Abstract
The goal of this study is to determine whether dermal fibroblasts lacking syndecan-1 (sdc1) show differences in integrin expression and function that could contribute to the delayed skin and corneal wound healing phenotypes seen in syndecan-1 (sdc-1) null mice. Using primary dermal fibroblasts, we show that after 3 days in culture no differences in α-smooth muscle actin were detected but sdc-1 null cells expressed significantly more αv and β1 integrin than wt cells. TGFβ1 treatment at day 3 increased αv- and β1- integrin expression in sdc-1 null cells at day 5 whereas wt cells showed increased expression only of αv-integrin. Using time-lapse studies, we showed that the sdc-1 null fibroblasts migrate faster than wt fibroblasts, treatment with TGFβ1 increased these migration differences, and treatment with a TGFβ1 antagonist caused sdc-1 null fibroblasts to slow down and migrate at the same rate as untreated wt cells. Cell spreading studies on replated fibroblasts showed altered cell spreading and focal adhesion formation on vitronectin and fibronectin coated surfaces. Additional time lapse studies with β1- and αv- integrin neutralizing antibodies, showed that wt fibroblasts expressing sdc-1 had activated integrins on their surface that impeded their migration whereas the null cells expressed αv-containing integrins which were less adhesive and enhanced cell migration. Surface expression studies showed increased surface expression of α2β1 and α3β1 on the sdc-1 null fibroblasts compared to wt fibroblasts but no significant differences in surface expression of α5β1, αvβ3, or αvβ5. Taken together, our data indicates that sdc-1 functions in the activation of αv-containing integrins and support the hypothesis that impaired wound healing phenotypes seen in sdc-1 null mice could be due to integrin-mediated defects in fibroblast migration after injury.
Keywords: integrins, fibroblasts, cell migration, syndecan-1
Introduction
Syndecans (sdcs) are a family of transmembrane proteoglycans which play a major role in tissue maintenance and regeneration (1, 2, 3). Via their heparan sulfate chains, they interact with heparin-binding growth factors like bFGF and TGFβ1 and distinct amino acids within their extracellular domain interact with specific integrin family members to affect cell adhesion and migration (4, 5). Sdc-1 deficient mice were initially shown to have signs of delayed healing in cornea and skin (6, 7) and subsequent studies have focused on the susceptibility of these mice to cancer, increase in inflammation, as well as their ability to repair coronary defects as summarized in Table 1. Detailed studies have been done on the keratinocytes from sdc-1 deficient mice (15) but there have been no reports on the sdc-1 null fibroblasts despite their importance in mediating wound healing. Our goal in this study was to determine whether dermal fibroblasts derived from the sdc-1 null mouse, like epidermal keratinocytes, show impaired regulation of integrin expression and function at the cell surface that could contribute to wound healing defects.
Table 1.
Phenotypes reported in Syndecan-1 Null Mice
| Reduced breast cancer tumor development |
Alexander, et al., 2000 [8] |
| McDermott, et al., 2007 [9] | |
| Increased inflammation | Gotte, et al., 2002 [10] |
| Gotte, et al., 2005 [6] | |
| Delayed corneal and skin wound healing |
Stepp, et al., 2002 [7] |
| Reduced bacterial pathogenesis | Park, et al., 2004 [11] |
| Haynes, et al., 2005 [12] | |
| Increased complications after coronary myocardial infarction |
Vanhoutte, et al., 2007 [13] |
| Increased severity of anti-GBM nephritis |
Rops, et al., 2007 [14] |
| Reduced migration and increased integrin function in keratinocytes |
Stepp, et al., 2007 [15] |
Research on the importance of sdc-1 function in wound healing has focused on increased expression of sdc-1 by wounded epidermal keratinocytes in the skin (7, 16) and the shedding of sdc-1 into the wound fluid after proteolytic cleavage (17). The functions of sdc-1 on mesenchymal cells during wound healing have not been directly addressed; research has focused on the roles played by sdc-4 and to a lesser extent sdc-2 in fibroblasts. Data show that the cytoplasmic domain of sdc-4 can regulate focal adhesion and stress fiber formation in dermal fibroblasts (18) by binding to the catalytic domain of protein kinase Cα (19). Additional studies show that the sdc-4 cytoplasmic domain is involved in mediating localization and activation of Rac1 to enhance directional or processive cell migration (20). The delayed skin wound healing phenotype in sdc-4 null mice has been reported to be due to defective fibroblast migration (21). Sdc-2 is induced in fibroblasts by TGFβ1 and has been shown to regulate matrix metalloproteinase expression and play a role in regulating TGFβ1 signaling in fibroblasts (22, 23).
In unwounded skin, fibroblasts in the dermis continuously synthesize and maintain the connective tissues that comprise their collagen scaffold; they rarely divide and have been called quiescent due to their slow proliferation rate. After wounding, growth factors derived from serum and cytokines secreted by inflammatory cells combine to activate quiescent fibroblasts adjacent to the wound bed. Transforming growth factor β1 (TGFβ1) is the founding member of a large family of growth factors that now includes important morphoregulatory proteins such as bone morphogenetic proteins (BMPs) and activins (24). TGFβ1 is induced in response to injury and upregulates extracellular matrix, integrin expression, and alters cell migration and differentiation (25, 26). Our studies use primary mouse fibroblasts obtained from the neonatal mouse dermis; culturing fibroblasts from the unwounded dermis in the presence of 10% serum also triggers their activation. Activated fibroblasts turn on expression of α-smooth muscle actin (αSMA), proliferate, and increase their expression of integrins.
Our previous report identified differences in α6β4-integrin mediated adhesion and altered TGFβ1 signaling in primary mouse keratinocytes (15). In this study, we set out to determine whether alterations in integrin expression and function are intrinsic to cells lacking sdc-1. Integrins support cell adhesion, spreading, and migration (27) and can be expressed on cell surfaces as inactive or active forms (28, 29). Over expression and/or enhanced activation of integrins can slow cell migration by increasing adhesion strength whereas reduced expression and/or failure to fully activate integrins can reduce adhesive strength and enhance migration. Using time-lapse migration studies we found that sdc-1 null dermal fibroblasts migrated faster than wt fibroblasts and had altered responses to addition or neutralization of TGFβ1 despite similarities in the regulation of αSMA expression in the two genotypes of fibroblasts. Analyses of integrin surface expression and localization as well as time lapse studies integrin antagonists prompt us to conclude that the accelerated migration rate in the sdc-1 null fibroblasts is due to differences in the regulation of expression and activation of αv-containing integrin heterodimers.
Materials and Methods
Antibodies Used
For immunoblots we used the following antibodies: αv integrin (AB1930; Chemicon International, Temecula, CA 92950), α5 integrin (5H10; BD Biosciences, San Jose, CA 95131). The β1 integrin antibody was an affinity purified rabbit polyclonal antibody made against the human cytoplasmic domain peptide (30). For α–smooth muscle actin (αSMA) the monoclonal A2547 (Sigma-Aldrich, St. Louis, MO 63103) was used. For microscopic localization of proteins, the antibodies listed above for biochemical analyses were used. For TGFβ1 neutralization studies, the antibody was obtained from R&D Systems (AB-101-NA; Minneapolis, MN 55413) and was used at 10 ng/mL. For BrdU analyses, we used the BrdU in situ labeling and detection kit 1 (11 296 736 001; Roche Applied Science) as specified by the manufacturer with modifications we have previously described (31).
Primary Mouse Fibroblast Cell Culture
The procedures used to obtain cells from mice were authorized and approved by the GWUMC Institutional Animal Care and Use Committee. Wt mice (BALB/c) were obtained from NCI-Frederick. Construction of sdc-1 deficient mice was described previously (7). Primary mouse fibroblasts were isolated from skin of newborn BALB/c or sdc-1 null mice as reported previously (29). For each experiment, primary fibroblasts were grown in DMEM with 10% serum, for the times indicated. For studies involving replating of fibroblasts onto specific matrix proteins, tissue culture plates were coated with either 10 mg/ml human plasma fibronectin (354008; BD Pharmingen) or 10 mg/ml human vitronectin (354238; BD Pharmingen) for 15 minutes at 37°C. For immunofluorescence microscopy, multi-well glass slides were coated overnight at 4°C with the indicated matrix ligands and then shifted to 37°C for 1 hr prior to replating cells.
Immunoblots
Wt or sdc-1 null fibroblasts were cultured from day1 to day 5 and fed every other day. 250 ml of M-Per protein extraction reagent (78503; Pierce Chemical Company, Rockland, IL 61105) with proteinase inhibitor (1: 100 dilution) (78415; Pierce Chemical Co.; inhibitor cocktail) was added to each of the 10 cm cell culture dishes, and the cells were harvested by scraping. Equal amounts of total protein of each extract were loaded to the 4-20% gel (EC6025BOX; Invitrogen,) and SDS-PAGE electrophoresis was performed at 140V. The proteins were transferred to PVDF membrane (IPVH15150; Millipore) at 300 mA for 1.5 hour, and the blot was blocked in blocking solution (TBS with 0.1% Tween 20 (TBST) and 10% milk) overnight at 4°C. Blots were subjected to ECL reaction (RPN2132; Amersham/GE Healthcare Services, Piscataway NJ, 08855), and chemiluminescence was detected using x-ray film. When appropriate, data were quantified using NIH Image.
Surface Biotinylation of Integrins
For biotinylation of cell surfaces, fibroblasts were grown in standard media for 3 days. Biotinylation was performed on adherent fibroblasts using EZ-Link NHS-Biotin (20217; Pierce Chemical Co.) as recommended with the exception that the biotin concentration was 0.5 mg/ml in PBS, and the reaction took place at 4°C for 1 h. To assure that integrins at the fibroblast basal surface of adherent cells were biotinylated efficiently, experiments were performed comparing integrin biotinylation in adherent and suspended fibroblasts and no differences were observed. After labeling, fibroblasts were washed briefly in PBS, their proteins extracted, and integrins were immunoprecipitated as described in (15). The efficiency of biotinylation was accessed by running 50 and 100 ng of total biotinylated protein extract from wt and null cell extracts, transferring the proteins to filters, and detecting biotin using horse radish peroxidase (HRP)-conjugated avidin (170-6528; Biorad, Hercules, CA). For all of the data presented, the biotinylation efficiencies were confirmed to be similar between wt and null keratinocytes. Experiments were repeated a minimum of 3 times on three separate sets of cells.
Immunofluorescence microscopy
Fibroblasts were replated onto uncoated or onto VN or FN coated glass 4 well chamber slides (154526; Lab Tek II Chamber Slide System, Nalge Nunc International Corp Naperville IL 60563) for either 4 or 16 h. The cells were then washed briefly and fixed either in 1) 4% paraformaldehyde in PBS for 20 m at room temperature and then permeabilized using 0.1% triton ×100 in PBS for 10 m or 2) in ice cold 50% methanol:50% acetone for 2 m followed by 20 m in 100% methanol. Indirect immunofluorescence was performed as described previously (Stepp, et al., 2002). For cytoskeleton and focal adhesion staining, the paraformaldehyde fixed cells were stained simultaneously with phalloidin (A-12379; AlexaFluor 488 phalloidin, Invitrogen) and antibody against vinculin (MAB3574; Chemicon/Millipore). For α5 and αv integrin immunolocalization, the methanol:acetone fixed cells were used with the appropriate integrin antibodies. Nuclei were visualized using DAPI (D21490; Invitrogen). Images were acquired at 40× magnification using a Nikon Fluorescent microscope equipped with a RT-Slider SPOT Camera. Adobe Photoshop 7.0 was used to manage images.
Cell Spreading Studies
For cell spreading studies day 3 cultured wt and sdc-1 null fibroblasts were harvested using 0.25% trypsin-EDTA (25200-056; Invitrogen), trypsin activity inhibited by addition of serum, and cells were resuspended in fibroblast media. Cell spreading was determined at various times after replating cells on plastic wells. Cells were fixed as described above for phalloidin studies and stained with an antibody recognizing actin (MAB 1501R, Chemicon/Millipore) followed by and an anti-mouse Alexa-488 conjugated secondary antibody (A11001; Molecular Probes/ Invitrogen) to visualize the cytoplasm and then low magnification images were obtained. The areas of no fewer than 100 cells were determined using Image Pro Plus software; experiments were performed 3 times and data expressed in arbitrary units.
Time-Lapse Studies
Cells were seeded on 24 well plates and allowed to grow for 2 days before imaging on an Olympus IX81 research microscope (Olympus America, Melville, NY 11747) equipped with a Proscan motorized stage (Prior Scientific Instruments Ltd., Rockland, MA 02370) and placed in a temperature and CO2 controlled chamber (LiveCell Incubation System, Neue Biosciences, Camp Hill PA, 17011). Using relief-contrast optics, 10× images were taken per well every 10 min for 16 h 40 m (100 images). For each variable, triplicate wells were tracked and images managed using Slidebook 4.0 Digital Microscopy Solutions software (Intelligent Imaging Innovations, Denver CO, 80216). Images were transferred to a workstation equipped with Metamorph image analysis software (Molecular Devices Corporations, Chicago, IL) where velocities of 20 cells were calculated using the track cell module. A visual basic program was written to allow us to choose the cells to track randomly from each field and to assist in data analysis. From each cell tracked, an average velocity, net displacement and total displacement were determined. The processive index (net displacement/ total displacement) was also calculated. To verify that there was no change in velocity over time for each experiment, we routinely assessed velocity over time for each cell tracked. For experiments involving measurement of cell velocity after replating, wt and sdc-1 null cells were initially plated out onto both 100 mm dishes for harvesting and onto 24 well plates for tracking.
For studies requiring blocking of integrin function using neutralizing antibodies, cells were treated at day 2 and tracked overnight. For the studies requiring addition of TGFβ1, cells were treated with 10ng/ml TGFβ1 at day 3 (30) and tracking was done 24 hours after treatment (day 4). Harvesting for immunoblot analysis was done 48 hours after TGFβ1 treatment (day 5). For the TGFβ1 neutralizing antibody studies, cells were also treated with antibody at day 3 and tracked at day 4.
Statistics
Data were subjected to statistical analyses using InStat software (Graphpad Software, Inc., San Diego, CA). Unless indicated, differences between groups were determined by two tail unpaired t-tests and data were considered significant for p values of less than or equal to 0.05. When standard deviations between groups were not equal, significance was determined using Welch corrected unpaired t tests and when the distributions of values within a data set were not Gaussian, significance was determined using the Mann-Whitney test. Bar graphs were generated using means and standard error of the mean (SEM) values.
Results
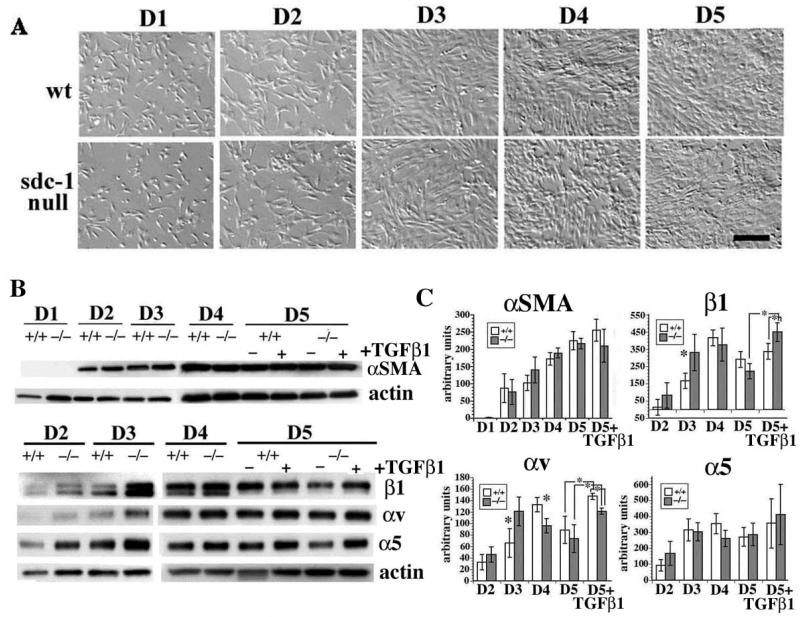
Although differences in αSMA expression were not seen, at day 3 sdc-1 null fibroblasts had increased integrin expression
To examine the activation of sdc-1 null fibroblasts, we analyzed the growth characteristics of the wt and sdc-1 null fibroblasts in primary cell culture. Dermal fibroblasts were prepared and equivalent numbers plated onto uncoated tissue culture plastic and maintained in DMEM medium supplemented with 10% serum. Presented in Figure 1A are relief contrast images of the fibroblasts; wt and sdc-1 null fibroblasts show typical fibroblast morphology. We then determined whether the primary wt and sdc-1 null fibroblasts had similar expression of activation markers αSMA, β1, α5, and αv integrins as a function of time after plating and addition of TGFβ1. Data are presented in Figure 1B and quantitated in 1C. We observed increased expression of αSMA in both wt and null fibroblasts as a function of time with levels increasing at each time point from day 1 to day 4; from day 4 to day 5, αSMA levels remained high and did not increase significantly; there was no difference in either the timing of the increase or in the amount of αSMA produced by the wt and the sdc-1 null fibroblasts. Likewise, for both genotypes, fibroblasts treated with TGFβ1 at day 3 showed no significant change in the expression of αSMA at day 5.
Figure 1. Primary cell cultures of sdc-1 null fibroblasts show transient up-regulation of total integrin expression compared to wt fibroblasts.
A. Relief contrast images of primary fibroblasts taken after the indicated number of days in culture showed that wt and sdc-1 null fibroblasts reach confluence between days 3 and 5. Bar = 15 μm. B. Immunoblot analyses of αSMA, as well as β1, αv, and α5 integrins with actin levels serving as loading controls. C. Quantification of the data presented in B using NIH-Image. A minimum of 3 different sets of fibroblasts were used for each protein quantified. Asterisks in C indicate data found to be significant at p <0.05 by Student’s t test.
Expression of β1, αv, and α5 integrins, expression in wt and null primary fibroblasts was below detectable at day 1. At day 2 in wt cells, β1- and αv-integrins increased, with expression of both peaking at day 4 at levels 4-8 fold higher than seen at day 2 before dropping slightly at day 5. TGFβ1 treatment at day 3 and analysis at day 5 showed an increase in αv integrin but β1 integrin levels did not vary after TGFβ1 treatment in wt cells. The sdc-1 null fibroblasts expressed higher levels of all three integrins assessed compared to wt cells at day 2 but these differences were not statistically significant. The peak level of expression of β1 and αv integrins in sdc-1 null cells was at day 3 with levels 2-5 fold higher than at day 2. Also, compared to wt cells, at day 3 there was significantly more β1 and αv integrin produced by the null fibroblasts but by days 4 and 5, these differences were no longer observed. When the sdc-1 null fibroblasts were treated with TGFβ1 and integrin expression assessed at day 5, they expressed significantly higher levels of β1 integrin compared to treated wt cells. By contrast, TGFβ1 -treated wt fibroblasts, increased expression only of αv integrin. In wt and sdc-1 null cells, α5 integrin was expressed similarly and showed an expression pattern distinct from those of β1 and αv integrins. It peaked at day 2 and remained at the same level regardless of genotype, time point or TGFβ1 treatment. Since we see similar levels of expression of αSMA, it appears that sdc-1 null fibroblasts are activated similar to wt fibroblasts. However, the fact that the sdc-1 null cells upregulate both families of integrins after TGFβ1 treatment suggests important differences in cell signaling between the two genotypes.
Syndecan-1 null fibroblasts migrate faster and have altered TGFβ1-mediated migration compared to wt fibroblasts
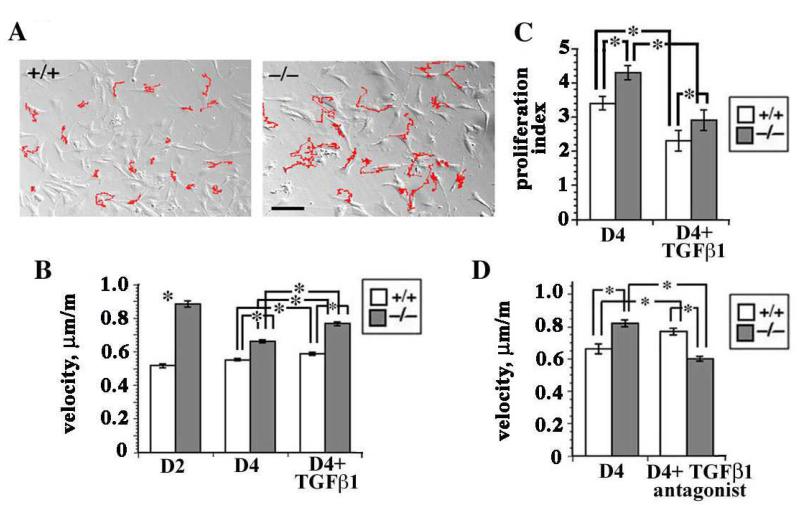
In Figure 1 we show that as dermal fibroblasts adapt to cell culture, they dramatically upregulate their expression of integrins, by day 3 the sdc-1 null cells expressed more αv- and β1-family integrins relative to wt cells, and while TGFβ1 treatment increased only αv integrin expression in wt cells, it increased both αv- and β1-family integrins in sdc-1 null cells. To determine if these differences in integrin expression and TGFβ1 responsiveness impact the ability of the cells to migrate spontaneously in cell culture, time lapse studies of randomly migrating wt and sdc-1 null fibroblasts were conducted. Shown in Figure 2A, the tracks traveled (in red) by the null fibroblasts were longer and the average velocities, shown in Figure 2B, were greater at days 2 and 4 than those of wt fibroblasts.
Figure 2. Primary sdc-1 null fibroblasts migrate faster than wt cells.
A. Time lapse studies of wt and sdc-1 null fibroblasts. The tracks (indicated in red) for the null fibroblasts were longer than those for the wt fibroblasts suggesting that the null cells were migrating faster. Bar = 10 μm B. The sdc-1 null cells migrate faster at days 2 and 4 as well as after TGFβ1 treatment; wt cells had similar velocities regardless of time point or TGFβ1 treatment. C. Proliferation indices showed that at day 4, the sdc-1 null fibroblasts proliferated faster than wt cells. Both genotypes responded to addition of TGFβ1 by reducing their proliferation rate. D. Antagonizing TGFβ1 activity using a TGFβ1 function blocking antibody inhibited null cell migration but had the opposite affect on wt cells. Data in B-D indicate mean values +/− SEMs for a minimum of 60 individual cells for each variable investigated; asterisks show data that were significantly different with p < 0.05 as determined using the student’s t test.
We showed previously in keratinocytes that TGFβ1 treatment altered cell proliferation and migration rates and enhanced total and surface integrin expression and cell migration rates (15). When we assessed fibroblast proliferation using BrdU in untreated as well as TGFβ1 treated cells at the same time points that cell migration was assessed, we found that the proliferation index in untreated sdc-1 null fibroblasts was significantly higher than in wt fibroblasts and TGFβ1 reduced the proliferation index of both wt and sdc-1 null dermal fibroblasts (Figure 2C). However, the sdc-1 null cells proliferated faster than the wt cells both before and after TGFβ1 treatment. 24 hours after treatment of day 4 cells with 10 ng/ml TGFβ1, the migration rates for sdc-1 null fibroblasts increased by 26% from 0.62 to 0.78 μm/m; the increase in wt fibroblasts was only 7%, from 0.54 to 0.58 μm/m (Figure 2B). Although both of these increases in cell migration were significant, the larger increase seen in the sdc-1 null cells suggests that cells without sdc-1 were more responsive to the cytokine.
The ability of cells to respond to exogenous, active TGFβ1 helps assess one aspect of TGFβ1-mediated signaling. Other important events known to mediate signal transduction include activation of latent, endogenously produced TGFβ1 at cell surfaces and binding of the activated growth factor to its receptor. To determine whether there are differences in endogenous TGFβ1-mediated cell migration between wt and sdc-1 null fibroblasts, we added a well characterized TGFβ1-neutralizing antibody to cultured cells at day 3 and 24 h later assessed cell migration rates; controls included fibroblasts incubated with a control antibody as well as untreated fibroblasts (Figure 2D). Neutralizing TGFβ1 activity in wt fibroblasts caused a significant increase in cell velocity of 20%; however the same treatment had the opposite effect in sdc-1 null fibroblasts decreasing null cell velocity by 26% to levels that were similar to untreated wt fibroblasts.
The increase in sdc-1 dermal fibroblast cell migration persists after replating cells onto uncoated or FN-coated tissue culture plastic but not onto VN
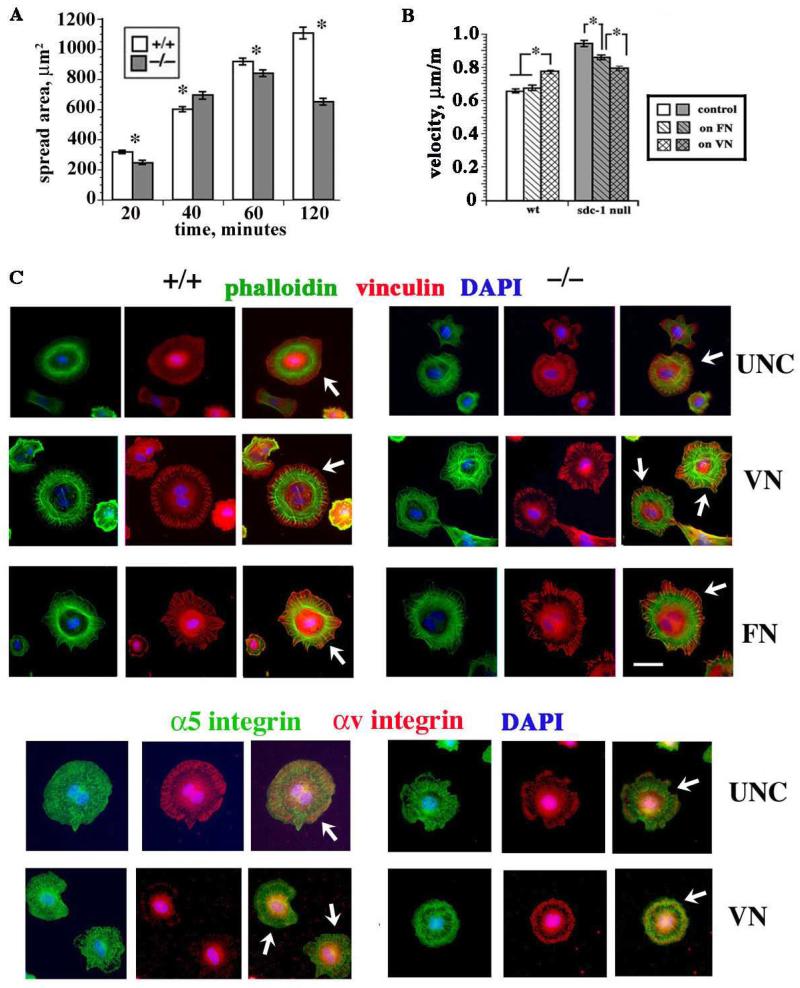
As seen in Figure 2B, a significant drop in cell migration rate was seen between days 2 and 4 in the sdc-1 null fibroblasts. This drop could be due to accumulation of matrix component(s) that slow their velocity or differences in expression and activation of integrins in sdc-1 null fibroblasts. We next performed cell adhesion and spreading assays on day 3 fibroblasts. Similar adhesion of wt and sdc-1 null fibroblasts to several different matrix ligands including collagen types I and IV, FN, and VN (data not shown) was observed. We then looked specifically at cell spreading from 20 to 120 m after replating. While both genotypes of fibroblasts spread at similar rates over the first 60 m, by 120 m, the wt fibroblasts were 1.8× more spread than were the null fibroblasts (Figure 3A).
Figure 3. Replating wt and sdc-1 null cells revealed differences in spreading, cell migration rates, and focal adhesions.
A. Cell spreading assay on cells replated on uncoated surfaces showed that by 120 min the wt fibroblasts were significantly more spread than the null fibroblasts. For each variable, the area of a minimum of 200 cells were measured after fixing and staining cells with an antibody against actin. B. Average velocities of both wt and sdc-1 null cells increased proportionately after replating onto uncoated tissue culture plastic. However, the sdc-1 null fibroblasts migrated faster than the wt cells except when replated on VN. Data in A and B show mean +/− SEMs; asterisks show data that are significantly different with p < 0.05 as determined using the Student’s t test in A and Mann Whitney test in B. C. Cells were replated on the indicated substrates and allowed to spread for 4 h and stained with phalloidin for F-actin or with the indicated antibodies. Arrows in the merged images indicate margins of cells where vinculin and αv-integrin containing focal adhesions form. Bar = 10 μm.
We then did time lapse studies after replating equal numbers of day 3 wt and sdc-1 null fibroblasts onto either tissue culture plastic or onto dishes that had been coated with 10 μg/ml FN or VN. Replating cells onto high concentrations of FN induces cells to use their fibronectin receptors, α5β1 integrin primarily in dermal fibroblasts, for their initial adhesion and migration whereas replating cells onto VN causes cells to use their VN receptors, primarily αvβ3 and αvβ5 integrins. As shown in Figure 3B, wt fibroblasts migrated faster after replating on either uncoated tissue culture plastic or FN coated dishes than they did after 4 days of continuous culture (compare data in Figure 3B to velocities in Figure 2B) and they migrated even faster on VN. Likewise, the sdc-1 null cells migrated faster after replating on tissue culture plastic compared to continuous culture but, unlike wt cells, they migrate slower on FN and even slower on VN. On VN, both genotypes migrated at the same rate.
When cells are placed in suspension during replating, cells become spherical and the well-ordered filaments that make up their cytoskeleton partially disassemble. Since replating cells induces rapid cytoskeletal reassembly, the slower rate of cell spreading seen for the null cells in Figure 3A suggested differences in the ability of wt and sdc-1 null cells to reassemble their cytoskeleton after replating. Cells were allowed to spread for 4 or 16 h after replating on uncoated, FN, or VN coated surfaces. After fixing, the cells were labeled for either F-actin (phalloidin) and focal adhesions (vinculin) or for one of the two components of a fibronectin receptor (α5 integrin) and vitronectin receptors (αv integrin). Data are presented in Figure 3C for cells 4 h after replating and in Figure 4 for cells 16 h after replating.
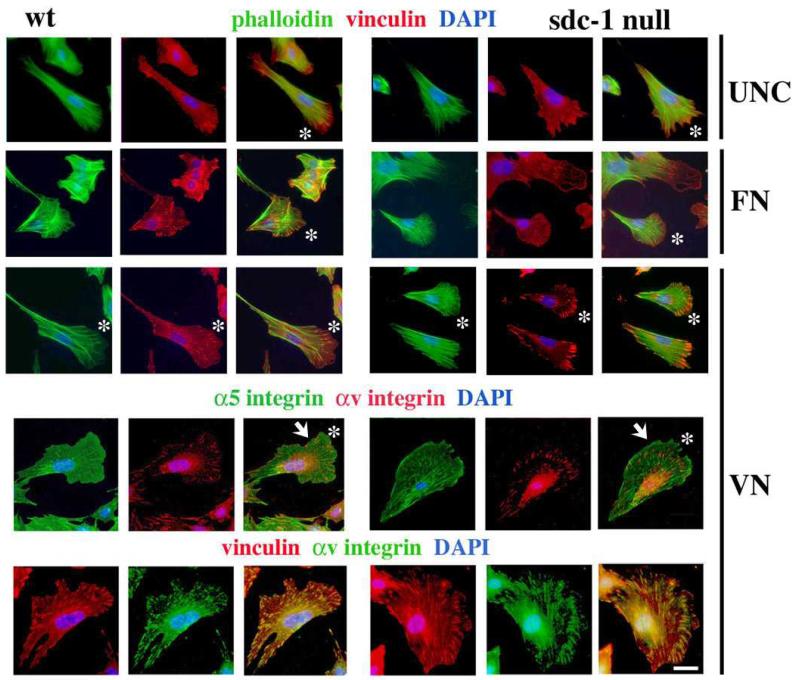
Figure 4. 16 hr after replating, vinculin and αv integrin containing focal adhesions were larger in sdc-1 null cells replated on VN.
Cells were replated on the indicated substrates and allowed to spread for 16 h prior to fixation and staining with phalloidin for F-actin or with the indicated antibodies. Asterisks in the merged images indicate leading edge lamellopodia. Note that when dermal fibroblasts are replated onto VN, focal adhesions are larger for the sdc-1 null cells compared to the wt cells (white arrows). Bar = 10 μm.
After 4 h (Figure 3C), dermal fibroblasts have spread and their actin cytoskeleton has partially reassembled. Compared to cells spreading on VN and FN, cytoskeletal reassembly was delayed on uncoated surfaces for both wt and sdc-1 null cells. Although the sdc-1 null cells spread slowly on uncoated surfaces, consistent with the data presented in Figure 3A, their actin cytoskeleton was better assembled and more vinculin was recruited to cell margins than seen in the wt cells. On VN and FN, for both wt and sdc-1 null cells, F-actin was localized to well-organized actin filaments occupying the space around the nucleus and occasionally inserted into vinculin-containing focal adhesion complexes at cell margins. In both wt and sdc-1 null cells, α5 integrin was distributed throughout the cytoplasm at 4 h after replating onto uncoated surfaces but αv integrin was recruited to focal adhesions at the cell periphery in both cell genotypes. Vinculin accumulated within focal adhesions in both wt and sdc-1 null cells after 4 h on VN and FN but αv integrin was sparse at cell margins in the wt cells on VN but not in the sdc-1 null cells (white arrows, Figure 3C). On wt cells replated onto uncoated surfaces, which contain low concentrations of VN and FN adsorbed from serum, αv integrin was assembled into larger focal adhesions compared to cells replated onto surfaces coated with high concentrations of VN. By contrast, sdc-1 null cells assembled large focal adhesions when replated on both uncoated surfaces and on surfaces coated with high concentrations of VN. Results for localization of α5 and αv integrin on FN-coated surfaces after 4 h were similar to those on uncoated surfaces with αv integrin prominent at focal adhesions (data not shown).
Shown in Figure 4 are cells stained for F-actin and vinculin after plating on uncoated, FN, and VN coated surfaces for 16 h. In order to minimize variability and to facilitate comparisons, we focused on cells having well-developed lamellopodia (white asterisks, Figure 4) with nuclei displaced towards their rear. Both the wt and sdc-1 null cells had elaborate F-actin networks on all three surfaces but there were differences in their assembly of focal adhesions which were substrate specific. For wt cells, focal adhesions were less well developed when cells were plated on VN compared to FN and uncoated surfaces (white arrows, Figure 4). For sdc-1 null cells, focal adhesions were more poorly developed in cells plated on FN, better developed on uncoated surfaces, and most developed on VN.
On VN coated surfaces, in wt cells, α5 integrin was diffusely localized within the extended lamellopodium all the way out to the leading edge; in sdc-1 null cells, α5 integrin was more filamentous within the lamellopodium and accumulated at the tip of the leading edge. As expected, αv integrin was detected in focal accumulations at the cell margins suggesting it’s presence in focal adhesions. Co-labeling of cells plated on VN for 16 h for vinculin and αv integrin showed colocalization of these proteins confirming that the sites of αv integrin localization are focal adhesions.
These results indicate that sdc-1 null cells assembled αv integrin-containing focal adhesions when replated on VN as early as 4 h and they did so at least as fast or faster than wt cells. The sdc-1 null cells migrated slower on surfaces coated with high concentrations of VN than they did on uncoated or FN coated surfaces; matrix-dependent slower migration correlated with larger focal adhesions. Wt cells migrated fastest on surfaces coated with high concentrations of VN; these more rapidly migrating cells had smaller focal adhesions. Despite the similarity between the velocities of wt and sdc-1 null cells replated onto VN coated surfaces (Figure 3B), their organization of αv-integrin and vinculin within focal adhesions was distinctly different.
Antagonizing the functions of both αv and β1 integrins in wt and sdc-1 null cells revealed differences in the ability of their integrins to mediate cell migration
At day 3 when sdc-1 cells migrated faster than wt cells, expression of both β1- and αv-integrin were both enhanced in sdc-1 null fibroblasts. To determine if the activity of integrins at the cell surface was also altered, wt and sdc-1 null fibroblasts were treated at day 3 with 25 μg/ml of two different integrin neutralizing antibodies (9EG7 against β1 and RMV-7 against αv) alone and in combination and migration rates were assessed 24 hr later. Both the αv- and β1-integrin targeted antibodies bind to integrin heterodimers on cell surfaces in their inactive and active conformations. The antibody antagonists affect only integrins on media-exposed plasma membrane. They cannot access integrins at the basal surface where integrins are bound to their underlying matrix and are transiently protected from the antagonist. However, cells are in constant motion in cell culture and basal membrane domains exchange rapidly with apical domains. Since antibody:integrin complexes are quickly internalized and sent to lysosomes for degradation, adding integrin antagonists to adherent cells growing in culture does not cause cell detachment but instead reduces the amount of a given integrin at the cell surface. Addition of the integrin antagonists can have several different effects on cells. If either the total number of integrin heterodimers and/or the activity of those integrins were above the optimal level required for supporting cell migration prior to addition of the antagonist, then adding the antagonist would cause cells to migrate faster. If the activity and/or amount of the integrins were below the optimal level, cells would migrate slower after treatment with an antagonist. Finally, if the activity and/or number of integrins targeted were in the optimal range for supporting cell migration, the antagonist would have no impact or only a slight effect on migration rates. Data presented in Figure 5A shows that compared to control IgG, wt fibroblasts treated with αv, β1, or both integrin antagonists increased their cell migration rate. This result suggests that, in wt cells, both classes of integrins are expressed and functioning at levels above those optimal for cell migration thus rendering the cells too adhesive. When levels of αv, β1, or both integrin families are reduced, cells were less adhesive and they migrated faster.
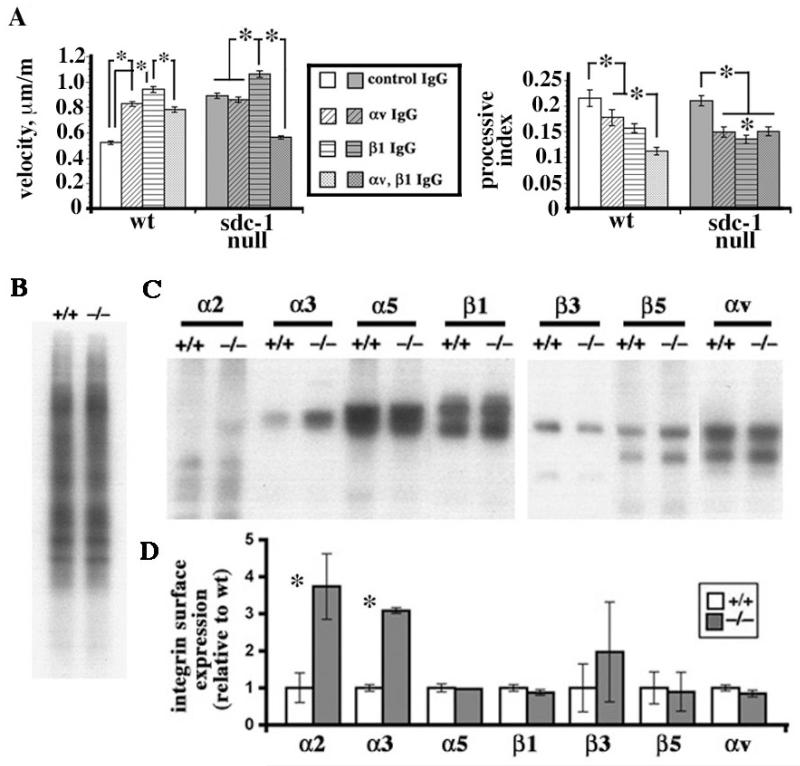
Figure 5. Integrin activation at cell surfaces is reduced in sdc-1 null cells compared to wt cells.
A. Addition of β1 and/or αv integrin function blocking antibodies alone or in combination to wt fibroblasts at day 2 in culture resulted in increased wt cell migration rates compared to IgG treated controls. Addition of β1 neutralizing antibodies alone increased null cell migration whereas addition of the αv antagonist had no affect. Adding both antibodies to the sdc-1 null cells significantly reduced their migration rate. The processive indices (PIs) of control wt and sdc-1 null cells were the same. However, the PI values for both wt and sdc-1 null cells dropped after addition of the β1 and/or αv integrin function blocking antibodies either alone or in combination. Data show mean velocities or mean PI values +/− SEMs; asterisks indicate data that were significant at the p < 0.05 levels using the Mann Whitney test. B. Biotinylated wt and sdc-1 null cells were normalized by protein level and their biotinylation efficiencies determined by running equal aliquots of total biotinylated cell extracts onto gels. There was no difference in the biotinylation efficiency of the wt and sdc-1 null fibroblasts. C. Equal aliquots of biotinylated cell extracts were immunoprecipitated using the antibodies against the indicated integrin subunits. Since biotin does not incorporate uniformly in the different integrin protein subunits, the α and β subunits that make up the integrin heterodimers are not apparent in each immunoprecipitation. Data were quantitated in D. Three independent sets of fibroblasts were used for these studies and experiments were repeated three times. Only α2β1 and α3β1 are expressed at elevated levels in the sdc-1 null cells.
Sdc-1 null fibroblasts treated with the inhibitory β1 integrin antibody migrated slightly faster than control IgG treated cells; thereby indicating that β1 integrin containing heterodimers were expressed and functioned at activities above the optimal level for mediating cell migration in sdc-1 null cells. The addition of the αv antagonist alone had no significant impact on the rate of sdc-1 null cell migration indicting that the levels and/or activities of the αv-containing integrins were in the optimal range for supporting cell migration. Compared to wt cells, the αv-integrins on sdc-1 null cells functioned to promote rapid migration. Although the migration rate of the sdc-1 null cells was maintained after reduction of the levels of either αv- or β1- integrin, when levels of both classes of integrins were reduced simultaneously, migration rates reduced for the sdc-1 null cells but were elevated for similarly treated wt cells. Thus, the overall activity of αv-integrins on the surface of sdc-1 null cells was less than that of wt cells.
Another way of assessing the roles of integrins in mediating cell migration is to evaluate the persistence index (PI) rather than the cell velocity. PI values assess net cell displacement/total cell displacement, thus giving a measure of the cell directionality, or processivity, and several recent studies show that PI is regulated primarily by β1 integrins via small GTPases, particularly Rac (30, 31). Calculations of PI values for the migration of wt and sdc-1 null cells after replating on uncoated tissue culture plastic in the presence of control IgG showed no significant differences (Figure 5B). In the wt cells, antagonizing integrin function using either αv- or β1- antibodies reduced PI values and using both inhibitors had a cumulative affect on PI values. In cells lacking sdc-1, αv- and β1- integrin antagonists also reduced PI values but when both integrin antagonists were added together, the reduction in PI was not cumulative as it was for the wt cells but identical to that seen with either inhibitor alone.
Despite the reduced activity of the αv-containing integrins on the sdc-1 null cells, their expression at the cell surface was equal to that in wt cells
Analyses of surface expression of mouse fibroblasts by flow cytometry would limit the integrin subunits analyzed to those for which extracellular domain directed antibodies recognizing specific mouse integrins exist. To study differences in integrin expression on fibroblast surfaces, we used an approach we successfully used for analysis of keratinocyte surface integrin expression (15). Day 3 wt and sdc-1 null fibroblast surfaces were biotinylated, proteins extracted, samples normalized based on protein content and biotinylation efficiency. Equivalent amounts of wt and sdc-1 null biotin-labeled cell extracts were immunoprecipitated using antibodies against α2, α3, α5, β1, αv, β3 and β5. Immunoprecipitated proteins were run onto gels, transferred to nitrocellulose, and the surface labeled proteins visualized with HRP-conjugated strepavidin. Data are presented in Figure 5B and show that although the levels of α2β1 and α3β1 integrins on the surface of sdc-1 null cells were ~3-fold higher than those of wt cells, the levels of αvβ3, αvβ5, and the major β1-family integrin on fibroblasts, α5β1, were the same on the wt and sdc-1 null cells. Thus, the reduced integrin activity in sdc-1 null cells using integrin antagonists was not due to reduced expression of integrins at the cell surface.
Discussion
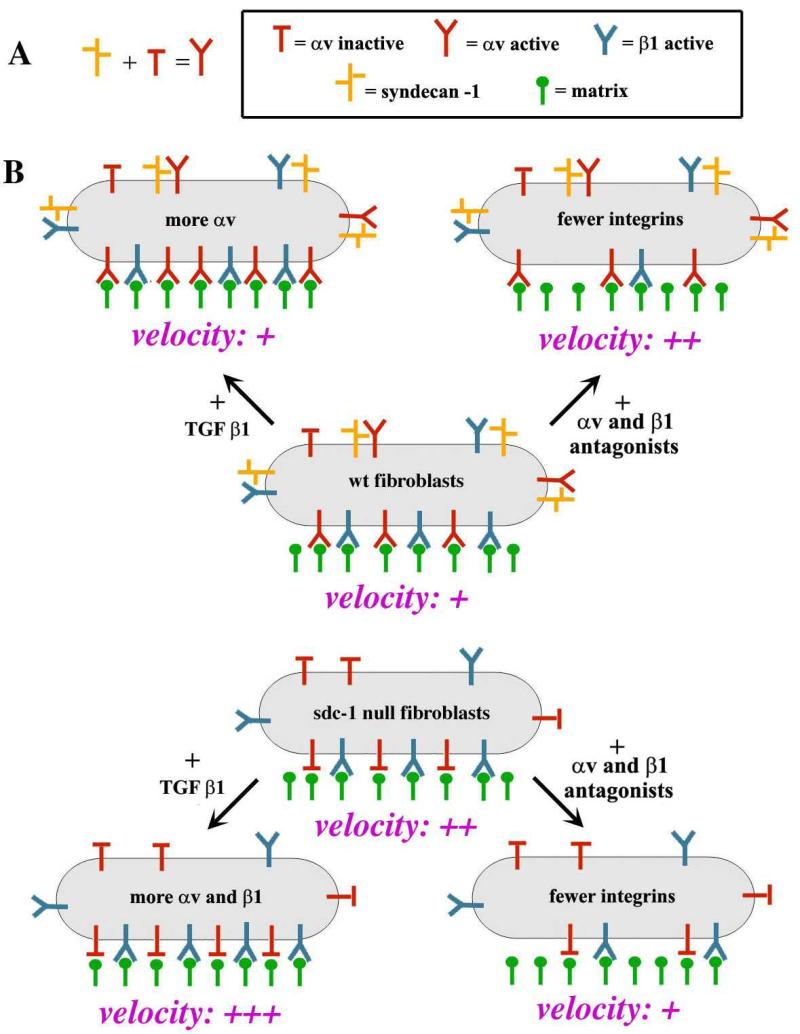
Syndecan-1 null primary dermal fibroblasts, like wt fibroblasts, proliferate well in culture and increase their expression of αSMA and integrins with similar kinetics. However, they show differences in migration, TGFβ1 responsiveness, and integrin expression and activity compared to wt cells. Exogenous TGFβ1 treatment increased αv-family integrin expression but enhanced the difference between wt and sdc-1 null cell migration rates. The reduced integrin activity in sdc-1 null cells was not caused by reduced expression of integrins at the cell surface and suggests that sdc-1 functions to mediate the conversion of αv-containing integrins from a less to a more active state on the surface of cells expressing them. These data are summarized in the model presented in Figure 6. Optimal migration speed during wound healing depends on the proper balance between adhesion and detachment (33, 34). Placing quiescent fibroblasts from the unwounded dermis in culture in the presence of serum caused a rapid 4-8 fold increase in integrin expression over a 5 day period. Our time lapse studies using integrin antagonists to assess cell migration showed that the activity of β1-family integrins in both wt and sdc-1 null cells tipped the balance in favor of slower migration rates and suggests that β1-family integrins are in an active state on sdc-1 null cells.
Figure 6. Model schematizing the differences in integrin activation on wt and sdc-1 null cells.
A. Data suggest that sdc-1 interacts either directly or indirectly with αv-containing integrins to increase their activation at the surface of fibroblasts. B. wt and sdc-1 null fibroblasts have similar surface localization of the major fibroblast integrins including α5β1 as well as αvβ3 and αvβ5. Treating fibroblasts with TGFβ1 increased αv-integrin expression in wt cells and enhanced migration rates by only 7% but in sdc-1 null fibroblasts, increased both αv- and β1- integrin expression and enhanced cell migration rates by 26%. In sdc-1 null cells, TGFβ1 increased overall expression of integrins but did not alter their activation state and cells migrated faster. Adding both αv- and β1- integrin antagonists reduces expression of integrins at the cell surface and caused wt cells to migrate faster by 43% and sdc-1 null cells to migrate 47% slower.
The role played by αv integrins in mediating wt and sdc-1 null fibroblast migration is more complex. In wt cells, αv integrins are present on cell surfaces at high levels and are active in mediating cell migration; adding the αv-antagonist interferes with αv-integrin expression and slows their migration. The same was not true in the sdc-1 null cells. Reducing the expression of αv-family integrins had no affect on sdc-1 null cell migration rates unless αv- and β1-family integrin levels were reduced simultaneously and even then, the impact of these reductions on cell migration rates were opposite to those seen in wt cells and the sdc-1 null cells migrated slower.
We have used two integrin antagonists in this study that impact entire classes of integrins. Fibroblasts express several different β1 family integrins including α1β1, α2β1, α3β1, α5β1, α10β1, and α11β1 but the dominant β1-containing integrin on primary mouse dermal fibroblasts is α5β1 (20). Dermal fibroblasts also express at least two different αv containing integrin heterodimers including αvβ3 and αvβ5. On both wt and sdc-1 null fibroblasts, αvβ5 was the major αv integrin present on the fibroblast surface (Figure 5C). Antagonizing αv- and/or β1- integrin function, will therefore affect the overall profile of integrins expressed on the cell surface.
The exact function(s) of syndecan-1 on fibroblast adhesion and migration has remained poorly understood as compared to syndecans-2 and −4. Sdc-4 has been extensively studied in mesenchymal cells where it localizes to focal adhesions and mediates directional cell migration via modulation of Rac1 and promotion of β1 integrin-dependent adhesion (1, 20, 35). Peptides derived from syndecan-2 promote β1 integrin dependent adhesion in fibroblasts (22), even though, like sdc-1, sdc-2 is not present within focal adhesions. Chen and colleagues have shown that sdc-2 can bind to TGFβ1 receptors and affect TGFβ1 signaling in renal fibroblasts, a finding consistent with a close relationship between syndecans and TGFβ1 signaling (23). Also, syndecan-1 has been shown to be transcriptionally activated after treatment of cells with TGFβ1 via a mechanism that involves protein kinase Cα (36).
We have shown previously in vivo that corneal and skin healing is delayed (7) and in vitro in cultured keratinocytes (15) and here using fibroblasts that cell migration rates are altered when sdc-1 is absent. Since sdc-1 does not localize within focal adhesions in fibroblasts or to the basal aspect of epithelial cells at the basement membrane junction during quiescence or reepithelialization (7), the migration defects reported in sdc-1 null mice and cells derived from them are exerted indirectly.
Adhesion assays have shown that sdc-1 can mediate cell adhesion via αvβ3 and αvβ5 integrins and peptides have been isolated which can interfere with this association via heparan sulfate independent mechanisms (5, 37). αv-family integrins, especially αvβ5 and αvβ6, have been shown to mediate binding and activation of latent forms of TGFβ1 (38). One of the mechanisms used to activate latent TGFβ1 involves binding of a latency associated protein to heparan sulfate side chains (39); our studies indicate that sdc-1 association with αv-containing integrins activates those integrins.
In wt keratinocytes that express α6β4, neutralizing β1 integrins dramatically reduced migration rates (15). We report here that in wt fibroblasts, antagonizing β1 integrin increased cell migration rates by 75% control values and further enhanced sdc-1 null cell migration by 16% control values. Antagonizing β1-integrin in wt and sdc-1 null fibroblasts dramatically reduced processivity from ~0.23 for both wt and null fibroblasts to 0.15 and 0.13 for wt and sdc-1 null fibroblasts respectively. This result is consistent with what is known about the important role played by β1 integrins in mediating directional migration in fibroblasts (33, 34) and demonstrates that sdc-1 null fibroblasts regulate their processivity via β1 integrin. In addition, antagonizing the αv-antibody also reduced both wt and sdc-1 null cell processivity. These results provide the first evidence suggesting that αvβ3 and/or αvβ5 integrin activity can regulate processive cell migration.
Normal wound healing involves interactions between epithelial and stromal cells that are mediated by integrins on their surface as well as by stromal extracellular matrix. The extracellular matrix serves a structural function and provides a reservoir for the storage and regulated release of cytokines important for wound healing. Sdc-1 has been shown to be upregulated in epithelia and stromal cells during wound healing via TGFβ1-induced PKCα activity (36). Keratinocytes and fibroblasts expressing sdc-1 regulate their migration differently than cells lacking sdc-1 and they do so by altering αv-integrin activation and expression.
While we have identified a role for sdc-1 in integrin activation in dermal fibroblasts during wound healing, additional studies are required to fully understand the mechanism used in sdc-1 null cells. We are currently examining the roles played by serum growth factors in mediating the migration differences seen in the sdc-1 null fibroblasts. The conversion of fibroblasts to αSMA positive myofibroblasts is driven by TGFβ1 and other growth factors and by growing cells in low serum, we can develop a better understanding of fibroblast activation and the impact of the loss of sdc-1. Integrin activation can occur via two well-characterized mechanisms termed “inside-out” or “outside in” activation (25, 26). We are currently investigating the mechanism used by sdc-1 to activate integrins by looking at whether recombinant sdc-1 ectodomain can reverse the integrin-mediated defects seen in sdc-1 null fibroblasts. While we show here for the first time important roles for sdc-1 in controlling migration and integrin activation in fibroblasts, more work must be done before a full understanding of how this proteoglycan functions to mediate proper wound healing can be achieved.
Acknowledgements
We would like to thank Caroline Alexander, Christophe Cataisson, Adam Glick, Melinda Larsen, Ulrike Lichti, Alan Rapraeger, and Stuart H. Yuspa and for advice and assistance at various stages of this project. This work was funded by NIH RO1-EY08512-17 and RO1-EY13559-05 (MAS).
References
- 1.Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–28. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 2003;4:926–37. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- 3.Perrimon N, Bernfield M. Cellular functions of proteoglycans--an overview. Semin Cell Dev Biol. 2001;12:65–7. doi: 10.1006/scdb.2000.0237. [DOI] [PubMed] [Google Scholar]
- 4.Beauvais DM, Rapraeger AC. Syndecans in tumor cell adhesion and signaling. Reprod Biol Endocrinol. 2004;2:3. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates αvβ3 integrin activity in human mammary carcinoma cells. J Cell Biol. 2004;167:171–81. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Götte M, Joussen AM, Klein C, Andre P, Wagner DD, Hinkes MT, Kirchhof B, Adamis AP, Bernfield M. Role of syndecan-1 in leukocyte-endothelial interactions in the ocular vasculature. Invest Ophthalmol Vis Sci. 2002;43:1135–41. [PubMed] [Google Scholar]
- 7.Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci. 2002;115:4517–31. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- 8.Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Genet. 2000;25:329–32. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 9.McDermott SP, Ranheim EA, Leatherberry VS, Khwaja SS, Klos KS, Alexander CM. Juvenile syndecan-1 null mice are protected from carcinogen-induced tumor development. Oncogene. 2007;26:1407–16. doi: 10.1038/sj.onc.1209930. [DOI] [PubMed] [Google Scholar]
- 10.Götte M, Bernfield M, Joussen AM. Increased leukocyte-endothelial interactions in syndecan-1-deficient mice involve heparan sulfate-dependent and -independent steps. Curr Eye Res. 2005;30:417–22. doi: 10.1080/02713680590956289. [DOI] [PubMed] [Google Scholar]
- 11.Park PW, Foster TJ, Nishi E, Duncan SJ, Klagsbrun M, Chen Y. Activation of syndecan-1 ectodomain shedding by Staphylococcus aureus α-toxin and β-toxin. J Biol Chem. 2004;279:251–8. doi: 10.1074/jbc.M308537200. [DOI] [PubMed] [Google Scholar]
- 12.Haynes A, 3rd, Ruda F, Oliver J, Hamood AN, Griswold JA, Park PW, Rumbaugh KP. Syndecan 1 shedding contributes to Pseudomonas aeruginosa sepsis. Infect Immun. 2005;73:7914–21. doi: 10.1128/IAI.73.12.7914-7921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanhoutte D, Schellings MW, Götte M, Swinnen M, Herias V, Wild MK, Vestweber D, Chorianopoulos E, Cortés V, Rigotti A, Stepp MA, Van de Werf F, Carmeliet P, Pinto YM, Heymans S. Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation. 2007;115:475–82. doi: 10.1161/CIRCULATIONAHA.106.644609. [DOI] [PubMed] [Google Scholar]
- 14.Rops AL, Götte M, Baselmans MH, van den Hoven MJ, Steenbergen EJ, Lensen JF, Wijnhoven TJ, Cevikbas F, van den Heuvel LP, van Kuppevelt TH, Berden JH, van der Vlag J. Syndecan-1 deficiency aggravates anti-glomerular basement membrane nephritis. Kidney Int. 2007;72:1204–15. doi: 10.1038/sj.ki.5002514. [DOI] [PubMed] [Google Scholar]
- 15.Stepp MA, Liu Y, Pal-Ghosh S, Jurjus RA, Tadvalkar G, Sekaran A, Losicco K, Jiang L, Larsen M, Li L, Yuspa SH. Reduced migration, altered matrix and enhanced TGFβ1 signaling are signatures of mouse keratinocytes lacking Sdc1. J Cell Sci. 2007;120:2851–63. doi: 10.1242/jcs.03480. [DOI] [PubMed] [Google Scholar]
- 16.Elenius K, Vainio S, Laato M, Salmivirta M, Thesleff I, Jalkanen M. Induced expression of syndecan in healing wounds. J Cell Biol. 1991;114:585–95. doi: 10.1083/jcb.114.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and −4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–24. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcox-Adelman SA, Denhez F, Goetinck PF. Syndecan-4 modulates focal adhesion kinase phosphorylation. J Biol Chem. 2002;277:32970–7. doi: 10.1074/jbc.M201283200. [DOI] [PubMed] [Google Scholar]
- 19.Lim ST, Longley RL, Couchman JR, Woods A. Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase Cα (PKCα) increases focal adhesion localization of PKCα. J Biol Chem. 2003;278:13795–802. doi: 10.1074/jbc.M208300200. [DOI] [PubMed] [Google Scholar]
- 20.Whiteford JR, Behrends V, Kirby H, Kusche-Gullberg M, Muramatsu T, Couchman JR. Syndecans promote integrin-mediated adhesion of mesenchymal cells in two distinct pathways. Exp Cell Res. 2007;313:3902–13. doi: 10.1016/j.yexcr.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Klass C, Woods A. Syndecan-2 regulates TGFβ1 signaling. J Biol Chem. 2004;279:15715–8. doi: 10.1074/jbc.C300430200. [DOI] [PubMed] [Google Scholar]
- 23.Munesue S, Yoshitomi Y, Kusano Y, Koyama Y, Nishiyama A, Nakanishi H, Miyazaki K, Ishimaru T, Miyaura S, Okayama M, Oguri K. A novel function of syndecan-2, suppression of matrix metalloproteinase-2 activation, which causes suppression of metastasis. J Biol Chem. 2007;282:28164–74. doi: 10.1074/jbc.M609812200. [DOI] [PubMed] [Google Scholar]
- 24.Massagué J, Cheifetz S, Ignotz RA, Boyd FT. Multiple type-beta transforming growth factors and their receptors. J Cell Physiol Suppl. 1987;5:43–7. doi: 10.1002/jcp.1041330409. [DOI] [PubMed] [Google Scholar]
- 25.Verrecchia F, Mauviel A. TGF-β signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–5. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim LT, Yamada KM. The regulation of expression of integrin receptors. Proc Soc Exp Biol Med. 1997;214:123–31. doi: 10.3181/00379727-214-44078. [DOI] [PubMed] [Google Scholar]
- 27.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 28.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 29.Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sta Iglesia DD, Gala PH, Qiu T, Stepp MA. Integrin expression during epithelial migration and restratification in the tenascin-C-deficient mouse cornea. J Histochem Cytochem. 2000;48:363–76. doi: 10.1177/002215540004800306. [DOI] [PubMed] [Google Scholar]
- 31.Pajoohesh-Ganji A, Pal-Ghosh S, Simmens SJ, Stepp MA. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cells. 2006;24:1075–86. doi: 10.1634/stemcells.2005-0382. [DOI] [PubMed] [Google Scholar]
- 32.Yuspa SH, Ben T, Patterson E, Michael D, Elgjo K, Hennings H. Stimulated DNA synthesis in mouse epidermal cell cultures treated with 12-O-tetradecanoyl-phorbol-13-acetate. Cancer Res. 1976;36:4062–8. [PubMed] [Google Scholar]
- 33.Moissoglu K, Schwartz MA. Integrin signaling in directed cell migration. Biol Cell. 2006;98:547–55. doi: 10.1042/BC20060025. [DOI] [PubMed] [Google Scholar]
- 34.Moissoglu K, Slepchenko BM, Meller N, Horwitz AF, Schwartz MA. In vivo dynamics of Rac-membrane interactions. Mol Biol Cell. 2006;17:2770–9. doi: 10.1091/mbc.E06-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- 36.Hayashida K, Johnston DR, Goldberger O, Park PW. Syndecan-1 expression in epithelial cells is induced by TGFβ through a PKA-dependent pathway. J Biol Chem. 2006;281:24365–74. doi: 10.1074/jbc.M509320200. [DOI] [PubMed] [Google Scholar]
- 37.McQuade KJ, Beauvais DM, Burbach BJ, Rapraeger AC. Syndecan-1 regulates αvβ5 integrin activity in B82L fibroblasts. J Cell Sci. 2006;119:2445–56. doi: 10.1242/jcs.02970. [DOI] [PubMed] [Google Scholar]
- 38.Sheppard D. Integrin-mediated activation of latent TGFβ. Cancer Metastasis Rev. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- 39.Chen Q, Sivakumar P, Barley C, Peters DM, Gomes RR, Farach-Carson MC, Dallas SL. Potential role for heparan sulfate proteoglycans in regulation of transforming growth factor-β (TGF-β) by modulating assembly of latent TGFβ-binding protein-1. J Biol Chem. 2007;282:26418–30. doi: 10.1074/jbc.M703341200. [DOI] [PubMed] [Google Scholar]