Abstract
Mitochondrial genome and functional alterations are related to various diseases including cancer. In all cases, the role of these organelles is associated with defects in oxidative energy metabolism and control of tumor-induced oxidative stress. The present study examines the involvement of mitochondrial DNA in cancer and in particular in breast cancer. Furthermore, since mitochondrial DNA is maternally inherited, hereditary breast cancer has been focused on.
Keywords: Mitochondrial DNA, Hereditary breast cancer, DNA variations, Polymorphism, Mitochondrial haplogroup.
INTRODUCTION
Mitochondria are small cytoplasmic organelles which produce cellular energy in the form of ATP molecules by oxidative phosphorylation (OXPHOS) and contain several copies of mitochondrial DNA (mtDNA). The mitochondrial genome, a 16,569 bp circular double-stranded molecule, encodes for 2 ribosomal RNAs (rRNAs), 22 transfer RNAs (tRNAs) and 13 protein coding genes (PCGs) [1]. mtDNA is more strongly subjected to nucleotide modifications than the nuclear genome, showing a rate supposed to be from 10 to 20 times higher than nuclear DNA [2, 3]. It was recently suggested that the high mutation rate in mtDNA may result from the small effective population size which is associated with effectively haploid inheritance [4]. An important aspect of these mutations is mtDNA organization within the dynamic mitochondrial network in protein–DNA complexes known as “nucleoids”, which are involved in DNA replication, repair, gene expression, segregation, and inheritance [5].
Indeed, mitochondrial alterations, which range from severe to mild missense mutations, are rapidly fixed by several mechanisms such as genetic random drift and/or natural selection [6, 7]. Moreover, the mitochondrial genome is not protected by histones, and some molecular alterations, including thymidine dimers, cannot be repaired by mtDNA repair systems [8].
It has also been hypothesized that the high rate of reactive oxygen species (ROS) is a condition that probably encourages cancer development [9-11]. Severe mtDNA mutations could inhibit OXPHOS and promote tumor development, while milder mutations could permit a cancer to adapt to a new environment [12]. Moreover, mtDNA mutations seem to play other roles in carcinogenesis, creating a tissue susceptibility to cancer and/or a metastatic potentiality [13].
Several studies have demonstrated that many cancers harbour somatic mutations which are involved in mitochondrial dysfunction [14-16]. An alteration in mitochondrial function can change the developmental and replicative status of the nucleus, influencing the glucose metabolic pathway and deregulating the cell cycle of the surrounding stromal cells [17, 18]. Indeed, the uptake of glucose is highly increased in cancer cells, and the metabolic pathway results in a greater percentage of piruvate which is converted to lactic acid and excreted as a cathabolic product. This metabolic pattern is referred to as aerobic glycolysis, also commonly known as the “Warburg effect”. [18]. The activity of specific tumor suppressors (i.e., protein p53) decreases glycolysis and stimulates mitochondrial respiration through the activation of specific proteins required for the assembly of the citochrome c oxidase (COX) complex. Thus, the loss of p53 results in an increasing glycolysis and a decreasing mitochondrial respiration, contributing to the Warburg effect [19].
It has also been shown that cellular aging can act by accumulating mitochondrial DNA alterations, enhancing the production of ROS and representing a critical factor in the tumoral process [20]. The incidence of cancer significantly increases with human aging because of a progressive decline of mitochondrial function [21]. It has been reported that cell cycle arrest upon inhibition of mitochondrial function in human cells is under control of the mitochondria damage checkpoint, also known as the mitocheckpoint control [10]. The mitocheckpoint permits cells to arrest the cell cycle in order to normalize mitochondrial function. Severe damage of mitochondria may allow cells to undergo senescence, which represents the last checkpoint before the cell decision to start apoptosis or, alternatively, tumorigenesis [10].
Mitochondria are known for their role in mediating the intrinsic apoptotic pathway, also known as “mitochondrial apoptosis”. It has been demonstrated that apoptosis occurs less frequently in cells harboring mitochondrial alterations regarding those genes involved in the apoptotic process, such as the ATP synthase subunit 6 gene (ATP6), supporting the idea that pathogenic mtDNA mutations seem to promote tumors by preventing apoptosis [22]. Additionally, apoptosis can also be mediated by an extrinsic apoptosis pathway which is started by an activation of the cell-surface tumor necrosis factor family named “death” receptors. Both intrinsic and extrinsic apoptoses are cross-linked and converge upon the activation of caspases which carry out the final apoptotic steps [23]. Consequently, a discrete number of therapeutic strategies have recently been based on targeting tumoral mitochondria in order to induce the apoptotic way [24-27].
Since mitochondria play an essential role in the control of tumor-induced oxidative stress, the integrity of the mitochondrial genome and its functionality is strongly involved to contrast the oncogenic process [28]. Among the extensive typologies of human cancers, hereditary breast cancer is the most diffused tumour amongst the female population worldwide and it has captured the greatest attention among the scientific community. In the last years many authors have investigated both the contribution of nuclear alterations as increasing factors of hereditary predisposition to breast cancer [29-31] and the several mitochondrial alterations retrieved in breast cancer samples as possible susceptibility markers of the carcinogenic process [32-37]. This review focuses on how genetic background could play a critical role in modifying an individual risk to breast cancer, and why modifications in mtDNA could be highly involved in breast cancer progression.
MITOCHONDRIAL DNA ALTERATIONS IN HUMAN CANCERS
The importance of mtDNA in cancer has been confirmed by the exchange of cancer cell mtDNA with pathogenic or normal mtDNA, resulting in alterations of cancer cell phenotypes [9, 11, 22]. Some recent insights have suggested that mtDNA mutations could be technical artifacts, due to nucleus-embedded mitochondrial sequences (NUMTs) ranging in size from ~50 bp to >15 kb [38, 39]. NUMTs, relocalized from the mitochondria to the nucleus during evolution, start to accumulate polymorphic mutations due to genetic drift [40, 41]. NUMTs are able to enter the nucleus especially under conditions of stress, as in case of tumorigenity. Since they have high similarity to authentic mtDNA, depending on the choice of primers NUMTs could be selectively amplified by PCR along with authentic mtDNA, allowing the misattribution of the mutations to those present in authentic tumoral mtDNA [42, 43]. These hypotheses are speculative and should not ignore the possibility that authentic mtDNA is mutated in tumors. Moreover, even if mutated DNA is an artefact derived from amplified NUMTs, it represents a useful marker of cancer and thus may have a clinical use [44-46].
In the past years, the whole human mtDNA or specific regions have been sequenced in many tumoral samples, revealing a very high frequency of mitochondrial alterations in various tissues and bodily fluids [32, 47, 48]. Mitochondrial DNA aberrations, including point mutations, instability of mono- or dinucleotide repeats, insertions, and deletions or quantitative alterations, have been found in solid tumors, such as breast, colon, head and neck cancer, stomach, liver, kidney, bladder, prostate, skin, lung cancer and several blood cancers [12, 35, 49].
In recent years, many studies have reported several mutations detected both in PCGs and in the main noncoding region, the displacement loop (D-loop). As the D-loop is responsible for replication control and transcription of the mitochondrial genome, mutations in this region modify mitochondrial genomic expression, deregulating mitochondrial metabolism and OXPHOS [50, 51]. In literature, some studies suggest how somatic mutations in the D-loop are involved in human carcinogenesis [52, 53]. For example, several studies reported that T146C, C150T and T152C polymorphisms occurred in tumor cell lines [54, 55]. Particularly, D-loop C150T was significantly indicated for an increased risk of cervical cancer and human papilloma virus (HPV) infection in Chinese women [56]. Moreover, the mitochondrial mutation T6777C, detected within cytochrome c oxidase subunit 1 (CO1), has been significantly linked to epithelial ovarian cancer [55]. It was also observed that elevated expressions of both CO1 and NADH dehydrogenase subunit 4 (ND4) were associated with gastric tumorigenesis and tumor dedifferentiation ex vivo [58]. An interesting study highlighted that a specific mtDNA mutation (A15296G) retrieved in cytochrome b (CYTB) was clonally detected in clinical samples of a leukemia patient, suggesting that this marker could play a role in cancer progression [59]. In an important insight, investigators focused also on the pathogenic mtDNA T8993G (within ATPase 6 of complex V) in prostate cancer and found that the T8993G mutation modifies mitochondrial ATPase synthesis. Importantly, these authors found that the mutant tumors also generated significantly more ROS, leading to an increase in DNA damage and hence tumor growth [9].
All these findings reveal that a few specific mitochondrial polymorphisms may be useful candidates for cancer biomarkers, but most somatic mitochondrial changes do not produce alterations in amino acids and their biologic functional contribution remains unclear. Mitochondria could play a causative role in an increasing risk of developing neoplastic lesions and progression, but further studies are required, perhaps by using experimental models including cybrids and analysis of large cohorts of patients. Furthermore, an accurate validation of tumor-associated mtDNA mutations by comparing normal and tumor samples followed by their detection in clinical samples should facilitate cancer prevention, early detection and therapeutic strategies.
MITOCHONDRIA AND BREAST CANCER
D-loop Alterations
Several studies have focused on the association between mitochondrial variants and breast cancer risk. The majority of mutations were specifically detected in the D-loop, and in NADH dehydrogenase, cytochrome oxidase and ATPase genes [60-63]. In tumoral samples, the D-loop represents one of the most important mitochondrial "hotspot" regions, harboring a large number of alterations which are significantly associated to breast cancer (Table 1). Among the alterations reported in literature over the past years, polycytidine stretch D310 (located between nucleotide 303 and 315 and interrupted by a T in position 310) has been frequently retrieved in tumoral samples, representing a potential starting point for the clonal expansion of malignant cells including breast cancer [64-71]. Indeed, D310 resulted the most significant mutation retrieved in breast samples [72].
Table 1.
Mitochondrial Variants Detected in Breast Cancer Tissues (nc= Noncoding; nr= Not reported; syn= Synonymous; *= Significant P-value).
| Gene | Nucleotide Position | Nucleotide Change | Amino Acid Change | P-value | Reference |
|---|---|---|---|---|---|
| 16S rRNA | 3197 | T>C | Nc | 0.03* | Bai et al., 2007 |
| ND1 | 3918 | G>A | Syn | nr | Parrella et al., 2001 |
| 4216 | T>C | Y-->H | 0.598 | Covarrubias et al., 2008 | |
| ATP6 | 9055 | G>A | A-->T | 0.005* | Bai et al., 2007 |
| ND3 | 10397 | A>G | syn | 0.030* | Fang et al., 2010 |
| 10398 | A>G | T -->A | 0.011* | Covarrubias et al., 2008 | |
| 10400 | C>T | syn | 0.040* | Fang et al., 2010 | |
| ND4 | 11719 | G>A | syn | 0.005* | Tommasi et al., personal data |
| 11900 | G>A | V-->M | nr | Parrella et al., 2001 | |
| TL2 | 12308 | A>G | nc | 0.84 | Covarrubias et al., 2008 |
| ND5 | 12344 | T>A | M-->K | nr | Parrella et al., 2001 |
| 13708 | G>A | A-->T | 0.0006* | Bai et al., 2007 | |
| CYTB | 14869 | G>A | syn | nr | Parrella et al., 2001 |
| D-Loop | 16093 | C>T | nc | nr | Parrella et al., 2001 |
| 16183 | A>C | nc | 0.03* | Tommasi et al., personal data | |
| 16278 | C>T | nc | 0.03* | Tommasi et al., personal data | |
| 16290 | C>T | nc | 0.002* | Sultana et al., 2012 | |
| 16292 | C>T | nc | 0.663 | Ma et al., 2011 | |
| 16293 | del-A | nc | 0.010* | Sultana et al., 2012 | |
| 16304 | T>C | nc | 0.252 | Czarnecka et al., 2010a | |
| 16390 | G>A | nc | 1.00 | Czarnecka et al., 2010a | |
| 16519 | T>C | nc | 0.036* | Bai et al., 2007 | |
| 153 | A>G | nc | 0.009* | Tommasi et al., personal data | |
| 195 | T>C | nc | 0.04* | Tommasi et al., personal data | |
| 225 | G>A | nc | 0.03* | Tommasi et al., personal data | |
| 226 | T>C | nc | 0.03* | Tommasi et al., personal data | |
| D310 | insC | nc | <0.0001* | Xu et al., 2012 |
Additional results have been reported about the role of the dinucleotide repeat polymorphism (CA)n in carcinogenesis risk. Tseng et al. [73] indicated a very low prevalence of the CA deletion in 60 breast subjects (1.6%), whereas another study conducted on more than 1000 cases evidenced that D-loop (CA)n polymorphism was not responsible for breast cancer risk but, conversely, should be associated with breast cancer survival [74].
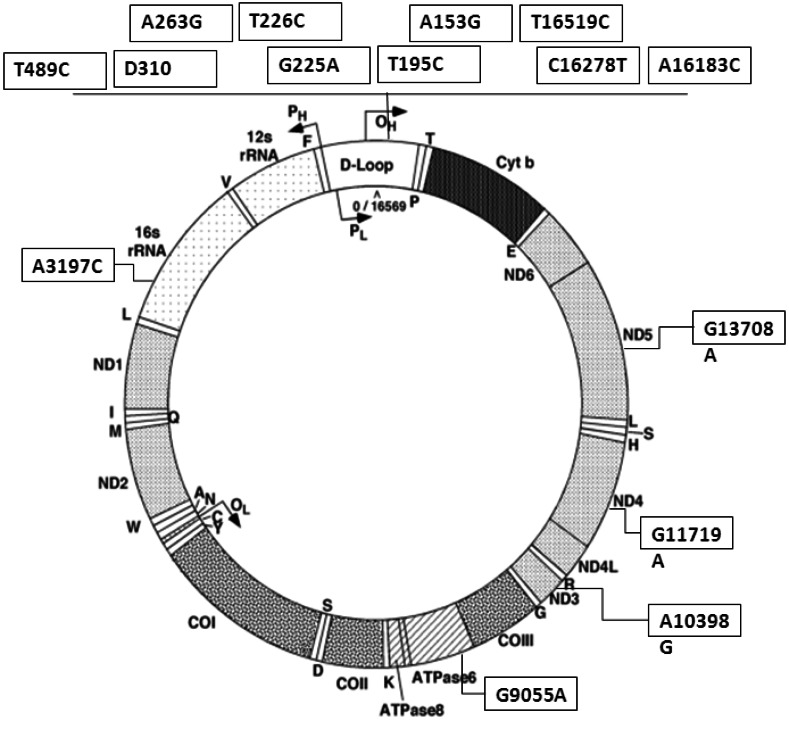
Other studies have focused on a few important variants significantly retrieved in breast tumoral subjects, such as C16290T, 16293del-A and T16519C [75, 76]. In particular, the mutation T16519C in the D-loop was found to increase breast cancer risk (P= 0.0366), either occurring singularly or in association with other mitochondrial PCGs alterations such as A10398G, G13368A or C14766T [75, 77]. There are only few data which consider D-loop in familial breast cancer patients [49]; this region resulted more frequently altered than that in sporadic breast cancer and the variations which seem to be more present in familial breast cancer are A263G, T489C and D310. Furthermore, in our hands, seven further loci are specifically associated with breast cancer familiarity (Fig. 1). Since scant data are available about the role of other mutations in breast carcinogenesis, further studies about the association between mitochondrial D-loop polymorphisms and breast cancer risk are needed.
Fig. (1).
Map of variations in D-loop and PCGs associated with familial breast cancer [49, 75 and Tommasi personal communication].
Mitochondrial PCGs Mutations
Recently, many authors have reported several alterations, detected in breast tissues, which are associated with cancer or mild breast pathologies [78, 79]. In particular, the most frequently mutated genes were ND1, ND3, ND4 and ND5 in a total of 6 missense substitutions and 5 synonymous alterations, of which 6 were significantly retrieved in breast cancer tissues (Table 1). Specifically, in the last studies the mtDNA variant G10398A, which results in a non-conservative substitution of threonine for alanine within the ND3 gene, has received the greatest attention. Many authors have reported that the presence of the 10398A allele has been significantly (P= 0.01) associated with an increased breast cancer risk in African-American women [80, 81]. Conversely, other studies found that mtDNA G10398A polymorphism did not result as a marker of breast cancer risk in African Americans, but individuals inheriting the A10398G polymorphism harbored a significant risk (P= 0.011) of developing breast cancer [77, 82, 83]. Additionally, an increased breast cancer risk (P= 0.03) associated with alcohol consumption was observed in a case-control study limited to carriers of the 10398G allele [84]. Moreover, the association of several variants resulted in a significant predictive breast cancer factor. Indeed, A10398G, together with some other mutations such as T4216C (P= 0.0009), G9055A (P= 0.0004), T16519C (P= 0.002) or A12308G (P= 0.0028), was found to increase the risk of a woman developing breast cancer [77, 85].
One of the most common somatic mtDNA deletions in tumoral cells (∆mtDNA4977) occurs between nucleotides 8.470 and 13.477 of the human mtDNA and includes 5 tRNA genes, 4 genes encoding subunits of NADH dehydrogenases, cytochrome oxidases subunit 3 (CO3) and ATPases genes. The major consequence is that ∆mtDNA4977 creates a smaller mtDNA molecule that leads to a decrease in energy production and to an abnormal ROS generation [86]. The ∆mtDNA4977, also called "the common deletion", seems to be associated with several mitochondrial encephalomyopathies including Pearson’s syndrome, Kearns-Sayre syndrome (KSS) and chronic progressive external ophthalmoplegia (CPEO) [87]. The "common deletion" has been studied in several human cells colonies [88-90] and also in liver [91] and breast tumors [73, 92].
The discovery that ∆mtDNA4977 occurs in low or discrete percentages in solid tumors, from 0.1% in colon tumors up to 30% in gastric and breast cancers [93] has led to controversial hypotheses. Some papers hypothesized that during carcinogenesis the cells containing mtDNA with no deletions are supported by a strong selection pressure compared to those with this severe alteration [91]. Other studies assumed that this deletion accumulates in many tissues during aging and has been used as a mtDNA damage biomarker [94]. Moreover, Shen and colleagues [95] found that ∆mtDNA4977 was implicated in the occurrence of breast and colorectal cancer, playing a role in modulating mtDNA contents in cancer cells.
Some researchers have performed a comparative analysis of expression levels of mitochondrial genes in benign and malignant breast tumors. Sharp et al. [96] found that the cytochrome c oxidase, subunit 2 (CO2) is expressed at significantly higher levels in carcinomas as compared to fibroadenomas, while no differences were found for ATPases or ND2 and ND4. The only author [75] who considered mitochondrial PCGs alterations in familial compared to sporadic breast cancer patients showed a significant increased frequency of variations in 16S rRNA, ATP6, ND3 and ND5; while the synonymous G11719A alteration in ND4 gene has been significantly associated to familial breast cancer by our studies still unpublished (Fig. 1).
Conclusively, even if many mitochondrial alterations have been detected and studied in breast cancer tissues, to date only a few mutations have been indicated as reliable biomarkers. One of these is the A10398G mutation which apparently remains one of the main independent predictors of the risk for breast carcinogenesis.
Mitochondria and Breast Cancer Susceptibility Genes
Genetic linkage studies have identified BRCA1 and BRCA2 genes (chromosomes 17q21 and 13q12, respectively) as validated susceptibility markers of hereditary breast cancer (HBC). In particular, the BRCA1 gene, when harboring germline mutations, confers a high susceptibility to breast and ovarian cancer predisposition and may account for a total of 10% of breast cancer incidence [97]. The Brca1 protein is involved in the control of genomic stability in the nucleus, such as in cell cycle regulation and checkpoint activation [98], by modulating specific transcriptional pathways and several highly specialized DNA repair processes [99, 100]. Brca1 is also implicated in the regulation of centrosomes, apoptosis, DNA binding and chromatin remodeling [101, 102].
Current advanced molecular technologies, including bi-directional sequencing and High Resolution DNA Melting Analysis, allow researchers to retrieve a widespread number of mutations in the BRCA1/2 genes of patients with a familial history of breast cancer [103, 104]. All the pathogenic mutations and unclassified variants retrieved in BRCA1/2 genes are reported in the Breast Cancer Core database (BIC: http://research.nhgri.nih.gov/bic). However, the pathogenic mutations found in BRCA1/2 genes account for only ~40% of familial breast cancer cases and there is a wide cohort of subjects harboring wild-type BRCA1/2 genes. It has therefore been suggested that other genes could be involved as predisposing factors to breast cancer [30, 105, 106]. Indeed, besides BRCA mutations, the current consensus is that modifications in BRCA1/2 genes allow the accumulation of other cellular defects. In the most recent studies, BRCA1/2 gene function in the DNA damage response pathway has led to the identification of a discrete number of susceptibility genes, including ATM, BRIP1, CASP8, CHEK2, NBN, PALB2, PTEN, TP53 and STK11 [29-31, 106-110].
Recently, genome wide association studies (GWAS) have also focused on the contribution of both BRCA1/2 gene alterations and genetic modifiers which could increase hereditary predisposition to breast cancer [31]. In the general population, breast carcinogenesis may also be attributed to rare mutations of other genetic modifiers identified by GWAS (i.e., 2q35, 5p12 and 19p13) which confer a moderate risk of cancer development [31, 97].
Furthermore, many findings have highlighted an interesting relationship between the nucleus and mitochondria, as it has been demonstrated that the majority of mitochondrial proteins are nuclear encoded and post-translationally imported in mitochondria [111]. Coene and colleagues [112] evidenced the nuclear, cytoplasmic and mitochondrial localization of Brca1 proteins in human cells. In particular, mitochondrial Brca1 proteins seem to have an antiproliferative activity on breast cancer cells [113]. Finally, Bandiera et al. [114] found a large number of nuclear-encoded miRNAs, named as “mitomiRs”, which resulted differently expressed in mitochondria and cytosol, showing that most mitochondrial miRNAs were both nuclear and mitochondrial-encoded targets. The nuclear/mitochondrial connection way had also been previously demonstrated by a diagnostic algorithm showing that the mitochondrial deletion ∆mtDNA4977 in association to alterations in nuclear genes, such as BRCA, ER and TP53 genes, led to a phenotypic expression of premature aging and breast cancer [115].
Several other nuclear genes that impact upon the carcinogenic process have been identified specifically in mitochondria, such as sirtuin proteins (Sirt3) belonging to the deacetylases protein family. Since human breast and other human cancer specimens exhibit reduced Sirt3 levels, it has been suggested that sirtuins act as mitochondrial tumor suppressors, modulating both aging and tumoral phenotype [116, 117]. All these findings have been recently confirmed by an interesting study that showed that changes in mtDNA can produce different expression levels of specific nuclear-encoded genes (i.e. MMP-9 and Col1a) which are capable of triggering the phenotype such as the one seen in malignant cells [118].
Moreover, the role of mitochondrial proteins in breast cancer development has been widely studied in recent years. A potential biomarker of the mitochondrial complex I subunit, NDUFS, resulted a strong indicator of breast cancer aggressiveness. It discriminated between normal and highly invasive breast carcinoma specimens, supporting a plausible mechanism involving mitochondrial dysfunction during the process of cancer cell transformation [119]. Moreover, a recent study found that the mitochondria of breast carcinoma expressed higher levels of mitochondrial fission protein dynamin-related protein 1 (Drp1), determining metastases to lymph nodes [37].
These findings show that nuclear mutated genes are responsible for only a part of hereditary breast cancer; the role played by mitochondrial modifiers in the general population highlighted the importance of these loci in breast carcinogenesis. Further studies should provide an opportunity to better understand the complicated relationship between genetic background and breast cancer etiology, describing the pathway through which they molecularly act.
Mitochondrial Haplogroups and Breast Cancer
Some studies have focused on studying the risk resulting from the association between a mitochondrial haplogroup and breast carcinogenesis. In a study conducted on a total of 416 subjects, Bai et al. [75] suggested that individuals classified as haplogroup K show a significant increase in the risk of developing breast cancer (P= 0.0004), whereas individuals bearing haplogroup U have a significant decrease in breast cancer risk (P= 0.0023).
The results obtained by Darvishi and colleagues [81] are more interesting. These authors analysed ~1000 complete human mtDNA sequences worldwide and 124 sporadic breast cancer patients from India, validating the exclusive presence of the pathogenic alteration G10398A (P= 0.01), which in literature is assigned to N haplotype. The apparent worldwide correlation in increased incidence rates of breast cancer and mtDNA haplogroup N distribution observed is interesting. These results were also confirmed by Gochhait et al. [120] who also observed the concomitant presence of 10398A in all 36 breast cancer patients in their study characterized by the N haplogroup.
Finally, Czarnecka et al. [61] concluded that haplogroup I assigned to 44 subjects among the Polish population is over-represented in individuals with breast cancer, whereas two studies agree that a total of 158 subjects from China harboring haplogroup M showed an increased carcinogenesis risk [121, 122].
In conclusion, mitochondrial dysfunction does appear to be a factor in cancer aetiology, an insight that may suggest new approaches for diagnosis and treatment. When comparing all the data on somatic mutations and haplogroup studies, no definitive results are provided by authors because the effect of the mitochondrial genetic background could be influenced by other features such as physiological conditions (i.e., hormonal state, age, sex) or geographical place of origin. Thus, the identification of significant mtDNA SNPs associated with breast cancer suggests that mitochondria may be involved in the pathogenetic mechanism of disease and cancer. To understand the etiology of the effect of mtDNA haplogroups or mitochondrial polymorphisms, many authors are looking for more nuclear and/or somatic mutations to determine if they play a critical role in breast carcinogenesis. Furthermore, mtDNA-SNPs association studies and haplogroup categorization are needed to obtain more pieces of this molecular puzzle.
ACKNOWLEDGEMENTS
S.W. contributed to performance and analysis of the text; A.P. contributed to design of the text; S.T. contributed to design and analysis. The authors would like to thank Caroline Oakley for manuscript revision. Partially funded by Fondazione Cassa di Risparmio di Puglia 2011.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Anderson S, Bankier AT, Barrell BG, De Bruijn MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJH, Staden R, Young IG. . Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 2.De Giorgi C, Saccone C. Mitochondrial genome in animal cells.Struture organization, and evolution. Cell Biophys. 1989;14(1):67–78. doi: 10.1007/BF02797392. [DOI] [PubMed] [Google Scholar]
- 3.Pesole G, Gissi C, De Chirico A, Saccone C. Nucleotide substitution rate of mammalian mitochondrial genomes. J. Mol. Evol. 1999;48(4):427–434. doi: 10.1007/pl00006487. [DOI] [PubMed] [Google Scholar]
- 4.Neiman M, Taylor DR. The causes of mutation accumulation in mitochondrial genomes. Proc. Biol. Sci. 2009;276(1660):1201–1209. doi: 10.1098/rspb.2008.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spelbrink JN. Functional organization of mammalian mitochondrial DNA in nucleoids history, recent de-velopments, and future challenges. IUBMB Life . 2010;62:19–32. doi: 10.1002/iub.282. [DOI] [PubMed] [Google Scholar]
- 6.Jenuth JP, Peterson AC, Fu K, Shoubridge EA. Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat. Genet . 1996;14(2):146–151. doi: 10.1038/ng1096-146. [DOI] [PubMed] [Google Scholar]
- 7.Chinnery PF, Thorburn DR, Samuels DC, White SL, Dahl HM, Turnbull DM, Lightowlers RN, Howell N. The inheritance of mitochondrial DNA heteroplasmy random drift selection or both Trends. Genet . 2000;16(11):500–505. doi: 10.1016/s0168-9525(00)02120-x. [DOI] [PubMed] [Google Scholar]
- 8.Strachan T, Read AP, editors. Ed. 29. West 35th Street. NY: Garland Sciences; 2003. The mitochondrial genome is a hotspot for pathogenic mutations, In Human Molecular Genetics; pp. 333–334. [Google Scholar]
- 9.Petros J A, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, Marshall FF, Wallace DC. mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl. Acad. Sci. U.S.A . 2005;102(3):719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh KK. Mitochondria damage checkpoint, aging, and cancer. 2006. Ann. N.Y. Acad. Sci. 2006;1067:182–190. doi: 10.1196/annals.1354.022. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science . 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 12.Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene. 2006;25(34):4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 13.Treuting PM, Linford NJ, Knoblaugh SE, Emond M, Morton JF, Martin GM, Rabinovitch PS, Ladiges WC. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63(8):813–822. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- 14.Ha PK, Tong BC, Westra WH, Sanchez-Cespedes M, Parrella P, Zahurak M, Sidransky D, Califano JA. Mitochondrial C-tract alteration in premalignant lesions of the head and neck a marker for progression and clonal proliferation. Clin. Cancer Res. 2002;8:2260–2265. [PubMed] [Google Scholar]
- 15.Dasgupta S, Yung RC, Westra WH, Rini DA, Brandes J, Sidransky D. l Following mitochondrial footprints through a long mucosal path to lung cancer. PLoS One . 2009;4(8):e6533–0. doi: 10.1371/journal.pone.0006533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma H, Singh A, Sharma C, Jain SK, Singh N. Mutations in the mitochondrial DNA D-loop region are frequent in cervical cancer. Cancer Cell Int. 2005;5:34–0. doi: 10.1186/1475-2867-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 18.Ericson NG, Kulawiec M, Vermulst M, Sheahan K, O'Sullivan J, Salk JJ, Bielas JH. Decreased mitochondrial DNA mutagenesis in human colorectal cancer. PLoS Genet. 2012;8(6):e1002689–0. doi: 10.1371/journal.pgen.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science . 2010;330(6009):1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 20.Gredilla R. DNA damage and base excision repair in mitochondria and their role in aging. J. Aging Res. 2011;257093:0–0. doi: 10.4061/2011/257093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh KK. Mitochondrial dysfunction is a common phenotype in aging and cancer. Ann. N.Y. Acad. Sci. 2004;1019:260–264. doi: 10.1196/annals.1297.043. [DOI] [PubMed] [Google Scholar]
- 22.Shidara Y, Yamagata K, Kanamori T, Nakano K, Kwong JQ, Manfredi G, Oda H, Ohta S. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 2005;65:1655–1663. doi: 10.1158/0008-5472.CAN-04-2012. [DOI] [PubMed] [Google Scholar]
- 23.Fogg VC, Lanning NJ, Mackeigan JP. Mitochondria in cancer at the crossroads of life and death. Chin. J. Cancer . 2011;30(8):526–539. doi: 10.5732/cjc.011.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berridge MV, Herst PM, Lawen A. Targeting mitochondrial permeability in cancer drug development. Mol. Nutr. Food Res. 2009;53(1):76–86. doi: 10.1002/mnfr.200700493. [DOI] [PubMed] [Google Scholar]
- 25.Gogvadze V. Targeting mitochondria in fighting cancer. Curr. Pharm. Des. 2011;17(36):4034–4046. doi: 10.2174/138161211798764933. [DOI] [PubMed] [Google Scholar]
- 26.Grimm D, Wehland M, Pietsch J, Infanger M, Bauer J. Drugs interfering with apoptosis in breast cancer. Curr. Pharm. Des . 2011;17(3):272–283. doi: 10.2174/138161211795049723. [DOI] [PubMed] [Google Scholar]
- 27.Constance JE, Lim CS. Targeting malignant mitochondria with therapeutic peptides. Ther. Deliv. 2012;3(8):961–979. doi: 10.4155/tde.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seoane M, Mosquera-Miguel A, Gonzalez T, Fraga M, Salas A, Costoya JA. The mitochondrial genome is a "genetic sanctuary" during the oncogenic process. PLoS One . 2011;6(8):e23327–0. doi: 10.1371/journal.pone.0023327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipollini G, Tommasi S, Paradiso A, Aretini P, Bonatti F, Brunetti I, Bruno M, Lombardi G, Schittulli F, Sensi E, Tancredi M, Bevilacqua G, Caligo MA. Genetic alterations in hereditary breast cancer. Ann. Oncol. 2004;15(1):I7–I13. doi: 10.1093/annonc/mdh651. [DOI] [PubMed] [Google Scholar]
- 30.Poumpouridou N, Kroupis C. Hereditary breast cancer beyond BRCA genetic analysis, PALB2 emerges. Clin. Chem. Lab. Med. 2011;50(3):423–434. doi: 10.1515/cclm-2011-0840. [DOI] [PubMed] [Google Scholar]
- 31.Shuen AY, Foulkes WD. Inherited mutations in breast cancer genes--risk and response. J. Mammary Gland Biol. Neoplasia . 2011;16(1):3–15. doi: 10.1007/s10911-011-9213-5. [DOI] [PubMed] [Google Scholar]
- 32.Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287(5460):2017–2019. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- 33.Parrella P, Xiao Y, Fliss M, Sanchez-Cespedes M, Mazzarelli P, Rinaldi M, Nicol T, Gabrielson E, Cuomo C, Cohen D, Pandit S, Spencer M, Rabitti C, Fazio VM, Sidransky D. Detection of mitochondrial DNA mutations in primary breast cancer and fine-needle aspirates. Cancer Res. 2001;61(20):7623–7626. [PubMed] [Google Scholar]
- 34.Rohan TE, Wong LJ, Wang T, Haines J, Kabat GC. Do alterations in mitochondrial DNA play a role in breast carcinogenesis. J. Oncol. 2010;604304:0–0. doi: 10.1155/2010/604304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatterjee A, Dasgupta S, Sidransky D. Mitochondrial subversion in cancer. Cancer Prev. Res. 2011;4(5):638–654. doi: 10.1158/1940-6207.CAPR-10-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace DC. Mitochondria and cancer. Nat. Rev. Cancer . 2012;12(10):685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, Abel PW, Tu Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene doi 10.1038/onc.2012.494. 2012 doi: 10.1038/onc.2012.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mourier T, Hansen A J, Willerslev E, Arctander P. The Human Genome Project reveals a continuous transfer of large mitochondrial fragments to the nucleus. Mol. Biol. Evol. 2001;18:1833–1837. doi: 10.1093/oxfordjournals.molbev.a003971. [DOI] [PubMed] [Google Scholar]
- 39.Ramos A, Barbena E, Mateiu L, del Mar González M, Mairal Q, Lima M, Montiel R, Aluja MP, Santos C. Nuclear insertions of mitochondrial origin database updating and usefulness in cancer studies. Mitochondrion . 2011;11:946–953. doi: 10.1016/j.mito.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Hazkani-Covo E, Zeller RM, Martin W. Molecular poltergeists mitochondrial DNA copies (numts) in sequenced nuclear genomes. PLoS Genet. 2010;6:e1000834–0. doi: 10.1371/journal.pgen.1000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lang M, Sazzini M, Calabrese FM, Simone D, Boattini A, Romeo G, Luiselli D, Attimonelli M, Gasparre G. Polymorphic Numts trace human population relationships. Hum. Genet. 2012;131:757–771. doi: 10.1007/s00439-011-1125-3. [DOI] [PubMed] [Google Scholar]
- 42.Parr RL, Maki J, Reguly B, Dakubo GD, Aguirre A, Wittock R. Robinson K, Jakupciak JP, Thayer RE. The pseudo-mitochondrial genome influences mistakes in heteroplasmy interpretation. BMC Genomics . 2006;7:185–0. doi: 10.1186/1471-2164-7-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellinger J, Müller DC, Müller SC, Hauser S, Heukamp LC, von Ruecker A, Bastian PJ, Walgenbach-Brunagel G. Circulating mitochondrial DNA in serum a universal diagnostic biomarker for patients with urological malignancies. Urol. Oncol. 2012;30(4):509–515. doi: 10.1016/j.urolonc.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Parr RL, Dakubo GD, Crandall KA, Maki J, Reguly B, Aguirre A, Wittock R, Robinson K, Alexander JS, Birch-Machin MA, Abdel-Malak M, Froberg MK, Diamandis EP, Thayer RE. Somatic mitochondrial DNA mutations in prostate cancer and normal appearing adjacent glands in comparison to age-matched prostate samples without malignant histology. J. Mol. Diagn. 2006;8:312–319. doi: 10.2353/jmoldx.2006.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maki J, Robinson K, Reguly B, Alexander J, Wittock R, Aguirre A, Diamandis EP, Escott N, Skehan A, Prowse O, Thayer RE, Froberg MK, Wilson MJ, Maragh S, Jakupciak JP, Wagner PD, Srivastava S, Dakubo GD, Parr RL. Mitochondrial genome deletion aids in the identifi-cation of false- and true-negative prostate needle core biopsy specimens. Am. J. Clin. Pathol. 2008;129:57–66. doi: 10.1309/UJJTH4HFEPWAQ78Q. [DOI] [PubMed] [Google Scholar]
- 46.Schon EA, Dimauro S, Hirano M. Human mitochondrial DNA roles of inherited and somatic mutations. Nat. Rev. Genet . 2012;13(12):878–890. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jerónimo C, Nomoto S, Caballero OL, Usadel H, Henrique R, Varzim G, Oliveira J, Lopes C, Fliss MS, Sidransky D. Mitochondrial mutations in early stage prostate cancer and bodily fluids. Oncogene . 2001;20(37):5195–5198. doi: 10.1038/sj.onc.1204646. [DOI] [PubMed] [Google Scholar]
- 48.Zhu W, Qin W, Wessel A, Puckett CL, Sauter E. Mitochondrial DNA mutations in breast cancer tissue and in matched nipple aspirate fluid. Carcinogenesis. 2005;26(1):145–152. doi: 10.1093/carcin/bgh282. [DOI] [PubMed] [Google Scholar]
- 49.Yu M, Shi Y, Zhang F, Zhou Y, Yang Y, Wei X, Zhang L, Niu R. Sequence variations of mitochondrial DNA D-loop region are highly frequent events in familial breast cancer. J. Biomed. Sci. 2008;15(4):535–543. doi: 10.1007/s11373-007-9229-4. [DOI] [PubMed] [Google Scholar]
- 50.Lee HC, Yin PH, Lin JC, Wu CC, Chen CY, Wu CW, Chi CW, Tam TN, Wei YH. Mitochondrial genome instability and mtDNA depletion in human cancers. Ann. N.Y. Acad. Sci. 2005;1042:109–122. doi: 10.1196/annals.1338.011. [DOI] [PubMed] [Google Scholar]
- 51.Zhao YB, Yang HY, Zhang XW, Chen GY. Mutation in D-loop region of mitochondrial DNA in gastric cancer and its significance. World J. Gastroenterol. 2005;11(21):3304–3306. doi: 10.3748/wjg.v11.i21.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee HC, Li SH, Lin JC, Wu CC, Yeh DC, Wei YH. Somatic mutations in the D-loop and decrease in the copy number of mitochondrial DNA in human hepatocellular carcinoma. Mutat Res. 2004;547(1-2):71–78. doi: 10.1016/j.mrfmmm.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Sharawat SK, Bakhshi R, Vishnubhatla S, Bakhshi S. Mitochondrial D-loop variations in paediatric acute myeloid leukaemia a potential prognostic marker. Br. J. Haematol. 2010;149(3):391–398. doi: 10.1111/j.1365-2141.2010.08084.x. [DOI] [PubMed] [Google Scholar]
- 54.Chen JZ, Kadlubar FF. Mitochondrial mutagenesis and oxidative stress in human prostate cancer. Journal of Environmental Science and Health. Part C Environmental Carcinogenesis & Ecotoxicology Reviews . 2004;22:1–12. doi: 10.1081/GNC-120037931. [DOI] [PubMed] [Google Scholar]
- 55.Yoneyama H, Hara T, Kato Y, Yamori T, Matsuura ET, Koike K. Nucleotide sequence variation is frequent in the mitochondrial DNA displacement loop region of individual human tumor cells. Mol. Cancer Res. 2005;3:14–20. [PubMed] [Google Scholar]
- 56.Zhai K, Chang L, Zhang Q, Liu B, Wu Y. Mitochondrial C150T polymorphism increases the risk of cervical cancer and HPV infection. Mitochondrion . 2011;11(4):559–563. doi: 10.1016/j.mito.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Permuth-Wey J, Chen YA, Tsai YY, Chen Z, Qu X, Lancaster JM, Stockwell H, Dagne G, Iversen E, Risch H, Barnholtz-Sloan J, Cunningham JM, Vierkant RA, Fridley BL, Sutphen R, McLaughlin J, Narod SA, Goode EL, Schildkraut JM, Fenstermacher D, Phelan CM, Sellers TA. Inherited variants in mitochondrial biogenesis genes may influence epithelial ovarian cancer risk. Cancer Epidemiol. Biomarkers Prev . 2011;20:1131–1145. doi: 10.1158/1055-9965.EPI-10-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma JT, Han CB, Zhou Y, Zhao JZ, Jing W, Zou HW. Altered expression of mitochondrial cytochrome c oxidase I and NADH dehydrogenase 4 transcripts associated with gastric tumorigenesis and tumor dedifferentiation. Mol. Med. Report . 2012;5(6):1526–1530. doi: 10.3892/mmr.2012.832. [DOI] [PubMed] [Google Scholar]
- 59.He L, Luo L, Proctor SJ, Middleton PG, Blakely EL, Taylor RW, Turnbull DM. Somatic mitochondrial DNA mutations in adult-onset leukaemia. Leukemia . 2003;17(12):2487–2491. doi: 10.1038/sj.leu.2403146. [DOI] [PubMed] [Google Scholar]
- 60.Wang CY, Wang HW, Yao YG, Kong QP, Zhang YP. Somatic mutations of mitochondrial genome in early stage breast cancer. Int. J. Cancer. 2007;121(6):1253–1256. doi: 10.1002/ijc.22822. [DOI] [PubMed] [Google Scholar]
- 61.Czarnecka AM, Krawczyk T, Plak K, Klemba A, Zdrozny M, Arnold RS, Kofler B, Golik P, Szybinska A, Lubinski J, Mossakowska M, Bartnik E, Petros JA. Mitochondrial genotype and breast cancer predisposition. Oncol Rep . 2010;24(6):1521–1534. doi: 10.3892/or_00001014. [DOI] [PubMed] [Google Scholar]
- 62.Czarnecka AM, Klemba A, Krawczyk T, Zdrozny M, Arnold RS, Bartnik E, Petros JA. Mitochondrial NADH-dehydrogenase polymorphisms as sporadic breast cancer risk factor. Oncol Rep. 2010;23(2):531–535. [PubMed] [Google Scholar]
- 63.Fendt L, Niederstätter H, Huber G, Zelger B, Dünser M, Seifarth C, Röck A, Schäfer G, Klocker H, Parson W. Accumulation of mutations over the entire mitochondrial genome of breast cancer cells obtained by tissue microdissection. Breast Cancer Res. Treat . 2011;128(2):327–336. doi: 10.1007/s10549-010-1092-8. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Cespedes M, Parrella P, Nomoto S, Cohen D, Xiao Y, Esteller M, Jeronimo C, Jordan RC, Nicol T, Koch WM, Schoenberg M, Mazzarelli P, Fazio VM, Sidransky D. Identification of a mononucleotide repeat as a major target for mitochondrial DNA alterations in human tumors. Cancer Res . 2001;61(19):7015–7019. [PubMed] [Google Scholar]
- 65.Parrella P, Seripa D, Matera MG, Rabitti C, Rinaldi M, Mazzarelli P, Gravina C, Gallucci M, Altomare V, Flammia G, Casalino B, Benedetti-Panici PL, Fazio VM. Mutations of the D310 mitochondrial mononucleotide repeat in primary tumors and cytological specimens. Cancer Lett. 2003;190(1):73–77. doi: 10.1016/s0304-3835(02)00578-5. [DOI] [PubMed] [Google Scholar]
- 66.Aral C, Kaya H, Ataizi-Celikel C, Akkiprik M, Sonmez O, Gulluoglu BM, Ozer A. A novel approach for rapid screening of mitochondrial D310 polymorphism. BMC Cancer. 2006;6:21–0. doi: 10.1186/1471-2407-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou YL, Niu RF, Shi YR. Study on gene control region of mitochondrial DNA in familial breast cancer. Zhonghua Yi Xue Yi Chuan Xue Za Zhi . 2007;24(5):529–532. [PubMed] [Google Scholar]
- 68.Yu M. Somatic mitochondrial DNA mutations in human cancers. Adv. Clin. Chem . 2012;57:99–138. doi: 10.1016/b978-0-12-394384-2.00004-8. [DOI] [PubMed] [Google Scholar]
- 69.Ma Y, Bai RK, Trieu R, Wong LJ. Mitochondrial dysfunction in human breast cancer cells and their transmitochondrial cybrids. Biochim. Biophys.Acta. 2010;1797(1):29–37. doi: 10.1016/j.bbabio.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei L, Zhao Y, Guo TK, Li PQ, Wu H, Xie HB, Ma KJ, Gao F, Xie XD. Association of mtDNA D-Loop polymorphisms with risk of gastric cancer in chinese population. Pathol. Oncol. Res. 2011;17(3):735–742. doi: 10.1007/s12253-011-9378-7. [DOI] [PubMed] [Google Scholar]
- 71.Santos-Jr GC, Góoes AC, Vitto Hd, Moreira CC, Avvad E, Rumjanek FD, Gallo CV. Genomic instability at the 13q31 locus and somatic mtDNA mutation in the D-loop site correlate with tumor aggressiveness in sporadic Brazilian breast cancer cases. Clinics (Sao Paulo) 2012;67(10):1181–1190. doi: 10.6061/clinics/2012(10)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu C, Tran-Thanh D, Ma C, May K, Jung J, Vecchiarelli J, Done SJ. Mitochondrial D310 mutations in the early development of breast cancer. Br. J. Cancer. 2012;106(9):1506–1511. doi: 10.1038/bjc.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tseng LM, Yin PH, Chi CW, Hsu CY, Wu CW, Lee LM, Wei YH, Lee HC. Mitochondrial DNA mutations and mitochondrial DNA depletion in breast cancer. Genes Chromosomes Cancer. 2006;45(7):629–638. doi: 10.1002/gcc.20326. [DOI] [PubMed] [Google Scholar]
- 74.Ye C, Gao YT, Wen W, Breyer JP, Shu XO, Smith JR, Zheng W, Cai Q. Association of mitochondrial DNA displacement loop (CA)n dinucleotide repeat polymorphism with breast cancer risk and survival among Chinese women. Cancer Epidemiol. Biomarkers Prev. 2008;17(8):2117–2122. doi: 10.1158/1055-9965.EPI-07-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bai RK, Leal SM, Covarrubias D, Liu A, Wong LJ. Mitochondrial genetic background modifies breast cancer risk. Cancer Res . 2007;67(10):4687–4694. doi: 10.1158/0008-5472.CAN-06-3554. [DOI] [PubMed] [Google Scholar]
- 76.Sultana GN, Rahman A, Shahinuzzaman AD, Begum RA, Hossain CF. Mitochondrial DNA mutations---candidate biomarkers for breast cancer diagnosis in Bangladesh. Chin. J. Cancer . 2012;31(9):449–454. doi: 10.5732/cjc.012.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Covarrubias D, Bai RK, Wong LJ, Leal SM. Mitochondrial DNA variant interactions modify breast cancer risk. J. Hum. Genet. 2008;53(10):924–928. doi: 10.1007/s10038-008-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jakupciak JP, Maggrah A, Maragh S, Maki J, Reguly B, Maki K, Wittock R, Robinson K, Wagner PD, Thayer RE, Gehman K, Gehman T, Srivastava S, Ngom A, Dakubo GD, Parr RL. Facile whole mitochondrial genome resequencing from nipple aspirate fluid using MitoChip v2. . BMC Cancer. 2008;8:95–0. doi: 10.1186/1471-2407-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plak K, Czarnecka AM, Krawczyk T, Golik P, Bartnik E. Breast cancer as a mitochondrial disorder (Review). Oncol. Rep. 2009;21(4):845–851. doi: 10.3892/or_00000293. [DOI] [PubMed] [Google Scholar]
- 80.Canter JA, Kallianpur AR, Parl FF, Millikan RC. Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res. 2005;65(17):8028–8033. doi: 10.1158/0008-5472.CAN-05-1428. [DOI] [PubMed] [Google Scholar]
- 81.Darvishi K, Sharma S, Bhat AK, Rai E, Bamezai RN. Mitochondrial DNA G10398A polymorphism imparts maternal Haplogroup N a risk for breast and esophageal cancer. Cancer Lett. 2007;249(2):249–255. doi: 10.1016/j.canlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 82.Setiawan VW, Chu LH, John EM, Ding YC, Ingles SA, Bernstein L, Press MF, Ursin G, Haiman CA, Neuhausen SL. Mitochondrial DNA G10398A variant is not associated with breast cancer in African-American women. Cancer Genet. Cytogenet. 2008;181(1):16–19. doi: 10.1016/j.cancergencyto.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Czarnecka AM, Krawczyk T, Zdrozny M, Lubinski J, Arnold RS, Kukwa W, Scinska A, Golik P, Bartnik E, Petros JA. Mitochondrial NADH-dehydrogenase subunit 3 (ND3) polymorphism (A10398G) and sporadic breast cancer in Poland. Breast Cancer Res. Treat. 2010;121(2):511–518. doi: 10.1007/s10549-009-0358-5. [DOI] [PubMed] [Google Scholar]
- 84.Pezzotti A, Kraft P, Hankinson SE, Hunter DJ, Buring J, Cox DG. The mitochondrial A10398G polymorphism, interaction with alcohol consumption, and breast cancer risk. PLoS One. 2009;4(4):e5356–0. doi: 10.1371/journal.pone.0005356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verma M, Naviaux RK, Tanaka M, Kumar D, Franceschi C. Singh K. Meeting report mitochondrial DNA and cancer epidemiology. Cancer Res . 2007;67(2):437–439. doi: 10.1158/0008-5472.CAN-06-4119. [DOI] [PubMed] [Google Scholar]
- 86.Peng TI, Yu PR, Chen JY, Wang HL, Wu HY, Wei YH, Jou MJ. Visualizing common deletion of mitochondrial DNA-augmented mitochondrial reactive oxygen species generation and apoptosis upon oxidative stress. Biochim. Biophys. Acta . 2006;1762(2):241–255. doi: 10.1016/j.bbadis.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Harding AE, Holt IJ, Cooper JM, Schapira AH, Sweeney M, Clark JB, Morgan-Hughes JA. Mitochondrial myopathies genetic defects. Biochem. Soc. Trans. 1990;18(4):519–522. doi: 10.1042/bst0180519. [DOI] [PubMed] [Google Scholar]
- 88.Dani MA, Dani SU, Lima SP, Martinez A, Rossi BM, Soares F, Zago MA, Simpson AJ. Less DeltamtDNA4977 than normal in various types of tumors suggests that cancer cells are essentially free of this mutation. Genet. Mol. Res. 2004;3(3):395–409. [PubMed] [Google Scholar]
- 89.Shenkar R, Navidi W, Tavaré S, Dang MH, Chomyn A, Attardi G, Cortopassi G, Arnheim N. The mutation rate of the human mtDNA deletion mtDNA4977. Am. J. Hum. Genet. 1996;59(4):772–780. [PMC free article] [PubMed] [Google Scholar]
- 90.Pavicic WH, Richard SM. Correlation analysis between mtDNA 4977-bp deletion and ageing. Mutat. Res. 2009;670(1-2):99–102. doi: 10.1016/j.mrfmmm.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 91.Wheelhouse NM, Lai PB, Wigmore SJ, Ross JA, Harrison DJ. Mitochondrial D-loop mutations and deletion profiles of cancerous and noncancerous liver tissue in hepatitis B virus-infected liver. Br. J. Cancer . 2005;92(7):1268–1272. doi: 10.1038/sj.bjc.6602496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bianchi MS, Bianchi NO, Bailliet G. Mitochondrial DNA mutations in normal and tumor tissues from breast cancer patients. Cytogenet. Cell Genet. 1995;71(1):99–103. doi: 10.1159/000134072. [DOI] [PubMed] [Google Scholar]
- 93.Radpour R, Fan AX, Kohler C, Holzgreve W, Zhong XY. Current understanding of mitochondrial DNA in breast cancer. Breast J. 2009;15(5):505–509. doi: 10.1111/j.1524-4741.2009.00767.x. [DOI] [PubMed] [Google Scholar]
- 94.Meissner C, Bruse P, Mohamed SA, Schulz A, Warnk H, Storm T, Oehmichen M. The 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain a useful biomarker or more? Exp. Gerontol. 2008;43(7):645–652. doi: 10.1016/j.exger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 95.Shen L, Fang H, Chen T, He J, Zhang M, Wei X, Xin Y, Jiang Y, Ding Z, Ji J, Lu J, Bai Y. Evaluating mitochondrial DNA in cancer occurrence and development. Ann. N.Y. Acad. Sci. 2010;1201:26–33. doi: 10.1111/j.1749-6632.2010.05635.x. [DOI] [PubMed] [Google Scholar]
- 96.Sharp MG, Adams SM, Walker RA, Brammar WJ, Varley JM. Differential expression of the mitochondrial gene cytochrome oxidase II in benign and malignant breast tissue. J. Pathol. 1992;168:163–168. doi: 10.1002/path.1711680203. [DOI] [PubMed] [Google Scholar]
- 97.Brankovic-Magic M, Dobricic J, Krivokuca A. Genetics of breast cancer contribution of BRCA1/2 genes alterations to hereditary predisposition. Vojnosanit Pregl. 2012;69(8):700–706. [PubMed] [Google Scholar]
- 98.Lee EY. Tumor suppressor genes and their alterations in breast cancer. Semin. Cancer Biol. 1995;6(3):119–125. doi: 10.1006/scbi.1995.0019. [DOI] [PubMed] [Google Scholar]
- 99.Deng CX, Wang RH. Roles of BRCA1 in DNA damage repair a link between development and cancer. Hum. Mol. Genet . 2003;12(1):R113–123. doi: 10.1093/hmg/ddg082. [DOI] [PubMed] [Google Scholar]
- 100.Somasundaram K. Breast cancer gene 1 (BRCA1) role in cell cycle regulation and DNA repair--perhaps through transcription. J. Cell Biochem. 2003;88(6):1084–1091. doi: 10.1002/jcb.10469. [DOI] [PubMed] [Google Scholar]
- 101.Wu J, Lu LY, Yu X. The role of BRCA1 in DNA damage response. Protein Cell. 2010;1(2):117–123. doi: 10.1007/s13238-010-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Medema RH, Macurek L. Checkpoint control and cancer. Oncogene . 2012;31(21):2601–2613. doi: 10.1038/onc.2011.451. [DOI] [PubMed] [Google Scholar]
- 103.Kwong A, Ng EK, Wong CL, Law FB, Au T, Wong HN, Kurian AW, West DW, Ford JM, Ma ES. Identification of BRCA1/2 founder mutations in Southern Chinese breast cancer patients using gene sequencing and high resolution DNA melting analysis. PLoS One. 2012;7(9):e43994–0. doi: 10.1371/journal.pone.0043994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pilato B, De Summa S, Danza K, Papadimitriou S, Zaccagna P, Paradiso A, Tommasi S. DHPLC/SURVEYOR nuclease a sensitive, rapid and affordable method to analyze BRCA1 and BRCA2 mutations in breast cancer families. Mol. Biotechnol. 2012;52(1):8–15. doi: 10.1007/s12033-011-9468-5. [DOI] [PubMed] [Google Scholar]
- 105.Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J, Rebbeck TR, Tonin P, Neuhausen S, Barkardottir R, Eyfjord J, Lynch H, Ponder BA, Gayther SA, Zelada-Hedman M. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families.The Breast Cancer Linkage Consortium. Am. J. Hum. Genet. 1998;62(3):676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang B, Beeghly-Fadiel A, Long J, Zheng W. Genetic variants associated with breast-cancer risk comprehensive research synopsis, meta-analysis, and epidemiological evidence. Lancet Oncol. 2011;12(5):477–488. doi: 10.1016/S1470-2045(11)70076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Desrichard A, Bidet Y, Uhrhammer N, Bignon YJ. CHEK2 contribution to hereditary breast cancer in non-BRCA families. Breast Cancer Res. 2011;13(6):R119–0. doi: 10.1186/bcr3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Easton D, Ford D, Peto J. Inherited susceptibility to breast cancer. Cancer Surv. 1993;18:95–113. [PubMed] [Google Scholar]
- 109.van der Groep P, van der Wall E, van Diest PJ. Pathology of hereditary breast cancer. Cell Oncol. (Dordr): 2011;34(2):71–88. doi: 10.1007/s13402-011-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma J, Cai H, Wu T, Sobhian B, Huo Y, Alcivar A, Mehta M, Cheung KL, Ganesan S, Kong AN, Zhang DD, Xia B. PALB2 interacts with KEAP1 to promote NRF2 nuclear accumulation and function. Mol. Cell. Biol. 2012;32(8):1506–1517. doi: 10.1128/MCB.06271-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Neupert W. Protein import into mitochondria. Annu. Rev. Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 112.Coene ED, Hollinshead M, Waeytens AA, Schelfhout VR, Eechaute WP, Shaw MK, Van Oostveldt PM, Vaux DJ. Phosphorylated BRCA1 is predominantly located in the nucleus and mitochondria. Mol. Biol. Cell. 2005;16(2):997–1010. doi: 10.1091/mbc.E04-10-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maniccia AW, Lewis C, Begum N, Xu J, Cui J, Chipitsyna G, Aysola K, Reddy V, Bhat G, Fujimura Y, Henderson B, Reddy ES, Rao VN. Mitochondrial localization, ELK-1 transcriptional regulation and growth inhibitory functions of BRCA1, BRCA1a, and BRCA1b proteins. J. Cell Physiol . 2009;219(3):634–641. doi: 10.1002/jcp.21708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bandiera S, Rüberg S, Girard M, Cagnard N, Hanein S, Chrétien D, Munnich A, Lyonnet S, Henrion-Caude A. Nuclear outsourcing of RNA interference components to human mitochondria. PLoS One. 2011;6(6):20746–0. doi: 10.1371/journal.pone.0020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hossein R, Houshmand M. Diagnostic algorithm for identification of individuals with hereditary predisposition to breast cancer. Lik Sprava . 2008;(1-2):103–108. [PubMed] [Google Scholar]
- 116.Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, Ozden O, Park SH, Singh KK, Abdulkadir SA, Spitz DR, Deng CX, Gius D. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17(1):41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park SH, Ozden O, Jiang H, Cha YI, Pennington JD, Aykin-Burns N, Spitz DR, Gius D, Kim HS. Sirt3 Mitochondrial ROS, Ageing, and Carcinogenesis. Int. J. Mol. Sci. 2011;12(9):6226–6239. doi: 10.3390/ijms12096226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jandova J, Janda J, Sligh JE. Changes in mitochondrial DNA alter expression of nuclear encoded genes associated with tumorigenesis. Exp. Cell. Res. 2012;318(17):2215–2225. doi: 10.1016/j.yexcr.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suhane S, Berel D, Ramanujan VK. Biomarker signatures of mitochondrial NDUFS3 in invasive breast carcinoma. Biochem. Biophys. Res. Commun . 2011;412(4):590–595. doi: 10.1016/j.bbrc.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gochhait S, Bhatt A, Sharma S, Singh YP, Gupta P, Bamezai RN. Concomitant presence of mutations in mitochondrial genome and p53 in cancer development - a study in north Indian sporadic breast and esophageal cancer patients. Int. J. Cancer . 2008;123(11):2580–2586. doi: 10.1002/ijc.23817. [DOI] [PubMed] [Google Scholar]
- 121.Fang H, Shen L, Chen T, He J, Ding Z, Wei J, Qu J, Chen G, Lu J, Bai Y. Cancer type-specific modulation of mitochondrial haplogroups in breast, colorectal and thyroid cancer. BMC Cancer . 2010;10:421–0. doi: 10.1186/1471-2407-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen L, Wei J, Chen T, He J, Qu J, He X, Jiang L, Qu Y, Fang H, Chen G, Lu J, Bai Y. Evaluating mitochondrial DNA in patients with breast cancer and benign breast disease. J. Cancer Res. Clin. Oncol. 2011;137(4):669–675. doi: 10.1007/s00432-010-0912-x. [DOI] [PubMed] [Google Scholar]