Abstract
Natural compounds containing fungal β-glucans have been used to improve general health for thousands of years in China and Japan. Lentinan, the backbone of β-(1, 3)-glucan with β-(1, 6) branches, is one of the active ingredients purified from Shiitake mushrooms and has been approved as a biological response modifier for the treatment of gastric cancer in Japan. Despite recent advances in chemotherapeutic agents, unresectable or recurrent gastric cancer remains an incurable disease, with survival rates being far from satisfactory. Recent clinical studies have shown that chemo-immunotherapy using lentinan prolongs the survival of patients with advanced gastric cancer, as compared to chemotherapy alone. In addition, trastuzumab, an antibody against HER2/neu growth factor receptor, has been used for the treatment of gastric cancer in combination with cytotoxic chemotherapeutic agents. Lentinan may exert a synergistic action with anti-cancer monoclonal antibodies to activate complement systems through the mechanism of antibody-dependent cellular cytotoxicity and complement dependent cytotoxicity. Because a better understanding of its biological activities should enable us to use lentinan more efficiently in the treatment of gastric cancer, immunological effects provided by β-glucans, a possible mode of action of lentinan, and its clinical application including future potential uses are discussed in the present review.
Keywords: Gastric cancer, β-glucan, Lentinan.
INTRODUCTION
Gastric cancer is the second most common cause of cancer-related death in the world [1, 2]. Although both incidence and mortality of this disease have decreased in developed countries, it is a significant problem in global health terms. In particular, unresectable advanced/recurrent gastric cancer remains an incurable disease [3]. Chemotherapy has been used in an attempt to both control cancer-related symptoms and improve survival [3-5]. Despite recent advances in chemotherapy for gastric cancer, the outcomes of anticancer therapy remain unsatisfactory, especially in terms of survival. Thus, further improvement of therapies for gastric cancer is necessary.
The medicinal qualities of mushrooms have been known for thousands of years and their consumption is an old tradition mainly in China and Japan [6, 7]. Although a number of fungal components have been implicated in these properties, β-glucans were actually identified as biologically active constituents [6, 8]. β-glucans are major cell wall structural components in fungi, yeast, certain bacteria, and cereal plants such as oats and barley [9, 10]. These polysaccharides are well known to be biological response modifiers (BRMs) which stimulate the immune system through activation of various immune cells including macrophages, dendritic cells, neutrophils, natural killer (NK) cells, and lymphocytes. BRMs have been used for cancer therapy in combination with cytotoxic-chemotherapeutic agents [11]. There are several reports describing in vivo administration of β-glucans as potentiating the host response against tumor development [12, 13]. In Japan, two types of β-glucans, krestin and lentinan, are licensed as drugs for gastric cancer treatment. Krestin, a protein-bound polysaccharide K (PSK) containing β-(1, 3)-glucan, is derived from Coriolus versicolor. This agent has been used clinically in postoperative treatment of resectable gastric cancer [14-16]. However, PSK is not a chemically pure β-glucan and the underlying mechanism is thus rather difficult to elucidate.
On the other hand, lentinan is purified β-glucan from Shiitake mushrooms [17, 18] and has been used in combination with oral fluoropyrimidines for treating gastric cancer in both adjuvant settings and far advanced tumor stages [19, 20]. In this review, we discuss the potential role and future uses of lentinan in the treatment of gastric cancer.
EFFECTS OF β-GLUCAN ON THE IMMUNE SYSTEM
β-glucans from fungi constitute a heterogeneous group of glucose polymers, consisting of a backbone of β-(1, 3)–linked β-D-glucopyranosyl units with β-(1, 6) linked side chains of varying distributions and lengths (Fig. 1). As β-glucans are not found in animals, they stimulate the immune system and induce innate immune responses, which protect us from attack by pathogenic microbes [6, 9]. The immunomodulatory effects of β-glucans are known to be inconsistent and variable, probably due to differences in the degree of branching, polymer length, and tertiary structures among β-glucans (Fig. 2). Certain glucans, including zymosan and lentinan appear to efficiently activate phagocytes [21]. Whereas neutrophils are effective against pyogenic bacteria, NK cells circulate in blood to lyse cancer and virus-infected cells. In addition, β-glucans stimulate macrophages to produce cytokines, local immunomodulators, and these in turn activate adaptive immunity against foreign antigens, which involves both B and T cells. B cells produce antibodies to mediate humoral immunity, whereas T cells induce cell-mediated immunity. The adaptive immune response also involves dendritic cells (DCs) derived from monocytes, and these present antigens to T cells for activation of immune responses. There are several reports indicating that DCs are functionally defective in tumor-bearing host [22, 23]. β-glucans were reported to enhance the antigen presenting function of DCs [24], thereby inducing tumor-specific cytotoxic T cells.
Fig. (1).
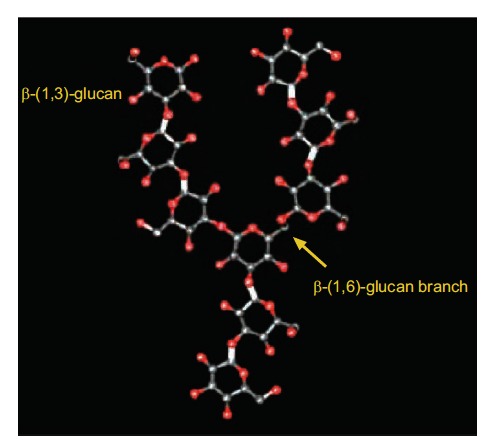
The structure of β-glucans [34]. β-glucans from fungi consist of a backbone of β-(1, 3)-glucan with various degrees of β-(1, 6) glucan branching.
Fig. (2).
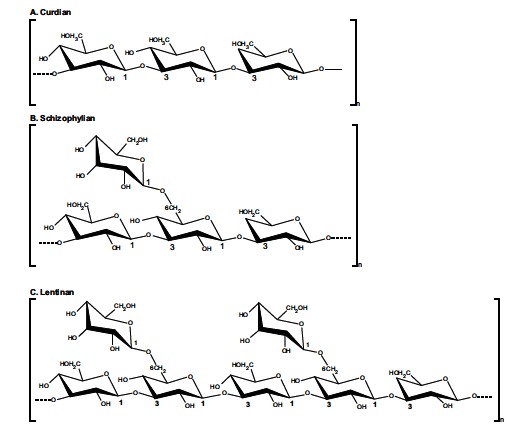
Examples of structures of microbial β-glucans showing the branching patterns of their repeating units [6].
In addition, when the constant region (Fc) of an immunoglobulin interacts with receptors for the Fc domain of IgG (Fc gamma R) on leucocytes, a variety of biological responses are triggered; phagocytosis, enhancement of antigen presentation, release of inflammatory mediators, and antibody-dependent cellular cytotoxicity (ADCC) [25, 26]. Fc gamma R (FcR) provides a critical link between specific humoral responses and cellular immunity. β-glucans were reported to enhance the expression of FcR [27] and the activation of complements [28, 29]. Therefore, β-glucans function actively in cooperation with anti-tumor monoclonal antibodies (mAbs) used in cancer treatment [30, 31].
POSSIBLE MECHANISMS OF ACTION OF LENTINAN
β-glucans are recognized via a number of cell surface receptors by the immune system as non-self molecules, inducing both innate and adaptive immune responses [6, 21]. Several receptors have been identified in humans, and these include Dectin-1, the toll-like receptor (TLR), complement receptor type 3 (CR3), scavenger receptors, and lactosylceramide (LacCer) (Fig. 3). Dectin-1, the C-type lectin family of receptors, is commonly expressed in macrophages, neutrophils, DCs, and some T-cells, but not in NK cells [32, 33]. Binding of Dectin-1 with β-glucans activates several signaling pathways to promote innate immune responses such as the induction of inflammatory cytokines, activation of phagocytosis, and reactive oxygen species (ROS) production [6, 34]. First, it might act synergistically with TLR to produce various cytokines such as interleukin (IL)-2 and IL-12 through activation of myeloid differentiation primary response gene 88 (MyD88) [35]. Another signaling pathway is mediated by spleen tyrosine kinase (Syk) [36, 37], which in turn activates the caspase recruitment domain 9-bcl10-malt1 complex (CARD9). This complex mediates the induction of NF-kβ [38], which also leads to productions of cytokines such as TNF-α and IL-12. In addition, Dectin-1 can trigger cellular responses to β-glucans independently of the TLRs, including phagocytosis and oxidative burst [33]. The CR3 receptor, leukocyte β2-integrin, consists of CD11b and CD18 domains and is expressed mainly on neutrophils, monocytes, and NK cells, but not macrophages [39, 40]. CR3 functions as an adhesion molecule and there is now evidence that it also activates Syk [41]. NK cells have no Dectin-1 receptor, so CR3 may be the major receptor for NK cells. CR3 on monocytes, granulocytes (neutrophils and eosinophils), and NK cells mediates adherence to inactivated C3b (iC3b)-opsonized targets and involves complement dependent cytotoxicity (CDC) [33]. However, CR3 can be triggered by iC3b deposited onto yeast or fungal cell walls [42], but not cancer cells [43]. This is because the activation of CR3 for CDC requires its dual ligation to both iC3b and cell wall β-glucan [44]. By using β-glucan in combination with anti-tumor mAbs activating complements, the coat of iC3b on tumor cells triggers CR3 dependent cytotoxicity by monocytes, granulocytes, and NK cells [45]. Without β-glucan, anti-tumor mAbs such as trastuzumab and rituximab have limited effector mechanisms including complement-mediated cell lysis and ADCC [46, 47]. The β-glucan primed cells, such as granulocytes and NK cells, then specifically recognize these complement-antibody complexes and kill the coated tumor cells more efficiently. Scavenger receptors located in myeloid and endothelial cells recognize a range of foreign cells, low density lipoprotein, and high-density lipoprotein [48, 49]. Signaling pathways are mediated by phosphatidylinositol-3 kinase (PI3K), leading to stimulation of Akt kinase, MAPK, and an endothelial nitric oxide synthase [50]. LacCer located in neutrophil and endothelial cells is a glycolipid containing a hydrophobic ceramide lipid and a hydrophilic sugar moiety [51]. It has been suggested that the interaction of β-glucan with this receptor can induce macrophage inflammatory protein-2 [52], the activation of NFκB [53], and neutrophil oxidative burst [54]. However, the mechanisms underlying these activities are unknown.
Fig. (3).
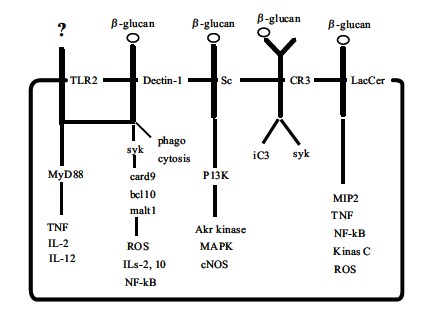
Possible fungal β-glucan mediated signal pathways [6].
Lentinan purified from Shiitake mushrooms has two β-(1, 6) side chains every five β-(1, 3)–linked backbone residues (Fig. 4). While the association between lentinan and scavenger receptors and LacCer has not yet been clarified, this immunomodulator is suspected to bind to human leukocytes through both complement receptors, CR1 (CD35) and CR3 (CD11b) as well as Dectin-1 (personal communication: Suga Y, et al). In cancer patients, it is well known that DCs are functionally defective [22, 23] and T-cell function as well as NK activity are also down-regulated [55, 56]. Mushiake et al described lentinan as activating DC function by increasing the number of tumor-infiltrating CD86+ cells in cancer-bearing mice [24]. The administration of lentinan was reported to stimulate the generation of both killer T cells and NK cells [12, 57, 58] and then restore the ratio of killer/suppressor T cells [59]. Lentinan up-regulated NK cell-mediated killing of tumor cells [60, 61] partly because of increased FcR expression [27], which might be involved in ADCC augmentation. The addition of lentinan activated either the classical or the alternative complement pathway [62] and eventually enhanced CDC and complement-dependent cell-mediated cytotoxicity via CR3. Taken together, these observations suggest that immunotherapy using lentinan may have synergistic effects with anti-cancer mAbs [63, 64].
Fig. (4).
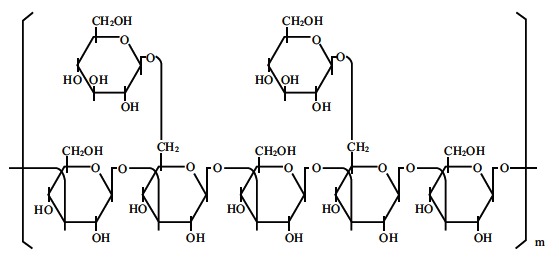
The structure of lentinan.
The activation of macrophages and monocytes by binding of lentinan to specific receptors induces IL-12 production [65], although the details of downstream signaling have not been fully elucidated. At the same time, lentinan decreases serum levels of IL-6 and PGE2 in patients with digestive tract cancer [66] and might prevent the Th2-dominant condition. As a result, lentinan induces Th1 polarization and improves the balance between Th1 and Th2. It has been noted that with the progression of cancer, the proportion of granulocytes increases in peripheral blood [15, 67]. Granulocytes reportedly suppress the antitumor activities of lymphocytes and lymphocyte-activated tumor cell killing [68, 69], such that the increased numbers of granulocytes promote tumor growth by antagonizing tumor-suppressing lymphocytes. The increase in granulocytes may be based on the G-CSF increment in cancer patients and the ratio of granulocytes/lymphocytes (G/L ratio) becoming higher in the advanced stage, as compared to the early stage [70, 71]. The G/L ratio was previously reported to correlate with prognosis in gastric cancer patients [72]. The administration of lentinan was found to decrease the serum G-CSF levels in cancer patients [59], which might eventually decrease the G/L ratio. Because the G/L ratio can easily be determined, even in a retrospective analysis, this ratio was chronologically determined as a parameter of its immunological effects in both a group of patients receiving chemotherapy alone and another group given chemotherapy in combination with lentinan [73]. At the start of chemotherapy, the G/L ratio was almost the same with or without lentinan treatment (Fig. 5A). However, at either 1 year after initiation of chemotherapy or 1 month before death (in cases in which the survival time was less than 1 year after starting chemotherapy), the G/L ratio of patients receiving lentinan was maintained at or below 2, which is significantly lower than those of patients who received chemotherapy alone (P < 0.001) (Fig. 5B).
Fig. (5).
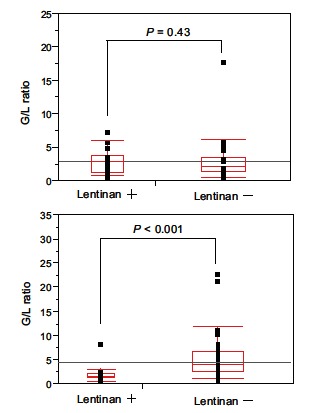
Comparison of the granulocyte/lymphocyte ratio between patients who received (Lentinan+) and those who did not (Lentinan-) using the x2 test [73]. A: Before therapy. B: At either 1 year after initiation of the S-1 based chemotherapy or 1 mo prior to death (cases in which the survival time was < 1 year after starting chemotherapy). P values of less than 0.05 were considered statistically significant. G/L ratio: Granulocyte/lymphocyte ratio.
CLINICAL APPLICATION OF LENTINAN IN THE TREATMENT OF GASTRIC CANCER
The clinical efficacy of lentinan has been reported in terms of survival in patients with unresectable and recurrent gastric cancer receiving an oral fluoropyrimidine (tegafur) [20]. A meta-analysis conducted by Oba et al showed that the addition of lentinan to chemotherapy prolonged the survival of patients with advanced gastric cancer as compared to chemotherapy alone [19]. Although the difference in median overall survival (OS) was statistically significant in this meta-analysis between patients with and without administration lentinan, the increased survivals (139 days versus 114 days; P = 0.011) were rather short, compared to those in a recent clinical study [74, 75]. This is explained by the fact that all 5 clinical studies used in this analysis were performed in the 1980s. Oral fluoropyrimidine, S-1, has since been newly combined with 2 modulating substances: gimeracil to inhibit dihydropyrimidine dehydrogenase and potassium oxonate to reduce gastrointestinal toxicities [76]. The anti-tumor effects of fluoropyrimidine are enhanced through biochemical modulation of folate metabolism modified by cisplatin [77], and combination therapy using S-1 and cisplatin reportedly achieves higher response rates than S-1 monotherapy [74, 75, 78]. In addition, taxane derivatives such as docetaxel and paclitaxel have a unique mechanism of action that differs from those of fluoropyrimidines and platinum compounds [79, 80], so that these compounds can be combined with either S-1 or S-1 plus cisplatin for the treatment of advanced gastric cancer [81-84]. As a result, in Japan, these S-1-based regimens are now widely used for the treatment of unresectable or recurrent gastric cancer. However, disease progression is still observed in some patients receiving S-1-based chemotherapy [75, 84, 85] and further improvement of chemotherapy is warranted. With the aim of evaluating the effect of chemo-immunotherapy using lentinan, 78 patients with unresectable/recurrent gastric cancer receiving S-1-based chemotherapy were retrospectively examined and their OS were then compared between treatments with versus without lentinan [73]. S-1-based chemotherapy was continued as long as possible. The median OS was significantly longer in the group that received chemo-immunotherapy with lentinan than in the chemotherapy alone group (689 days [95% CI, 467–2324 days]) versus 565 days [95% CI, 323–662 days]), P = 0.0406) (Fig. 6). One-, two-, and five-year survival rates were better in the group that received lentinan than in the group that received chemotherapy alone, (91.3% versus 59.4%, 45.7% versus 32.7%, 10.0% versus 0%, respectively). These data on S-1-based chemotherapy support the results of a meta-analysis which showed that the addition of lentinan to chemotherapy prolonged the survival of patients with advanced gastric cancer, as compared to chemotherapy alone.
Fig. (6).
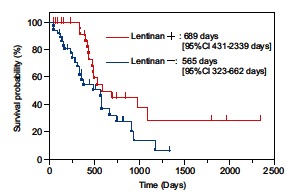
Kaplan-Meier curves of overall survival [73]. Lentinan+: The group that received chemo-immunotherapy with lentinan. Lentinan-: The group that received S-1 based chemotherapy alone. OS: overall survival.
Furthermore, recent advances have led to the development of targeted therapies that specifically inhibit the growth of cancer cells [26, 30, 86]. The human epidermal growth factor receptor 2 (HER2) is a member of the ErbB family that plays an important role in promoting oncogenic transformation and tumor growth [87, 88]. Approximately 20-30% of all breast cancers overexpress HER2 [89]. Antibodies against the HER2/neu growth factor receptor prevent the growth of breast carcinoma cells in vitro and in vivo [90, 91] and target treatment of HER2-positive metastatic breast cancer has been shown to demonstrate favorable efficacy [92, 93]. Trastuzumab, a humanized IgG1 antibody specific for the cellular proto-oncogene HER2/neu, was also approved for the treatment of HER-2 positive gastric cancer [94]. There is evidence supporting a role for trastuzumab in mediating ADCC [95]. The interaction between the Fc domain of trastuzumab and FcR on effecter cells has shown major ADCC involvement and IL-12 enhances cytotoxicity against mAb-coated tumor cells [96, 97]. NK cytotoxicity via ADCC is probably one of the mechanisms of action of trastuzumab, but its mode of action includes CDC and complement-dependent cell-mediated cytotoxicity [98, 99]. It has also been reported that platinum compounds exert a synergistic action with trastuzumab in preclinical models of gastric cancer [100]. The objective of a study administering trastuzumab for gastric cancer (ToGA) was to show that addition of this mAb to chemotherapy using capecitabine and cisplatin significantly improved survival in patients with advanced gastric cancer as compared with chemotherapy alone (13.8 months [95% CI, 12-16]) versus 11.1 months [95% CI, 10-13]), P = 0.0046) [94]. The binding of lentinan to leukocytes could induce IL-12 production and enhance the anti-tumor effects of mAbs through augmented ADCC and complement mediated cytotoxicity via CR3 activation. An in vivo study clearly demonstrated lentinan to significantly suppress tumor growth in combination with trastuzumab (Fig. 7) [64]. Considering these properties of lentinan, its synergistic action with targeting cancer therapy might be expected when this immunomodulator is used in combination with trastuzumab plus cytotoxic chemotherapeutic agents for patients with HER-2 positive gastric cancer.
Fig. (7).
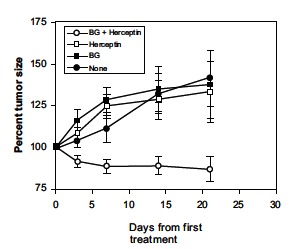
Synergy of β-glucan with anti-HER2 antibody (Herceptin) against human breast carcinoma BT474 xenografts in nude mice [64]. In contrast to controls (solid circles), Herceptin (open squares), or β-glucan controls (BG: solid squares), the combination of Herceptin and β-glucan (open circles) significantly suppressed tumor growth (P = 0.002).
CONCLUSION
Chemo-immunotherapy in combination with S-1-based chemotherapy and lentinan might be among the potential candidates for standard treatment of patients with unresectable or recurrent advanced gastric cancer. In Japan, a phase III study comparing therapy using S-1/lentinan with S-1 alone is now under-way. Moreover, as targeted therapy has been applied for gastric cancer, the role of lentinan as an enhancer of the effect of mAb therapy should be examined in clinical settings.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Hohenberger P, Gretshel S. Gastric cancer. Lancet. 2003;362:305–315. doi: 10.1016/s0140-6736(03)13975-x. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dorce GM, Anderson WF. Patterns of cancer incidence mortality and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi R, Scartozzi M, Romagnoli E, Antognoli S, Cascinu S. Gastric cancer treatment: A system review. Oncol. Rep. 2004;11:911–916. [PubMed] [Google Scholar]
- 4.Wohler SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Annals Oncol. 2004;15:1585–1595. doi: 10.1093/annonc/mdh422. [DOI] [PubMed] [Google Scholar]
- 5.Sastre J, Garcia-Saenz JA, Diaz-Rubio E. Chemotherapy for gastric cancer. World J. Gastroenterol. 2006;12 :204–213. doi: 10.3748/wjg.v12.i2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Seviour R. Medicinal importance of fungal β--(1, 3), (1, 6)-glucans. Mycol. Res. 2007:635–652. doi: 10.1016/j.mycres.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Raymond K. β-glucans in the treatment of diabetes and associated cardiovascular risks. Vascul. Health Risk Manag. 2008;4 :1265–1272. doi: 10.2147/vhrm.s3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetvicka V. Glucan-immunostimulant adjuvant potential drug. World J. Clin. Oncol. 2011;2 :115–119. doi: 10.5306/wjco.v2.i2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy EA, Davis JM, Carmichael MD. Immune modulating effects of β-glucan. Curr. Opi.Clin. Nutr. Metab. Care. 2010;13 :656–661. doi: 10.1097/MCO.0b013e32833f1afb. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka K, Tanaka Y, Suzuki T, Mizushima T. Protective effect of β-(1,3→1.6)-D-glucan against irritant-induced gastric lesions. Br. J. Nutr. 2011;106 :475–485. doi: 10.1017/S0007114511000365. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto J, Teramukai S, Nakazato H, Sato Y, Uchino J, Taguchi T. Efficacy of adjuvant immunochemotherapy with OK-432 for patients with curatively resected gastric cancer: A meta-analysis of centrally randamized controlled clinical trials. J. Immunother. 2002;25 :405–412. doi: 10.1097/00002371-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hamuro J, Rollinghoff M, Wagner H. β-(1, 3) glucan-mediated augmentation of alloreactive murine cytotoxic T-lymphocytes in vivo. Cancer Res. 1978;38:3080–3085. [PubMed] [Google Scholar]
- 13.Chan GC, Chan WK, Sze DM. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009;2 :25–29. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Lancet. 1994;343 :1122–1126. doi: 10.1016/s0140-6736(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 15.Toge T, Yamaguchi Y. Protein-bound polysaccharide increases survival in resected gastric cancer cases stratified with a preoperative granulocyte and lymphocyte count. Oncol. Rep. 2000;7 :1157–1161. doi: 10.3892/or.7.5.1157. [DOI] [PubMed] [Google Scholar]
- 16.Oba K, Teramaki S, Kobayashi M, Matsui T, Kodera Y, Sakamoto J. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curative resection of gastric cancer. Cancer Immunol. Immunother. 2007;56 :905–911. doi: 10.1007/s00262-006-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chihara G, Maeda Y, Hamuro J, Sasaki T, Fukuoka F. Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk.) Sing. Nature. 1969;222:687–688. doi: 10.1038/222687a0. [DOI] [PubMed] [Google Scholar]
- 18.Chihara G, Hamuro J, Maeda Y, Arai Y, Fukuoka F. Fractionation and purification of the polysaccharides with marked antitumor activity especially lentinan, from Lentinus edodes. Cancer Res. 1970;30:2776–2781. [PubMed] [Google Scholar]
- 19.Oba K, Kobayashi M, Matsui T, Kodera Y, Sakamoto J. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 2009;29 :2739–2746. [PubMed] [Google Scholar]
- 20.Nakano H, Namatame K, Nemoto H, Motohashi H, Nishiyama K, Kumada K. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: Effect on prolongation of survival and improvement of quality of life. Hepato-Gastroenterol. 1999;46 :2662–2668. [PubMed] [Google Scholar]
- 21.Brown GD, Gordon S. Fungal β-glucans and mammalian immunity. Immunity. 2003;19 :311–315. doi: 10.1016/s1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 22.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin. Cancer Res. 2000;6 :1755–1766. [PubMed] [Google Scholar]
- 23.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogl M, Ferri E, DellaCuna GR, Tura S, Baccarani M, Lemoli RM. Dendritic cells are functionally defective in multiple myeloma. Blood. 2002;100 :230–237. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- 24.Mushiake H, Tsunoda T, Nukatsuka M, Shimao K, Fukushima M, Tahara H. Dendritic cells might be one of key factors for eliciting antitumor effect by chemoimmunotherapy in vivo. Cancer Immunol. Immunother. 2005;54:120–126. doi: 10.1007/s00262-004-0585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.vandeWinkel JG, Capel PJ. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol. Today. 1993;14:215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 26.Carter P. Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer. 2001;1:118–129. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- 27.Tani M, Tanimura H, Yamaue H, Iwahashi M, Tsunoda T, Tamai M, Noguchi K, Arii K. In vitro generation of activated natural killer cells and cytotoxic macrophages with lentinan. Eur. J. Clin. Pharmacol. 1992;42:623–627. doi: 10.1007/BF00265926. [DOI] [PubMed] [Google Scholar]
- 28.Czop JK, Austen KF. A β-glucan inhibitable receptor on human monocytes its identity with the phagocytic receptor for particular activators of alternative complement pathway. J. Immunol. 1985;134:2588–2593. [PubMed] [Google Scholar]
- 29.Okuda T, Yoshioka Y, Ikekawa T, Chihara G, Nishioka K. Anti-complementary activity of antitumor polysaccharides. Nature. 1972;238:59–60. doi: 10.1038/newbio238059a0. [DOI] [PubMed] [Google Scholar]
- 30.Clynes RA, Towers TL, Presta LG, Ravtech JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 31.Modak S, Koehne G, Vickers A, O’Reilly RJ, Cheung N. Rituximab therapy of lymphoma is enhanced by orally administered (1-3) (1-4)-D-β-glucan. Leukemia. Res. 2005;29:679–683. doi: 10.1016/j.leukres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Pomares LM, Wong SYC, Gordon S. Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown GD. Dectin-1: A signaling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 34.Goodridge HS, Wolf AJ, Underhill DM. β-glucan recognition by the innate immune system. Immunol. Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kataoka K, Muta T, Yamazaki S, Takeshige K. Activation of macrophages by linear (1, 3)-β-D-glucans. J. Biol. Chem. 2002;277:36825–36831. doi: 10.1074/jbc.M206756200. [DOI] [PubMed] [Google Scholar]
- 36.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, ReiseSousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Young SH, Fraser DG, Shi X, Castranova V. Molecular mechanism of tumor necrosis factor-α production in 1, 3-β-glucan-activated macrophages. J. Biol. Chem. 2001;276:20781–20787. doi: 10.1074/jbc.M101111200. [DOI] [PubMed] [Google Scholar]
- 39.Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complment receptor type 3 (CD11b/CD18) J. Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 40.Xia Y, Ross GD. Generation of recombinant fragments of CD11b expressing the functional β-glucan-binding lectin site of CR3 (CD11b/CD18) J. Immunol. 1999;162:7285–7293. [PubMed] [Google Scholar]
- 41.Li B, Allendorf DJ, Hansen R, Marroquin J, Ding C, Cramer DE, Yan J. Yeast β-glucan amplifies phagocyte killing of iC3b-opsonized tumor cell via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathway. J. Immunol. 2006;177:1661–1669. doi: 10.4049/jimmunol.177.3.1661. [DOI] [PubMed] [Google Scholar]
- 42.Cain JA, Newman SL, Ross GD. Role of complement receptor type three and secure opsonins in the neutrophil response to yeast. Complement. 1987;4:75–86. doi: 10.1159/000463011. [DOI] [PubMed] [Google Scholar]
- 43.Ross GD, Cain JA, Myones BL, Newman SL, Lachmann PJ. Specificity of membrane complement receptor type three (CR3) for β-glucan. Complement. 1987;4:61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- 44.Vetvicka V, Thornton BP, Ross GD. Soluble β-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J. Clin. Invest. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allendorf DJ, Yan J, Ross GD, Hansen RD, Baran JT, Subbarao K, Wang L, Haribabu B. C5a-mediated leukotriene B-amplified neutrophil chemotaxis is essential in tumor immunotherapy facilitated by anti-tumor monoclonal antibody and β-glucan. J. Immunol. 2005;174:7050–7056. doi: 10.4049/jimmunol.174.11.7050. [DOI] [PubMed] [Google Scholar]
- 46.Wang SC, Zhang L, Hortobagyi GN, Hung MC. Targeting HER2: Recent developments and further directions for breast cancer patients. Semin. Oncol. 2001;28:21–29. doi: 10.1053/sonc.2001.29724. [DOI] [PubMed] [Google Scholar]
- 47.Gelderman K, Tomlinson AS, Ross GD, Gorter A. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 2004;25:158–164. doi: 10.1016/j.it.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Rice PJ, Kelley JL, Kogan G, Ensley HE, Kalbfleisch JH, Browder IW, Williams DL. Human monocytes scavenger receptors are pattern recognition receptors for (1-3)-β-D-glucans. J. Leukoc. Biol. 2002;72:140–146. [PubMed] [Google Scholar]
- 49.Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, delaLleraMoya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J. Clin. Invest. 2005;115:969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High Density Lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinase. J.Biol. Chem. 2003;278:9142–9149. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- 51.Zimmerman JW, Lindermuth J, Fish PA, Palace GP, Stevenson TT, DeMong DE. A novel carbohydrate-glycosphingolipid interaction between a β-(1-3)-glucan immunomodulator PGG-glucan and lactosylceramide of human leukocytes. J. Biol. Chem. 1998;273:22014–22020. doi: 10.1074/jbc.273.34.22014. [DOI] [PubMed] [Google Scholar]
- 52.Hahn PY, Evans SE, Kottom TJ, Standing JE, Pagano RE, Limper AH. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 2003;278:2043–2050. doi: 10.1074/jbc.M209715200. [DOI] [PubMed] [Google Scholar]
- 53.Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV. Pneumocystis cell wall β-glucan stimulates alveolar epithelial cell chemokine generation through nuclear factor-Kβ -dependent mechanism. Am. J. Respir. Cell. Mol. Biol. 2005;32:490–497. doi: 10.1165/rcmb.2004-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakshull E, Brunke-Reese D, Lindermuth J, Fisette L, Nathans RS, Crowley JJ, Tufts JC, Zimmerman J, Mackin W, Adams DS. PGG-glucan a soluble β-(1, 3)-glucan enhances the oxidative burst response microbicidal activity and activates an NF-Kβ-like factor in human PMN: Evidence for a glycosphingolipid β-(1. 3)-glucan receptor. Immunopharmacol. 1998;41:89–107. doi: 10.1016/s0162-3109(98)00059-9. [DOI] [PubMed] [Google Scholar]
- 55.Haba S, Hamaoka T, Takatsu K, Kitagawa M. Selective suppression of T-cell activity in tumor-bearing mice and its improvement by lentinan a potent antitumor polysaccharide. Int. J. Cancer. 1976;18:93–104. doi: 10.1002/ijc.2910180113. [DOI] [PubMed] [Google Scholar]
- 56.Langstein H, Norton A. Mechanisms of cancer cachexia. Hematol. Oncol. Clin. North Am. 1991;5:103–203. [PubMed] [Google Scholar]
- 57.Hamuro J, Rollinghoff M, Wagner H. Induction of cytotoxic peritoneal exudates cells by T-cell immune adjuvant of the β-1, 3-glucan-type lentinan and its analogs. Immunology. 1980;39:551–559. [PMC free article] [PubMed] [Google Scholar]
- 58.Peter G, Karoly V, Imre B, Janos F, Kaneko Y. Effects of lentinan on cytotoxic functions of human lymphocytes. Immunopharmac. Immunotoxic. 1988;10:157–163. doi: 10.3109/08923978809014330. [DOI] [PubMed] [Google Scholar]
- 59.Matsuoka H, Seo Y, Wakasugi H, Saito T, Tomoda H. Lentinan potentiates immunity and prolongs the survival time of some patients. Anticancer Res. 1997;17:2751–2756. [PubMed] [Google Scholar]
- 60.Miyakosi H, Aoki T. Acting mechanisms of lentinan in human. Int. J. Immunopharmac. 1984;6:373–379. doi: 10.1016/0192-0561(84)90057-2. [DOI] [PubMed] [Google Scholar]
- 61.Oka M, Hazama S, Suzuki M, Wang F, Wadamori K, Iizuka N, Takeda S, Akitomi Y, Ohba Y, Kajiwara K, Suga T, Suzuki T. In vitro and in vivo analysis of human leukocyte binding by the antitumor polysaccharide lentinan. Int. J. Immunopharmac. 1996;18:211–216. doi: 10.1016/0192-0561(95)00115-8. [DOI] [PubMed] [Google Scholar]
- 62.Hamuro J, Hadding U, Bitter-Suermann D. Solid phase activation of alternative pathway of complement by β-1, 3-glucans and its possible role for tumor regressing activity. Immunology. 1978;34:695–705. [PMC free article] [PubMed] [Google Scholar]
- 63.Herlyn D, Kaneko Y, Powe J, Aoki T, Koprowski H. Monoclonal antibody-dependent murine macrophage-mediated cytotoxicity against human tumors is stimulated by lentinan. Jpn. J. Cancer Res. 1985;76:37–42. [PubMed] [Google Scholar]
- 64.Cheung NKV, Modak S, Vickers A, Knuckles B. Orally administered β-glucans enhance anti-tumor effects of monoclonal antibodies. Cancer Immunol. Immunother. 2002;51:557–564. doi: 10.1007/s00262-002-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murata Y, Shimamura T, Tagami T, Takatsuki F, Hamuro J. The skewing to Th1 induced by lentinan is directed through the distinctive cytokine production by macrophages with elevated intracellular glutathione content. Int. Immunophamacol. 2002;2 :673–689. doi: 10.1016/s1567-5769(01)00212-0. [DOI] [PubMed] [Google Scholar]
- 66.Yoshino S, Tabata T, Hazama S, Iizuka N, Yamamoto K, Hirayama M, Tangoku A, Oka M. Immunoregulatory effects of the antitumor polysaccharide lentinan on Th1/Th2 balance in patients with digestive cancer. Anticancer Res. 2000;20 :4707–4712. [PubMed] [Google Scholar]
- 67.Tabuchi T, Ubukata H, Sato S, Nakata I, Goto Y, Watanabe Y, Hashimoto T, Mizuta T, Adachi M, Soma T. Granulocytapheresis as a possible cancer treatment. Anticancer Res. 1995;15:985–990. [PubMed] [Google Scholar]
- 68.Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J. Immunol. 1985;134:230–234. [PubMed] [Google Scholar]
- 69.Shau HY, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol. 1988;141:4395–4402. [PubMed] [Google Scholar]
- 70.Donskov F, vonderMasse H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J. Clin. Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 71.Liu H, Tabuchi T, Takemura A, Kasuga T, Motohashi G, Hiraishi K, Katano M, Nakada I, Ubukata H, Tabuchi T. The granulocyte/lymphocyte ratio as an independent predictor of tumor growth metastasis and progression its clinical application. Mol. Med. Rep. 2008;1:699–704. doi: 10.3892/mmr_00000016. [DOI] [PubMed] [Google Scholar]
- 72.Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric. Cancer. 2010;13:170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 73.Ina K, Furuta R, Kataoka T, Kayukawa S, Yoshida T, Miwa T, Yamamura Y, Takeuchi Y. Lentinan prolonged the survival of patients with unresectable or recurrent gastric cancer receiving S-1-based chemotherapy. World J. Clin. Oncol. 2011;2:339–343. doi: 10.5306/wjco.v2.i10.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lenz HJ, Lee FC, Haller DG, Singh D, Benson AB, Strumberg D, Yanagihara R, Yao JC, Phan AT, Ajani JA. Extended safety and efficacy data on S-1 plus cisplatin in patients with untreated advanced gastric carcinoma in a multicenter phase II study. Cancer. 2007;109:33–40. doi: 10.1002/cncr.22329. [DOI] [PubMed] [Google Scholar]
- 75.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kibayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for first line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 76.Shirasaka T, Shimamoto Y, Ohshino H, Yamaguchi M, Kato T, Yonekura K, Fukushima M. Development of a novel form of an oral 5-fluorouracil derivative (S1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulations. Anticancer Drugs. 1996;7:548–557. doi: 10.1097/00001813-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 77.Scanlon KJ, Newmann EM, Lu Y, Priest DG. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc. Natl. Acad. Sci. USA. 1986;83:8923–8925. doi: 10.1073/pnas.83.23.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iwase H, Shimada M, Tsuzuki T, Horiuchi Y, Kumada S, Haruta J, Yamaguchi T, Sugihara M, Ina K, Kusugami K, Goto H. A phase II multicentric trial of S-1 combined with 24 h-infusion of cisplatin in patients with advanced gastric cancer. Anticancer Res. 2005;25:1297–1302. [PubMed] [Google Scholar]
- 79.Ohtsu A, Boku N, Tamura F, Muro K, Shimada Y, Saigenji K, Akazawa S, Kitajima M, Kanamaru R, Taguchi T. An early phase II study of a 3-hour infusion of paclitaxel for advanced gastric cancer. Am. J. Clin. Oncol. 1998;21:416–419. doi: 10.1097/00000421-199808000-00021. [DOI] [PubMed] [Google Scholar]
- 80.Sulkes A, Smyth J, Sessa C, Drinx LY, Vermorken JB, Kaye S, Wanders J, Franklin H, LeBail N, Verweij J. Docetaxel (Taxotere) in advanced gastric cancer: results of a phase II clinical trial. EORTIC Early Clinical Trials Group. Br. J. Cancer. 1994;70:380–38. doi: 10.1038/bjc.1994.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamaguchi K, Shimamura T, Hyodo I, Koizumi W, Narahara H, Komatsu Y, Kato T, Saitoh S, Akiya T, Munakata M, Miyata Y, Takiuchi H, Nakano S, Esaki T, Kinjo F, Sakata Y. Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer. Br. J. Cancer. 2006;94:1803–1808. doi: 10.1038/sj.bjc.6603196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sato Y, Takayama T, Sagawa T, Takahashi Y, Ohnuma H, Okubo S, Shintani N, Tanaka S, Kida M, Sato Y, Ohta H, Miyanishi K, Sato T, Takimoto R, Kobune M, Yamaguchi K, Hirata K, Niitsu Y, Kato J. Phase II study of S-1 docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother. Pharmacol. 2010;66:721–728. doi: 10.1007/s00280-009-1215-2. [DOI] [PubMed] [Google Scholar]
- 83.Ina K, Furuta R, Kataoka T, Kayukawa S, Iwase H. Manag. Gastric Cancer. InTech; 2011. Prospective study of the triple combination chemotherapy consisting of paclitaxel, S-1 and 24-hour infusion of cisplatin (PSC) against inoperable highly advanced gastric cancer; pp. 119–126. [Google Scholar]
- 84.Iwase H, Shimada M, Tsuzuki T, Ina K, Sugihara M, Haruta J, Shinoda M, Kumada T, Goto H. A phase II multi-study of triple therapy with paclitaxel S-1 and cisplatin in patients with advanced gastric cancer. Oncology. 2011;80:76–83. doi: 10.1159/000328746. [DOI] [PubMed] [Google Scholar]
- 85.Ina K, Kataoka T, Takeuchi Y, Fukuoka T, Miwa T, Nishio T, Furuta R, Masaki A, Mori F, Kayukawa S, Nagao S, Ando T, Goto H. Pathological complete response induced by the combination therapy of S-1 and 24-h infusion of cisplatin in two cases initially diagnosed as inoperable advanced gastric cancer. Oncol. Rep. 2008;20:259–264. [PubMed] [Google Scholar]
- 86.Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, Saburi Y, Miyamoto T, Takemoto S, Suzushima H, Tsukasaki K, Nosaka K, Fujiwara H, Ishitsuka K, Inagaki H, Ogura M, Akinaga S, Tomonaga M, Tobinai K, Ueda R. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J. Clin. Oncol. 2012;30 :837–842. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- 87.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizers human breast tumor cells to tumor necrosis factor. Mol. Cell Biol. 1989;9 :1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hudis CA. Trastuzumab-mechanism of action and use in clinical practice. N. Eng. J. Med. 2007;357 :39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 89.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235 :177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 90.Lewis GD, Figari I, Fendly B, Wong WL, Carter P, Gorman C, Shepard M. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol. Immunother. 1993;37 :255–263. doi: 10.1007/BF01518520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baselga I, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the anti-tumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58 :2825–2831. [PubMed] [Google Scholar]
- 92.Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ. Phase II study of receptor enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2 overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol. 1998;16 :2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 93.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Eng. J. Med. 2001;344 :783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 94.Bang YJ, VanCutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer (ToGA): A phase III open-label randomised controlled trial. Lancet. 2010;376 :687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 95.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, Jeannin JF, Coudert B. Trastuzumab-based treatment of HER2-positive breast cancer. An antibody-dependent cellular cytotoxicity mechanism. Br. J. Cancer. 2006;94 :259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, Neri TM, Ardizzoni A. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER2/neu-positive metastatic breast cancer. J. Clin. Oncol. 2008;26 :1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 97.Perihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J. Clin. Invest. 2002;110 :983–992. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harjunpaa A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: Direct complement killing is superior to cellular effector mechanisms. Scand. J. Immunol. 2000;51 :634–641. doi: 10.1046/j.1365-3083.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 99.Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borlerim GM, Bernasconi S, Tedesco F, Rambaldi A, Introna M. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95 :3900–3908. [PubMed] [Google Scholar]
- 100.Kim SY, Kim HP, Kim YJ, Ohdo Y, Im SA, Lee D, Jong HS, Kim TY, Bang YJ. Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int. J. Oncol. 2008;32 :89–95. [PubMed] [Google Scholar]


