Abstract
THAP1 mutations have been shown to be the cause of DYT6. A number of different mutation types and locations in the THAP1 gene have been associated with a range of severity and dystonia phenotypes, but, as yet, it has been difficult to identify clear genotype phenotype patterns. Here, we screened the THAP1 gene in a further series of dystonia cases and evaluated the mutation pathogenicity in this series as well as previously reported mutations to investigate possible phenotype-genotype correlations. THAP1 mutations have been identified throughout the coding region of the gene, with the greatest concentration of variants localized to the THAP1 domain. In the additional cases analyzed here, a further two mutations were found. No obvious, indisputable genotype-phenotype correlation emerged from these data. However, we managed to find a correlation between the pathogenicity of mutations, distribution, and age of onset of dystonia. THAP1 mutations are an important cause of dystonia, but, as yet, no clear genotype-phenotype correlations have been identified. Greater mutation numbers in different populations will be important and mutation-specific functional studies will be essential to identify the pathogenicity of the various THAP1 mutations. © 2012 Movement Disorder Society
Keywords: THAP1, dystonia, DYT6, mutations, phenotype, genotype
Primary dystonias are often inherited as monogenic traits, but the inheritance can be complicated by reduced expression and phenocopies within families. Originally, dystonia was classified purely on clinical features, but the classification and understanding of the dystonias has grown dramatically over the last decade with the application of genetic testing. At least 17 distinct, likely Mendelian primary dystonias have been identified.1–11
The most common inherited dystonia is DYT1, encoded by the TorsinA8 gene. The DYT1 phenotype has been described extensively with variable clinical presentation and progression. A three-nucleotide deletion (GAG) in exon 5 accounts for almost all DYT1 cases. Three other mutations have been described in TorsinA in isolated cases and, with their pathogenicity, are still unclear for two of them.12–14 THAP1-associated DYT6 patients present with a wide variety of sites of onset and severity. Missense, nonsense, and frameshift mutations have been described in all three exons of the THAP1 gene, but they tend to be concentrated in the THAP domain. THAP1 binds to the core promoter of TorsinA, and wild-type THAP1 represses the expression of TorsinA, whereas Dyt6-associated mutant THAP1 results in decreased repression of TorsinA and increased expression.15–21 Recent studies have emphasized the fact that THAP1 mutations occur frequently with oromandibular and laryngeal dystonia, but focal, segmental, and generalized dystonia have all been described. There have been no reported THAP1 genotype/phenotype predictors that have been associated with the range of clinical features.
Therefore, we screened the THAP1 gene in a further series of dystonia cases from the United Kingdom and performed a meta-analysis of published cases to investigate a possible genotype/phenotype in THAP1-associated dystonia.
Patients and Methods
Patients and Screening of the THAP1 Gene
The THAP1 gene was analyzed in a group of 150 DYT1-negative, characterized patients with various forms of primary dystonia. All patients gave informed consent, and ethics approval was obtained from the joint medical and ethics committee at the National Hospital for Neurology and Neurosurgery (07/Q0502/2). Patients were assessed and followed up by movement disorder specialists. The THAP1 gene was analyzed in this series by Sanger sequencing, as previously described (RefSeq NM_018105.2).19 The control series that were sequenced consisted of 176 healthy UK white individuals who were older than 50 years and neurologically healthy, 40 North London Jewish controls, and 68 Indian control individuals.
Review of Published THAP1 Data
A comprehensive literature review of all reported THAP1 mutations was performed to include as many patients as possible. Data were analyzed from 100 patients published in the literature since the discovery of DYT6-associated dystonia until August 2011.22–35
Evaluation of Pathogenicity of Mutations Using Computational Prediction
Two algorithms were used to evaluate the effect of amino-acid substitutions on the function of THAP1: polymorphism phenotyping (polyphen) and sorting intolerance from tolerance (SIFT).36 Prediction is based on empirical rules applied to the sequence, phylogenetic, and structural information characterizing the amino-acid substitution (http://genetics.bwh.harvard.edu/pph/ and http://sift.jcvi.org). The Human Splicing Finder tool37 was used to evaluate mutations that potentially affect splicing (http://www.umd.be/HSF/).
Statistical Analysis
We used descriptive statistics to present demographic, clinical, and other characteristics of patients overall and by dystonia type. Demographic and clinical characteristics of patients by dystonia type were examined using analysis of variance (Scheffe's post hoc) for continuous variables and the chi-square test for categorical variables. We calculated Cox's proportional hazards models with dystonia as the outcome and dystonia age of onset as the time-to-event variable. In an initial model, dystonia type was the time-constant predictor (generalized dystonia as the reference). In a subsequent model, we considered pathogenicity of the mutation (benign as the reference) as the time-constant predictor. IBM/SPSS software was used (version 19; SPSS, Inc., Chicago, IL).
Results
Clinical details of patients are presented in Table 1. Among patients with dystonia that we screened, we identified three variants. These included one novel nonsense mutation p.R29X (c.85C>T) and one missense mutation:p. N136S:(c.407A>G) in the heterozygous and in homozygous state, respectively. The homozygous case was found to have been included in a previous study.19 None of these variations were found among control chromosomes.
TABLE 1.
Clinical detais of patients with dystonia screened for THAP1 mutations
| Patients | n = 150 |
|---|---|
| Women (%) | 80 (53) |
| Median age at onset, years (range) | 28 (2–54) |
| Median age at examination, years (range) | 39 (14–58) |
| Median duration of dystonia, years (range) | 25 (0–40) |
| Distribution of dystonia (%) | |
| Focal | 35 (23) |
| Segmental | 53 (35) |
| Multifocal | 22 (15) |
| Generalized | 40 (27) |
Patient 1
The patient that carries the R29X mutation is a 27-year-old woman with generalized dystonia, which affects her arms, trunk (mildly), and both feet. She also has marked oromandibular dystonia with protrusion of the tongue. Her symptoms began around the age of 7. Initially, her leg was affected, then her handwriting, and, at the age of 11, her speech was involved. Although her tongue was markedly affected, swallowing was preserved. There was no family history.
Patient 2
The patient that carries the N136S mutation in the heterozygous state is a 36-year-old right-handed woman that noticed a pain in her neck at the age of 30, which got worse over the next 4 years. At the age of 34, she noticed a bit of a head shake and she developed a right torticollis. There is no evident dystonia in other parts of her body. There is no family history of dystonia. She responds well to botulinum toxin injections.
Literature Review
One hundred patients (60 females and 40 males) were included in our study group. In these patients, 63 different mutations have been identified (Fig. 1). The majority of these mutations are missense (66%), and the rest of them are small insertions/deletions, nonsense mutations, and splice-site mutations. In total, 77% of these were found to be probably and computationally possibly pathogenic, and 23% of them were computationally benign. The THAP domain (exons 1 and 2) contained 66% of mutations (Fig. 1). All descriptive characteristics of our study group are given in Table 2. We further analyzed these patients according to type of dystonia (Table 2).
FIG. 1.
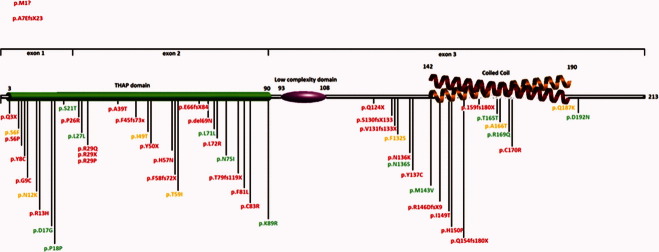
THAP1 mutations distributed throughout the gene. Each color represents different pathogenicity according to polyphen prediction program. Red: probable pathogenic; Yellow: possibly pathogenic; green: benign. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
TABLE 2.
Clinical characteristics of patients published in the literature with THAP1 mutations and classification of mutations according to computational prediction
| First Symptom (%) | Pathogenicity of Mutation (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Age at Onset Years (SD) | Family History (%) | Gender Distribution (Females) (%) | Speech Involvement (%) | Limb | Cervical | Cranial | Laryngeal | Benign | Possibly Damaging | Probably Damaging | |
| Patients (total number = 100) | 24.4 (18.6) | 62 | 60 | 58 | 44 | 31 | 15 | 10 | 23 | 11 | 66 |
| Type of dystonia | |||||||||||
| Generalized (37%) | 11.7 (8.7) | 78.4 | 56.8 | 54.1 | 62.2 | 18.9 | 13.5 | 5.4 | 2.7 | 13.5 | 83.8 |
| Segmental (30%) | 24.7 (16.5) | 56.7 | 50 | 50 | 40 | 30 | 20 | 10 | 23.3 | 6.7 | 70 |
| Multifocal (6%) | 19 (19) | 100 | 66.7 | 33.3 | 100 | 0 | 0 | 0 | 0 | 16.7 | 83.3 |
| Focal (27%) | 42.8 (15.7) | 37 | 77.8 | 18.5 | 11.1 | 55.6 | 14.8 | 18.5 | 55.6 | 11.1 | 33.3 |
SD, standard deviation.
An important result of our study is the effect of age at onset on the distribution of dystonia. Compared to patients with generalized dystonia, those with segmental (hazards ratio [HR] = 12.93; 95% confidence interval [CI] = 3.13–22.7; P = 0.004), multifocal (borderline, possibly resulting from a very small number of cases; HR = 7.27; 95% CI = −5.03−24.0; P = 0.07), and focal (HR = 31.08; 95% CI = 20.98–41.19; P = 0.000) dystonia had later age of onset (Fig. 2).
FIG. 2.
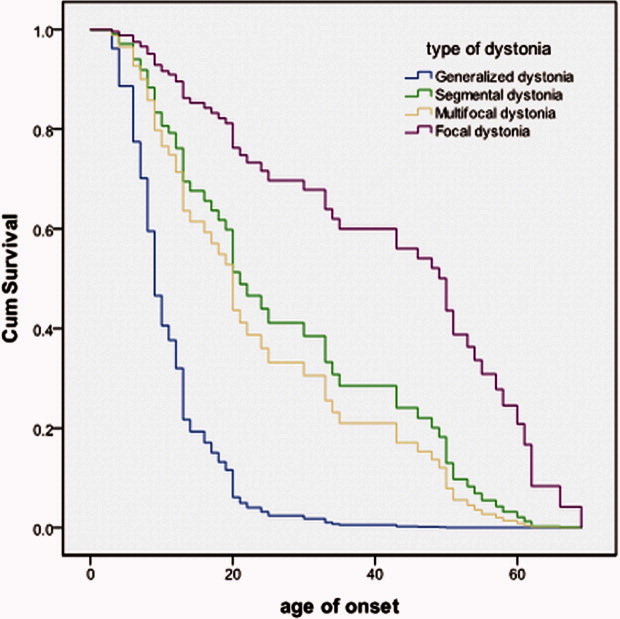
Survival curves based on Cox's analysis comparing DYT6 dystonia age of onset in patients grouped by dystonia type. Y-axis: 1-cumulative survival/probability of developing DYT6 dystonia. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Overall, no significant differences were observed between groups of patients with different types of dystonia and gender. In focal dystonia, though, we noticed that 77.8% of patients are women and 22.2% are men and that more benign variants have been found in women (70%), compared to men. In the generalized type of dystonia, 83.8% of patients harbor likely damaging mutations versus 4.3% that present with benign variants. On the other hand, in focal dystonia, 65.2% harbor benign variants versus 13.6% with likely damaging mutations (P < 0.0001). Limb onset is also associated significantly with more-pathogenic mutations (77.7% likely damaging versus 9.1% benign) versus cervical onset that benign and likely damaging mutations share the same percentage (41.9% and 48.4%, respectively). Compared to benign mutations, those with possibly damaging mutations had a slightly earlier age of dystonia onset (HR = 13.9; 95% CI = 1.07-26.8; P = 0.03), whereas those with probably damaging mutations had much earlier age of onset (HR = 28.8; 95% CI = 20.3-37.4; P = 0.000) (Fig. 3).
FIG. 3.
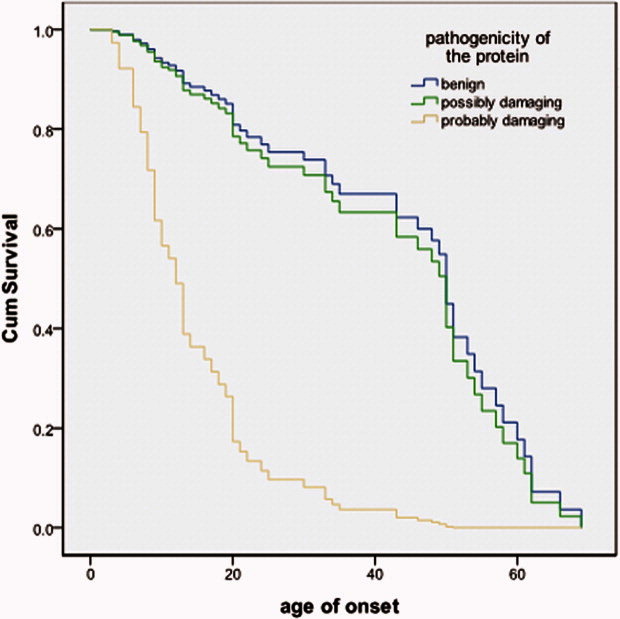
Survival curves based on Cox's analysis comparing DYT6 dystonia age of onset in patients grouped by mutation pathogenicity. Y-axis: 1-cumulative survival/probability of developing DYT6 dystonia. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion and Conclusions
We have sequenced the THAP1 gene in a series of British dystonia patients as well as analyzing the previous THAP1 reports. Two mutations were identified, consistent with previous reports and at a similar frequency of 1% to 2% of dystonia cases. This work shows that there were no specific THAP1 mutations that consistently led to a severe or mild dystonia phenotype. This is the result of the large variety of THAP1 mutations that have been described, with few in more than one families and only one, the F45fs73X mutation, in a significant number of cases.23, 28
However, it is clear that THAP1 mutations influence several characteristics that include distribution of dystonia and age at onset. Patients with computationally pathogenic mutations, based on SIFT and POLYPHEN, frequently have generalized dystonia with earlier age at onset and positive family history. In a more simplistic way, this means that the probability of developing dystonia, that expresses survival endpoint, at a younger age is higher for patients with computationally pathogenic mutations, compared to the other groups. However, in silico investigations are not as good as true functional investigation.38 Prediction is based on factors such as three-dimensional protein structure, polarity, homology, and species conservation. These programs do not take into account other important factors, such as supramolecular interactions with homologous molecules.38 Overall, a correct phenotype is predicted in almost 70% of the cases, showing that the level of confidence is relatively high for research purposes.39
In a large number of cases with THAP1 mutations, cervical dystonia was the presenting feature, in complete accord with all previous studies.36–38 More specifically, we observed that cervical dystonia usually emerges in middle age, is sporadic, and develops primarily in females. However, a large number of cervical dystonia cases harbor computationally benign variants. The main question remains: Can these variants cause the disorder or are they rare benign variants that have nothing to do with the dystonia. In most adult-onset dystonia families, inheritance does not appear to be Mendelian, but is rather consistent with a multifactorial trait.41 The main hypothesis today is that a number of common genes underlie the pathophysiological mechanisms shared by the various forms of adult-onset focal dystonia, and that additional genes and environmental triggers determine the clinical, neurophysiological, and imaging differences described in the various forms of dystonia.
To tackle the difficulty with the small number of cases, ideally, each mutation would be functionally characterized to investigate the degree of transcriptional dysregulation of TorsinA and the other consequences of a defective THAP1 protein.16 The analysis of further mutations and families will also be important, and recently, a web-based database has been created to allow the inclusion of mutation reports to increase the THAP1 dataset.43 More clinical and molecular data will be needed to elucidate the complex genotype/phenotype correlations associated with DYT6.
Acknowledgments
The authors thank the patients and families for their essential contribution to this research.
References
- 1.Asmus F, Gasser T. Inherited myoclonus-dystonia. Adv Neurol. 2004;94:113–119. [PubMed] [Google Scholar]
- 2.Asmus F, Salih F, Hjermind LE, et al. Myoclonus-dystonia due to genomic deletions in the epsilon-sarcoglycan gene. Ann Neurol. 2005;58:792–797. doi: 10.1002/ana.20661. [DOI] [PubMed] [Google Scholar]
- 3.Bandmann O, Valente EM, Holmans P, et al. Dopa-responsive dystonia: a clinical and molecular genetic study. Ann Neurol. 1998;44:649–656. doi: 10.1002/ana.410440411. [DOI] [PubMed] [Google Scholar]
- 4.Brashear A, Dobyns WB, de Carvalho Aguiar P, et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain. 2007;130:828–835. doi: 10.1093/brain/awl340. [DOI] [PubMed] [Google Scholar]
- 5.Camargos S, Scholz S, Simon-Sanchez J, et al. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7:207–215. doi: 10.1016/S1474-4422(08)70022-X. [DOI] [PubMed] [Google Scholar]
- 6.Chouery E, Kfoury J, Delague V, et al. A novel locus for autosomal recessive primary torsion dystonia (DYT17) maps to 20p11.22-q13.12. Neurogenetics. 2008;9:287–293. doi: 10.1007/s10048-008-0142-4. [DOI] [PubMed] [Google Scholar]
- 7.Grotzsch H, Pizzolato GP, Ghika J, et al. Neuropathology of a case of dopa-responsive dystonia associated with a new genetic locus, DYT14. Neurology. 2002;58:1839–1842. doi: 10.1212/wnl.58.12.1839. [DOI] [PubMed] [Google Scholar]
- 8.Ozelius LJ, Hewett JW, Page CE, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 9.Rainier S, Thomas D, Tokarz D, et al. Myofibrillogenesis regulator 1 gene mutations cause paroxysmal dystonic choreoathetosis. Arch Neurol. 2004;61:1025–1029. doi: 10.1001/archneur.61.7.1025. [DOI] [PubMed] [Google Scholar]
- 10.Valente EM, Bentivoglio AR, Cassetta E, et al. DYT13, a novel primary torsion dystonia locus, maps to chromosome 1p36.13—36.32 in an Italian family with cranial-cervical or upper limb onset. Ann Neurol. 2001;49:362–366. [PubMed] [Google Scholar]
- 11.Wider C, Melquist S, Hauf M, et al. Study of a Swiss dopa-responsive dystonia family with a deletion in GCH1: redefining DYT14 as DYT5. Neurology. 2008;70:1377–1383. doi: 10.1212/01.wnl.0000275527.35752.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Defazio G, Matarin M, Peckham EL, et al. The TOR1A polymorphism rs1182 and the risk of spread in primary blepharospasm. Mov Disord. 2009;24:613–616. doi: 10.1002/mds.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabakci K, Hedrich K, Leung JC, et al. Mutations in DYT1: extension of the phenotypic and mutational spectrum. Neurology. 2004;62:395–400. doi: 10.1212/01.wnl.0000113024.84178.f7. [DOI] [PubMed] [Google Scholar]
- 14.Leung JC, Klein C, Friedman J, et al. Novel mutation in the TOR1A (DYT1) gene in atypical early onset dystonia and polymorphisms in dystonia and early onset parkinsonism. Neurogenetics. 2001;3:133–143. doi: 10.1007/s100480100111. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser FJ, Osmanoric A, Rakovic A, et al. The dystonia gene DYT1 is repressed by the transcription factor THAP1 (DYT6) Ann Neurol. 2010;68:554–559. doi: 10.1002/ana.22157. [DOI] [PubMed] [Google Scholar]
- 16.Gavarini S, Cayrol C, Fuchs T, et al. Direct interaction between causative genes of DYT1 and DYT6 primary dystonia. Ann Neurol. 2010;68:549–553. doi: 10.1002/ana.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohmann K, Uflacker N, Erogullari A, et al. Identification and functional analysis of novel THAP1 mutations. Eur J Hum Genet. 2012;20:171–175. doi: 10.1038/ejhg.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osmanovic A, Dendorfer A, Erogullari A, et al. Truncating mutations in THAP1 define the nuclear localization signal. Mov Disord. 2011;26:1565–1567. doi: 10.1002/mds.23611. [DOI] [PubMed] [Google Scholar]
- 19.Tamiya G. Transcriptional dysregulation: a cause of dystonia? Lancet Neurol. 2009;8:416–418. doi: 10.1016/S1474-4422(09)70087-0. [DOI] [PubMed] [Google Scholar]
- 20.Muller U. A molecular link between dystonia 1 and dystonia 6? Ann Neurol. 2010;68:418–420. doi: 10.1002/ana.22183. [DOI] [PubMed] [Google Scholar]
- 21.Cheng FB, Ozelius LJ, Wan XH, et al. THAP1/DYT6 sequence variants in non-DYT1 early-onset primary dystonia in China and their effects on RNA expression. J Neurol. 2012;259:342–347. doi: 10.1007/s00415-011-6196-5. [DOI] [PubMed] [Google Scholar]
- 22.Bonetti M, Barzaghi C, Brancati F, et al. Mutation screening of the DYT6/THAP1 gene in Italy. Mov Disord. 2009;24:2424–2427. doi: 10.1002/mds.22861. [DOI] [PubMed] [Google Scholar]
- 23.Bressman SB, Raymond D, Fuchs T, Heiman GA, Ozelius LJ, Saunders-Pullman R. Mutations in THAP1 (DYT6) in early-onset dystonia: a genetic screening study. Lancet Neurol. 2009;8:441–446. doi: 10.1016/S1474-4422(09)70081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng FB, Wan XH, Feng JC, Wang L, Yang YM, Cui LY. Clinical and genetic evaluation of DYT1 and DYT6 primary dystonia in China. Eur J Neurol. 2011;18:497–503. doi: 10.1111/j.1468-1331.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- 25.Clot F, Grabli D, Burbaud P, et al. Screening of the THAP1 gene in patients with early-onset dystonia: myoclonic jerks are part of the dystonia 6 phenotype. Neurogenetics. 2011;12:87–89. doi: 10.1007/s10048-010-0264-3. [DOI] [PubMed] [Google Scholar]
- 26.De Carvalho Aguiar P, Fuchs T, Borges V, et al. Screening of Brazilian families with primary dystonia reveals a novel THAP1 mutation and a de novo TOR1A GAG deletion. Mov Disord. 2010;25:2854–2857. doi: 10.1002/mds.23133. [DOI] [PubMed] [Google Scholar]
- 27.Djarmati A, Schneider SA, Lohmann K, et al. Mutations in THAP1 (DYT6) and generalised dystonia with prominent spasmodic dysphonia: a genetic screening study. Lancet Neurol. 2009;8:447–452. doi: 10.1016/S1474-4422(09)70083-3. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs T, Gavarini S, Saunders-Pullman R, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–288. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- 29.Houlden H, Schneider SA, Paudel R, et al. THAP1 mutations (DYT6) are an additional cause of early-onset dystonia. Neurology. 2010;74:846–850. doi: 10.1212/WNL.0b013e3181d5276d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paisan-Ruiz C, Ruiz-Martinez J, Ruibal M, et al. Identification of a novel THAP1 mutation at R29 amino-acid residue in sporadic patients with early-onset dystonia. Mov Disord. 2009;24:2428–2429. doi: 10.1002/mds.22849. [DOI] [PubMed] [Google Scholar]
- 31.Puschmann A, Xiao J, Bastian RW, Searcy JA, Ledoux MS, Wszolek ZK. An African-American family with dystonia. Parkinsonism Relat Disord. 2011;17:547–550. doi: 10.1016/j.parkreldis.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider SA, Ramirez A, Shafiee K, et al. Homozygous THAP1 mutations as cause of early-onset generalized dystonia. Mov Disord. 2011;26:858–861. doi: 10.1002/mds.23561. [DOI] [PubMed] [Google Scholar]
- 33.Sohn AS, Glockle N, Doetzer AD, et al. Prevalence of THAP1 sequence variants in German patients with primary dystonia. Mov Disord. 2010;25:1982–1986. doi: 10.1002/mds.23207. [DOI] [PubMed] [Google Scholar]
- 34.Van Gerpen JA, Ledoux MS, Wszolek ZK. Adult-onset leg dystonia due to a missense mutation in THAP1. Mov Disord. 2010;25:1306–1307. doi: 10.1002/mds.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao J, Zhao Y, Bastian RW, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;74:229–238. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flanagan SE, Patch AM, Ellard S. Using SIFT and PolyPhen to predict loss-of-function and gain-of-function mutations. Genet Test Mol Biomarkers. 2010;14:533–537. doi: 10.1089/gtmb.2010.0036. [DOI] [PubMed] [Google Scholar]
- 37.Desmet FO, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tchernitchko D, Goossens M, Wajcman H. In silico prediction of the deleterious effect of a mutation: proceed with caution in clinical genetics. Clin Chem. 2004;50:1974–1978. doi: 10.1373/clinchem.2004.036053. [DOI] [PubMed] [Google Scholar]
- 39.Zou M, Baitei EY, Alzahrani AS, et al. Mutation prediction by PolyPhen or functional assay, a detailed comparison of CYP27B1 missense mutations. Endocrine. 2011;40:14–20. doi: 10.1007/s12020-011-9489-7. [DOI] [PubMed] [Google Scholar]
- 40.Evatt ML, Freeman A, Factor S. Adult-onset dystonia. Handb Clin Neurol. 2011;100:481–511. doi: 10.1016/B978-0-444-52014-2.00037-9. [DOI] [PubMed] [Google Scholar]
- 41.Sharma N, Franco RA, Jr, Kuster JK, et al. Genetic evidence for an association of the TOR1A locus with segmental/focal dystonia. Mov Disord. 2010;25:2183–2187. doi: 10.1002/mds.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Defazio G. The epidemiology of primary dystonia: current evidence and perspectives. Eur J Neurol. 2010;17(Suppl 1):9–14. doi: 10.1111/j.1468-1331.2010.03053.x. [DOI] [PubMed] [Google Scholar]
- 43.Blanchard A, Ea V, Roubertie A, et al. DYT6 dystonia: review of the literature and creation of the UMD Locus-Specific Database (LSDB) for mutations in the THAP1 gene. Hum Mutat. 2011;32:1213–1224. doi: 10.1002/humu.21564. [DOI] [PubMed] [Google Scholar]


