Abstract
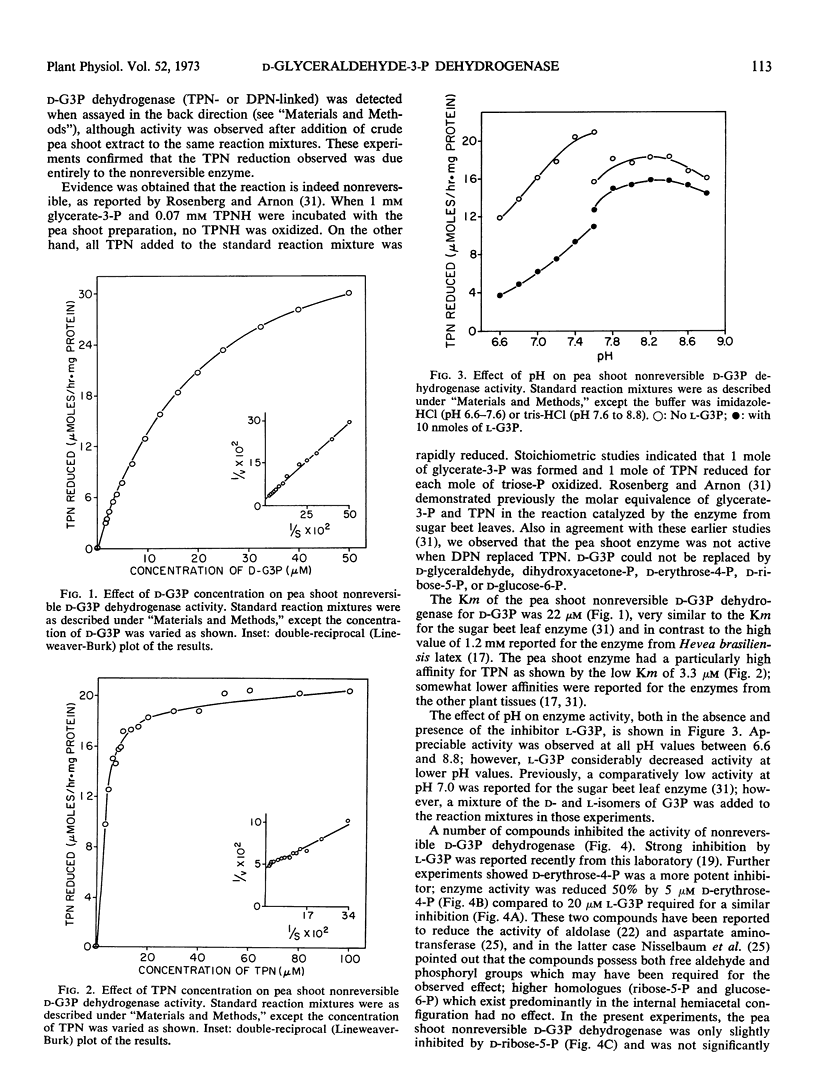
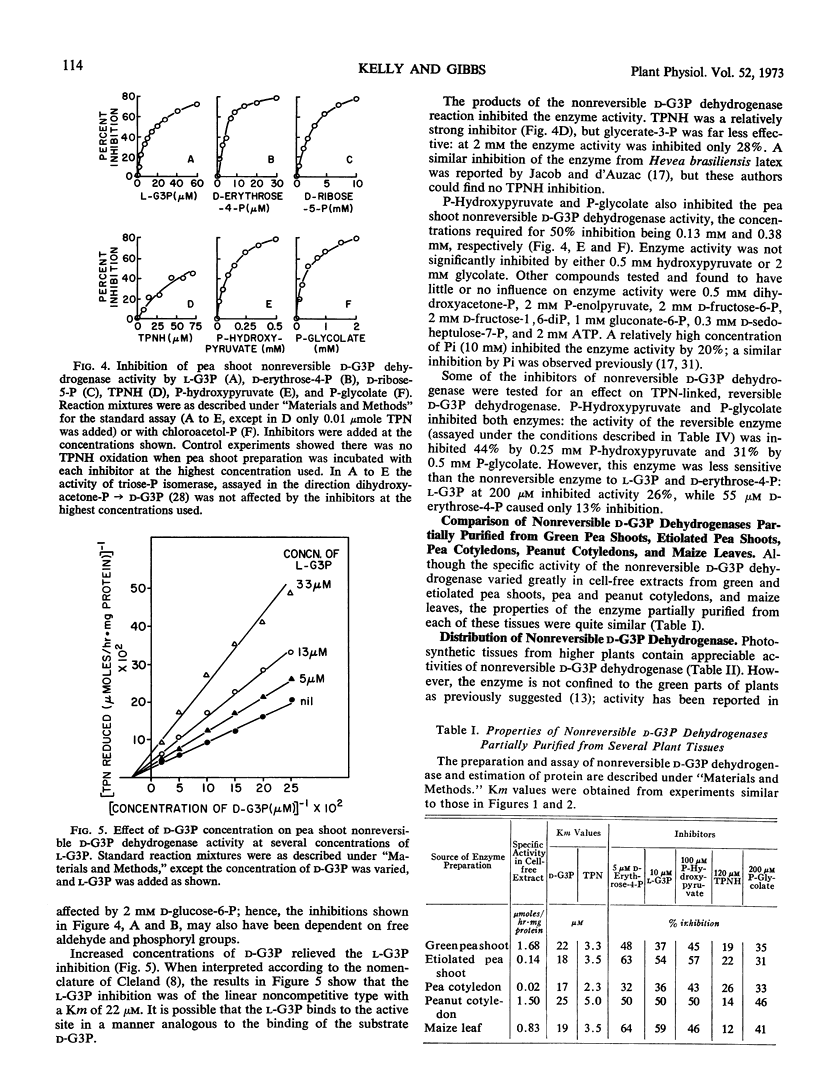
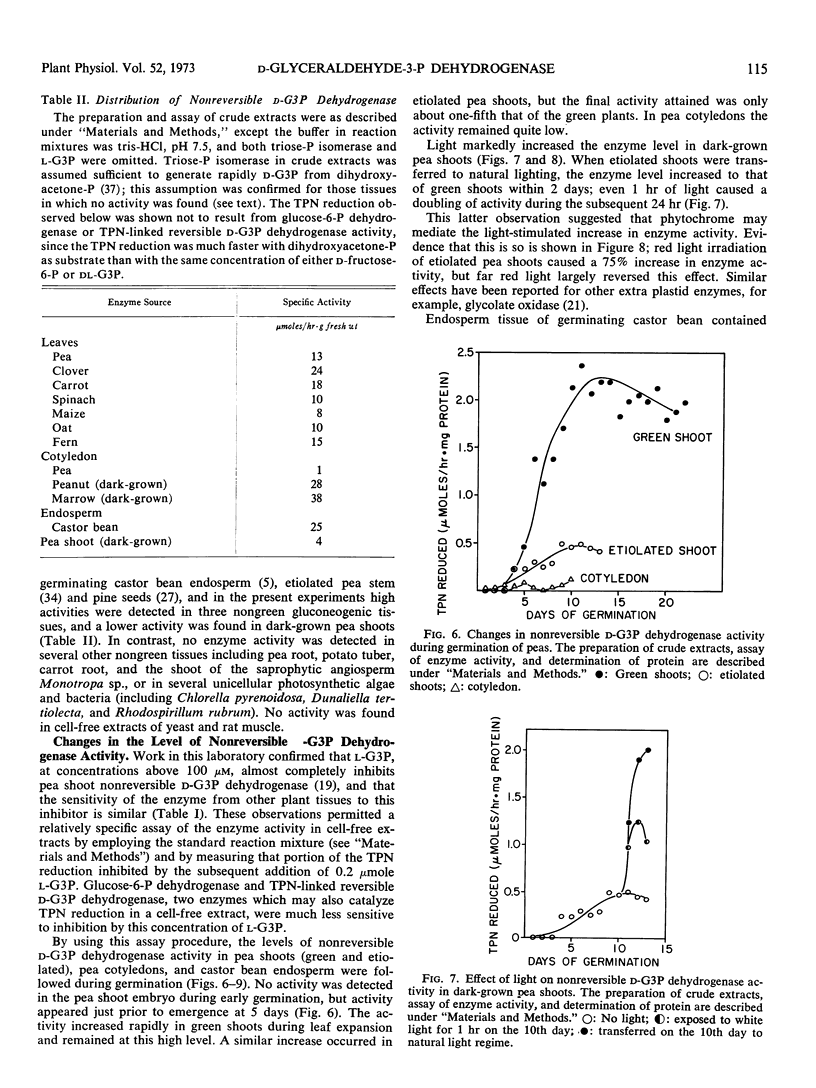
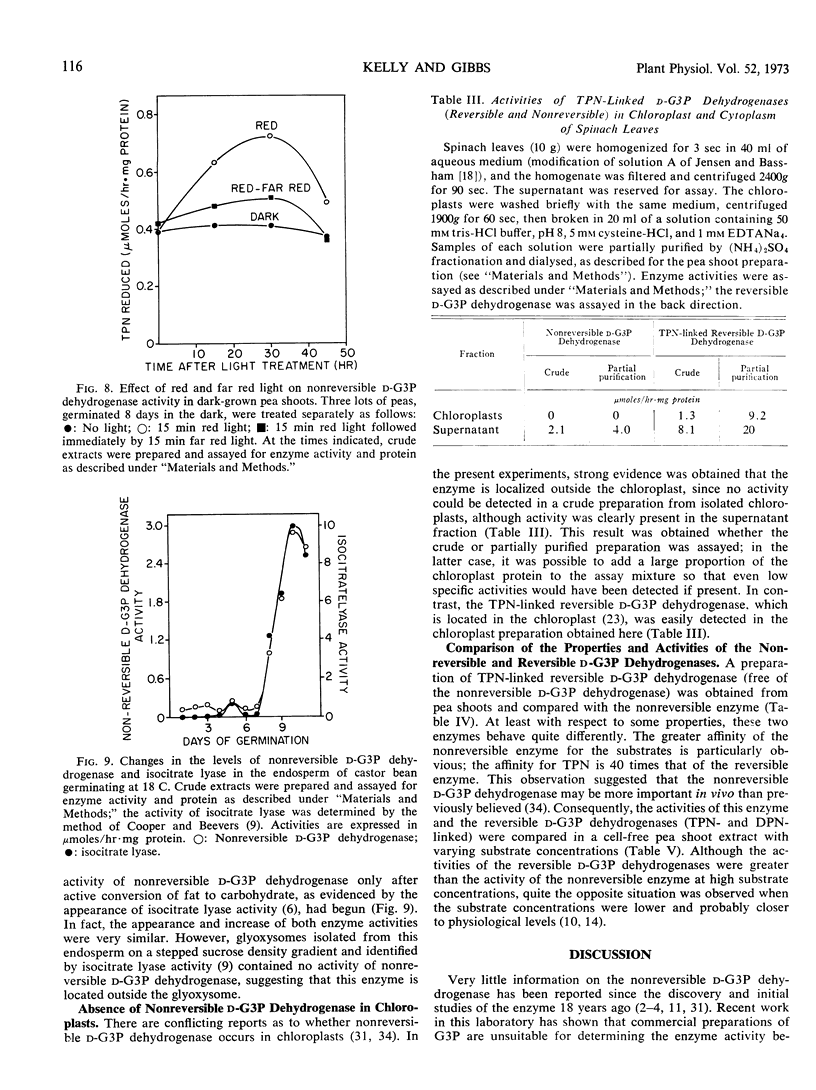
Preparations of TPN-linked nonreversible d-glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.9), free of TPN-linked reversible d-glyceraldehyde 3-phosphate dehydrogenase, have been obtained from green shoots, etiolated shoots, and cotyledons of pea (Pisum sativum), cotyledons of peanut (Arachis hypogea), and leaves of maize (Zea mays). The properties of the enzyme were similar from each of these sources: the Km values for d-glyceraldehyde 3-phosphate and TPN were about 20 μm and 3 μm, respectively. The enzyme activity was inhibited by l-glyceraldehyde 3-phosphate, d-erythrose 4-phosphate, and phosphohydroxypyruvate. Activity was found predominantly in photosynthetic and gluconeogenic tissues of higher plants. A light-induced, phytochrome-mediated increase of enzyme activity in a photosynthetic tissue (pea shoots) was demonstrated. Appearance of enzyme activity in a gluconeogenic tissue (endosperm of castor bean, Ricinus communis) coincided with the conversion of fat to carbohydrate during germination. In photosynthetic tissue, the enzyme is located outside the chloroplast, and at in vivo levels of triose-phosphates and pyridine nucleotides, the activity is probably greater than that of DPN-linked reversible d-glyceraldehyde 3-phosphate dehydrogenase. Several possible roles for the enzyme in plant carbohydrate metabolism are considered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., BANDURSKI R. S., GREINER C. M., JANG R. The metabolism of hexose and pentose phosphates in higher plants. J Biol Chem. 1953 Jun;202(2):619–634. [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):237–244. doi: 10.1016/0005-2744(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Barnett R. C., Stafford H. A., Conn E. E., Vennesland B. Phosphogluconic Dehydrogenase in Higher Plants. Plant Physiol. 1953 Jan;28(1):115–122. doi: 10.1104/pp.28.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Carpenter W. D., Beevers H. Distribution and Properties of Isocitritase in Plants. Plant Physiol. 1959 Jul;34(4):403–409. doi: 10.1104/pp.34.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Effer W. R., Ranson S. L. Some effects of oxygen concentration on levels of respiratory intermediates in buckwheat seedlings. Plant Physiol. 1967 Aug;42(8):1053–1058. doi: 10.1104/pp.42.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M. The Respiration of the Pea Plant. Oxidation of Hexose Phosphate and Pentose Phosphate by Cell-free Extracts of Pea Leaves. Plant Physiol. 1954 Jan;29(1):34–39. doi: 10.1104/pp.29.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGEMAN R. H., ARNON D. I. Changes in glyceraldehyde phosphate dehydrogenase during the life cycle of a green plant. Arch Biochem Biophys. 1955 Aug;57(2):421–436. doi: 10.1016/0003-9861(55)90304-0. [DOI] [PubMed] [Google Scholar]
- HORECKER B. L., SMYRNIOTIS P. Z. Purification and properties of yeast transaldolase. J Biol Chem. 1955 Feb;212(2):811–825. [PubMed] [Google Scholar]
- Harvey M. J., Brown A. P. Nicotinamide cofactors of intact chloroplasts isolated on a sucrose density gradient. Biochim Biophys Acta. 1969 Jan 14;172(1):116–125. doi: 10.1016/0005-2728(69)90096-6. [DOI] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- Jacob J. L., D'Auzac J. La glyćeraldehyde-3-phosphate déshydrogénalse du latex d'Hevea brasiliensis. Comparaison avec son homologue phosphorylante. Eur J Biochem. 1972 Dec 4;31(2):255–265. doi: 10.1111/j.1432-1033.1972.tb02528.x. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Turner J. F. The regulation of pea-seed phosphofructokinase by 6-phosphogluconate, 3-phosphoglycerate, 2-phosphoglycerate and phosphoenolpyruvate. Biochim Biophys Acta. 1970 Jun;208(3):360–367. doi: 10.1016/0304-4165(70)90207-2. [DOI] [PubMed] [Google Scholar]
- Klein A. O. Persistent photoreversibility of leaf development. Plant Physiol. 1969 Jun;44(6):897–902. doi: 10.1104/pp.44.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. Y., Martinez-de Dretz G., Bacila M., Marinello E., Horecker B. L. Labeling of the active site of aldolase with glyceraldehyde 3-phosphate and erythrose 4-phosphate. Biochem Biophys Res Commun. 1968 Mar 27;30(6):665–672. doi: 10.1016/0006-291x(68)90564-0. [DOI] [PubMed] [Google Scholar]
- Nisselbaum J. S., Sweetman L., Kopelovich L. Regulation of aspartate aminotransferase isozymes by glyceraldehyde-3-phosphate. Adv Enzyme Regul. 1972;10:273–287. doi: 10.1016/0065-2571(72)90018-0. [DOI] [PubMed] [Google Scholar]
- Norton I. L., Hartman F. C. Haloacetol phosphates. A comparative study of the active sites of yeast and muscle triose phosphate isomerase. Biochemistry. 1972 Nov 21;11(24):4435–4441. doi: 10.1021/bi00774a003. [DOI] [PubMed] [Google Scholar]
- OESPER P., MEYERHOF O. The determination of triose phosphate isomerase. Arch Biochem. 1950 Jun;27(1):223–233. [PubMed] [Google Scholar]
- ROSENBERG L. L., ARNON D. I. The preparation and properties of a new glyceraldehyde-3-phosphate dehydrogenase from photosynthetic tissues. J Biol Chem. 1955 Nov;217(1):361–371. [PubMed] [Google Scholar]
- Robinson J. M., Stocking C. R. Oxygen evolution and the permeability of the outer envelope of isolated whole chloroplasts. Plant Physiol. 1968 Oct;43(10):1597–1604. doi: 10.1104/pp.43.10.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman M. D., Gibbs M. D-glyceraldehyde 3-phosphate dehydrogenases of higher plants. Plant Physiol. 1968 Nov;43(11):1805–1812. doi: 10.1104/pp.43.11.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter J. C., Davies D. D. The isolation and characterization of 3-phosphoglycerate dehydrogenase from peas. Biochem J. 1968 Oct;109(5):743–748. doi: 10.1042/bj1090743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C. R., Larson S. A chloroplast cytoplasmic shuttle and the reduction of extraplastid NAD. Biochem Biophys Res Commun. 1969 Oct 8;37(2):278–282. doi: 10.1016/0006-291x(69)90731-1. [DOI] [PubMed] [Google Scholar]
- Turner D. H., Blanch E. S., Gibbs M., Turner J. F. Triosephosphate Isomerase of Pea Seeds. Plant Physiol. 1965 Nov;40(6):1146–1150. doi: 10.1104/pp.40.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENKATARAMAN R., RACKER E. Mechanism of action of transaldolase. I. Crystalization and properties of yeast enzyme. J Biol Chem. 1961 Jul;236:1876–1882. [PubMed] [Google Scholar]


