whether E12 and E47 and/or EBF1 promote B cell lineage restriction by collaborating with Pax5 or whether these factors are components of distinct transcriptional circuits important for acquiring and perhaps maintaining B cell identity.
In the thymus, the T cell program is initiated when the earliest defined T cell precursors (ETPs) encounter ligands for the Notch receptor family (Sambandam et al., 2005). Stimulation of Notch1 on ETPs by the Notch ligand delta-like-4 (DL4) promotes the expression of T-cell-affiliated transcription factors including TCF1 (encoded by Tcf7), which in turn promotes the expression of many genes required for T cell function (Weber et al., 2011). However, early T cell development is also controlled by the zinc finger transcription factor GATA3. Indeed, GATA3 is essential for very early T cell development in the thymus beginning at the ETP stage (Hendriks et al., 1999; Taghon et al., 2007), and optimal Notch1 expression may require GATA3 (Wei et al., 2011). Suppression of the T cell fate in B cells is thought to occur through the Pax5-driven repression of Notch1 (Souabni et al., 2002). However, we showed previously that EBF1 prevents myeloid and T cell differentiation when introduced into Pax5−/− progenitors (Pongubala et al., 2008). The latter observation suggests that Pax5-independent transcriptional pathways may also regulate B cell lineage restriction, while also raising questions about the mechanism(s) employed by EBF1 to constrain T cell differentiation.
Here, we utilize a series of gain- and loss-of-function approaches to uncover the transcriptional mechanism underpinning EBF1-mediated suppression of T cell development. Our findings indicate that EBF1 limits early T cell differentiation by directly repressing Gata3 transcription and suggest that EBF1 silences Gata3 expression by promoting repressive histone modifications across Gata3 regulatory regions. These data identify a transcriptional circuit critical for preventing T cell differentiation and adopting the B cell fate.
Results
EBF1 Suppresses T Cell Differentiation in B-Cell-Lineage-Biased Lymphoid Progenitors
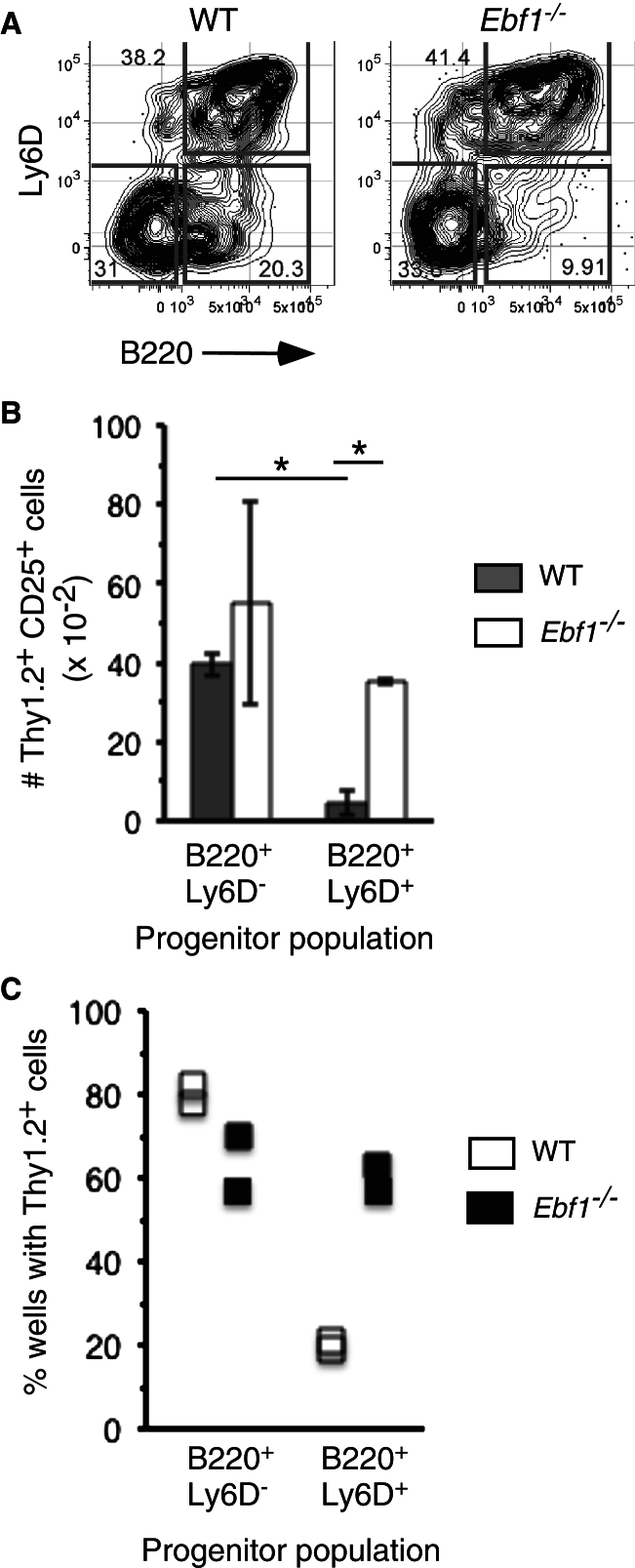
Lymphoid-biased progenitors in the bone marrow (BM), also referred to as common lymphoid progenitors (CLPs) (Kondo et al., 1997), can be subdivided into several subpopulations. More mature B-cell-lineage-biased progenitors within this heterogeneous population are also termed pre-pro-B cells and are characterized by progressive loss of T cell potential coincident with expression of the surface proteins B220 and/or Ly6D (Inlay et al., 2009; Rumfelt et al., 2006). Other researchers have employed a λ5 transgene to mark B-cell-lineage-biased precursors in these pools (Mansson et al., 2008). Given the rarity of these cells (less than 0.2% of all BM cells) and the diverse approaches used to resolve these populations, we developed a flow cytometric strategy based on differential surface expression of B220 and Ly6D on lymphoid-biased progenitors defined previously as Lineage(Lin)−CD19−IL-7Rα+Flt3+Sca1loc-Kitlo (Allman et al., 2003). With this approach we resolved three populations of IL-7Rα+Flt3+Sca1loc-Kitlo cells defined as B220−Ly6D−, B220+Ly6D−, and B220+Ly6D+ in both wild-type and Ebf1−/− mice (Figure 1A and Figure S1A available online). Consistent with past work (Rumfelt et al., 2006), coexpression of B220 and Ly6D correlated with increased Ebf1, Pax5, and Rag1 expression (Figure S1B), and when sorted from wild-type mice, the B220+Ly6D+ subset possessed fewer cells with T cell lineage potential (Figure 1B; Inlay et al., 2009; Rumfelt et al., 2006). Notably, however, although Ebf1−/− B220+Ly6D+ precursors were not substantially altered in number or phenotype compared to their wild-type counterparts (Figure 1A), they exhibited increased T cell lineage potential in both bulk and single-cell cultures (Figures 1B and 1C). These data extend past analyses (Zandi et al., 2008) by showing that EBF1 constrains T cell potential as B-cell-lineage-biased B220+Ly6D+ progenitors give rise to B-cell-lineage-restricted precursors.
Figure 1.
Ebf1-Deficient B-Cell-Lineage-Biased Precursors Possess Increased T Lymphoid Potential
(A) BM cells from chimeras reconstituted with B6 or B6.Ebf1−/− fetal liver 12 weeks previously were stained with antibodies to the host-specific determinant CD45SJL and the antibodies shown in Figure S1. 2 × 106 events were collected on an LSR2 flow cytometer and gated as in Figure S1.
(B) 200 WT or Ebf1−/− B220+Ly6D− or B220−Ly6D+ cells derived from the indicated chimeras were sorted onto pre-established OP9-DL4 cells supplemented with IL-7, FL, and SCF. Seven days later, cells were counted and analyzed for cell surface marker expression. The mean number of viable (DAPI−) CD45+ early T cell lineage (Thy1.2+CD25+) cells in triplicate cultures is shown. Error bars indicate SEMs, *p < 0.005. Data are representative of three separate experiments.
(C) 96 single WT or Ebf1−/− B220+Ly6D− or B220+Ly6D+ cells were sorted onto pre-established OP9-DL4 cells in flat-bottom 96-well plates with IL-7, FL, and SCF. Wells containing cell growth were counted and analyzed on day 10. Data are representative of two separate experiments. See also Figure S1.
EBF1 Overrides Overt T Cell Induction by Notch1 and TCF1
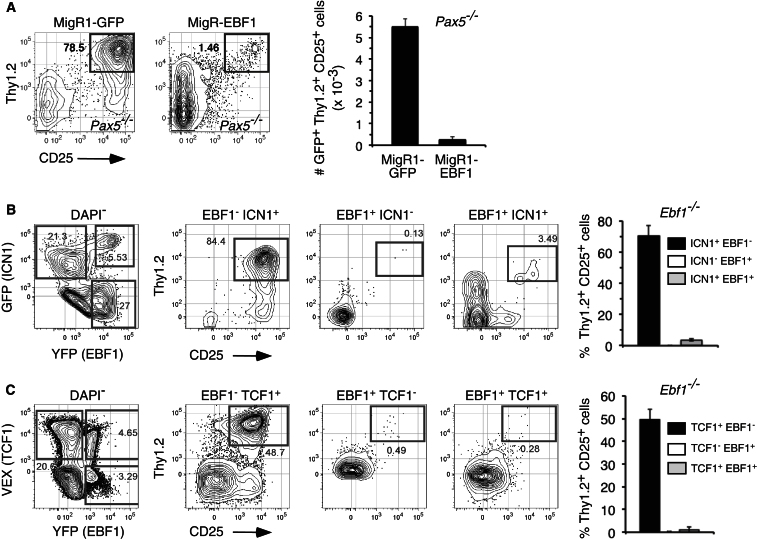
To test directly whether EBF1 prevents very early T cell differentiation independently of Pax5, we sorted BM progenitors from Pax5−/− fetal liver, transduced these cells with control or EBF1-expressing retrovirus, and then resorted transduced (GFP+) cells onto OP9-DL4 stromal cells. As shown (Figure 2A), despite stimulation with DL4, upon enforced EBF1 expression, Pax5−/− progenitors generated substantially fewer T lineage cells compared to controls. Enforcing EBF1 expression also prevented T cell development from Ebf1−/− and Pax5−/− LSKs transduced with an active allele for Notch1 (ICN1) (Figures 2B and S2). Likewise, EBF1 prevented T cell differentiation from progenitors transduced with the Notch1 target Tcf7 (encoding TCF1) (Figure 2C; Weber et al., 2011). These data indicate that EBF1 can override T-cell-lineage-promoting signals mediated by the Notch1-TCF1 pathway and further support the notion that EBF1 limits cell-intrinsic T cell potential in lymphoid precursors independently of Pax5.
Figure 2.
Notch1 and TCF1 Fail to Prevent Inhibition of Early T Cell Lineage Differentiation by EBF1
(A) LSK (Lin−Sca1+c-kit+) cells were sorted from e14.5 Pax5−/− fetal livers and transduced with control MigR1 or MigR-EBF1 virus. Viable GFP+ cells were sorted 24 hr after transduction onto pre-established OP9-DL4 cells with IL-7, FL, and SCF. Seven days later, cultures were stained and analyzed for absolute numbers of DAPI−CD45+GFP+Thy1.2+CD25+ cells. Data in right-most graph are means and SEMs from each group.
(B) e14.5 Ebf1−/− fetal liver LSKs were isolated and cotransduced with MigY-EBF1 and MigR-ICN1 viruses and plated on OP9 stromal cells. A day later, cells were washed and replated on OP9s in fresh media supplemented with IL-7, FL, and SCF. On day 7, cultures were stained and analyzed for relative contribution of DAPI−CD45+ single-transduced (GFP+YFP− or GFP−YFP+) or double-transduced (GFP+YFP+) cells that coexpressed Thy1.2 and CD25.
(C) e14.5 Ebf1−/− fetal liver LSKs were isolated and cotransduced with MigY-EBF1 and TCF1-VEX viruses as in (B). Plots were gated on DAPI−CD45+ single- or double-transduced cells. All graphs show means ± SEMs of triplicate samples. Data are representative of three separate experiments. See also Figure S2.
EBF1 Represses the Generation of Gata3 Transcripts
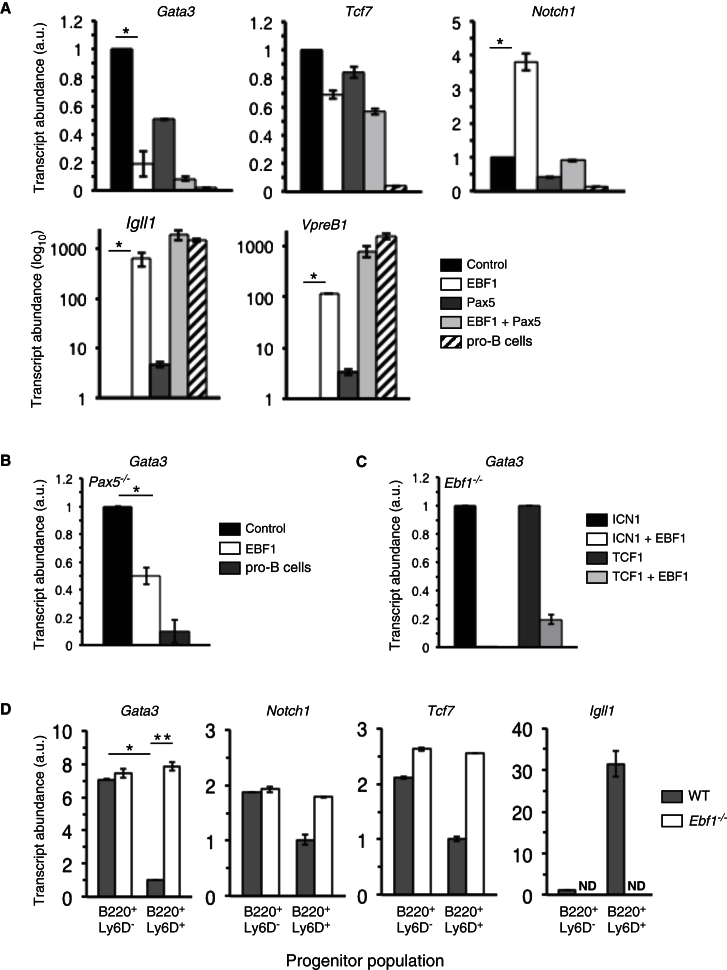
To test whether EBF1 represses the expression of essential T cell lineage genes, we transduced an Ebf1−/− progenitor cell line corresponding to pre-pro-B cells with EBF1 (Pongubala et al., 2008) and analyzed changes in gene expression on Affymetrix microarrays. In these experiments, transcripts for Gata3 declined reproducibly, whereas Notch1 and Tcf7 transcript levels as well as the T cell lineage regulator Bcl11b were not altered or increased slightly (Figure S3A). We repeated these analyses but with quantitative RT-PCR and locus-specific probes in Ebf1−/− progenitors transduced with virus encoding EBF1 and/or Pax5. Transduction with EBF1 or Pax5 led to the expected upregulation of transcripts for Igll1 and Vpreb1 encoding components of the pre-BCR, and cotransduction with EBF1 and Pax5 resulted in a synergistic increase in abundance of these transcripts (Figure 3A). Consistent with past work (Souabni et al., 2002), transduction with Pax5 reduced Notch1 transcript abundance to 50% of controls. Of note, EBF1 transduction did not perturb Tcf7 transcription substantially and appeared to drive a modest increase in Notch1 expression that was overridden by coexpression of Pax5. In sharp contrast, EBF1 transduction consistently induced an 80% decline in Gata3 transcript abundance (Figure 3A, top left). Pax5 transduction, by comparison, led to only a modest reduction in Gata3 transcripts, and cells cotransduced with EBF1 and Pax5 mirrored transduction with EBF1 alone. Introduction of EBF1 into a Pax5−/− pro-B cell line (Schaniel et al., 2002) also repeatedly resulted in a 50% decline in Gata3 transcripts (Figure 3B), and EBF1 expression continued to promote the downregulation of Gata3 transcripts in cells cotransduced with ICN1 or TCF1 (Figure 3C).
Figure 3.
EBF1 Represses Gata3 Transcript Levels
(A) Ebf1−/− lymphoid progenitors were transduced with MigR1, MiY-EBF1, or Mig-Pax5 virus alone or cotransduced with MiY-EBF1 and Mig-Pax5 and plated on OP9 cells supplemented with IL-7, FL, and SCF. 50,000 DAPI−YFP+GFP−, YFP−GFP+, or YFP+GFP+ cells were sorted 24 hr later for RNA isolation. Transcript levels of the indicated genes were assayed by qRT-PCR. Expression levels in MigR1-transduced samples were set to 1 and sorted pro-B cells (B220+CD43+AA4.1+CD19+) were included as an additional control. Error bars indicate SEMs, *p < 0.001.
(B) 2 × 107Pax5−/− pro-B cells were transduced with control MigR1 or MigR-EBF1 virus and assayed for relative levels of Gata3 transcripts 24 hr later by sorting on DAPI−GFP+ cells as in (A). Starting cell numbers were high due to the refractory nature of these cells to retroviral transduction. Error bars indicate SEMs, *p < 0.01.
(C) Ebf1−/− pre-pro-B cells were single transduced with Mig-ICN1 or TCF1-VEX or cotransduced with MigY-EBF1 and MigR-ICN1 or MigY-EBF1 and TCF1-VEX viruses. Seven days later, RNA was isolated and assayed for relative expression of Gata3 transcripts as in (A).
(D) 50,000 B220+Ly6D− or B220+Ly6D+ BM cells were sorted from chimeras established with WT or Ebf1−/− fetal liver progenitors as in Figure 1A. cDNA was prepared and qRT-PCR performed as in (A). Expression levels for the indicated genes in WT B220+Ly6D+ cells were arbitrarily set to 1, except for Igll1 detection where B220+Ly6D− cells were employed. ND, signal not detected. Data are means ± SEMs of triplicate samples. Error bars indicate SEMs, *p < 0.001, **p < 0.01.
All data are representative of three separate experiments. See also Figure S3.
Next, we analyzed Gata3, Notch1, and Tcf7 transcript abundance in wild-type and Ebf1−/− Lin−CD19−IL-7Rα+Flt3+Sca1loc-KitloB220+ BM lymphoid progenitors subdivided based on differential Ly6D surface expression (see Figure 1). Remarkably, wild-type B220+Ly6D+ progenitors exhibited a 7- to 10-fold decrease in Gata3 transcripts compared to cells within the less mature B220+Ly6D− fraction (Figure 3D), indicating that decreased Gata3 expression correlates with loss of T cell lineage potential. Furthermore, Ebf1−/− B220+Ly6D+ precursors exhibited a robust increase in Gata3 transcripts compared to their wild-type counterparts to levels found in wild-type B220+Ly6D− progenitors. In contrast, we observed only a modest increase in Notch1, Tcf7 transcripts in B220+Ly6D+ precursors lacking Ebf1. As expected, expression of the canonical EBF1 target Igll1 was severely decreased in Ebf1−/− B220+Ly6D+ progenitors (Figure 3D, right-most panel). These data, together with the data in Figure 1, show that deletion of Ebf1 results in increased Gata3 transcripts and a corresponding increase in T cell lineage potential in B-cell-lineage-biased lymphoid progenitors, implicating EBF1-mediated repression of Gata3 as an important event in early B cell development.
Mechanisms of EBF1-Mediated Gata3 Repression
Given that early B cell development involves collaborative interactions between EBF1 and the E12 and E47 transcription factors encoded by the Tcf3 gene (Lin et al., 2010; Sigvardsson et al., 1997), we tested whether EBF1-driven downregulation of Gata3 mRNA expression required Tcf3-encoded proteins. For these experiments we employed 4-hydroxytamoxifen (4-OHT)-responsive fusion proteins consisting of either the E47 or EBF1 coding regions fused to a mutated ligand-binding domain of estrogen receptor-α (referred to as E47:ER or EBF1:ER, respectively) (Kikuchi et al., 2008; Xu and Kee, 2007). These constructs were introduced into Tcf3−/− or Ebf1−/− progenitors and routinely resulted in nearly 100% GFP+ cells (not shown). Notably, whereas induction of E47:ER in Tcf3−/− progenitors led to only a 2-fold decrease in Gata3 transcripts 12 hr later (Figure S3B), induction of EBF1:ER expression resulted in a 5-fold decrease in Gata3 mRNA within 12 hr, similar to experiments with Ebf1−/− progenitors (Figure S3C). Moreover, after only 4 hr, induction of EBF1:ER in Tcf3−/− progenitors led to a significant loss in Gata3 transcript abundance (Figure S3C, left). As expected, in this system induced EBF1:ER caused robust increases in Igll1 transcripts (Figure S3C, right). These data indicate that silencing of GATA3 expression by EBF1 does not strictly require E2a proteins.
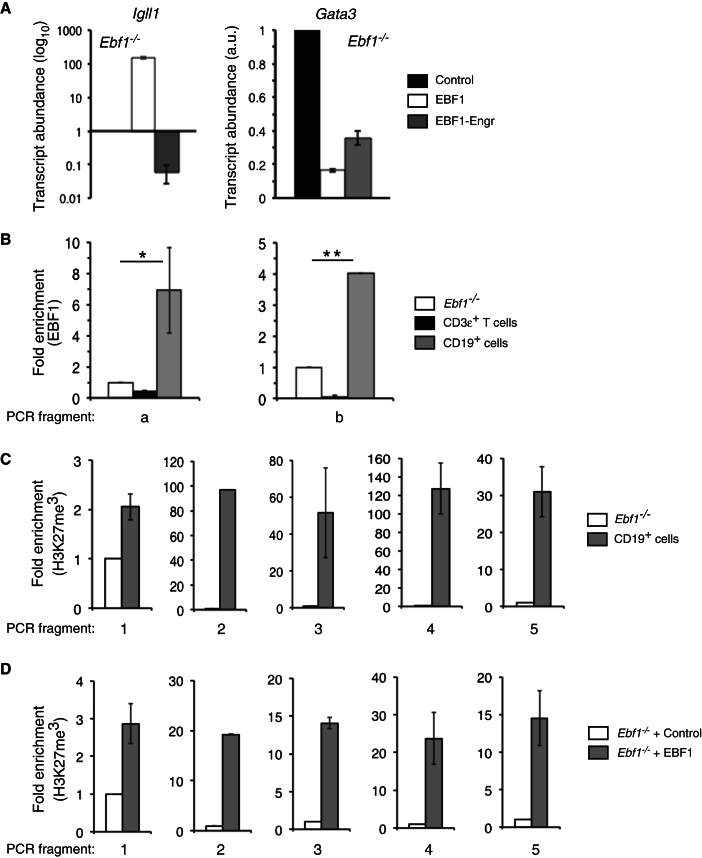
We also adopted several approaches that together indicate that EBF1 represses Gata3 expression directly. First, we tested whether inhibition of protein synthesis with cycloheximide (CHX) prevents EBF1-driven decreases in Gata3 transcripts. For these experiments we employed Tcf3−/− and Ebf1−/− progenitors containing 4-OHT-regulated EBF1:ER. Gata3 downregulation still occurred when transduced Tcf3−/− or Ebf1−/− progenitors were preincubated with CHX for 8 hr before adding 4-OHT (Figure S3D). Likewise, increases in Igll1 transcripts were also intact in CHX-pretreated cells (Figure S3E). To test further the possibility that EBF1 mediates silencing of Gata3 indirectly by activating an unknown transcriptional repressor, we determined whether conversion of EBF1 into an obligate repressor prevented EBF1-mediated downregulation of Gata3. To this end we fused the DNA binding domain of EBF1 to the Drosophila Engrailed repression domain (Fang et al., 2007; Vickers and Sharrocks, 2002). The resulting EBF1-Engrailed protein readily repressed transcription of Igll1 when introduced into Ebf1−/− pre-pro-B cells (Figure 4A, left). However, EBF1-Engrailed continued to decrease transcript abundance for Gata3 similar to wild-type EBF1 (Figure 4A, right). Together these data suggest that EBF1 regulates Gata3 mRNA levels directly.
Figure 4.
EBF1 Binds to the Gata3 Locus and Induces Repressive Histone Modifications
(A) Ebf1−/− lymphoid progenitors were transduced with a construct encoding an EBF1-Engrailed fusion protein, wild-type EBF1, or control virus. After 12 hr, GFP+ cells were harvested, cDNA prepared, and Igll1 or Gata3 mRNA levels determined with Taqman primer-probe sets. The data are normalized to transcript levels for either Igll1 or Gata3 in cells transduced with MigR1 control virus.
(B) ChIP via anti-EBF1 was performed with the indicated cell types including CD19+ BM lymphocytes and CD3ε+ thymocytes. qRT-PCR was performed to amplify immunoprecipitated DNA with flanking primers to sites a or b; detection signals were normalized to input DNA. Data are expressed as fold enrichment over Ebf1−/− cells, which served as negative control.
(C) H3K27me3 modifications within sites 1–5 in CD19+ BM lymphocytes relative to Ebf1−/− cells.
(D) Ebf1−/− cells were transduced with control MigR1 or MigR-EBF1 virus and plated on OP9 cells in presence of IL-7, FL, and SCF. 24 hr later, DAPI−GFP+ cells were sorted, fixed, and processed for ChIP. Relative H3K27me3 enrichment at sites 1–5 in EBF1 versus control-transduced samples is shown. Data are means ± SEMs of triplicate samples. Error bars indicate SEMs, *p < 0.01, **p < 0.001. Data are representative of four separate experiments.
See also Figures S3 and S4 and Table S1.
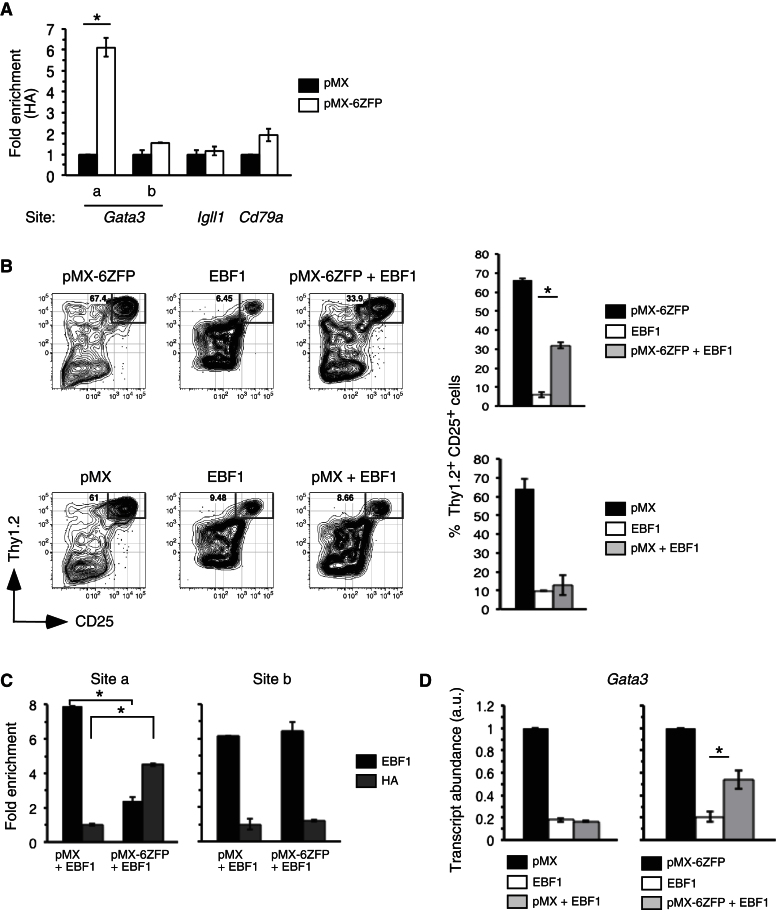
We utilized the ECR-browser database (http://ecrbrowser.dcode.org) to determine whether the Gata3 locus contains conserved EBF1 binding sequences. We noted two such sites within the Gata3 locus, one upstream of the Gata3 promoter 1b (“site a,” ∼2,350 bases from the transcriptional start site 1b) and a second site within the second intron (“site b,” ∼3,290 bases downstream of transcriptional start site 1b) (Figure S4A). Notably, past data indicate that the intronic regions of Gata3 play fundamental roles in regulating its expression (Hwang et al., 2002). We performed ChIP experiments with EBF1 antibodies and PCR primers flanking either putative EBF1 binding site (Table S1). Target populations included CD19+ BM B lineage cells, the Ebf1−/− progenitor cell line, and wild-type CD3ε+ splenic T cells. We observed a 4- to 6-fold enrichment in EBF1 binding at each site in CD19+ BM cells but not Ebf1−/− progenitors or peripheral T cells (Figure 4B). We also failed to detect EBF1 binding from a gene desert region on mouse chromosome 6 in Ebf1−/− progenitors and CD19+ BM cells (not shown). In addition, a 24 base pair biotin-labeled DNA probe encompassing “site a” led to formation of a protein-DNA complex in electrophoretic mobility shift assays using nuclear extracts from 293T cells transfected with a His6-tagged version of EBF1, which was super-shifted by an anti-histidine antibody. This complex failed to form when we used a probe in which the putative EBF1 site was mutated and was reduced substantially upon inclusion of an unlabeled wild-type Cd79a probe containing a canonical EBF1 binding site (Figure S4B). These data further support a model whereby EBF1 mediates the direct repression of Gata3 transcription.
To probe the mechanism underlying repression of Gata3, we tested whether EBF1 promotes epigenetic changes proximal to EBF1 binding sites associated with transcriptional repression. First, we explored the possibility that EBF1 promotes DNA methylation of CpG residues. Though the Gata3 promoter and second intron both contain prominent CpG islands adjacent to the EBF1 binding sites (Attema et al., 2007), by sodium bisulfite sequencing we did not detect changes in the methylation status of these regions in multipotent lymphoid progenitors versus pro-B cells (not shown). Because gene silencing can be mediated by histone modifications independently of DNA methylation (Kondo et al., 2008), we also evaluated repression-associated histone-methylation signatures upon ectopic EBF1 expression. We performed ChIP experiments with a H3K27me3-specific antibody, because stable transcriptional silencing is tightly associated with trimethylation of H3K27 (Table S1; Barski et al., 2007). CD19+ BM cells were highly enriched for H3K27me3 marks in the Gata3 locus, although this was not the case for Ebf1−/− progenitors (Figure 4C). Again, the gene desert region on chromosome 6 was included as an additional negative control (data not shown). Furthermore, transduction of Ebf1−/− progenitors with EBF1 readily induced the acquisition of H3K27me3 marks on chromatin surrounding both EBF1 binding sites (Figure 4D). These data suggest that EBF1 orchestrates epigenetic changes at the Gata3 locus by directly or indirectly recruiting polycomb (PcG) group complexes that impart repressive H3K27me3 modifications to this region.
Functional Relevance of Gata3 Repression
We sought to test whether ectopic expression of progenitors with GATA3 would bypass EBF1-mediated suppression of T cell differentiation. To this end we cotransduced Ebf1−/− progenitors with EBF1 and GATA3 before sorting these cells on OP9-DL4 cultures. However, consistent with past work indicating that transduction of lymphoid progenitors with GATA3 readily induces apoptosis (Taghon et al., 2007), there was a progressive decline in viable GATA3-positive cells after transduction (Figure S5A). We also considered generating transgenic mice as an alternative approach to achieving increased GATA3 expression. However, given that Gata3 expression is regulated by distal enhancer elements (Hosoya-Ohmura et al., 2011), we concluded that this approach was unlikely to succeed. Consequently, we tested whether blocking EBF1 binding to Gata3 regulatory sites prevented EBF1-driven suppression of early T cell differentiation. Indeed, we reasoned that a failure of EBF1 to bind to and repress the Gata3 locus should result in increased Gata3 expression, thereby providing an alternative approach for increasing GATA3 expression in lymphoid progenitors. To this end, we designed a retroviral construct (pMX-6ZFP) to express a synthetic hexa-modular Zinc-finger protein (6ZFP) specific for an 18 base region including the last seven nucleotides of the 8 base core EBF1 binding site between exons 1a and 1b (“site a”) of the Gata3 locus (Figure S5B). We were unable to engineer a Zn-finger protein to block binding of EBF1 to “site b” because of inadequate target site overlap requirements (see Experimental Procedures). The individual modular domains of 6ZFP were linked via a polydactyl zinc finger assembly strategy for recognizing extended sequences (Gonzalez et al., 2010). The nucleotide and predicted amino acid sequences for 6ZFP are shown in Table S2. A VP64 transcriptional activation domain was included to counter any potential stalling effect of 6ZFP on endogenous transcription (Beerli et al., 2000), and a hemagglutinin (HA) tag was added for assessing 6ZFP binding to particular DNA segments. Control constructs included retroviruses lacking a polypeptide encoding insert (pMX) and a second construct encoding an unrelated nonfunctional peptide (pMX-SS). Expression of 6ZFP was tracked via cotranslation of GFP from an IRES element. Significantly, after transduction of wild-type LSKs with pMX-6ZFP, the 6ZFP protein readily occupied “site a” as predicted, but we were unable to detect occupancy of 6ZFP at “site b” or at canonical EBF1 binding sites within the Cd79a or Igll1 loci (Figure 5A). In contrast, pMX-transduced cells failed to exhibit 6ZFP enrichment at any of these sites.
Figure 5.
Inhibition of EBF1 Binding to the Gata3 Locus Rescues T Cell Differentiation
(A) ChIP experiments with HA antibodies were performed on GFP+ progenitors transduced 7 days previously with pMX-6ZFP or pMX (control) by site-specific PCR primers.
(B) Sorted e14.5 Pax5−/− fetal liver LSKs were cotransduced with pMX-ZF and MigY-EBF1 viruses and then added to OP9 stromal cells. pMX and MigY-EBF1 cotransduced cells served as control. After 24 hr, cells were washed and replated on OP9-DL4 stromal cells in fresh media supplemented with IL-7, FL, and SCF. On day 7, cultures were stained and analyzed for frequencies of Thy1.2+CD25+ cells among DAPI−CD45+ single- or double-transduced cells.
(C) Sorted e14.5 Ebf1−/− fetal liver LSKs were cotransduced with pMX and MigY-EBF1 or pMX-ZF and MigY-EBF1 viruses, added to OP9 stromal cells. After 7 days, viable YFP+GFP+ cells were sorted, fixed, and subjected to ChIP with either EBF1 or HA antibodies. Relative enrichment of EBF1 versus 6ZFP in transduced Ebf1−/− cells at “site a” is shown, with ChIP results for “site b” included as controls for EBF1 and 6ZFP occupancy. Data are expressed as fold enrichment over nontransduced Ebf1−/− cells.
(D) Single- or double-transduced cells from pMX and MigY-EBF1 transduced samples as well as pMX-ZF and MigY-EBF1 transduced samples were sorted for RNA 24 hr after infection and analyzed for relative expression of Gata3 by qRT-PCR. Data are means ± SEMs of triplicate samples. Error bars indicate SEMs, *p < 0.001. Data are representative of two separate experiments.
Pax5−/− progenitors were transduced with YFP-marked EBF1 virus and/or one of the aforementioned pMX constructs and then cultured on OP9-DL4 stromal cells for 7 days. As shown (Figure 5B), whereas EBF1 expression alone led to the expected reduction in Thy1.2+CD25+ T lineage cells, cotransduction with EBF1 and pMX-6ZFP but not with EBF1 and either control pMX constructs restored numbers of T lineage cells to 50% of that observed in cells transduced with pMX-6ZFP alone (pMX-SS data not shown). As expected, 6ZFP expression led to decreased enrichment for EBF1 between exons 1a and 1b without affecting EBF1 binding between exons 2 and 3 (Figure 5C). Moreover, cotransduction with pMX-6ZFP and EBF1 restored Gata3 mRNA levels to 50% of EBF1-transduced cells (Figure 5D), and pMX-6ZFP expression alone increased Gata3 mRNA levels 2-fold compared to cells transduced with pMX (not shown).
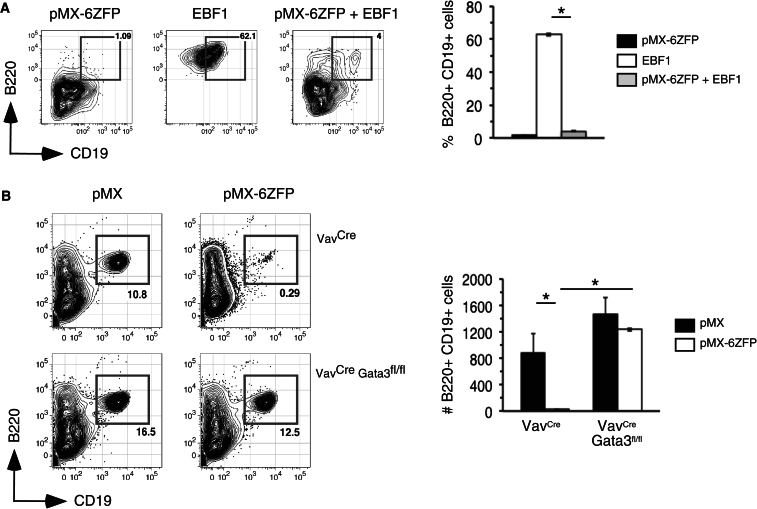
Notably, the increased levels of Gata3 induced with 6ZFP expression correlated with decreased differentiation of B220+CD19+ B lineage cells from wild-type fetal liver cells cotransduced with EBF1 (Figure 6A), suggesting that elevated Gata3 mRNA levels, in part due to reduced EBF1 occupancy within “site a,” perturbed early B cell development. To test this hypothesis directly, we introduced pMX or pMX-6ZFP into BM LSKs and CLPs sorted from either VavCre transgenics or VavCreGata3fl/fl mice and then sorted GFP+ cells from these cultures onto OP9 stromal cells. Strikingly, although 6ZFP prevented B cell differentiation when introduced into GATA3-competent lymphoid progenitors, early B cell differentiation was restored upon deletion of Gata3 (Figure 6B). Altogether these findings indicate that direct EBF1-mediated repression of Gata3 transcription is critical for extinguishing T cell lineage choice and allowing lymphoid progenitors to adopt the B cell fate.
Figure 6.
Decreased GATA3 Expression Is Essential for Early B Cell Development
(A) Sorted e14.5 WT fetal liver LSKs were transduced with only pMX-6ZFP or MigY-EBF1 or both viruses, plated on OP9s, and analyzed for frequencies of viable B220+CD19+ cells on day 7. Double-transduced cells were identified as viable (DAPI−) GFP+YFP+ cells.
(B) Sorted CD45B6+Flt3+ LSKs and CLPs from B6.CD45SJL adults previously reconstituted with BM cells from C57BL/6 (CD45B6) backcrossed VavCre or VavCreGata3fl/fl mice were transduced with pMX or pMX-6ZFP. Equal numbers of GFP+ cells were sorted into triplicate cytokine supplemented OP9 stromal cultures, and B cell differentiation was assessed 7 days later. Graphical data are means ± SEMs of triplicate samples.
p < 0.001.
Repression of GATA3 in T-Lineage-Committed Cells
Finally, to probe whether EBF1 can repress Gata3 in T-lineage-committed cells, we assessed whether survival of GATA3-dependent Tcf3−/− 1.F9 thymoma cells (Xu and Kee, 2007) is perturbed upon introducing EBF1. Transduction of 1.F9 cells with EBF1 led to a 5-fold reduction of Gata3 transcript abundance in these cells (Figure S6A). Whereas control virus did not affect viability over 8 days, introducing EBF1 decreased cell viability to less than 20% within 4 days (Figure S6B). Consistent with past data (Xu and Kee, 2007), loss of viability correlated with accumulation of the active form of proapoptotic caspase 3 (Figures S6C). Moreover, both cell death and active caspase 3 generation were reversed upon enforced coexpression of EBF1 and GATA3 in 1.F9 cells (Figures S6D and S6E). These data further support a model whereby EBF1 constrains T cell differentiation by silencing Gata3 expression and further suggest that EBF1 may limit T cell development by perturbing GATA3-dependent survival mechanisms.
Discussion
Our results provide insights into the transcriptional circuitry responsible for generating early B-cell-lineage-restricted precursors. Whereas deletion of Ebf1 in B-cell-lineage-biased lymphoid progenitors catalyzed an increase in Gata3 transcript abundance concurrent with a rejuvenation in their T cell potential, enforcing EBF1 repressed T cell differentiation and Gata3 mRNA levels, even in Pax5-deficient progenitors and despite ectopic Notch1 and TCF1 activity. Perhaps most notably, perturbation of EBF1 binding at a Gata3 regulatory region interfered with EBF1-driven suppression of early T cell differentiation, concomitantly preventing B cell differentiation due to increased Gata3 expression.
EBF1 is an indispensible component of the transcriptional regulatory framework required for generating pro-B cells. Within this network of transcription factors, E2a proteins activate the expression of Ebf1 and Foxo1, and subsequently EBF1 works in concert with E12 and E47 and Foxo1 to promote Pax5 expression (Lin et al., 2010). Although these previous studies demonstrate synergism between these transcription factors to activate certain key B-cell-specific molecules, they also raise questions about how specific components of this network might collaborate to constrain alternative fates. Because Pax5 is essential for establishing and maintaining B cell identity (Cobaleda et al., 2007; Mikkola et al., 2002), one view would be that B cell lineage restriction is mediated chiefly and perhaps exclusively by Pax5. However, our results highlight the ability of EBF1 to suppress T cell differentiation of Pax5-deficient progenitors without an immediate decline in Notch1 transcripts. Furthermore, unlike many genes that are upregulated by EBF1, our data suggest that EBF1 represses Gata3 transcription without input from E2a proteins. Therefore, we propose that B cell lineage restriction is controlled by two or more separate transcriptional pathways, with Pax5 and EBF1 repressing Notch1 and Gata3 expression, respectively. Moreover, in light of past data indicating that E2a proteins are unique among B cell regulatory transcription factors in their ability to repress erythroid differentiation (Ikawa et al., 2004), B cell lineage commitment may be facilitated by a variety of transcriptional and temporally distinct mechanisms. In this model, E2a proteins would silence erythroid and perhaps myeloid potentials as lymphoid-biased progenitors develop, EBF1 would silence T cell potential as well as residual myeloid potentials during adoption of the B cell fate, and Pax5 would silence alternative fates after the generation of pro-B cells. This viewpoint is consistent with recent data suggesting that suppression of alternative fates during T cell commitment also requires the activity of multiple transcriptional pathways (Zhang et al., 2012). Further work, including an evaluation of the potential role played by EBF1 in maintaining mature B cell identity and the role of other transcriptional regulators and cofactors in this process, may shed additional light on whether maintenance of the B cell fate also requires input from multiple transcription factors.
Although the role of GATA3 in peripheral T cell differentiation has been studied intensively (Amsen et al., 2007; Fang et al., 2007), recent studies together with our findings emphasize the essential role played by GATA3 in very early stages of T cell development. In the thymus, Notch1 signaling in early T lineage cells induces the expression of additional regulators including Hes1 and Tcf7. In turn, TCF1 promotes the expression of numerous genes required for T cell development and function (Weber et al., 2011), but Tcf7-deficient mice continue to generate T cells, albeit with decreased numbers (Schilham et al., 1998). By contrast, Gata3-deficient progenitors fail to generate T lineage cells beyond the ETP stage of early thymocyte development (Hendriks et al., 1999; Taghon et al., 2007). Indeed, recent data suggest that GATA3 activity may be essential for optimal Notch1 expression in ETPs, as shown by the fact that GATA3 binds to the Notch1 promoter in early thymocytes and that deletion of Gata3 in thymocytes reduces Notch1 transcripts (Wei et al., 2011). Moreover, GATA3 occupies distinct sets of regulatory regions at different stages of thymocyte development (Zhang et al., 2012), suggesting that GATA3 regulates many diverse aspects of T cell development downstream of Notch1 activation. The latter viewpoint is supported by our data showing that EBF1 prevents T cell differentiation despite ectopic Notch1 activity or enforced TCF1 expression. Altogether, these collective observations indicate that Gata3 constitutes a prime target for regulatory pathways that silence the T cell fate in early B lineage cells.
The mechanisms governing transcriptional activation of EBF1 target genes have been studied intensively (Hagman et al., 1995; Maier et al., 2004; Sigvardsson et al., 2002). By contrast, how EBF1 represses transcription of certain loci is only beginning to be addressed (Gao et al., 2009). Recent analyses of EBF1 occupancy of genes across the B cell genome suggest that the vast majority of EBF1 targets are located in transcriptionally active regions (Treiber et al., 2010). Therefore, the default mode for EBF1 may be as a transcriptional activator. However, other EBF1 target loci were found in transcriptionally inactive regions, and EBF1 has also been shown to downregulate the expression of the E-protein regulators Id2 and Id3 (Thal et al., 2009). Therefore, EBF1 may repress additional important loci during establishment and maintenance of the B cell fate.
The use of synthetic Zn fingers such as our 6ZFP construct to evaluate the role of defined cis elements may prove useful in studying other region-specific protein-DNA interactions in a wide variety of biological and experimental contexts. However, interpretation of data derived from this approach could be confounded by off-target DNA binding events. Here, 6ZFP did not appear to bind to alternative EBF1 binding sites within the Gata3, CD79a, or Igll1 locus and led to the predicted outcomes in Gata3 mRNA levels and T and B cell differentiation. Nonetheless, 6ZFP may bind to additional undefined elements across the genome. We considered performing ChIP-seq experiments to determine the identity of these alternative elements, but we suspect strongly that the functional relevance of these sites would be obscure at best. Indeed, recent genome-wide analyses of DNA occupancy by EBF1 suggest that endogenous transcription factors may also bind to a wide range of elements with unknown functional relevance (Treiber et al., 2010). Therefore, although synthetic proteins containing six tandem Zn fingers such as 6ZFP may prove to possess minimal off-target effects (Gabriel et al., 2011), validating the specificity of such proteins with a particular biological outcome may require the use of cells bearing appropriate mutations.
Finally, although the precise molecular details remain unclear, we hypothesize that the switch between activator and repressor functions of EBF1 at different loci reflects differential spatio-temporal interactions of EBF1 with coactivator and corepressor complexes, which in turn would be dictated by diverse proximal and/or distal cis elements. Our results show that EBF1 binding within the Gata3 locus is associated with repressive H3K27me3 marks on nearby chromatin. These results implicate EBF1 in the active recruitment of polycomb group (PcG) repressor complexes. Currently the chief mediator of H3K27me3 modifications in eukaryotes is the protein enhancer of zeste 2 (Ezh2), a member of the polycomb repressor complex 2 (PRC2) (Cao and Zhang, 2004). PRC2 activity in turn attracts PRC1, which prevents transcriptional initiation by RNA polymerase II (Dellino et al., 2004). Therefore, it is tempting to suggest that EBF1 binding at the Gata3 locus directly or indirectly initiates mobilization of PRC2 complexes to this region, thereby blocking interactions with coactivators and stabilizing chromatin modifications associated with long-term transcriptional silencing. Assuming that this sequence of events is indeed contingent on sequence-specific corepressors, competitive blockade of their cognate DNA sites via our Zn-finger protein strategy might neutralize repressive effects of EBF1 at the Gata3 locus. Furthermore, given that histone modification is a dynamic and reversible process (Tagoh et al., 2004; Zhang et al., 2012), it will be informative to test whether induced deletion of EBF1 in B-lineage-committed cells causes the reversal of trimethylation on H3K27 residues in Gata3 regulatory regions, possibly leading to Gata3 expression. The consequences of such aberrant expression would give us further clues into the biological significance of Gata3 silencing in B cells.
Experimental Procedures
Mice
C57BL/6 (B6) and B6.Ly5SJL females (6–8 weeks of age) were from the NCI animal facility (Frederick, MD). Ebf1+/− mice were kindly provided by B. Kee (University of Chicago) with permission from R. Grosschedl (Max Planck Institute, Freiburg). Pax5+/− mice were kindly provided by M. Busslinger (Research Institute of Molecular Pathology, Vienna). Gata3fl/fl and VaviCre mice were kindly provided by J. Zhu (National Institutes of Health) and D. Kioussi (National Institute for Medical Research, London), respectively. All animal experiments were performed according to protocols approved by the Office of Regulatory Affairs of the University of Pennsylvania in accordance with guidelines set forth by NIH and by the local ethics committee at the University of Oxford and the United Kingdom Home Office.
Cell Culture
Primary progenitors and progenitor cell lines were cultured as described previously (Pongubala et al., 2008; Xu and Kee, 2007). Details are provided in the Supplemental Information.
Plasmids
A detailed description of all retroviral plasmids is available in the Supplemental Information. For expression of synthetic Zn-finger polypeptides, the predicted 6ZFP mini-gene sequence was synthesized by Blue Heron Biotech, cloned into the control pMX-VP64-IRES-GFP retroviral construct (C. Barbas, Scripps Research Institute), and renamed pMX-6ZFP. A plasmid eliciting translation of an unrelated nonfunctional peptide (pMX-SS) was also provided by the Barbas lab. A nuclear localization signal sequence (NLS) and hemagglutinin (HA) tag are also included in the pMX vector backbone.
Flow Cytometry
Stained single-cell suspensions were stained and analyzed as described previously (Pongubala et al., 2008). Details of antibodies and flow cytometers used are available in the Supplemental Information.
Generation of Fetal Liver Chimeras
B6.Ly5SJL hosts were irradiated (900R) 6 hr before intravenous transfer of day 14.5 fetal liver cells isolated from B6 or Pax5−/− or Ebf1−/− embryos.
T Lymphoid Differentiation Assays
Bulk and clonal assays measuring T cell differentiation were performed with OP9-DL4 stromal cells as described previously (Weber et al., 2011) and in the Supplemental Information.
Retroviral Transduction
High-titer virus was generated by a CaPO4 transfection protocol (Pear et al., 1993). Cultures were supplemented with fresh medium at 12 hr after infection and harvested for downstream applications at indicated time points after infection.
Gene Expression Analyses
For quantitative RT-PCR, RNA was purified from indicated cell types with the QIAGEN RNeasy Mini Kit and reverse transcribed to cDNA with GE first-strand cDNA synthesis kit. Real-time PCR was performed with inventoried TaqMan probes for indicated genes and analyzed on an ABI Prism 7300 system (Applied Biosystems). 18S rRNA served as endogenous control for all samples. Relative transcript abundance was determined with the ΔΔCt method. Microarray analyses are described in the Supplemental Information.
Chromatin Immunoprecipitation Assays
CD3ε+ thymocytes and CD19+ BM cells were column selected (Miltenyi Biotec). In case of control vector and EBF1-transduced Ebf1−/− cells or cells cotransduced with EBF1 and pMX/pMX-6ZFP/pMX-SS viruses, viable GFP+ or YFP+GFP+ cells were sorted either 24 hr or 7 days after infection as indicated. ChIP was performed on indicated cell types (2 × 106 cells/assay) with the ChIP-IT kit (Active Motif). In brief, cells were fixed for 10 min at room temperature with 1% formaldehyde, treated with glycine stop-fix solution, then lysed with dounce-homogenizer. Pelleted nuclei were digested in presence of protease inhibitor cocktail and chromatin was enzymatically sheared. Sheared chromatin was immunoprecipitated with 4 μg anti-EBF1 (a kind gift from R. Grosschedl) or anti-H3K27me3 (07-449, Millipore) or anti-HA (ab9110, Abcam). After washing, bound chromatin was eluted and treated for reversal of crosslinking. After proteinase K digestion, DNA was immediately used in quantitative real-time PCR (SYBR Green, ABI). PCR primer sets are outlined in Table S1. A negative control primer set (Active Motif, catalog number 71011) for an 82 base pair gene desert region on mouse chromosome 6 was included in each immunoprecipitation. Nonenrichment for EBF1 or anti-H3K27me3 at this site served as a ChIP specificity control.
Zinc Finger Protein Design
A 48 base pair (bp) sequence spanning the EBF1 binding “site a” (as confirmed by ChIP assays) in Gata3 genomic locus was selected. Using the publicly available online resource called Zinc Finger Tools (http://www.scripps.edu/mb/barbas/zfdesign/zfdesignhome.php), an 18 bp potential target site including the EBF1 binding site was identified and the amino acid sequence of a hexa-modular zinc finger protein (6ZFP) predicted to bind to this site was designed (Table S2). Because of unmet target site overlap requirements, designing 6ZFP for a target site covering the intronic EBF1 binding “site b” was avoided as recommended (Mandell and Barbas, 2006).
Statistical Analysis
The means of each data set were analyzed by Student’s t test, with a two-tailed distribution assuming equal sample variance.
Acknowledgments
We thank A. Bhandoola, R. Sen, and M. De Obaldia for helpful discussions and/or critically reviewing this manuscript and J. Hagman, B. Kee, R. Grosschedl, and M. Busslinger for providing critical reagents and/or mice. We also gratefully acknowledge the expert technical assistance provided by C. Mauvais and support from the Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource. This work was supported by a grant from the National Institutes of Health (AI052861) to D.A. and a program grant (H4RPLK0) from the Medical Research Council, UK, to S.E.W.J.
Published: May 16, 2013
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2013.01.014.
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Accession Numbers
The microarray data are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/gds) under the accession number GSE46004.
Supplemental Information
References
- Allman D., Sambandam A., Kim S., Miller J.P., Pagan A., Well D., Meraz A., Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- Amsen D., Antov A., Jankovic D., Sher A., Radtke F., Souabni A., Busslinger M., McCright B., Gridley T., Flavell R.A. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attema J.L., Papathanasiou P., Forsberg E.C., Xu J., Smale S.T., Weissman I.L. Epigenetic characterization of hematopoietic stem cell differentiation using miniChIP and bisulfite sequencing analysis. Proc. Natl. Acad. Sci. USA. 2007;104:12371–12376. doi: 10.1073/pnas.0704468104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A., Cuddapah S., Cui K., Roh T.Y., Schones D.E., Wang Z., Wei G., Chepelev I., Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Beerli R.R., Dreier B., Barbas C.F., 3rd Positive and negative regulation of endogenous genes by designed transcription factors. Proc. Natl. Acad. Sci. USA. 2000;97:1495–1500. doi: 10.1073/pnas.040552697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M. Transcriptional control of early B cell development. Annu. Rev. Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- Cao R., Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 2004;14:155–164. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Cobaleda C., Jochum W., Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449:473–477. doi: 10.1038/nature06159. [DOI] [PubMed] [Google Scholar]
- Dellino G.I., Schwartz Y.B., Farkas G., McCabe D., Elgin S.C., Pirrotta V. Polycomb silencing blocks transcription initiation. Mol. Cell. 2004;13:887–893. doi: 10.1016/s1097-2765(04)00128-5. [DOI] [PubMed] [Google Scholar]
- Fang T.C., Yashiro-Ohtani Y., Del Bianco C., Knoblock D.M., Blacklow S.C., Pear W.S. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel R., Lombardo A., Arens A., Miller J.C., Genovese P., Kaeppel C., Nowrouzi A., Bartholomae C.C., Wang J., Friedman G. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat. Biotechnol. 2011;29:816–823. doi: 10.1038/nbt.1948. [DOI] [PubMed] [Google Scholar]
- Gao H., Lukin K., Ramírez J., Fields S., Lopez D., Hagman J. Opposing effects of SWI/SNF and Mi-2/NuRD chromatin remodeling complexes on epigenetic reprogramming by EBF and Pax5. Proc. Natl. Acad. Sci. USA. 2009;106:11258–11263. doi: 10.1073/pnas.0809485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez B., Schwimmer L.J., Fuller R.P., Ye Y., Asawapornmongkol L., Barbas C.F., 3rd Modular system for the construction of zinc-finger libraries and proteins. Nat. Protoc. 2010;5:791–810. doi: 10.1038/nprot.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J., Lukin K. Transcription factors drive B cell development. Curr. Opin. Immunol. 2006;18:127–134. doi: 10.1016/j.coi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Hagman J., Gutch M.J., Lin H., Grosschedl R. EBF contains a novel zinc coordination motif and multiple dimerization and transcriptional activation domains. EMBO J. 1995;14:2907–2916. doi: 10.1002/j.1460-2075.1995.tb07290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks R.W., Nawijn M.C., Engel J.D., van Doorninck H., Grosveld F., Karis A. Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. Eur. J. Immunol. 1999;29:1912–1918. doi: 10.1002/(SICI)1521-4141(199906)29:06<1912::AID-IMMU1912>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Hosoya-Ohmura S., Lin Y.H., Herrmann M., Kuroha T., Rao A., Moriguchi T., Lim K.C., Hosoya T., Engel J.D. An NK and T cell enhancer lies 280 kilobase pairs 3′ to the gata3 structural gene. Mol. Cell. Biol. 2011;31:1894–1904. doi: 10.1128/MCB.05065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E.S., Choi A., Ho I.C. Transcriptional regulation of GATA-3 by an intronic regulatory region and fetal liver zinc finger protein 1. J. Immunol. 2002;169:248–253. doi: 10.4049/jimmunol.169.1.248. [DOI] [PubMed] [Google Scholar]
- Ikawa T., Kawamoto H., Wright L.Y., Murre C. Long-term cultured E2A-deficient hematopoietic progenitor cells are pluripotent. Immunity. 2004;20:349–360. doi: 10.1016/s1074-7613(04)00049-4. [DOI] [PubMed] [Google Scholar]
- Inlay M.A., Bhattacharya D., Sahoo D., Serwold T., Seita J., Karsunky H., Plevritis S.K., Dill D.L., Weissman I.L. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K., Kasai H., Watanabe A., Lai A.Y., Kondo M. IL-7 specifies B cell fate at the common lymphoid progenitor to pre-proB transition stage by maintaining early B cell factor expression. J. Immunol. 2008;181:383–392. doi: 10.4049/jimmunol.181.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M., Weissman I.L., Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Shen L., Cheng A.S., Ahmed S., Boumber Y., Charo C., Yamochi T., Urano T., Furukawa K., Kwabi-Addo B. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat. Genet. 2008;40:741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- Lin Y.C., Jhunjhunwala S., Benner C., Heinz S., Welinder E., Mansson R., Sigvardsson M., Hagman J., Espinoza C.A., Dutkowski J. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat. Immunol. 2010;11:635–643. doi: 10.1038/ni.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier H., Ostraat R., Gao H., Fields S., Shinton S.A., Medina K.L., Ikawa T., Murre C., Singh H., Hardy R.R., Hagman J. Early B cell factor cooperates with Runx1 and mediates epigenetic changes associated with mb-1 transcription. Nat. Immunol. 2004;5:1069–1077. doi: 10.1038/ni1119. [DOI] [PubMed] [Google Scholar]
- Mandel E.M., Grosschedl R. Transcription control of early B cell differentiation. Curr. Opin. Immunol. 2010;22:161–167. doi: 10.1016/j.coi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Mandell J.G., Barbas C.F., 3rd Zinc Finger Tools: custom DNA-binding domains for transcription factors and nucleases. Nucleic Acids Res. 2006;34(Web Server issue):W516–W523. doi: 10.1093/nar/gkl209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansson R., Zandi S., Anderson K., Martensson I.L., Jacobsen S.E., Bryder D., Sigvardsson M. B-lineage commitment prior to surface expression of B220 and CD19 on hematopoietic progenitor cells. Blood. 2008;112:1048–1055. doi: 10.1182/blood-2007-11-125385. [DOI] [PubMed] [Google Scholar]
- Mikkola I., Heavey B., Horcher M., Busslinger M. Reversion of B cell commitment upon loss of Pax5 expression. Science. 2002;297:110–113. doi: 10.1126/science.1067518. [DOI] [PubMed] [Google Scholar]
- Miyamoto T., Iwasaki H., Reizis B., Ye M., Graf T., Weissman I.L., Akashi K. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev. Cell. 2002;3:137–147. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- Nutt S.L., Heavey B., Rolink A.G., Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- Pear W.S., Nolan G.P., Scott M.L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongubala J.M., Northrup D.L., Lancki D.W., Medina K.L., Treiber T., Bertolino E., Thomas M., Grosschedl R., Allman D., Singh H. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat. Immunol. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]
- Rumfelt L.L., Zhou Y., Rowley B.M., Shinton S.A., Hardy R.R. Lineage specification and plasticity in CD19- early B cell precursors. J. Exp. Med. 2006;203:675–687. doi: 10.1084/jem.20052444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambandam A., Maillard I., Zediak V.P., Xu L., Gerstein R.M., Aster J.C., Pear W.S., Bhandoola A. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat. Immunol. 2005;6:663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- Schaniel C., Bruno L., Melchers F., Rolink A.G. Multiple hematopoietic cell lineages develop in vivo from transplanted Pax5-deficient pre-B I-cell clones. Blood. 2002;99:472–478. doi: 10.1182/blood.v99.2.472. [DOI] [PubMed] [Google Scholar]
- Schilham M.W., Wilson A., Moerer P., Benaissa-Trouw B.J., Cumano A., Clevers H.C. Critical involvement of Tcf-1 in expansion of thymocytes. J. Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- Sigvardsson M., O’Riordan M., Grosschedl R. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity. 1997;7:25–36. doi: 10.1016/s1074-7613(00)80507-5. [DOI] [PubMed] [Google Scholar]
- Sigvardsson M., Clark D.R., Fitzsimmons D., Doyle M., Akerblad P., Breslin T., Bilke S., Li R., Yeamans C., Zhang G., Hagman J. Early B-cell factor, E2A, and Pax-5 cooperate to activate the early B cell-specific mb-1 promoter. Mol. Cell. Biol. 2002;22:8539–8551. doi: 10.1128/MCB.22.24.8539-8551.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souabni A., Cobaleda C., Schebesta M., Busslinger M. Pax5 promotes B lymphopoiesis and blocks T cell development by repressing Notch1. Immunity. 2002;17:781–793. doi: 10.1016/s1074-7613(02)00472-7. [DOI] [PubMed] [Google Scholar]
- Taghon T., Yui M.A., Rothenberg E.V. Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3. Nat. Immunol. 2007;8:845–855. doi: 10.1038/ni1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagoh H., Schebesta A., Lefevre P., Wilson N., Hume D., Busslinger M., Bonifer C. Epigenetic silencing of the c-fms locus during B-lymphopoiesis occurs in discrete steps and is reversible. EMBO J. 2004;23:4275–4285. doi: 10.1038/sj.emboj.7600421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal M.A., Carvalho T.L., He T., Kim H.G., Gao H., Hagman J., Klug C.A. Ebf1-mediated down-regulation of Id2 and Id3 is essential for specification of the B cell lineage. Proc. Natl. Acad. Sci. USA. 2009;106:552–557. doi: 10.1073/pnas.0802550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber T., Mandel E.M., Pott S., Györy I., Firner S., Liu E.T., Grosschedl R. Early B cell factor 1 regulates B cell gene networks by activation, repression, and transcription- independent poising of chromatin. Immunity. 2010;32:714–725. doi: 10.1016/j.immuni.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Vickers E.R., Sharrocks A.D. The use of inducible engrailed fusion proteins to study the cellular functions of eukaryotic transcription factors. Methods. 2002;26:270–280. doi: 10.1016/S1046-2023(02)00031-2. [DOI] [PubMed] [Google Scholar]
- Weber B.N., Chi A.W., Chavez A., Yashiro-Ohtani Y., Yang Q., Shestova O., Bhandoola A. A critical role for TCF-1 in T-lineage specification and differentiation. Nature. 2011;476:63–68. doi: 10.1038/nature10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G., Abraham B.J., Yagi R., Jothi R., Cui K., Sharma S., Narlikar L., Northrup D.L., Tang Q., Paul W.E. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011;35:299–311. doi: 10.1016/j.immuni.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Kee B.L. Growth factor independent 1B (Gfi1b) is an E2A target gene that modulates Gata3 in T-cell lymphomas. Blood. 2007;109:4406–4414. doi: 10.1182/blood-2006-08-043331. [DOI] [PubMed] [Google Scholar]
- Yang Q., Jeremiah Bell J., Bhandoola A. T-cell lineage determination. Immunol. Rev. 2010;238:12–22. doi: 10.1111/j.1600-065X.2010.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi S., Mansson R., Tsapogas P., Zetterblad J., Bryder D., Sigvardsson M. EBF1 is essential for B-lineage priming and establishment of a transcription factor network in common lymphoid progenitors. J. Immunol. 2008;181:3364–3372. doi: 10.4049/jimmunol.181.5.3364. [DOI] [PubMed] [Google Scholar]
- Zhang J.A., Mortazavi A., Williams B.A., Wold B.J., Rothenberg E.V. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell. 2012;149:467–482. doi: 10.1016/j.cell.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.