Abstract
There is growing evidence that drugs of abuse alter processing of emotional information in ways that could be attractive to users. Our recent report that Δ9-tetrahydrocannabinol (THC) diminishes amygdalar activation in response to threat-related faces suggests that THC may modify evaluation of emotionally-salient, particularly negative or threatening, stimuli. In this study, we examined the effects of acute THC on evaluation of emotional images. Healthy volunteers received two doses of THC (7.5 and 15 mg; p.o.) and placebo across separate sessions before performing tasks assessing facial emotion recognition and emotional responses to pictures of emotional scenes. THC significantly impaired recognition of facial fear and anger, but it only marginally impaired recognition of sadness and happiness. The drug did not consistently affect ratings of emotional scenes. THC' effects on emotional evaluation were not clearly related to its mood-altering effects. These results support our previous work, and show that THC reduces perception of facial threat. Nevertheless, THC does not appear to positively bias evaluation of emotional stimuli in general
Keywords: Tetrahydrocannabinol, facial emotion recognition, emotions, humans, behavior, drugs, drug abuse, cannabinoids
Introduction
Emerging research suggests that drugs of abuse can alter evaluation of emotional stimuli in ways that could be desirable to users. Specifically, these drugs may positively bias perception of salient stimuli in the environment – either by making bad things seem less bad, or by making good things seem better. Such influences on emotional evaluation may be an important unstudied factor underlying the positively reinforcing properties of drugs of abuse. We recently found that a moderate dose of Δ9-tetrahydrocannabinol (THC), the main psychoactive component of cannabis, selectively decreases amygdalar activation elicited by threatening (i.e. angry/fearful), relative to non-threatening (i.e. happy), faces (Phan et al., 2008). Dampened amygdalar reactivity to threatening faces often corresponds to impaired recognition of threat-related facial emotions, and could be indicative of an altered perception of emotional stimuli more generally (Adolphs et al., 1994; Ohman, 2005). However, THC' effects on evaluation of emotional stimuli have not been thoroughly investigated.
THC' psychoactive properties derive primarily from its actions at cannabinoid type-1 receptors (CB1Rs) (Cooper and Haney, 2009; D'Souza et al., 2004). CB1Rs are distributed extensively throughout the brain, including the amygdala (Katona et al., 2001; Mackie, 2005). The amygdala plays an important role in mood regulation and perception and processing of emotional information (Price and Drevets, 2010; Vuilleumier and Pourtois, 2007), and it is potently activated by emotionally-salient, particularly threatening, stimuli. Anxiolytic drugs dampen this reactivity, most notably in response to fearful faces (Del-Ben et al., 2011; Paulus et al., 2005), and there is evidence that CB1Rs may mediate some of the therapeutic effects of non-cannabinoid anxiolytic drugs, such as benzodiazepines (Garcia-Gutierrez and Manzanares, 2010). Although few studies have been carried out specifically examining the involvement of CB1Rs in emotional processing in humans, in a recent comprehensive review of the animal literature concerning the function of the endocannabinoid system in cognition and emotion, Zanettini et al. (2011) concluded that ‘endocannabinoid signaling may change the impact of environmental influences on emotional and cognitive behavior rather than selectively affecting any specific behavior’ (for additional reviews of the animal literature see Bambico et al., 2009; Moreira and Wotjak, 2010). Taken together, these data illustrate a potential mechanism by which THC can influence the evaluation of emotional stimuli. Indirect evidence suggests that THC is likely to affect facial emotion recognition. For instance, we previously found that a moderate dose of THC (7.5 mg) reduces amygdalar reactivity to angry and fearful faces (Phan et al., 2008), and a similar trend has been observed by another group using only fearful faces and a slightly higher dose (10 mg) of THC (Bhattacharyya et al., 2010; Fusar-Poli et al., 2009). This profile resembles those of several other drugs of abuse, including alcohol (Gilman et al., 2008), benzodiazepines (Paulus et al., 2005), and +/-3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) (Bedi et al., 2009). More importantly, each of these other drugs has also been shown to impair recognition of threat-related facial emotions (Bedi et al., 2010; Blair and Curran, 1999; Borrill et al., 1987; Craig et al., 2009; Zangara et al., 2002; but see Coupland et al., 2003, who found that diazepam produced non-specific emotion recognition impairments). Based on this evidence, we predicted that THC would impair recognition of threat-related facial emotions.
In addition to altering perception of certain facial emotions, there is also evidence that drugs of abuse can alter the perceived emotionality of other types of salient stimuli in the user' environment. Indeed, Knowles and Duka (2004) found that social drinkers rate pictures of positive and neutral scenes more positively when they are under the influence of alcohol, and Gospic et al. (2008) found that the opioid drug remifentanil increases the perceived positivity of pictures of neutral scenes; neither drug was found to affect ratings of pictures of negative scenes, however. Furthermore, we recently found that d-amphetamine increases positivity and arousal ratings of scenes independently of their valence and emotional salience (Wardle and de Wit, 2012). Although some studies have failed to detect effects of drugs of abuse (i.e. alcohol and diazepam) on self-reported evaluation of emotional scenes (Patrick et al., 1996; Stritzke et al., 1995), changes in physiological responses to these stimuli were observed, perhaps indicating more subtle influences on emotional processing. For example, reduced skin-conductance responses and startle-reactivity to emotional scenes have been reported following treatment with alcohol (Donohue et al., 2007; Stritzke et al., 1995), diazepam (Patrick et al., 1996), and nicotine (Cinciripini et al., 2006). Thus, there is some indication that drugs of abuse can modulate processing of a variety of salient stimuli, suggesting that THC' effects on emotional evaluation may extend beyond facial emotion recognition.
It is possible that drugs' effects on evaluation of emotional stimuli are related to their mood-altering properties. Support for this idea comes from research showing that non-pharmacologically-induced positive mood states enhance recognition of facial happiness (Trevisani et al., 2008), and individuals with lower anxiety are less accurate in recognizing facial fear than higher anxiety individuals (Surcinelli et al., 2006). Previous drug studies have largely overlooked the question of whether drugs' effects on emotional evaluation are related to their acute effects on mood. It is therefore of interest to investigate this potential link, as these relationships may be useful for understanding how drugs of abuse influence behavior.
In the present study, we examined the effects of acute, oral THC on two measures of emotional evaluation: 1) recognition of facial emotions, and 2) emotional responses to pictures of scenes with varying emotional content. Based on our previous findings and those with other drugs of abuse, we hypothesized that THC would impair recognition of facial anger and fear, and perhaps also increase recognition of facial happiness. Secondarily, we hypothesized that THC would increase the perceived positivity of positive and neutral scenes. Finally, we predicted that these effects would be greatest among those participants who most enjoyed the drug.
Methods and Materials
Subjects
Healthy occasional cannabis users (N=25), aged 18–35 years, were recruited from the University of Chicago and surrounding community via posters, advertisements, and word-of-mouth referrals. Cannabis users were eligible if they had used the drug more than 10 times in their lives, but were not currently using it more than three times per week – to reduce the likelihood of tolerance (D'Souza et al., 2008) – and if they reported no serious cannabis-related adverse events. Participants underwent an in-person psychiatric interview and physician-supervised physical examination including an electrocardiogram, and they completed a health questionnaire with detailed information on current and lifetime drug use. Exclusion criteria included current Axis I DSM-IV disorder including substance dependence (American Psychiatric Association, 1994) other than tobacco dependence. Volunteers were also excluded if they had a lifetime history of psychosis or mania, less than a high school education, lack of fluency in English, a body mass index outside of 19–26 kg/m2, high blood pressure (>140/90), abnormal electrocardiogram, daily use of any medication other than birth control, or were pregnant, lactating, or planning to become pregnant in the next three months.
Design
This study utilized a three-session, double-blind, placebo-controlled, within-subjects design. During each of the three four-hour experimental sessions, participants received a capsule containing 7.5 or 15 mg THC or placebo in random order before performing tasks assessing evaluation of emotional images. These doses of THC are within the range used recreationally, and the oral route of administration was selected to ensure reliable and stable subjective and behavioral effects throughout the study tasks (Wachtel and de Wit, 2000). The 7.5 and 15 mg doses produce blood plasma THC levels of roughly 4 and 8 ng/mL respectively, at about the time the study tasks were administered (Wachtel et al., 2002). In the tasks, participants were required to identify emotions expressed in photographs of faces, and to rate their emotional responses to pictures of emotional (both positive and negative valence) and unemotional (neutral valence) scenes. The primary outcome measures included facial emotion identification accuracy, and positivity, negativity, and arousal ratings of the pictures. Secondary outcome measures included subjective mood and drug effect ratings and cardiovascular measures. These data were obtained as part of a larger study investigating the effects of THC on memory; memory results will be presented separately. This study was approved by the University of Chicago Biological Sciences Division Institutional Review Board. All study procedures were in accordance with the 1975 Declaration of Helsinki, revised in Seoul 2008.
Procedure
Qualifying participants attended a one-hour orientation session to explain procedures and risks associated with the study, provide informed consent, and practice study tasks and questionnaires. All participants were blinded to the treatments they received in order to control for any expectancies they may have regarding THC' effects on mood and behavior. To facilitate blinding, participants were informed that they might receive a placebo, stimulant (e.g. amphetamine), sedative/tranquilizer (e.g. Valium), or a marijuanalike drug. They were instructed to consume their normal amounts of caffeine and nicotine before sessions, but to abstain from using alcohol and prescription and over-the-counter drugs for 24 hours, other recreational drugs for two days, and marijuana for seven days before each session. Participants were told that they would be tested for drug use before each session to verify abstinence, and were instructed to get adequate sleep (i.e. 7-8 hours) and not to eat solid food for two hours before experimental sessions. Compliance with pre-session instructions was confirmed by a research assistant before each session, using self-report and biochemical assessments for alcohol, illicit drug use, and pregnancy (see below).
Participants were tested individually in comfortably furnished rooms with a television and VCR, magazines, and a computer for administering questionnaires and tasks. They were allowed to watch television and movies, or read when no measures were being obtained, but they were not allowed to sleep, work, or study, and they had no access to cell phones or internet. Subjects were allowed to choose from a selection of movies with relatively unemotional content. Upon arrival for sessions, participants first completed compliance measures including breath alcohol levels (Alco-sensor III, Intoximeters, St Louis, MO) and urine drug (ToxCup, Branan Medical Co., Irvine, CA) and pregnancy tests (women only, using an hCG assay; Aimstrip, Craig Medical, Vista, CA). They then completed pre-capsule mood ratings and cardiovascular measurements were collected (see Dependent measures, below), and subsequently ingested capsules containing THC (7.5 or 15 mg) or placebo with 100 mL water. For the remainder of the four-hour visit, participants completed subjective mood and drug effect ratings, and provided cardiovascular measurements every 30 minutes. At 90 minutes and 120 minutes after capsule ingestion, tasks assessing emotional responses to pictures of emotional scenes and facial emotion recognition, respectively, were administered. The tasks were timed to coincide with peak drug effects, as orally-administered THC has a Tmax of roughly 140 min (Wachtel et al., 2002) and a four-hour half-life (Marinol® package insert). At the end of the session, participants completed an end-of-session questionnaire, and were allowed to leave once residual subjective and cardiovascular drug effects subsided. All participants were fully debriefed at study completion.
Dependent Measures
Study Tasks
Facial Emotion Recognition
This task, adapted from Harmer et al. (2004), measures facial emotion identification ability using standardized faces taken from the Pictures of Facial Affect set (Ekman and Friesen, 1976). In this version, participants viewed images of facial emotions morphed by 10% increments along a continuum between 10% and 100% emotional expression (Young et al., 1997) and were instructed to classify the face as angry, fearful, happy, sad, or neutral according to the emotion it expressed. All responses were self-paced. We employed equal numbers of angry, fearful, happy, and sad faces. The same four actors (two male and two female) were depicted as conveying each emotion at all 10 intensity levels (i.e. 40 emotive faces from each actor). Thus, there were a total of 160 emotive faces. Each face was presented on a computer screen for 500 milliseconds in randomized order. The primary outcome measure was recognition accuracy, calculated as the percent of correctly identified morphed faces from each emotion category. For comparison with our previous data on THC' effects on amygdalar reactivity to emotional faces (Phan et al., 2008), we first examined recognition of only unambiguous (i.e. 100% emotion intensity) facial emotions. This analysis involved 16 stimuli – four faces from each emotion category.
Emotional Pictures
This task measures participants' self-reported emotional responses to pictures of emotionally salient and non-salient scenes. Participants viewed 60 standardized photographs from the International Affective Picture System (IAPS) (Lang et al., 1999) depicting negative/unpleasant, neutral, and positive/pleasant scenes (20 each valence, according to normative ratings). Pictures were presented on a computer monitor for three seconds each. Each trial began with a fixation cross, and the picture was presented when the participant pressed a key. Participants rated each picture for perceived valence and arousal at their own pace using Likert scales from one to nine. Valence was defined as how positive and negative the picture made them feel, and arousal was defined as how stimulated, excited, or awake they felt in response to the picture (Lang et al., 1993). Positive and negative pictures were matched on extremity of valence and arousal based on normative ratings (Lang et al., 1999). Different ratings-matched sets of pictures were presented across the three sessions, in random order. Outcome measures included subjective ratings of positivity, negativity, and arousal response to pictures from individual valence categories.
Subjective Drug Effect and Mood Measures
Subjective drug effects and mood were assessed at baseline, and every 30 minutes after capsule ingestion using the Drug Effects Questionnaire (DEQ) (Fischman and Foltin, 1991), Visual Analog Scales (VAS) (Folstein and Luria, 1973), Positive Affect and Negative Affect Schedule (PANAS; Watson et al., 1988), Addiction Research Center Inventory (ARCI) (Martin et al., 1971) including marijuana scale (Chait et al., 1985), and Hallucinogen Rating Scale (HRS) (Strassman et al., 1994), as well as with an end-of-session questionnaire. The ARCI was administered only at baseline, and 90 minutes and 180 minutes post-capsule, and the HRS was only administered at 180 minutes post-capsule to reduce participant burden. Together, these questionnaires provided a comprehensive assessment of the drug' subjective and mood-altering effects.
Drug Effects Questionnaire (DEQ)
The DEQ consists of five questions concerning current drug effects – specifically, how much participants feel a drug effect, dislike the effect, like the effect, feel high, and want more of the drug (Fischman and Foltin, 1991). Participants rated their responses on 100 mm sliding scales from ‘not at all /neutral’ to ‘very much’ participants were instructed to select ‘not at all /neutral’ if they had not yet received a capsule (i.e. baseline DEQ ratings all equaled zero).
Visual Analog Scales (VAS)
The VAS consists of seven adjectives used to assess individual dimensions of subjective mood – anxious, stimulated, confused, sedated, excited, angry, and elated (Folstein and Luria, 1973). Participants rated their responses on 100 mm sliding scales from ‘not at all’ to ‘extremely’.
Positive Affect and Negative Affect Schedule (PANAS)
The PANAS (Watson et al., 1988) consists of 10 negative and 10 positive mood terms rated on five-point Likert-type response scales from 1 (very slightly or not at all) to 5 (extremely). PANAS positive and negative scale scores each range between 10 and 50. The PANAS was chosen as a composite measure of self-reported positive and negative affect.
Addiction Research Center Inventory (ARCI)
The ARCI (Martin et al., 1971) is a 53-item true-false questionnaire with six empirically-derived scales that are sensitive to the effects of several classes of abused drugs. The PCAG (Pentobarbital-Chlorpromazine Group) scale is used as a measure of sedation; the MBG (Morphine-Benzedrine Group) scale is used as a general measure of drug-induced euphoria; the BG (Benzedrine Group) scale is used as a stimulant scale and consists mainly of items relating to intellectual efficiency and energy; the LSD (Lysergic Acid Diethylamide) scale is used as a measure of dysphoria and somatic symptoms; the A (amphetamine) scale is used as a measure of amphetamine-related effects; and the M (marijuana) scale is used as a measure of cannabis-related effects (Chait et al., 1985).
Hallucinogen Rating Scale (HRS)
The HRS (Strassman et al., 1994) is a 100-item questionnaire that measures hallucinogenic drug experiences. Participants rated the drug' effects on scales from 0 (not at all) to 4 (extremely), and individual items were summed to produce scores on six dimensions: intensity (e.g. ‘waxing and waning of experience’), somesthesia (e.g. ‘body feels different’), affect (e.g. ‘awe’), perception (e.g. ‘change in skin sensitivity’), cognition (e.g. ‘new thoughts or insights ’), and volition (e.g. ‘able to let go ’). The HRS was used to assess peak subjective drug effects across an entire session.
End-of-Session Questionnaire (ESQ)
The ESQ is designed to assess participants' retrospective perception of drug effects, and consists of questions including: 1) desire to take the drug again ‘yes/no’, and 2) best guess of drug received (e.g. stimulant, sedative, marijuana-like drug, or placebo).
Cardiovascular Measures
Blood pressure (BP) and heart rate (HR) were measured at baseline, and every 30 minutes post-capsule, using a portable digital blood pressure monitor (AND Medical / Life Source, San Jose, CA).
Drugs
THC (Marinol® (dronabinol); Solvay Pharmaceuticals, Marietta, GA) capsules were placed in opaque size 00 capsules in doses of 7.5 or 15 mg, with dextrose filler. Placebo capsules contained only dextrose. Capsules were administered in counterbalanced order under double-blind conditions.
Statistical Analyses
We first confirmed that repeated testing did not affect performance in the facial emotion recognition task or emotional pictures task. We also examined all data for normality. In cases where there were substantive departures from normality, log transformations were applied, and where non-normality appeared to be due to a single data point, outliers were substituted for three standard deviations from the mean. Because these corrections did not affect the significance of any of the measures, only uncorrected results are reported for ease of interpretation.
Facial Emotion Recognition
THC' effects on facial emotion recognition were first assessed using a two-way repeated measures ANOVA (dose × emotion) for unambiguous (i.e. 100% emotion intensity) angry, fearful, happy, and sad faces. This analysis was used for comparison with our previous imaging study which employed only 100% emotional intensity faces. Then, to more closely examine our a priori hypothesis that THC would especially alter recognition of threat-related expressions, we also planned to determine the drug' effects on recognition of each emotion individually using separate one-way repeated measures ANOVA. Because we expected THC' effects to increase dose-dependently, linear main effects of drug dose are reported. Similar analyses were carried out with all morphed faces combined (i.e. from 10-100% emotional intensity). Significant effects of dose (p<.05) were followed up with post hoc LSD pairwise comparisons between each dose of THC and placebo to determine dose-dependence. Alpha was set at p=.025 to control for the inflated risk of Type I error resulting from two comparisons with placebo.
Emotional Pictures
THC' effects on self-reported ratings of positive, negative, and arousal responses to pictures of emotional and unemotional scenes was examined using two-way repeated measures ANOVA with drug dose and valence category (i.e. negative, neutral, positive; according to normative ratings) as within-subject factors. Because we had a priori hypotheses that THC would especially alter ratings of pictures of certain valence (e.g. positive, neutral) (Gospic et al., 2008; Knowles and Duka, 2004), we planned to conduct one-way ANOVA followed by pairwise comparisons within each valence category individually. Because we expected THC' effects to increase dose-dependently, linear main effects of drug dose are reported. Alpha was set at p=.025 for pairwise comparisons with placebo as described above. One participant' data was excluded due to non-compliance with task instructions, leaving n=24 for emotional pictures analyses.
Subjective Drug Effect and Mood Measures
THC' effects on subjective mood state ratings were analyzed using one-way repeated measures ANOVA, with drug dose as the within-subject repeated measure, and change from pre-capsule scores for each subject averaged across each post-capsule time (+30, +60, +90, +120, +150, and +180 minutes). Significant main effects of dose were followed by pairwise comparisons as necessary to determine dose-dependency. Where violations of sphericity were apparent in ANOVA, Greenhouse–Geisser corrected results are presented. Alpha was set at p=.025 for pairwise comparisons with placebo. Separate analyses were conducted for each scale of the DEQ, VAS, PANAS, ARCI (administered at baseline, +90 minutes and +180 minutes only), and HRS (administered at +180 minutes only). A similar approach was taken for BP and HR responses.
Relation Between Subjective Response to THC and Altered Evaluation of Emotional Images
We performed exploratory analyses to investigate the possibility that THC' effects on evaluation of emotional images were related to its acute, mood-altering effects. Bivariate correlational analyses of drug effect (drug minus placebo) were undertaken using subjective measures and stimulus-response measures (i.e. facial emotion recognition, picture valence and arousal ratings) for each dose of THC separately. For these analyses, we subtracted change-from-baseline DEQ ‘dislike’ from DEQ ‘like’ ratings obtained at the time point immediately preceding the respective stimulus-response assessment as a comprehensive indicator of drug preference. Similar analyses were performed using VAS anxiety scores. These two subjective measures were selected because positive mood and anxiety have been linked to altered recognition of certain facial emotions (Surcinelli et al., 2006; Trevisani et al., 2008), and because positive mood and anxiety changes are two of the most commonly-reported effects of THC. Alpha was set liberally at p=.05 for correlational analyses, given their exploratory nature and to facilitate generation of novel hypotheses for future investigation.
Results
Participants
A total of 25 participants (11 female) completed the study. Of these, 18 self-identified as White (three Hispanic), three as Black or African American, two as Asian, and one as Asian/White; one participant did not identify their race. All participants were current or past occasional recreational cannabis users (Table 1). Fifteen reported current nicotine use; nine of these were daily smokers (mean: 38 cigarettes/week, SD: 30.0, range: 14 to 105/week), and six were occasional smokers (mean: 1.8 cigarettes/week, SD: 1.17, range: 1 to 4/week). All participants reported current use of alcohol (Table 1), and several reported some other recreational drug use (Supplementary Table 1).
Table 1.
Participants' Demographics and Past-Month Drug Use.
| Mean | SD | Range | |
|---|---|---|---|
| Age (years) | 24.36 | 4.56 | 18–35 |
| Body mass index | 22.45 | 2.04 | 19–26 |
| Education (years) | 15.48 | 1.45 | 12–18 |
| Caffeine (cups/day) | 1.64 | 1.08 | 0–5 |
| Alcohol (drinks/week) | 7.12 | 4.20 | 0–14 |
| Cannabis (times/month) | 2.12 | 2.54 | 0–8 |
Effects of THC on Facial Emotion Recognition
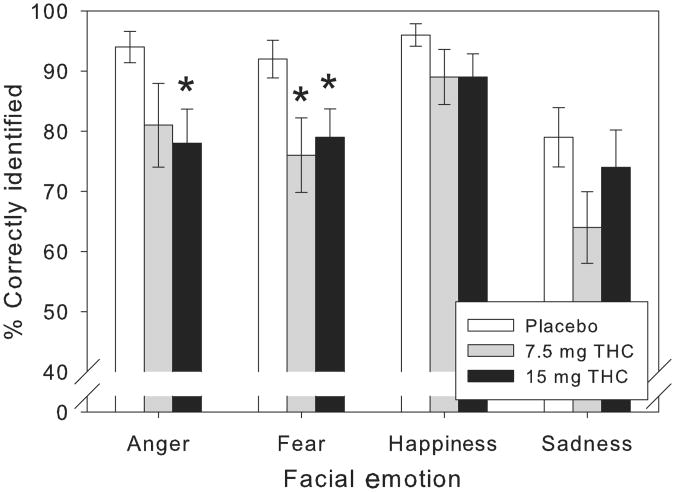
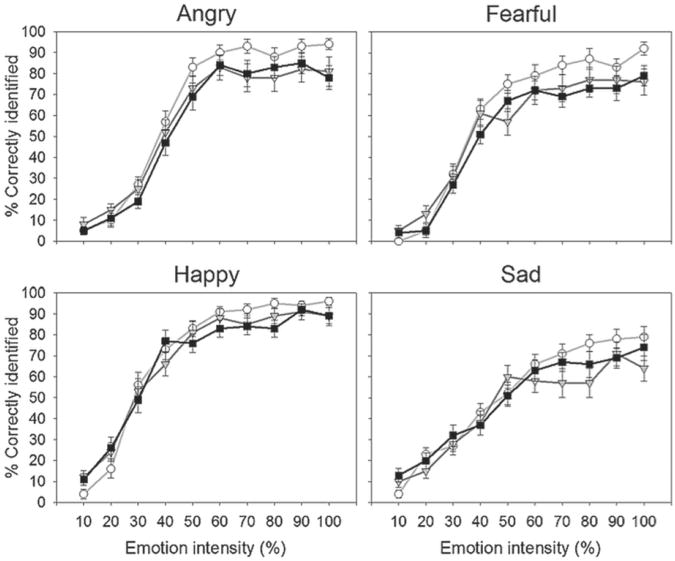
THC impaired recognition of unambiguous facial emotions overall in a dose-dependent fashion (F1,24=9.74, p=.005, MSE=134.90, ηp2=.29). There were no significant interactions between drug condition and facial emotion category, however, suggesting that the drug' effects were not exclusive for certain emotions. Nonetheless, consistent with our predictions, there was some evidence that THC predominantly impaired recognition of threat-related emotions: planned examination of individual facial emotions indicated that THC significantly impaired recognition of unambiguous anger at the higher dose (F1,24=7.28, p=.013, MSE=439.58, ηp2=.23) and fear at both doses (F1,24=6.69, p=.016, MSE=315.63, ηp2=.22), but it only marginally impaired recognition of unambiguous happiness (p=.07) (Figure 1). In addition, despite lack of a linear effect of THC dose on recognition of unambiguous sadness, visual inspection of the data suggests that there was some evidence of impairment at the lower dose, and this was confirmed by exploratory pairwise comparison with placebo (t24=2.77, p=.01). THC mostly impaired emotion recognition of highly intense emotional faces, whereas it left that of lower emotional intensity faces largely unchanged (Figure 2). Indeed, when we included data from faces of all levels of emotional intensity (i.e. 10–100%), there was a significant linear dose × linear intensity interaction (F1,24=16.80, p<.001, MSE=135.32,ηp2=.41). With all levels of emotional intensity included, the main effect of THC was somewhat less robust (F1,24=7.10, p=.014, MSE=51.35, ηp2=.23), but an analogous profile remained evident for the individual emotions. Only the higher dose of THC significantly impaired recognition of facial fear when analyses were extended to include ambiguous (i.e. lower emotional intensity) faces (F1,24=11.51, p=.002, MSE=69.53, ηp2=.32). Similarly, for morphed angry faces, the higher dose of THC produced only trend-level recognition impairments, although the dose-dependent linear function remained significant (F1,24=5.11, p=.033, MSE=152.78, ηp2=.18). The drug still did not clearly affect recognition of facial happiness or sadness when all levels of emotional intensity were included in analyses.
Figure 1.
Mean (± SEM) percent correctly identified unambiguous (100% emotional intensity) angry, fearful, happy, and sad faces after placebo and Δ9-tetrahydrocannabinol (THC) (7.5 and 15 mg). THC significantly impaired recognition of unambiguous anger (15 mg) and fear (7.5 and 15 mg).
*p<.025 compared with placebo, post hoc pairwise comparisons.
Figure 2.
Mean (± SEM) percent correctly identified angry, fearful, happy, and sad faces of varying emotional intensity after placebo and Δ9-tetrahydrocannabinol (THC) (7.5 and 15 mg). Open circles represent placebo; gray triangles represent 7.5 mg THC; black squares represent 15 mg THC.
Effects of THC on Ratings of Emotional Pictures
Self-reported ratings of positive and negative responses to the pictures were consistent with the valence categories to which they were assigned, as positive pictures were rated most positively and negative pictures were rated most negatively (Table 2). Both positive and negative pictures were rated as more arousing than neutral pictures, regardless of dose (all t23s>6.10, ps<.001). Although the positive and negative pictures were equated on arousal according to normative ratings, participants rated negative pictures as slightly, but significantly, more arousing than positive pictures in the placebo condition (t23=4.33, p<.001).
Table 2.
Ratings of Emotional Pictures.
| Subjective rating | Negative pictures | Neutral pictures | Positive pictures | |
|---|---|---|---|---|
| Placebo | 1.72 ± .15 | 3.55 ± .33 | 5.70 ± .36 | |
| Positivity | 7.5 mg | 1.96 ± .25 | 3.70 ± .29 | 5.63 ± .30 |
| 15 mg | 1.90 ± .20 | 4.00 ± .33 | 5.81 ± .37 | |
|
| ||||
| Placebo | 6.67 ± .25 | 1.93 ± .13 | 1.83 ± .14 | |
| Negativity | 7.5 mg | 6.71 ± .30 | 2.61 ± .27 | 2.28 ± .30 |
| 15 mg | 6.51 ± .27 | 2.42 ± .22 | 1.87 ± .17 | |
|
| ||||
| Placebo | 5.38 ± .32 | 2.98 ± .25 | 4.43 ± .29 | |
| Arousal | 7.5 mg | 5.67 ± .37 | 3.69 ± .35 | 4.98 ± .34 |
| 15 mg | 5.26 ± .31 | 3.34 ± .32 | 4.70 ± .38 | |
Data are means ± SEM.
THC did not consistently influence participants' ratings of emotional pictures, and there were no interactions of dose effects on positivity, negativity, or arousal ratings with valence category. There was some indication that THC altered participants' evaluation of neutral pictures, but these effects unexpectedly appeared to be largely limited to the lower dose. Specifically, despite a lack of linear main effects of dose on ratings, visual inspection of the data suggests that participants tended to rate neutral pictures as slightly more negative and arousing in the lower dose condition than they did in the placebo condition. Indeed, exploratory pairwise comparisons revealed increased negativity (t23=2.49, p=.021), and arousal (t23=2.27, p=.033) ratings of neutral pictures at the 7.5 mg dose. THC also tended to increase arousal ratings of positive pictures (t23=2.27, p=.033, exploratory pairwise comparison with placebo), but again this effect was mostly restricted to the 7.5 mg dose, and neither dose substantially affected arousal ratings of negative pictures. Finally, neither dose of THC clearly altered positivity ratings of pictures of any valence.
Effects of THC on Subjective and Cardiovascular Measures
Subjective mood and cardiovascular measures before drug administration were stable across the three sessions (drug condition and session order). THC increased ratings on scales of subjective drug effect and mood, including DEQ ratings of ‘feel’, ‘dislike’, ‘like’, ‘high’, and ‘want more’, VAS ratings of ‘anxious’, ‘stimulated’, ‘confused’, ‘sedated’, and ‘elated’, scores on ARCI PCAG, MBG, BG, LSD, M, and A scales, and scores on HRS scales designed to assess intensity of drug experience, somesthesia, and changes to affect, perception, and cognition (all ps≤.05). THC also increased diastolic BP and HR. Mean change from baseline scores for DEQ, VAS, PANAS, ARCI, and HRS scales, and cardiovascular measures are included in Supplementary Table 2. Although most effects increased dose-dependently, DEQ ratings of ‘like’ and ‘want more’, VA S ratings of ‘stimulated’, and scores on ARCI MBG and A scales were slightly higher at the 7.5 mg dose. Notably, the positive mood-enhancing effects did not increase along with dose. Not only were DEQ ‘dislike’ ratings higher at the 15 mg dose, and DEQ ‘like’ ratings highest at the 7.5 mg dose, but anxiety was also only evident at the 15 mg dose. Moreover, of the 25 study participants, 15 indicated a desire to take the 7.5 mg dose again, whereas only 11 out of 25 did so at the 15 mg dose. Thus, subjective responses to oral THC were not consistently positive in this sample of participants. Further, participants' subjective and cardiovascular responses to the drug were not related to gender or cannabis use experience (data not shown). Just over half of the participants identified THC as marijuana-like (7.5 mg: 56%; 15 mg: 52%). They identified the 7.5 and 15 mg doses of THC as a sedative 24% and 28% of the time, respectively, and as a stimulant 16% of the time (both doses). Sixty-eight percent of participants correctly identified the placebo (20% incorrectly identified it as a sedative drug, and 12% incorrectly identified it as a marijuana-like drug).
Relation Between Subjective Response to THC and Altered Evaluation of Emotional Images
We conducted exploratory correlational analyses to determine whether there was any relation between THC' effects on mood and its effects on facial emotion recognition or ratings of emotional pictures. For these analyses, we examined two measures of subjective drug response: 1) drug preference – calculated as liking minus disliking; and 2) anxiety. Change from placebo scores was calculated for each dose of THC separately. Although no correlations remained significant when corrected for multiple comparisons, there were some interesting trends. For instance, the higher dose of THC tended to impair recognition of facial happiness most in those participants who least enjoyed its effects (i.e. positive correlation between drug preference score and morphed happiness recognition accuracy: r=.40). Additionally, there was also some indication that participants' preference for THC' effects was related to the drug' ability to reduce the perceived negativity of negative emotional scenes (i.e. negative correlations between drug preference score and negativity ratings of negative pictures: r = −.52 and r = −.41 for the 7.5 mg dose and the 15 mg dose, respectively). Contrary to expectations, greater THC-induced anxiety appeared to be associated with greater impairments in fear recognition (i.e. negative correlation between anxiety and morphed fear recognition accuracy at the 15 mg dose: r = −.39). It is noteworthy that participants' ratings of drug disliking and liking were not negatively correlated as might be expected, suggesting that some participants simultaneously both disliked and liked THC' effects. Further, anxiety ratings were not correlated with drug preference, indicating that THC-induced anxiety was not necessarily perceived as subjectively negative.
Discussion
This study examined the effects of acute, oral THC on evaluation of emotional images, and was based on our previous research showing that THC reduces amygdalar reactivity to threatening faces (Phan et al., 2008). As hypothesized, THC impaired recognition of threat-related facial emotions. This impairment was most pronounced for facial fear, and then for anger, but recognition of happiness and sadness remained relatively intact. THC did not consistently affect the perceived valence or arousal of pictures of emotional scenes, but neutral pictures were rated as slightly more negative and arousing following the lower dose. Thus, while THC reduced perception of threat-related facial emotions, it did not appear to positively bias evaluation of emotional images in general. The drug produced its prototypic mood-altering effects, and these were not uniformly positive: participants reported both disliking and liking the drug' effects, which included both anxiety and elation.
The finding that THC impaired recognition of intense facial fear and anger, but not happiness, is concordant with our previous pharmaco-imaging study, in which we demonstrated that THC preferentially blunted neural activity in the amygdala elicited by unambiguous fearful and angry, relative to happy, faces (Phan et al., 2008). The current study expanded on our previous work in several ways. Here, we included a second, higher, dose of THC (i.e. 15 mg), and extended our examination to incorporate a less-threatening negative emotion (i.e. sadness), as well as faces depicting various intensities of emotion. Emotion recognition impairments generally increased dose-dependently, but recognition of sadness tended to be impaired by only the lower dose of THC. Importantly, THC' impairing effects on facial emotion recognition were most evident with respect to intensely emotional faces, suggesting that impairments were not related simply to a reduced ability to process complex stimuli. These data are also consistent with those reported by another group who found a tendency for THC (10 mg) to reduce amygdalar reactivity to 100% intensity fearful faces, with lesser effects seen for 50% intensity faces (Bhattacharyya et al., 2010; Fusar-Poli et al., 2009); although, seemingly paradoxically in that study, THC also potentiated skin-conductance responses to fearful faces – an outcome interpreted as indicative of a heightened response to threatening stimuli. This paradox between dampened amygdalar reactivity and increased physiological indicators of emotional response remains to be resolved. Our facial emotion recognition results are also broadly in agreement with studies showing that heavier cannabis users exhibit less amygdalar reactivity to emotional, particularly threat-related, faces (Cornelius et al., 2010; Gruber et al., 2009), and add to a growing literature suggesting that drugs that act at CB1Rs can uniquely influence processing of certain types of emotional information (Horder et al., 2009, 2010, 2011). Furthermore, our data fit with those reported for alcohol (Borrill et al., 1987; Craig et al., 2009), benzodiazepines (Blair and Curran, 1999), and MDMA (Bedi et al., 2010), all of which reduce perception of threat-related facial emotions. That this effect appears to be common across multiple drugs of abuse raises the question of whether it may indirectly contribute to these drugs' attractiveness to some users.
In contrast to its effects on facial emotion recognition, THC did not clearly alter ratings of pictures of emotional scenes. The finding that THC did not increase positivity ratings of pictures of any valence is consistent with some previous studies with different drugs of abuse (Patrick et al., 1996; Stritzke et al., 1995); although drug-induced increases in positivity ratings of pictures have also been reported (Gospic et al., 2008; Knowles and Duka, 2004; Wardle and de Wit, 2012). THC did slightly increase negativity and arousal ratings of neutral pictures in this sample, as well as arousal ratings of positive pictures. These effects were small, however, and they were unexpectedly largely limited to the lower dose. A more systematic examination will undoubtedly be necessary to fully determine the implications of these findings.
Study participants varied widely in their subjective responses to THC. Some individuals reported roundly liking the drug' effects, while others strongly disliked its effects, and the drug produced mood changes ranging from anxiety to elation. In order to assess the extent to which THC' effects on evaluation of emotional images were related to its subjective effects, we conducted exploratory correlational analyses. These analyses provided some suggestion that positive subjective responses to THC were related to its ability to positively bias perception. For instance, individuals who most preferred the drug tended to exhibit the greatest drug-induced reductions in negative response to negative pictures at both doses. Further, individuals who least preferred the drug tended to exhibit the greatest drug-induced impairments in recognizing facial happiness at the higher dose. These profiles would be consistent with the idea that drug-induced positive mood states correspond to a more positively biased perception of one' environment. Although other studies indicate that anxious individuals identify facial fear more accurately than non-anxious individuals (Surcinelli et al., 2006), we found that, greater THC-induced anxiety tended to correspond to greater impairments in fear recognition at the higher dose. The reason for this apparent discrepancy between effects of drug-induced anxiety and trait anxiety on evaluation of facial fear is not yet clear, but it could indicate that drug-induced acute mood state changes involve different mechanisms than more stable mood traits. Despite these interesting trends, these correlations are limited by the small size of our sample, and the failure to survive correction for multiple comparisons. Investigation of the relation between drugs' effects on mood and evaluation of emotional stimuli will be an interesting future direction of research. It is also worth noting that there is evidence that drugs can bias processing of emotional information independently of acute mood alterations (Browning et al., 2007; Harmer et al., 2003, 2004, 2006, 2008). Indeed, Harmer et al. (2009) have proposed that antidepressant drugs' early effects on emotional processing form the basis of later mood state improvements. Thus, even though drugs' acute effects on mood may not predict their effects on evaluation of emotional stimuli, their effects on emotional evaluation could be predictive of behavioral changes that will arise following repeated use.
There are several points that should be kept in mind when interpreting these data. First, either expectancies or other contextual conditions could have influenced THC' effects on evaluation of emotional images, just as they can alter subjective mood response to drugs (Doty and de Wit, 1995; Kirk et al., 1998; Metrik et al., 2009). Second, the effects of oral THC may not be entirely generalizable to whole-plant cannabis, which contains additional chemicals that could modulate THC' subjective effects (Zuardi et al., 1982; but see Ilan et al., 2005 and Karschner et al., 2011), and which is normally ingested by the smoked route (Wachtel et al., 2002). Another limitation of the present study was the absence of THC plasma level data, as individual variations in pharmacokinetics may have contributed to the variable subjective responses observed. Nonetheless, the oral route produces relatively consistent and reliable effects, perhaps even more than smoking, and THC produces very similar subjective effects to whole-plant cannabis (Wachtel et al., 2002). The heterogeneity of our participants' subjective responses to THC was probably not related to differences in experience using cannabis, as prior use did not predict correct identification of the THC capsules as being ‘marijuana-like’, or preference for the drug' effects (data not shown). Prior use also did not modulate THC' effects on performance of the study tasks (data not shown). However, it remains possible that individuals with less experience of using cannabis (e.g. fewer than 10 times lifetime) might exhibit a more homogeneous subjective response profile (e.g. more consistent anxiety response) (Bhattacharyya et al., 2010; Fusar-Poli et al., 2009). Finally, this study provides only suggestive evidence of the relation between mood and evaluation of emotional stimuli, and a larger sample size including a broad range of subjective responses to the drug (i.e. highly positive and highly negative) would be valuable to address this issue.
In summary, we found that THC acutely impairs recognition of threat-related facial emotions at moderately strong doses, but does not consistently influence recognition of non-threat-related facial emotions or the perceived emotionality of pictures of emotional scenes. These results demonstrate that although THC can diminish perception of threat, it does not appear to positively bias evaluation of emotional stimuli in general, as might be expected from a euphorigenic drug. The finding that THC reduced recognition of facial fear and anger is consistent with our previous observation that THC reduces amygdalar reactivity to fearful and angry faces, and illustrates a novel mechanism that may contribute to the positively reinforcing effects of cannabis. Specifically, this property could increase the drug' appeal to certain users, as a reduced sensitivity to anxiety-provoking emotional signals in others may facilitate social interactions, especially among individuals with social inhibition. Further research into the effects of THC and other drugs of abuse on evaluation of social and non-social emotional stimuli will be necessary to determine the extent to which such alterations occur, and will serve to improve our understanding of their role in promoting drug use.
Supplementary Material
Acknowledgments
The authors are grateful to Margaret Wardle for her insight and statistical advice.
Funding: This work was supported by NIDA DA02812 and (to MEB) NIDA T32 DA007255.
Footnotes
Conflict of interest: The authors declare that they do not have any conflict of interest.
References
- Adolphs R, Tranel D, Damasio H, et al. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bambico FR, Duranti A, Tontini A, et al. Endocannabinoids in the treatment of mood disorders: Evidence from animal models. Curr Pharm Des. 2009;15:1623–1646. doi: 10.2174/138161209788168029. [DOI] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an ‘Empathogen’? Effects of +/-3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol Psychiatry. 2010;68:1134–1140. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, et al. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl) 2009;207:73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Curran HV. Selective impairment in the recognition of anger induced by diazepam. Psychopharmacology (Berl) 1999;147:335–338. doi: 10.1007/s002130051177. [DOI] [PubMed] [Google Scholar]
- Borrill JA, Rosen BK, Summerfield AB. The influence of alcohol on judgement of facial expression of emotion. Br J Med Psychol. 1987;60:71–77. [PubMed] [Google Scholar]
- Browning M, Reid C, Cowen PJ, et al. A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol. 2007;21:684–690. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- Chait LD, Fischman MW, Schuster CR. ‘Hangover’ effects the morning after marijuana smoking. Drug Alcohol Depend. 1985;15:229–238. doi: 10.1016/0376-8716(85)90002-x. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, et al. The effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine Tob Res. 2006;8:379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Actions of delta-9-tetrahydrocannabinol in cannabis: Relation to use, abuse, dependence. Int Rev Psychiatry. 2009;21:104–112. doi: 10.1080/09540260902782752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Aizenstein HJ, Hariri AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav. 2010;35:644–646. doi: 10.1016/j.addbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland NJ, Singh AJ, Sustrik RA, et al. Effects of diazepam on facial emotion recognition. J Psychiatry Neurosci. 2003;28:452–463. [PMC free article] [PubMed] [Google Scholar]
- Craig LC, Attwood AS, Benton CP, et al. Effects of acute alcohol consumption and alcohol expectancy on processing of perceptual cues of emotional expression. J Psychopharmacol. 2009;23:258–265. doi: 10.1177/0269881108092126. [DOI] [PubMed] [Google Scholar]
- Del-Ben CM, Ferreira CA, Sanchez TA, et al. Effects of diazepam on BOLD activation during the processing of aversive faces. J Psychopharmacol. 2011;26:443–451. doi: 10.1177/0269881110389092. [DOI] [PubMed] [Google Scholar]
- Donohue KF, Curtin JJ, Patrick CJ, et al. Intoxication level and emotional response. Emotion. 2007;7:103–112. doi: 10.1037/1528-3542.7.1.103. [DOI] [PubMed] [Google Scholar]
- Doty P, de Wit H. Effect of setting on the reinforcing and subjective effects of ethanol in social drinkers. Psychopharmacology (Berl) 1995;118:19–27. doi: 10.1007/BF02245245. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Ranganathan M, Braley G, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect [slides] Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the visual analog mood scale. Psychol Med. 1973;3:8. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Manzanares J. The cannabinoid CB1 receptor is involved in the anxiolytic, sedative and amnesic actions of benzodiazepines. J Psychopharmacol. 2010;24:757–765. doi: 10.1177/0269881109106910. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, et al. Why we like to drink: A functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospic K, Gunnarsson T, Fransson P, et al. Emotional perception modulated by an opioid and a cholecystokinin agonist. Psychopharmacology (Berl) 2008;197:295–307. doi: 10.1007/s00213-007-1032-4. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: An FMRI study. Drug Alcohol Depend. 2009;105:139–153. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Bhagwagar Z, Perrett DI, et al. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003;28:148–152. doi: 10.1038/sj.npp.1300004. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Heinzen J, O'Sullivan U, Ayres RA, et al. Dissociable effects of acute antidepressant drug administration on subjective and emotional processing measures in healthy volunteers. Psychopharmacology (Berl) 2008;199:495–502. doi: 10.1007/s00213-007-1058-7. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, et al. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O'Sullivan U, Favaron E, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, et al. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Horder J, Browning M, Di Simplicio M, et al. Effects of 7 days treatment with the cannabinoid type 1 receptor antagonist, rimonabant, on emotional processing. J Psychopharmacol. 2011;26:125–132. doi: 10.1177/0269881111400649. [DOI] [PubMed] [Google Scholar]
- Horder J, Cowen PJ, Di Simplicio M, et al. Acute administration of the cannabinoid CB1 antagonist rimonabant impairs positive affective memory in healthy volunteers. Psychopharmacology (Berl) 2009;205:85–91. doi: 10.1007/s00213-009-1517-4. [DOI] [PubMed] [Google Scholar]
- Horder J, Harmer CJ, Cowen PJ, et al. Reduced neural response to reward following 7 days treatment with the cannabinoid CB(1) antagonist rimonabant in healthy volunteers. Int J Neuropsychopharmacol. 2010;13:1103–1113. doi: 10.1017/S1461145710000453. [DOI] [PubMed] [Google Scholar]
- Ilan AB, Gevins A, Coleman M, et al. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol. 2005;16:487–496. doi: 10.1097/00008877-200509000-00023. [DOI] [PubMed] [Google Scholar]
- Karschner EL, Darwin WD, McMahon RP, et al. Subjective and physiological effects after controlled Sativex and oral THC administration. Clin Pharmacol Ther. 2011;89:400–407. doi: 10.1038/clpt.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of gabaergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JM, Doty P, de Wit H. Effects of expectancies on subjective responses to oral delta-9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1998;59:287–293. doi: 10.1016/s0091-3057(97)00414-0. [DOI] [PubMed] [Google Scholar]
- Knowles SK, Duka T. Does alcohol affect memory for emotional and non-emotional experiences in different ways? Behav Pharmacol. 2004;15:111–121. doi: 10.1097/00008877-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention, University of Florida; 1999. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, et al. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. In: Pertwee RG, editor. Cannabinoids Handbook of Experimental Pharmacology. Vol. 168. Heidelberg: Springer-Verlag; 2005. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, et al. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Metrik J, Rohsenow DJ, Monti PM, et al. Effectiveness of a marijuana expectancy manipulation: Piloting the balanced-placebo design for marijuana. Exp Clin Psychopharmacol. 2009;17:217–225. doi: 10.1037/a0016502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Wotjak CT. Cannabinoids and anxiety. Curr Top Behav Neurosci. 2010;2:429–450. doi: 10.1007/7854_2009_16. [DOI] [PubMed] [Google Scholar]
- Ohman A. The role of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Berthot BD, Moore JD. Diazepam blocks fearpotentiated startle in humans. J Abnorm Psychol. 1996;105:89–96. doi: 10.1037//0021-843x.105.1.89. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, et al. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, et al. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- Stritzke WG, Patrick CJ, Lang AR. Alcohol and human emotion: A multidimensional analysis incorporating startle-probe methodology. J Abnorm Psychol. 1995;104:114–122. doi: 10.1037//0021-843x.104.1.114. [DOI] [PubMed] [Google Scholar]
- Surcinelli P, Codispoti M, Montebarocci O, et al. Facial emotion recognition in trait anxiety. J Anxiety Disord. 2006;20:110–117. doi: 10.1016/j.janxdis.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Trevisani DP, Johnson SL, Carver CS. Positive mood induction and facial affect recognition among students at risk for mania. Cognit Ther Res. 2008;32:639. doi: 10.1007/s10608-007-9140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, de Wit H. Naltrexone does not block the subjective effects of oral delta-9-tetrahydrocannabinol in humans. Drug Alcohol Depend. 2000;59:251–260. doi: 10.1016/s0376-8716(99)00127-1. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, Elsohly MA, Ross SA, et al. Comparison of the subjective effects of delta-9-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Wardle MC, de Wit H. Effects of amphetamine on reactivity to emotional stimuli. Psychopharmacology (Berl) 2012;220:143–153. doi: 10.1007/s00213-011-2498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Young AW, Rowland D, Calder AJ, et al. Facial expression megamix: Tests of dimensional and category accounts of emotion recognition. Cognition. 1997;63:271–313. doi: 10.1016/s0010-0277(97)00003-6. [DOI] [PubMed] [Google Scholar]
- Zanettini C, Panlilio LV, Alicki M, et al. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangara A, Blair RJ, Curran HV. A comparison of the effects of a beta-adrenergic blocker and a benzodiazepine upon the recognition of human facial expressions. Psychopharmacology (Berl) 2002;163:36–41. doi: 10.1007/s00213-002-1120-4. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, et al. Action of cannabidiol on the anxiety and other effects produced by delta-9-thc in normal subjects. Psychopharmacology (Berl) 1982;76:245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.