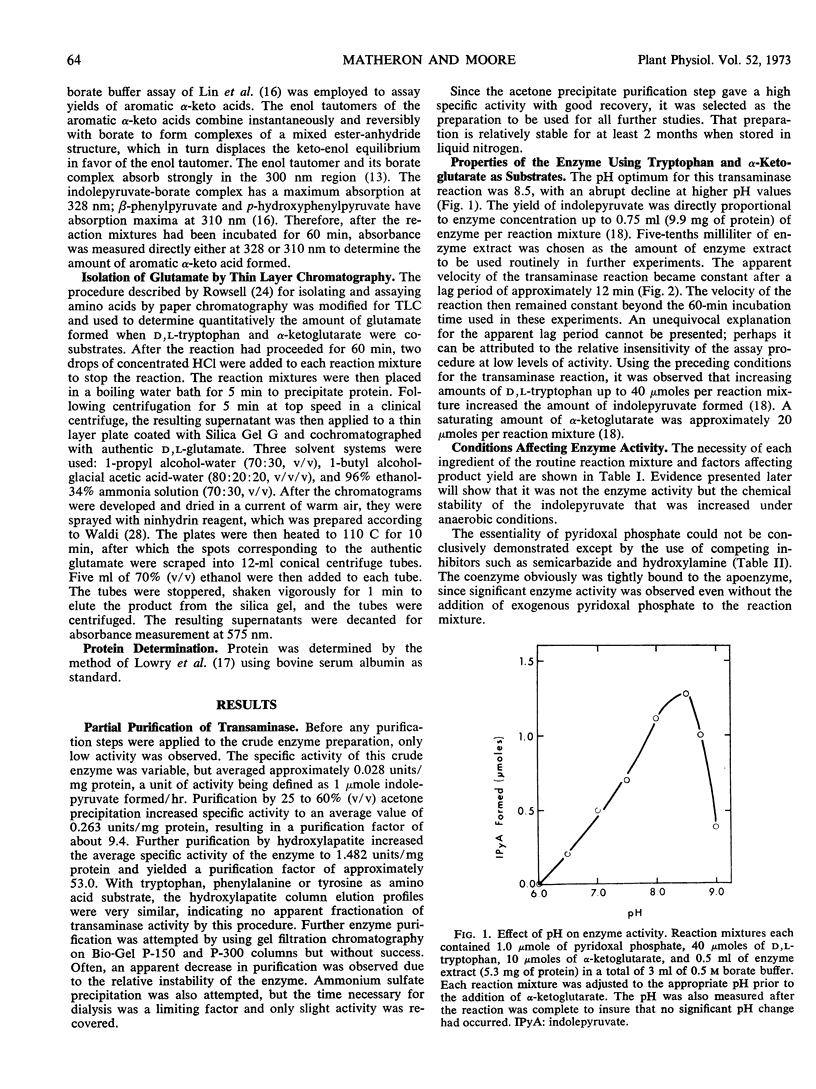
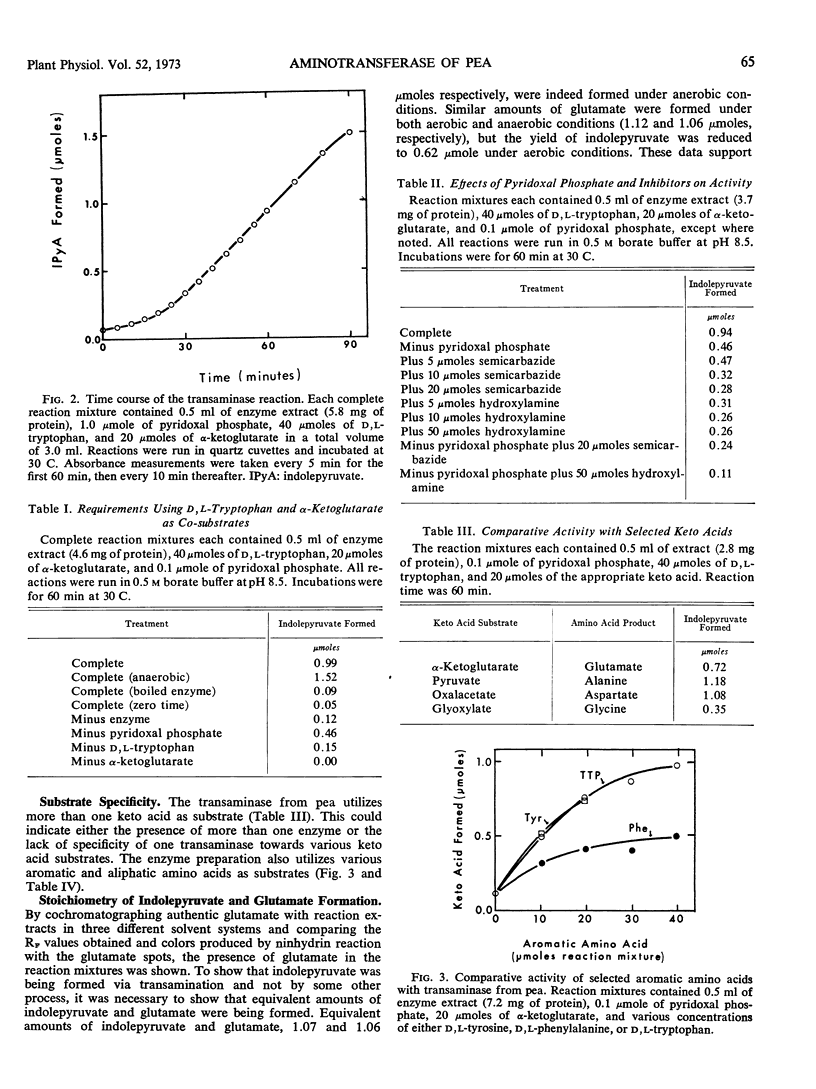
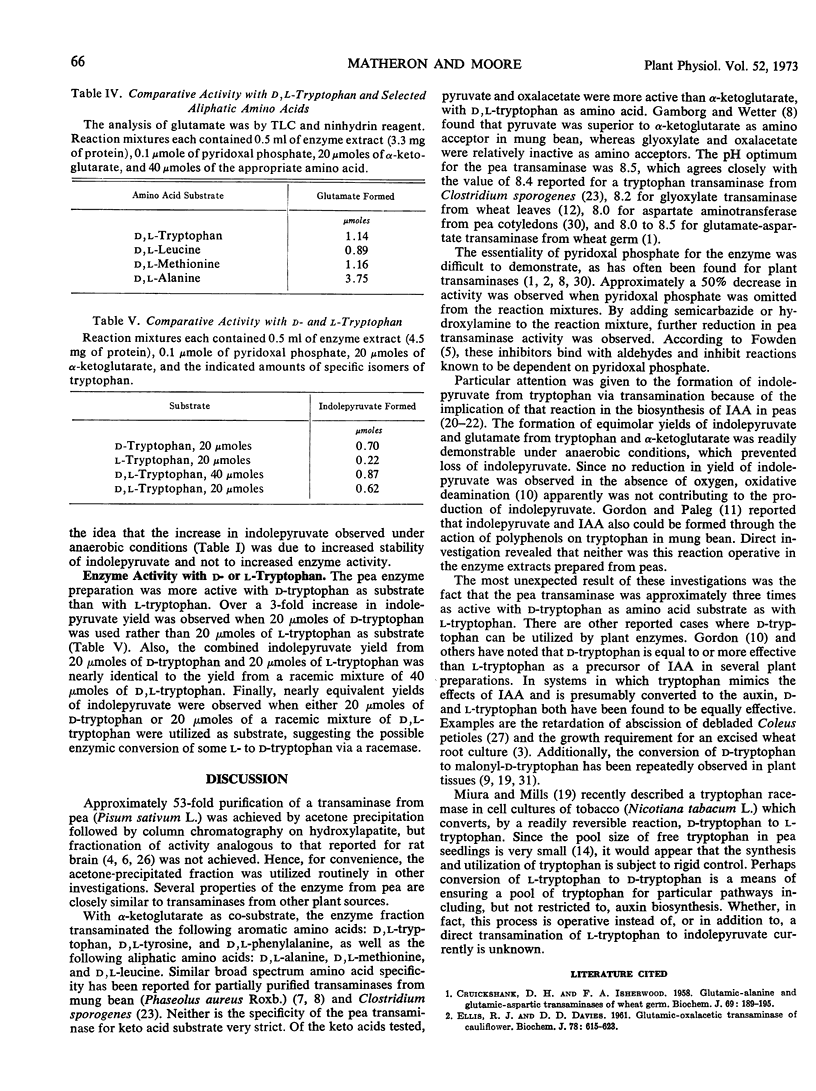
Abstract
A transaminase (aminotransferase, EC 2.6.1) fraction was partially purified from shoot tips of pea (Pisum sativum L. cv. Alaska) seedlings. With α-ketoglutarate as co-substrate, the enzyme transaminated the following aromatic amino acids: d,l-tryptophan, d,l-tyrosine, and d,l-phenylalanine, as well as the following aliphatic amino acids: d,l-alanine, d,l-methionine, and d,l-leucine. Of other α-keto acids tested, pyruvate and oxalacetate were more active than α-ketoglutarate with d,l-tryptophan. Stoichiometric yields of indolepyruvate and glutamate were obtained with d,l-tryptophan and α-ketoglutarate as co-substrates. The specific activity was three times higher with d-tryptophan than with l-tryptophan.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRUICKSHANK D. H., ISHERWOOD F. A. Glutamic-alanine and glutamic-aspartic transaminases of wheat germ. Biochem J. 1958 Jun;69(2):189–195. doi: 10.1042/bj0690189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS R. J., DAVIES D. D. Glutamic-oxaloacetic transaminase of cauliflower. 1. Purification and specificity. Biochem J. 1961 Mar;78:615–623. doi: 10.1042/bj0780615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONNUM F., HAAVALDSEN R., TANGEN O. TRANSAMINATION OF AROMATIC AMINO ACIDS IN RAT BRAIN. J Neurochem. 1964 Feb;11:109–118. doi: 10.1111/j.1471-4159.1964.tb06747.x. [DOI] [PubMed] [Google Scholar]
- Gabay S., Huang K. H. Studies of brain aminotransferase and psychotropic agents. I. Interaction of B-B'-iminodipropionitrile and rat brain aromatic aminotransferases. Biochem Pharmacol. 1969 Apr;18(4):767–775. doi: 10.1016/0006-2952(69)90047-1. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L. Transamination in plants. The specificity of an aminotransferase from mung bean. Can J Biochem. 1965 Jun;43(6):723–730. doi: 10.1139/o65-083. [DOI] [PubMed] [Google Scholar]
- Good N. E., Andreae W. A. Malonyltryptophan in Higher Plants. Plant Physiol. 1957 Nov;32(6):561–566. doi: 10.1104/pp.32.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. A., Paleg L. G. Formation of auxin from tryptophan through action of polyphenols. Plant Physiol. 1961 Nov;36(6):838–845. doi: 10.1104/pp.36.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Waygood E. R. Glyoxylate aminotranferases from wheat leaves. Can J Biochem. 1968 Aug;46(8):771–779. doi: 10.1139/o68-118. [DOI] [PubMed] [Google Scholar]
- LIN E. C., PITT B. M., CIVEN M., KNOX W. E. The assay of aromatic amino acid transaminations and keto acid oxidation by the enol borate-tautomerase method. J Biol Chem. 1958 Sep;233(3):668–673. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence J. M., Grant D. R. Nitrogen Mobilization in Pea Seedlings. II. Free Amino Acids. Plant Physiol. 1963 Sep;38(5):561–566. doi: 10.1104/pp.38.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura G. A., Mills S. E. The Conversion of d-Tryptophan to l-Tryptophan in Cell Cultures of Tobacco. Plant Physiol. 1971 Apr;47(4):483–487. doi: 10.1104/pp.47.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. C., Shaner C. A. Biosynthesis of Indoleacetic Acid from Tryptophan-C in Cell-free Extracts of Pea Shoot Tips. Plant Physiol. 1967 Dec;42(12):1787–1796. doi: 10.1104/pp.42.12.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil S. R., DeMoss R. D. Tryptophan transaminase from Clostridium sporogenes. Arch Biochem Biophys. 1968 Sep 20;127(1):361–369. doi: 10.1016/0003-9861(68)90237-3. [DOI] [PubMed] [Google Scholar]
- Sukanya N. K., Vaidyanathan C. S. Aminotransferases of Agrobacterium tumefaciens. Transamination between tryptophan and phenylpyruvate. Biochem J. 1964 Sep;92(3):594–598. doi: 10.1042/bj0920594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANGEN O., FONNUM F., HAAVALDSEN R. SEPARATION AND PURIFICATION OF AROMATIC AMINO ACID TRANSAMINASES FROM RAT BRAIN. Biochim Biophys Acta. 1965 Jan;96:82–90. doi: 10.1016/0005-2787(65)90612-x. [DOI] [PubMed] [Google Scholar]
- Valdovinos J. G., Perley J. E. Metabolism of tryptophan in petioles of coleus. Plant Physiol. 1966 Dec;41(10):1632–1636. doi: 10.1104/pp.41.10.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON D. G., KING K. W., BURRIS R. H. Transamination reactions in plants. J Biol Chem. 1954 Jun;208(2):863–874. [PubMed] [Google Scholar]


