Abstract
Autophagy and endocytosis are two evolutionarily conserved catabolic processes that comprise vesicle trafficking events for the clearance of the sequestered intracellular and extracellular cargo. Both start differently but end in the same compartment, the lysosome. Mounting evidences from the last years have established the involvement of proteins sensitive to intracellular Ca2+ in the control of the early autophagic steps and in the traffic of autophagic, endocytic and lysosomal vesicles. However, this knowledge is based on dispersed outcomes that do not set up a consensus model of the Ca2+-dependent control of autophagy and endocytosis. Here, we will provide a critical synopsis of insights from the last decade on the involvement of Ca2+-sensor proteins in the activation of autophagy and in fusion events of endocytic vesicles, autophagosomes and lysosomes.
Keywords: Autophagy, calcium, endocytosis, lysosomes, membrane fusion.
INTRODUCTION
Lysosomes are ubiquitous organelles that degrade material sequestered by two main dynamic processes: autophagy and endocytosis. Both processes comprise a complex traffic of vesicles that finally ends with the clearance of their contents by the lysosomal acid hydrolases.
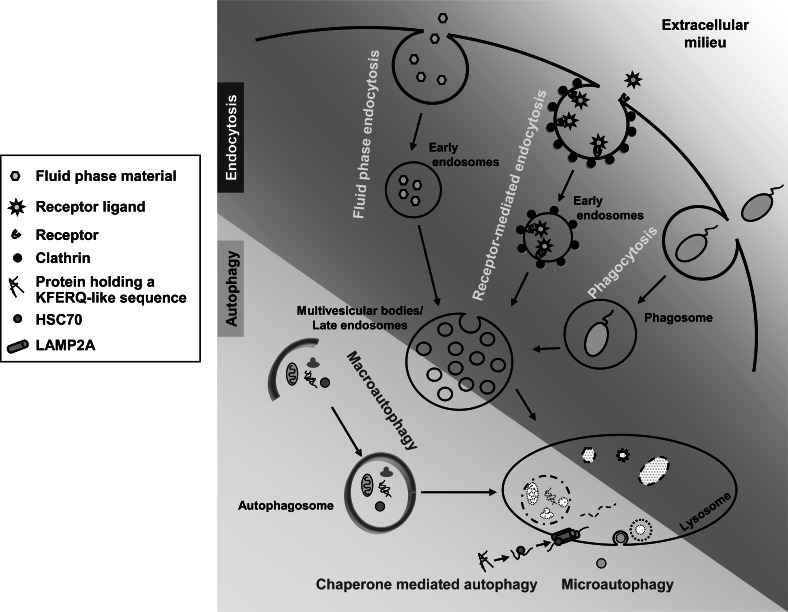
Autophagy is an important pathway responsible for the turnover of intracellular macromolecules and even whole organelles [1]. At least three different forms of autophagy coexist in the cell (Fig. 1): microautophagy, chaperone-mediated autophagy and macroautophagy. Microautophagy involves the internalization of cytosolic components by various modifications of the lysosomal membrane [2]. It has been mainly characterized in yeast and it is still poorly understood in eukaryotic cells. Chaperone-mediated autophagy is a more selective form of autophagy by which specific amino acid motifs in cytosolic proteins (KFERQ-like) are recognized by a chaperone (HSC70) that binds to isoform A of lysosome-associated membrane protein type 2 (LAMP2A). This allows, with the help of other chaperones at the lysosome, such as HSP90 and the lysosomal isoform of HSC70, the unfolding and subsequent translocation of the specific substrate proteins into the lysosomal lumen [3]. Finally, macroautophagy is the most prominent and best studied of these three forms and hence it will be simply called autophagy. It starts with the formation of a cup-shaped vesicle, called phagophore, whose origin is still a matter of conjecture, that engulfs cytoplasmic material and closes, thus generating a double membrane vacuole, the autophagosome [4]. Several compartments, including mitochondria [5, 6], plasma membrane [7], Golgi complex [8] and endosomes [9], appear to contribute proteins and lipids to the phagophore [10], but the most accepted origin of this structrue is the endoplasmic reticulum (ER) [11-13]. Once formed, the autophagosome undergoes a maturation process by fusing with late endosomes/lysosomes to acquire proteolytic competence [14]. Analysis of autophagy in yeast led to the identification of a series of autophagy-related genes (ATGs), most of them essential for autophagosome formation and whose mammalian homologues are well identified [15]. Many reviews have already discussed the functions of these genes (e.g. [1, 15, 16]), and here we will only provide a brief summary of those mentioned in the text. They include UNC-51 Like Kinase (ULK1) (whose yeast homologue is ATG1), ATG13, FIP200 (ATG17) and ATG101, all of which form a complex involved in the initiation of the phagophore, and WIPI1 (ATG18), which is involved in the nucleation of the autophagosomal membrane. In addition, its elongation is controlled by two complexes. The first is formed by the ATG7-mediated binding of ATG12 and ATG5, which later oligomerize with ATG16L (ATG16). The second is formed by Beclin 1 (ATG6), phosphatidylinositol 3-kinase class III (VPS34), p150 (VPS15) and ATG14L (ATG14). Beclin 1 is a tumor suppressor that under nutrient rich conditions is bound to protein B-cell lymphoma/leukemia 2 (Bcl-2). Under starvation, JNK1 phosphorylates Bcl-2, from which Beclin 1 dissociates and interacts with the above mentioned second complex involved in the elongation of the autophagosomal membrane. Other Beclin 1 partners appear to inhibit, such as Bcl-XL, or to activate, such as Activating molecule in Beclin 1-regulated autophagy (Ambra), autophagosome formation and others, such as Bif1 and Ultraviolet irradiation resistance-associated gene, VPS38 (UVRAG), induce the fusion of autophagosomes with lysosomes. Finally, we should also mention here LC3 (ATG8). Its cytosolic form (LC3-I) can covalently bind to phosphatidylethanolamine under a series of reactions catalyzed by ATG4, ATG7 and ATG3, forming LC3-II that associates to the autophagosomal membrane.
Fig. (1).
Main endocytic and autophagic pathways. Upper part depicts from left to right: i) fluid phase endocytic uptake of extracellular fluid containing small molecules; ii) receptor-mediated endocytic uptake of specific ligands, generally within clathrin-coated vesicles; and iii) phagocytic uptake of solid particles such as bacteria. Lower part represents from left to right: i) macroautophagy of cytosolic components including organelles; ii) chaperone mediated autophagy of proteins harboring KFERQ-related sequences; iii) microautophagy of cytosolic material. See text for further details.
Endocytosis is the process whereby extracellular and plasma membrane materials are internalized and transported to lysosomes by vesicles [17]. During this endocytic traffic, early endosomes undergo maturation and budding/scission events, thereby generating larger and more acidic multivesicular bodies/late endosomes, which are subsequently delivered to lysosomes for the final degradation of the endocytosed cargo (see Fig. 1). One of the best-characterized forms of endocytosis is receptor-mediated endocytosis, responsible for the selective internalization of specific ligands recognized by their receptors at the cell surface [17]. Phagocytosis and fluid phase endocytosis are other forms of endocytosis in which structures and molecules of variable size are engulfed by the cell [18]. Different proteins are involved in all these endocytic processes that together coordinate the specific and non-specific uptake of extracellular material into the cell and their subsequent transport to lysosomes. Therefore, and although their early steps are differently governed, autophagy and endocytosis can converge at a pre-lysosomal step or at the lysosomes to form hybrid organelles called, respectively, amphisomes or amphilysosomes [17, 19].
Ca2+ is a second messenger that is involved in the regulation of several physiological cell functions, such as gene transcription, metabolism, secretion and apoptosis, and perturbations in its homeostasis have been implicated in various pathological processes, such as disorders of the nervous system, cardiac and vascular pathologies and diabetes mellitus [20, 21]. Insights from the last years have deciphered some mechanisms that link Ca2+ with signalling and trafficking steps related with autophagy and endocytosis, but several details still remain unknown. Here we will review, consecutively, the role of Ca2+ in the regulation of: i) autophagy, ii) endocytosis, and iii) their final convergence into lysosomes for the degradation of the material taken up by these two processes.
1. INVOLVEMENT OF CA2+ IN THE REGULATION OF AUTOPHAGY
1.1. Cytosolic Ca2+ Signaling in Autophagy
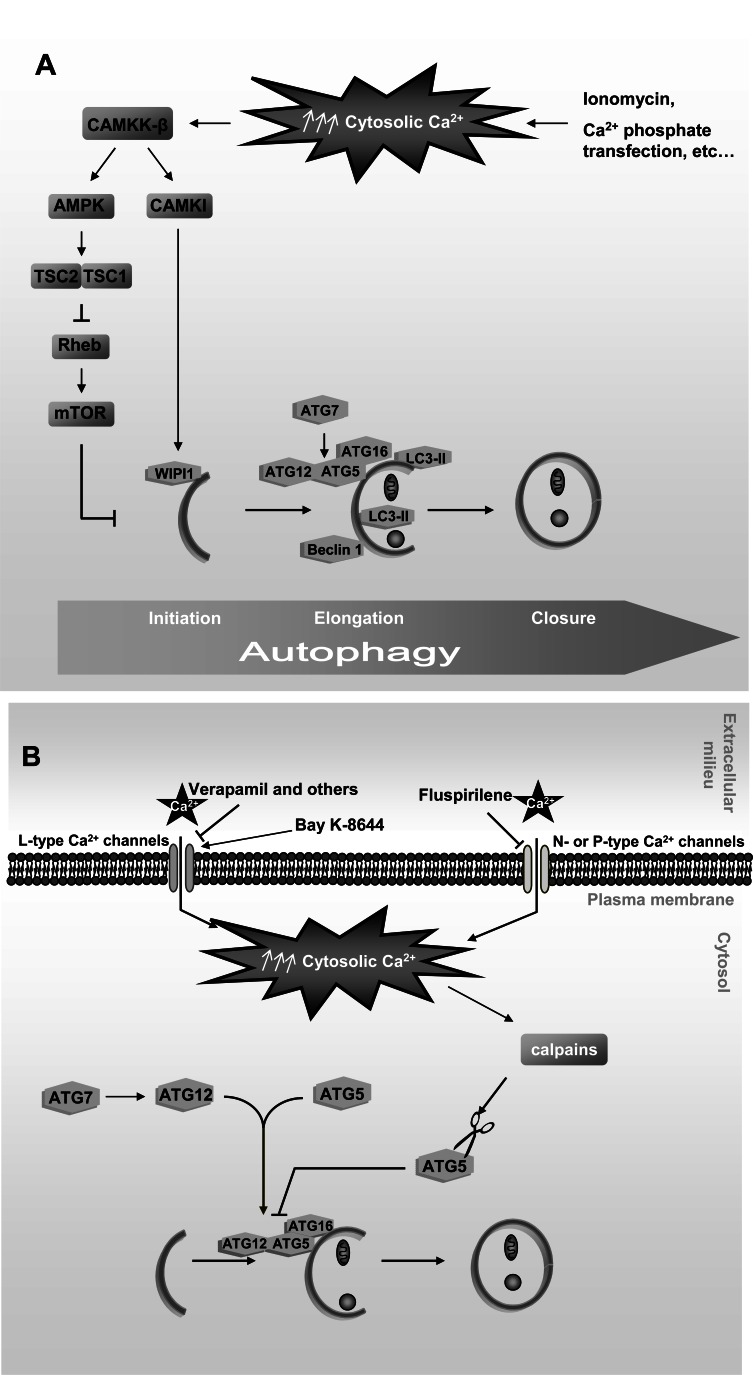
Direct evidence that cytosolic Ca2+ signaling activates autophagy was provided in a study performed in MCF-7, NIH3T3 and HeLa cells, where increasing cytosolic Ca2+ levels with pharmacological agents such as ionomycin induced autophagy in a Beclin 1- and ATG7-dependent manner [22] (see Fig. 2A). Autophagy was activated by a signaling pathway, involving Ca2+/calmodulin-dependent kinase kinase-beta (CAMKK-β) and AMP-activated protein kinase (AMPK), which inhibits the serine-threonine kinase mammalian target of rapamycin (mTOR). This inhibition of mTOR occurs via the GTPase activating protein Tuberous Sclerosis Complex (TSC1/2) and its substrate, the Ras-family GTP binding protein Rheb that directly regulates the activity of mTOR [23]. This was also confirmed in HEK293 cells transfected with amyloid-β and using resveratrol, a naturally existing polyphenol that increases cytosolic Ca2+. Under these conditions, the CAMKK-β-AMPK signalling pathway becomes activated and inhibits mTOR, leading to the autophagic degradation of amyloid-β [24]. Moreover, autophagy activation by resveratrol has been reported to occur in MCF-7 cells by a non conventional mechanism independent from canonical Beclin 1 [25].
Fig. (2).
Cytosolic Ca2+ effects on autophagy. A. Cytosolic Ca2+ induces autophagy in non-excitable cells: Rise of cytosolic Ca2+ produced by different drugs and Ca2+ phosphate-mediated transient transfections activates the CAMKK-β-AMPK-mTOR and CAMKK-β-CAMKI signalling pathways that induce autophagy through various protein targets implicated in this process. B. Cytosolic Ca2+ inhibits autophagy in excitable cells: Antagonists of L-, N- or P-type Ca2+ channels (verapamil, fluspirilene etc…), and an agonist of L-type Ca2+ channels (Bay K-8644) modify cytosolic Ca2+ levels and consequently affect the activity of the Ca2+-dependent proteases calpains, including their ATG5 cleavage that inhibits autophagy. See text for further details.
However, it has been reported that Ca2+ can also induce autophagy via WIPI1 by an alternative pathway downstream of CAMKK-β that activates Ca2+/calmodulin-dependent protein kinase I (CAMKI) and bypasses AMPK [26]. Further support for the involvement of cytosolic Ca2+ in the induction of autophagy was derived from transfection experiments with calcium-phosphate precipitates in which it was observed that these precipitates activate autophagy in a Beclin 1- and ATG5-dependent way [27].
However, other results are in conflict with those described above, since they support an inhibitory effect of cytosolic Ca2+ on autophagy (see Fig. 2B). Thus, using Ca2+ channel antagonists, such as verapamil, which inhibit a family of Ca2+-activated cysteine proteases, the calpains, autophagy was activated by a pathway independent of mTOR [28], whereas Ca2+ channel agonists inhibit autophagy via the cleavage of ATG5 by calpains, which in turn decreases the formation of the ATG12-ATG5 conjugate that is indispensable for the formation of autophagosomes [29].
Therefore, whether rises in the cytosolic Ca2+ activate or inactivate autophagy is still a matter of discussion. Of note, studies supporting inactivation of autophagy by cytosolic Ca2+ are based on the modulation of voltage-dependent Ca2+ channels (L-, N- or P-type Ca2+ channels) that exist only in excitable cells [28, 29], whereas activation of autophagy by cytosolic Ca2+ has been reported in non-excitable cells [22, 26, 27]. Given that in excitable cells cytosolic Ca2+ is mainly provided from the extracellular space by voltage-activated channels, whereas in non-excitable cells it is mainly released from intracellular stores via second messengers (such as inositol 1,4,5-trisphosphate (IP3)) [26], it is possible that different Ca2+-sensor proteins in both groups of cells activate distinct signalling routes that lead to opposite autophagic responses.
1.2. Regulation of Autophagy by ER-Derived Ca2+
Earlier studies demonstrated a role of Ca2+ storage within cell compartments in autophagy stimulation [30]. Since then, the importance of ER-derived Ca2+ for the autophagic activity has been confirmed by several experimental evidences. The ER lumen constitutes both the main intracellular Ca2+ store and the major site in the secretory pathway for the proper folding of proteins, which is carried out by a group of chaperones, most of them Ca2+-dependent [31-33]. Therefore, disturbances in Ca2+ homeostasis inside the ER cause stress that compromises the functionality of this organelle and of the cell.
1.2.1. Autophagic Response to the Inhibition of ER Ca2+-ATPases by Thapsigargin
The first direct evidence of a possible connection between Ca2+ efflux from the ER and autophagy came from the observation of an induction of autophagy by thapsigargin [34]. This compound hampers the Ca2+ transport into the ER through Ca2+-ATPase pumps, rendering this store depleted of Ca2+ and, subsequently, provokes ER stress [34, 35]. Several evidences indicate that Ca2+ rather than ER stress is important for the induction of autophagy by thapsigargin, since its effect is abolished by the potent cell permeant Ca2+ chelator BAPTA-AM (1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid (acetoxy methyl ester)) [22, 36]. In fact, thapsigargin causes ER stress only after prolonged treatments (reviewed in [37]), while autophagy activation is evident at short times. Moreover, thapsigargin is able to induce autophagy in cells deficient in the unfolded protein response [38] and other compounds that deplete Ca2+ from the ER induce autophagy without altering the unfolded protein response [39]. All these data support the contribution of ER-derived Ca2+ to the activation of autophagy independently of ER stress.
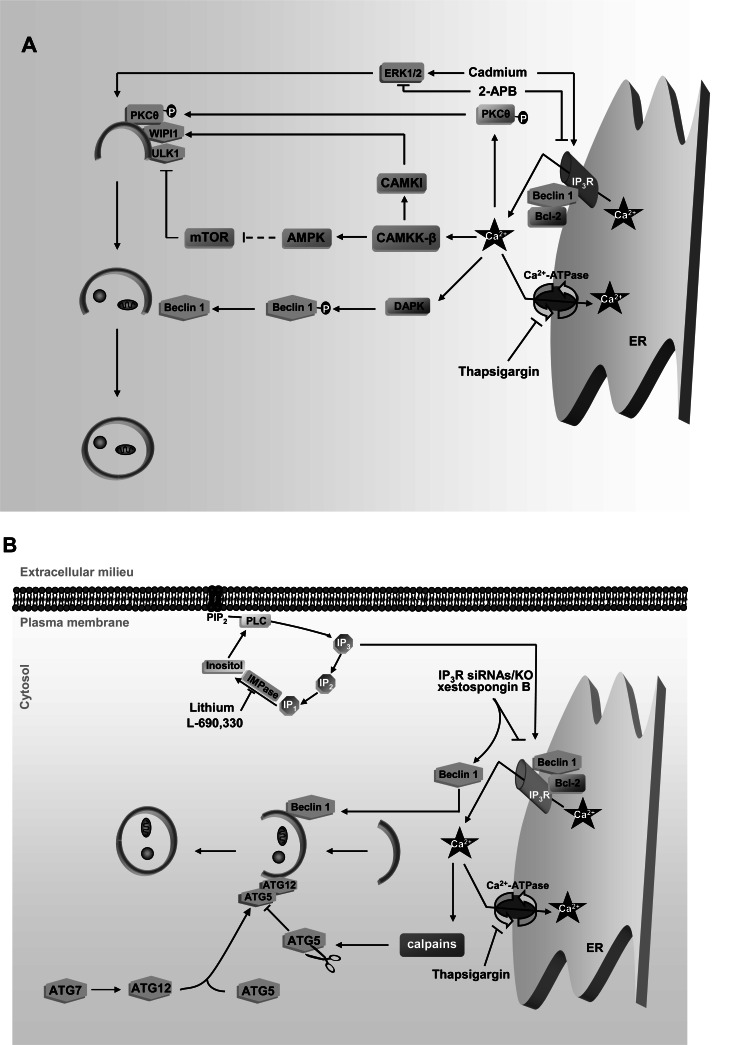
The Ca2+-dependent activation of autophagy by thapsigargin has been reported to occur in simple eukaryotes such as Dictyostelium [40], as well as in a wide range of mammalian cells (lymphocytes, hepatocytes and fibroblasts are some examples) [22, 36, 38, 41]. In Dictyostelium ATG1 is shown to be required [40], whereas in mammalian cells this Ca2+-dependent autophagy activation has been described to occur either via CAMKK-b-AMPK-mTOR signalling [22] that activates the mammalian homologue of ATG1, ULK1 (according to [42] and our unpublished results). Other possibilities for this autophagy activation include the participation of CAMKK-β-CAMKI [36, 41] or a Ca2+-dependent phosphorylation of PKCθ that recruits this PKC isoform to the autophagic vesicles [38] (see Fig. 3A).
Fig. (3).
ER-derived Ca2+ effects on autophagy. A. Under starvation conditions, Ca2+ derived from the ER activates autophagy: ER depletion of Ca2+ by thapsigargin induces autophagy via the same signalling pathways from fig. 2A, and by a Ca2+-dependent phosphorylation of PKCθ that directs this kinase to autophagosomes. Ca2+ release from the ER through the IP3R is inhibited with 2-APB and induced with Cadmium and this inhibits and activates, respectively, autophagy via ERK1/2 signalling. Ca2+-dependent phosphorylation of Beclin 1 by DAPK also induces autophagy. B. Under full nutrient conditions, Ca2+ derived from the ER restrains autophagy: Thapsigargin inhibits autophagy via ATG5 cleavage by calpains. As regards IP3R function, inhibitors of inositol monophosphatases (IMPase), such as Lithium and L-690,330, which prevent IP3 generation and, hence, Ca2+ release through IP3R, induce autophagy. Also the inhibition of IP3R function with xestospongin B and knockdown/knockout of IP3R dissociates Beclin 1 from Bcl-2-IP3R complex and stimulates autophagy. IP: inositol 4 monophosphate; IP2: inositol 4,5 bisphosphate. See text for further details.
However, other studies have shown the opposite effect of thapsigargin [28, 30, 43, 44], and, as mentioned before, one of these studies ascribed this inhibition of autophagy to the Ca2+-dependent activation of calpains [28]. It seems that, in general and in accordance with what it was indicated in the previous section, in excitable cells autophagy is inhibited by thapsigargin, suggesting a negative role of ER-derived Ca2+ and hence of the Ca2+ supplied to the cytosol in this process. However, examples of non-excitable cells where autophagy is inhibited [28, 30] or activated [22, 38, 41, 45] are also observed. Given the diversity of the experimental conditions employed (0.01 to 5 μM of thapsigargin, for 15 min to 24 h), these differences could be due to side effects unrelated with the ER-derived Ca2+, since, for example, the use of BAPTA-AM in some of these studies does not rule out the involvement of Ca2+ present in other organelles. In fact, thapsigargin treatments at high concentrations and/or during prolonged times inhibit for example Ca2+-ATPase pumps at the Golgi complex [46]. Therefore, whether the Ca2+ released by thapsigargin from the ER activates or inhibits autophagy in non-excitable cells is still under debate.
Apart from Ca2+-ATPase pumps that control Ca2+ entry to the ER lumen, Ca2+ homeostasis in this organelle is also affected by Ca2+ release through the IP3 receptor (IP3R), an aspect that we discuss below.
1.2.2. Regulation of Autophagy by IP3R-Dependent Ca2+ Release from the ER
Efflux of Ca2+ from the ER is mainly regulated by interaction of the second messenger IP3 with IP3R, resulting in the formation of a Ca2+ release channel at the ER [47]. IP3 is generated through the cleavage of phosphatidylinositol 4, 5-bisphosphate (PIP2) by phospholipase C (PLC), which can be activated by inositol recycled from inositol monophosphate by dephosphorylation [48]. Inhibitors of this inositol monophosphatase, such as Lithium, induce autophagy, suggesting a negative role of IP3 in the regulation of autophagy (see Fig. 3B) [49, 50]. In accordance with this observation, various reports suggest that Ca2+ release through IP3R prevents autophagy, since inhibitors of this receptor, such as xestospongin B or dexamethasone, or the knockdown/knockout of all three IP3R isoforms induce autophagy [50-53]. This negative effect on autophagy of the Ca2+ released to the cytosol through IP3R appears to be only relevant under nutrient rich conditions, because in this situation, but not under starvation [51], the knockout of the three IP3R isoforms decreases mTOR activity and results in an increase of basal autophagy [52].
Moreover, this channel has been associated with two autophagy-related proteins, Bcl-2 and Beclin 1, which interact with IP3R forming a complex. Although Bcl-2 is not necessary for the in vitro binding of Beclin 1 to IP3R, it is indispensable for the complex formation in a cellular context and under full nutrient conditions [54]. However, starvation releases Beclin 1 from the complex with IP3R/Bcl-2 [54, 55] and this dissociation, which is a basic condition to activate autophagy, occurs when Beclin 1 is phosphorylated by the death-associated protein kinase (DAPK) [56]. Of note, interactors of Beclin 1, such as Bcl-XL and the nutrient deprivation factor NaF-1, are also part of this complex and are released from Beclin 1 and IP3R under starvation conditions [57-60]. Also, inhibition of IP3R by its knockdown or by xestospongin B disrupts the complex and leads to autophagy activation [50, 55]. Thus, IP3R probably acts as a scaffold to recruit proteins of the autophagic machinery under nutrient rich conditions.
As for the role of these autophagy-related proteins in IP3R function as a Ca2+ channel, it also seems to be dependent on the nutritional state of the cell, at least for the autophagy inducer Beclin 1. Under full nutrient conditions this protein does not affect Ca2+ release through IP3R [55], whereas under starvation Beclin 1 enhances the release of Ca2+ from the ER by IP3R in response to IP3 [54]. Moreover, Bcl-2, which inhibits autophagy by recruiting Beclin 1 to IP3R, reduces Ca2+ release through IP3R by a still unknown mechanism [61-64].
In conclusion, the impact of Ca2+ discharge from the ER through IP3R on autophagy appears to depend on two factors: the nutritional state of the cell and the scaffold properties of this channel to recruit autophagy-related proteins. Under full nutrient conditions, IP3R sequesters proteins essential for autophagy activation that do not affect Ca2+ release through this channel, whereas under starvation conditions these proteins are liberated and this increases both autophagy and Ca2+ release.
Other drugs that increase (Cadmium) or inhibit (2-aminoethoxydiphenyl borate) Ca2+ efflux from the ER via IP3R, produce a similar effect (activation or inhibition, respectively) on autophagy via extracellular signalling-regulated kinase (ERK1/2) [65] (see Fig. 3A). However, these chemicals are not necessarily specific for IP3R. For example, 2-aminoethoxydiphenyl borate is not a selective inhibitor of IP3R, because it also alters the activity of store-operated Ca2+ channels and Endoplasmic Reticulum Ca2+-ATPase (SERCA) pumps at the plasma membrane [66, 67] and activates mTOR and AMPK in a CAMKK-β independent manner (our unpublished results). Thus, probably the effect of these drugs on autophagy may not be exclusively due to the Ca2+ derived from the ER through IP3R.
Overall, Ca2+ release from the ER through this channel appears to induce autophagy in starved cells, but to inhibit it under full nutrient conditions. As all these studies have been performed in non-excitable cells, this conclusion, at least under starvation conditions, is in agreement with the studies that proposed a role of cytosolic Ca2+ inducing autophagy in these cells.
1.3. Mitochondrial Link Between ER Derived Ca2+ and Autophagy
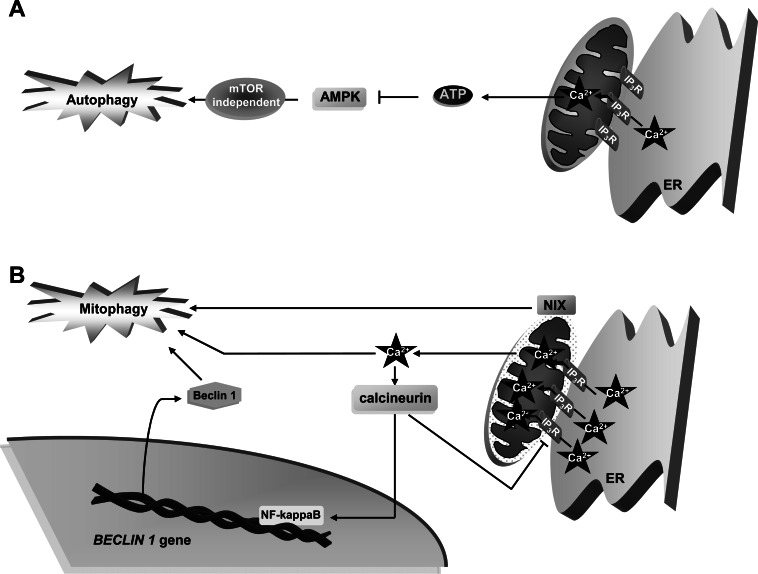
IP3R is also found at ER-mitochondrial contact sites, since these two organelles are often found in close connection [50]. Thus, a blockage in Ca2+ release from the ER also alters Ca2+ homeostasis in mitochondria. The close proximity of ER and mitochondria is essential for an efficient transport of Ca2+ from the ER to mitochondria and the subsequent activation of Ca2+-dependent mitochondrial enzymes that participate in ATP production, such as pyruvate dehydrogenase (PDH), two enzymes of the Krebs cycle (isocitrate dehydrogenase and ketoglutarate dehydrogenase), and the F1F0 ATPase. Activation of PDH occurs by its dephosphorylation produced by the Ca2+-dependent stimulation of the PDH phosphatase (PDP). Although some cells, such as hepatocytes, express a PDP isoform whose activity is Ca2+-independent [68], the Ca2+-dependent activation of PDH by PDP seems to be a key step in many cells to supply them with NADH and ATP [69-72]. Thus, in HEK-293 cells that express PDP with Ca2+-dependent activity, when a moderate extent of Ca2+ (in the low micromolar range) is delivered to mitochondria, ATP increases, AMPK is inhibited and this restrains autophagy by an mTOR-independent signalling pathway [51] (see Fig. 4A).
Fig. (4).
Effects of mitochondrial Ca2+ on autophagy. A. Mitochondrial Ca2+ inhibits autophagy: A moderate transfer of Ca2+ from the ER to mitochondria through IP3R, triggers ATP production that subsequently inactivates AMPK-dependent autophagy. B. Under stress, mitochondrial Ca2+ can activate autophagy: Mitochondria overloaded with Ca2+ are permeabilized and damaged. This promotes mitophagy and also activates calcineurin, which enhances NF-KappaB-mediated transcription of Beclin 1. Also, NIX buried in the outer mitochondrial membrane induces Ca2+ transfer from the ER to mitochondria and activates mitophagy. See text for further details.
On the contrary, under situations that may induce cell death, such as oxidative stress, a massive entry of Ca2+ (in the millimolar range) into mitochondria occurs as a consequence of its depolarization. This provokes the disruption of the integrity of the mitochondrial outer membrane and a rise in mitochondrial permeability [73, 74]. In most cells, these stress events provoke a specific autophagy (called mitophagy), which selectively degrades damaged mitochondria to preserve a healthy mitochondrial pool [75, 76] (see Fig. 4B).
Indirect links between mitochondrial Ca2+ overload and autophagy are provided by some proteins. The proapoptotic proteins Bcl-2 and adenovirus E1B 19-kDa-interacting protein 3 (BNIP3) and BNIP3-like, also known as NIX, participate in mitophagy induction in various cell types, including tumors, and localize on the outer mitochondrial membrane [77]. As NIX has been reported to trigger Ca2+ transfer from the ER to mitochondria in cardiac cells under stress conditions that may induce cell death [78], it is possible that this BNIP3-like protein uses this action to activate autophagy. However, further experiments are needed to confirm whether autophagy induction by these two proteins is due to an effect of NIX on mitochondrial Ca2+ overload and to generalize these observations to other cell types.
Moreover, permeabilization of mitochondrial membranes under Ca2+ overload inside this organelle activates the cytosolic Ca2+-dependent phosphatase calcineurin [79], which further promotes autophagy (see Fig. 4B) by dephosphorylation and inhibition of IP3R, constituting in this way a negative feedback to control Ca2+ release and to preserve mitochondrial homeostasis [65, 80]. Since calcineurin has been reported to be essential for the activation of NF-kappaB [81], a nuclear factor that, among other effects, enhances the transcription of Beclin 1 and induces autophagy [82], it is possible that this effect also contributes to the activation of autophagy observed in the mitochondrial Ca2+-mediated activation of calcineurin (see Fig. 3B).
In summary, mitochondrial Ca2+ regulates autophagy in two opposite ways. Moderate Ca2+ levels provided from the ER within mitochondria produce ATP that represses autophagy via inhibition of AMPK. On the other hand, when cells run into stress conditions, an excessive mitochondrial Ca2+ upload occurs that activates mitophagy by mechanisms involving pro-apoptotic proteins and probably calcineurin.
Taken together the different Ca2+ stores in non-excitable cells, it seems that this cation and its sensor proteins in the cytosol induce autophagy when cells encounter conditions that require this process. Ca2+ release from the ER and mitochondria to the cytosol activates autophagy under stress conditions, whereas in healthy state, the storage of this cation inside these two organelles maintains low levels of autophagy. Thus, Ca2+ seems to participate in the adaptation of the autophagic level of the cells to their physiological state. As for excitable cells, although less attention has been paid to the Ca2+ impact on their autophagy, cytosolic Ca2+ seems to have the opposite effect on autophagy, probably because, as pointed above, the characteristics of the Ca2+-sensor proteins implicated in autophagy in these cells are different from the corresponding proteins in non-excitable cells.
2. INVOLVEMENT OF CA2+ IN ENDOCYTOSIS
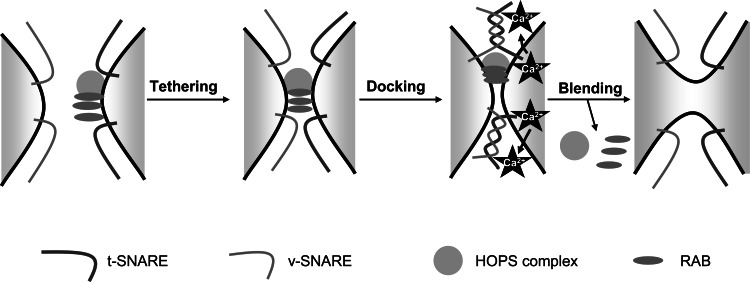
During endocytosis, Ca2+ appears to be relevant in fusion/fission events [83, 84]. There are two different types of fusion: homotypic (early endosomes) and heterotypic (late endosomes-lysosomes), and their basic steps comprise: tethering, docking and, finally, blending of the membrane bilayers. Tethering starts with the binding of a complex of proteins including RABs and HOPS to the target membrane. Subsequently, membrane docking is promoted by the phosphoinositide (PIP)-dependent association of soluble NSF attachment protein receptors (SNAREs) to the two opposite membranes (v- for vesicle and t- for target) that finally culminate their fusion (Fig. 5) [85-88]. In spite of their differences, fission and fusion events share several biochemical similarities and, for instance, RAB proteins and PIPs regulate both processes [89]. After docking, a release of luminal Ca2+ from endolysosomal compartments is thought to trigger fusion/fission events near Ca2+ release sites [83, 84, 90, 91]. This concept was evidenced for the first time using the intracellular Ca2+ chelators BAPTA and ethylene glycol tetraacetic (EGTA). In membrane fusion assays, BAPTA but not EGTA inhibits the fusion of late endosomes with early endosomes [92], lysosomes [93] or yeast vacuoles [84]. As at their maximal concentrations (10 mM) BAPTA binds Ca2+ in less time (0.3 μs) than EGTA (1.2 ms) [94], and since the Ca2+ diffusion rate in the cytosol is 20 nm/ms [95], this selective inhibition leads to postulate that the Ca2+ release source is situated at 20 nm or less from the site where fusion occurs, a reasonable distance to consider the lumen of vesicles committed to a fusion event as the source of this Ca2+. In fact, the depletion of luminal Ca2+ from these vesicles has the same effect on their fusion than BAPTA [93, 96].
Fig. (5).
Main steps in the fusion of endocytic vesicles. First, the HOPs complex, RABs and other proteins are recruited to the target vesicle in order to allow tethering with the other vesicle. Subsequently, v- and t-SNAREs interact to allow the appropriate docking of the two opposite membranes. Ca2+ release from the target vesicle occurs at this stage to facilitate the blending of the two membranes. See text for further details.
It is believed that specific endolysosomal Ca2+-sensors transduce these Ca2+ signalling into a fusion response. The best studied sensor is calmodulin, which has been shown to be crucial in homotypic [92, 97] and heterotypic [84] fusions. Ca2+ binding to calmodulin leads to interactions between this protein and specific targets, such as calmodulin-dependent kinase II (CAMKII) [97] or a complex formed by early endosome antigen 1 (EEA1) [92] and the SNARE protein SYNTAXIN 13 [98], to promote early endosome fusions. Moreover, calmodulin has the ability to dislocate EEA1 from early endosomal membranes [92]. Thus, Ca2+/calmodulin may not only play the role of recruiting fusion effectors to early endosomes, but it can also recycle tethering molecules such as EEA1.
Apoptosis-linked gene-2 (ALG-2), has been also proposed as a Ca2+-sensor for later fusion events in the endolysosomal system through its Ca2+-dependent interaction with the transient receptor potential cation channel, mucolipin subfamily 1 (TRPML1) [99], a putative endolysosomal ion channel involved in the transport of Ca2+ and other ions from the lysosomal lumen to the cytosol [100-102]. Since the release of Ca2+ from the lumen of vesicles is essential for their fusion, this channel may provide Ca2+ from endolysosomes for their fusion with endosomes and autophagic vacuoles [100, 103-106], hence the importance of lysosomal Ca2+ in these processes that we will discuss in the following section.
3. ROLE OF ENDOLYSOSOMAL CA2+ IN AUTOPHAGY AND ENDOCYTOSIS
Fusion of autophagosomes and endosomes with lysosomes to deliver their respective cargo constitutes a late step in autophagy and endocytosis. Both groups of fusions share certain features, such as the involvement of RAB7 and the AAA ATPase SKD (Vacuolar protein sorting 4/suppressor of K+ transport growth defect 1) in their regulation [107, 108]. Although in comparison to the early stages of autophagy and endocytosis these late steps remain poorly understood, it is known that Ca2+ is a key player [83, 84]. Here below, we will review recent advances focused on the involvement of Ca2+ derived from lysosomes in the fusion of these organelles with autophagosomes and endosomes.
3.1. Endolysosomal Ca2+ Channels
The best characterized Ca2+ channels present in lysosomal and late endosomal membranes are TRPMLs [100, 101, 106, 109]. Three isoforms (1, 2 and 3) have been identified in database searches [109] and mutations in the gene encoding TRPML1 provoke type IV mucolipidosis [110], a lysosomal storage disease. Although TRPML1 showed Ca2+-related features in endolysosomal compartments, such as Ca2+ permeability [101, 102], its consideration as a reliable Ca2+ channel is still under debate [111-113]. However, TRPML1, and TRPML2 as well, have been reported to heteromultimerize with TRPML3, which is the most accepted isoform to function as a Ca2+ channel [102, 114-116], and to control its lysosomal localization [117].
While there are no experimental evidences for a direct involvement of the two other isoforms in autophagy, recent data have shown that TRPML3 is localized on autophagosomal membrance, where it induces autophagy under stress conditions [105, 118], and also at the plasma membrane and early endosomal membranes, where it inhibits endocytosis [118, 119].
Two other candidates to function as lysosomal/endosomal Ca2+ release channels have recently emerged: Transient receptor potential cation channel, subfamily M, member 2 (TRPM2) and two-pore channels (TPCs). TRPM2, whose expression is restricted to specific cells, like pancreatic β cells, is mainly expressed at the plasma membrane, but it has been also localized on lysosomes, where it has been proposed to regulate luminal Ca2+ release [120]. TPC1 and TPC2 appear to be exclusively localized on early/late endosomes and lysosomes, respectively [121-123]. Both TRPM2 and TPCs are reported to be regulated by NAADP, a well-known endogenous second messenger that releases Ca2+ from acidic compartments [121, 124, 125].
Although NAADP-regulated TRPM/TPCs channels can release Ca2+ from endolysosomal compartments, knowledge on their specific role in autophagy and endocytosis remains rudimentary. In this regard, it has been suggested that Ca2+ release through NAADP-sensitive channels contributes, at least, to fusions between lysosomes and endosomes, since these channels are localized on these organelles [121-123].
Lysosomal Ca2+ is also regulated by pH. In fact, disruption of lysosomal pH by lysosomotropic agents, like bafilomycin A1, chloroquine diphosphate or nigericin, prevents Ca2+ storage in the lysosomal lumen and arrests the fusion of lysosomes with autophagosomes [126]. Therefore, an acidic pH is crucial to maintain high levels of Ca2+ in the lysosomal lumen, a requirement to induce fusions between lysosomes and endosomes or autophagosomes upon Ca2+ release from the lumen of these vesicles. In accordance with this concept, an in vitro study with isolated autophagosomes and lysosomes revealed that fusion between both organelles requires a minimum of 250 μM of calcium chloride [127].
3.2. Ca2+-Dependent Effectors of Endolysosomal Fusions
Another physiological feature of Ca2+ that is relevant in autophagy and endocytosis consists on its ability to promote the fusion of vesicles by inducing local segregations of specific lipids such as phosphatidic acid [128, 129]. Several in vivo and in vitro data support that these lipid domains are stabilized by proteins that bind to the membranes [130-132]. The best studied of these proteins belong to the SNARE machinery. First, this protein complex triggers docking of vesicles, which provokes a quick luminal Ca2+ release. Subsequently, Ca2+-binding proteins (that we will discuss below: see Table 1) are activated, probably organizing a scaffold upon the membranes that initiates the fusion processes. Finally, after dissipation of the Ca2+ gradient, these proteins remain activated until fusion is accomplished [90].
Table 1.
Ca2+-Dependent Effectors Involved in the Fusions Between Lysosomes, Autophagosomes and/or Endosomes.
| Ca2+-dependent effectors | Organelles participating in the fusion event | Molecular details of their role | References |
|---|---|---|---|
| ALG-2 | Late endosomes and lysosomes | Interacts with TRPML1 channel | [99] |
| Annexin A1 | Early endosomes | Requires Ca2+ to induce fusion in vitro | [141] |
| Annexin A2 | Early endosomes | Mediates membrane interactions between early endosomes | [142] |
| Annexin A5 | Autophagosomes and lysosomes | Translocates, under starvation, to lysosomes in a Ca2+-dependent way | [136] |
| Annexin A6 | Late endosomes and lysosomes | Requires Ca2+ and calpains for fusion | [143] |
| Calmodulin | Late endosomes and lysosomes Early endosomes |
Its binding to Ca2+ leads to interactions with specific targets | [84] |
| CAMKII | Early endosomes | Calmodulin target | [97] |
| EEA1 | Early endosomes | Interacts with calmodulin and SYNTAXIN 13 | [92] |
| HRS | Early endosomes Autophagosomes and lysosomes |
Inhibits fusion when Ca2+ release abolishes its interaction with SNAREs | [134] [133] [98] |
| SYNTAXIN 13 | Early endosomes | Interacts with Ca2+/calmodulin to promote early endosome fusions | [98] |
A peculiar protein from the SNARE complex is Hepatocyte responsive serum phosphoprotein (HRS), a Ca2+-sensitive protein associated to early endosomes. When bound to a still undefined SNARE protein on membranes of early endosomes, HRS prevents homotypic membrane fusions, thus negatively regulating the fusogenic function of SNAREs [133]. Ca2+ release from the endosomal lumen dissociates HRS from the SNARE complex and abolishes this effect, enabling in this way endocytic fusion [134].
On the other hand, this protein has been also shown to partially colocalize with autophagosomes and to promote their maturation [135]. Somewhat related to these findings, a Ca2+-binding protein, annexin A5, has been shown to be recruited to lysosomal membranes in a Ca2+ dependent way, to induce autophagosome fusion with lysosomes and to inhibit endocytosis [136, 137]. The similarity between the roles on autophagy and endocytosis of HRS and annexin A5, together with the TRPML3 channel reported in the previous section (that also promotes autophagy and inhibits endocytosis), suggests that Ca2+ release from the autophagic/ endolysosomal vesicles may control the role of these proteins in autophagy and endocytosis.
Calmodulin has been also proposed to be a Ca2+-sensor of SNAREs. The first evidences of this role were obtained in yeast, where calmodulin was identified within a protein complex involved in homotypic vacuole fusion [93, 138]. In mammalian cells, an implication of calmodulin in homotypic and heterotypic fusions was also proposed [84, 92, 97, 139, 140].
Moreover, some members of the annexin family are associated with fusion events in the endolysosomal system. In vitro studies showed the requirement of annexin A1 in fusions between early endosomes in a Ca2+-dependent manner [141], whereas in vivo analysis attributed to annexins A2, A5 and A6 the abilities to mediate the fusions of early endosomes [142], autophagosomes/ lysosomes [136], and late endosomes/lysosomes, respectively [143].
Overall, Ca2+-dependent effectors of fusions between autophagosomes, endosomes and lysosomes belong to a wide range of subgroups such as SNAREs, EF-hand proteins and annexins, with some common characteristics, including the requirement of Ca2+ binding. However, the molecular mechanisms by which they control these events are still poorly understood.
CONCLUSIONS
Growing evidences support that Ca2+ controls endocytosis and autophagy. Its effect on autophagy occurs both at the level of the signalling pathways that initiate it or, later, when autophagosomes fuse with endolysosomal compartments.
The effect of Ca2+ on autophagy depends on the cell type, since excitable and non-excitable cells exhibit opposite autophagic responses (inhibition or activation, respectively) to this cation. Although less attention has been paid to excitable cells, Ca2+ rise within them restrains autophagy and this effect is mainly due to the activation of calpains that cleave proteins essential for autophagy. To decide whether other Ca2+-sensor proteins, specific or not for these cells, are also involved in this effect requires further work that would help to better understand the autophagic behavior of these cells.
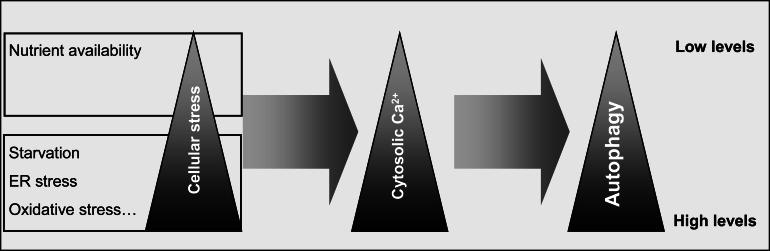
In non-excitable cells, the effect of Ca2+ on autophagy depends on the nutritional state of the cells and, probably, on the Ca2+ levels within the cytosol. Under full nutrient conditions, Ca2+ levels in the cytosol are low and maintain a basal autophagy. Starvation and stress conditions induce a rise of cytosolic Ca2+ originated, respectively, from the ER and mitochondria overloaded with Ca2+. Subsequently, these conditions trigger autophagy via various pathways that depend on Ca2+-sensor proteins (Fig. 6). Thus, in non-excitable cells, Ca2+ seems to play a protective role by adapting autophagic activity to extracellular conditions. Therefore, manipulation of intracellular Ca2+ levels in situations of defective autophagy may be useful to recuperate cellular homeostasis.
Fig. (6).
Possible relationships between nutrient availability, cell stress, cytosolic Ca2+ levels and autophagy. Starvation and an increased cell stress correlate with a high level of cytosolic Ca2+ generated from the ER and/or mitochondria and this induces autophagy. On the other hand, when nutrients are available to cells and no stress occurs, cytosolic Ca2+ remains at a low level and, consequently, basal autophagic activity is maintained.
Concerning endocytosis, the traffic of endocytic vesicles is controlled by Ca2+ derived from their lumen and, subsequently, Ca2+-sensor proteins transduce this Ca2+ signalling into fusion events.
Finally, the convergence of the autophagic and endocytic vesicles to lysosomes shares several features that depend on Ca2+ originated from lysosomes/late endosomes and on proteins that are subsequently activated by this cation. However, the involvement of Ca2+ and its effects on sensor proteins in these final autophagic and endocytic stages remain poorly understood. Although various members of these proteins have been identified, further investigations are needed to identify new Ca2+ effectors and their role in the regulation of the different steps of autophagy and endocytosis.
ACKNOWLEDGEMENTS
We thank Eva Pérez-Jiménez for helpful suggestions. Work in the authors´ lab is supported by the Spanish Ministry of Science and Innovation (BFU2008-00186), the Fundación Marató TV3 (Ref. 100130) and the Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER).
LIST OF ABBREVIATIONS
- ALG-2
= Apoptosis-linked gene-2
- AMPK
= AMP-activated protein kinase
- ATGs
= Autophagy-related genes
- BAPTA-AM
= 1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid (acetoxy methyl ester)
- Bcl-2
= Protein B-cell lymphoma/leukemia 2
- BNIP3
= Bcl-2 and adenovirus E1B 19-kDa-interacting protein 3
- CAMK
= Ca2+/calmodulin-dependent protein kinase
- CAMKK-β
= Ca2+/calmodulin-dependent kinase kinase-beta
- DAPK
= Death-associated protein kinase
- EEA1
= Early endosome antigen 1
- EGTA
= Ethylene glycol tetraacetic
- ER
= Endoplasmic reticulum
- ERK1/2
= Extracellular signalling-regulated kinase
- HRS
= Hepatocyte responsive serum phosphoprotein
- IP3
= Inositol 1,4,5-trisphosphate
- IP3R
= Inositol 1,4,5-trisphosphate receptor
- PIP
= Phosphoinositide
- mTOR
= Mammalian target of rapamycin
- PDH
= Pyruvate dehydrogenase
- PDP
= PDH phosphatase
- SNARE
= Soluble NSF attachment protein receptors
- TRPM2
= Transient receptor potential cation channel, subfamily M, member 2
- TRPML
= Transient receptor potential cation channel, mucolipin subfamily
- TPCs
= Two-pore channels
- ULK1
= UNC-51 Like Kinase
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Knecht E, Aguado C, Cárcel J, Esteban I, Esteve JM, Ghislat G, Moruno JF, Vidal JM, Sáez R. Intracellular protein degradation in mammalian cells: recent developments. Cell Mol. Life Sci. 2009;66(15):2427–2443. doi: 10.1007/s00018-009-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mijaljica D, Prescott M, and Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011;7(7):673–682. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- 3.Cuervo AM. Chaperone-mediated autophagy: selectivity pays off. Trends Endocrinol. Metab. 2010;21(3):142–150. doi: 10.1016/j.tem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, and Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 5.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim P K, Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141(4):656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mari M, Griffith J, Rieter E, Krishnappa L, Klionsky DJ, Reggiori F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J. Cell Biol. 2010;190(6):1005–1022. doi: 10.1083/jcb.200912089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein D C. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat. Cell Biol. 2010;12(8):747–757. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto H, Kakuta S, Watanabe T M, Kitamura A, Sekito T, Kondo-Kakuta C, Ichikawa R, Kinjo M, Ohsumi Y. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J. Cell Biol. 2012;198(2):219–233. doi: 10.1083/jcb.201202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longatti A, and Tooze SA. Recycling endosomes contribute to autophagosome formation. Autophagy. 2012;8(11) doi: 10.4161/auto.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuervo AM. The plasma membrane brings autophagosomes to life. Nat. Cell Biol. 2010;12(8):735–737. doi: 10.1038/ncb0810-735. [DOI] [PubMed] [Google Scholar]
- 11.Axe EL, Walker S A, Manifava M, Chandra P, Roderick H L, Habermann A, Griffiths G, Ktistakis N T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008;182(4):685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat. Cell Biol. 2009;11(12):1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- 13.Yla-Anttila P, Vihinen H, Jokitalo E, Eskelinen E L. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy. 2009;5(8):1180–1185. doi: 10.4161/auto.5.8.10274. [DOI] [PubMed] [Google Scholar]
- 14.Noda T, Fujita N, and Yoshimori T. The late stages of autophagy: how does the end begin? Cell Death Differ. 2009;16(7):984–990. doi: 10.1038/cdd.2009.54. [DOI] [PubMed] [Google Scholar]
- 15.Klionsky DJ, Cregg J M, Dunn W A, Jr, Emr S D, Sakai Y, Sandoval I V, Sibirny A, Subramani S, Thumm M, Veenhuis M, Ohsumi Y. A unified nomenclature for yeast autophagy-related genes. Dev. Cell. 2003;5(4):539–545. doi: 10.1016/s1534-5807(03)00296-x. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima N, Yoshimori T, and Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011;27(10):107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 17.Doherty GJ, and McMahon HT. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 18.Swanson J A. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol. Cell Biol. 2008;9(8):639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fader CM, and Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 2009;16(1):70–78. doi: 10.1038/cdd.2008.168. [DOI] [PubMed] [Google Scholar]
- 20.Berridge M J, Bootman MD, and Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 21.Missiaen L, Robberecht W, van den Bosch L, Callewaert G, Parys J B, Wuytack F, Raeymaekers L, Nilius B, Eggermont J, De Smedt H. Abnormal intracellular ca(2+)homeostasis and disease. Cell Calcium. 2000;28(1):1–21. doi: 10.1054/ceca.2000.0131. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen I S, Jäättelä M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell. 2007;25(2):193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Sarbassov DD, Ali SM, and Sabatini DM. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005;17(6):596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Vingtdeux V, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010;285(12):9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15(8):1318–1329. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- 26.Pfisterer S G, Mauthe M, Codogno P, Proikas-Cezanne T. Ca2+/calmodulin-dependent kinase (CaMK) signaling via CaMKI and AMP-activated protein kinase contributes to the regulation of WIPI-1 at the onset of autophagy. Mol. Pharmacol. 2011;80(6):1066–1075. doi: 10.1124/mol.111.071761. [DOI] [PubMed] [Google Scholar]
- 27.Gao W, Ding W X, Stolz D B, Yin X M. Induction of macroautophagy by exogenously introduced calcium. Autophagy. 2008;4(6):754–761. doi: 10.4161/auto.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams A, Sarkar S, Cuddon P, Ttofi E K, Saiki S, Siddiqi F H, Jahreiss L, Fleming A, Pask D, Goldsmith P, O'Kane C J, Floto R A, Rubinsztein D C. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2008;4(5):295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia HG, Zhang L, Chen G, Zhang T, Liu J, Jin M, Ma X, Ma D, Yuan J. Control of basal autophagy by calpain1 mediated cleavage of ATG5. Autophagy. 2010;6(1):61–66. doi: 10.4161/auto.6.1.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon PB, Holen I, Fosse M, Røtnes J S, Seglen P O. Dependence of hepatocytic autophagy on intracellularly sequestered calcium. J. Biol. Chem. 1993;268(35):26107–26112. [PubMed] [Google Scholar]
- 31.Berridge M J. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32(5-6):235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 32.Bernales S, Papa FR, and Walter P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 33.Momoi T. Conformational diseases and ER stress-mediated cell death: apoptotic cell death and autophagic cell death. Curr. Mol. Med. 2006;6(1):111–118. doi: 10.2174/156652406775574596. [DOI] [PubMed] [Google Scholar]
- 34.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback J A, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell Biol. 2006;26(24):9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thastrup O, Cullen P J, Drøbak B K, Hanley M R, Dawson A P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. USA. 1990;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfisterer SG, Mauthe M, Codogno P, Proikas-Cezanne T. Ca2+/Calmodulin-dependent Kinase Signaling via CaMKI and AMPK Contributes to the Regulation of WIPI-1 at the Onset of Autophagy. Mol. Pharmacol. 2011 doi: 10.1124/mol.111.071761. [DOI] [PubMed] [Google Scholar]
- 37.Puzianowska-Kuznicka M, and Kuznicki J. The ER and ageing II: calcium homeostasis. Ageing Res. Rev. 2009;8(3):160–172. doi: 10.1016/j.arr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Sakaki K, Wu J, Kaufman R J. Protein kinase Ctheta is required for autophagy in response to stress in the endoplasmic reticulum. J. Biol. Chem. 2008;283(22):15370–15380. doi: 10.1074/jbc.M710209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoyer-Hansen M, and Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14(9):1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 40.Lam D, Kosta A, Luciani M F, Golstein P. The inositol 1,4,5-trisphosphate receptor is required to signal autophagic cell death. Mol. Biol. Cell. 2008;19(2):691–700. doi: 10.1091/mbc.E07-08-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grotemeier A, Alers S, Pfisterer S G, Paasch F, Daubrawa M, Dieterle A, Viollet B, Wesselborg S, Proikas-Cezanne T, Stork B. AMPK-independent induction of autophagy by cytosolic Ca2+ increase. Cell Signal. 2010;22(6):914–925. doi: 10.1016/j.cellsig.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, Kundu M, Viollet B, Guan K L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yitzhaki S, Hochhauser E, Porat E, Shainberg A. Uridine-5'-triphosphate (UTP) maintains cardiac mitochondrial function following chemical and hypoxic stress. J. Mol. Cell Cardiol. 43(5):653–662. doi: 10.1016/j.yjmcc.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 44.Ganley IG, Wong P M, Gammoh N, Jiang X. Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest. Mol. Cell. 2011;42(6):731–743. doi: 10.1016/j.molcel.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfisterer SG, Mauthe M, Codogno P, Proikas-Cezanne T. Ca2+/Calmodulin-dependent Kinase Signaling via CaMKI and AMPK Contributes to the Regulation of WIPI-1 at the Onset of Autophagy. Mol. Pharmacol. 2011;80(6):1066–1075. doi: 10.1124/mol.111.071761. [DOI] [PubMed] [Google Scholar]
- 46.Dode L, Andersen J P, Vanoevelen J, Raeymaekers L, Missiaen L, Vilsen B, Wuytack F. Dissection of the functional differences between human secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and 2 isoenzymes by steady-state and transient kinetic analyses. J. Biol. Chem. 2006;281(6):3182–3189. doi: 10.1074/jbc.M511547200. [DOI] [PubMed] [Google Scholar]
- 47.Patterson RL, Boehning D, and Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 48.Brandt I, De Vriendt K, Devreese B, Van Beeumen J, Van Dongen W, Augustyns K, De Meester I, Scharpé S, Lambeir A M. Search for substrates for prolyl oligopeptidase in porcine brain. Peptides. 2005;26(12):2536–2546. doi: 10.1016/j.peptides.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Sarkar S, Floto R A, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook L J, Rubinsztein D C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005;170(7):1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Criollo A, Maiuri M C, Tasdemir E, Vitale I, Fiebig A A, Andrews D, Molgó J, Díaz J, Lavandero S, Harper F, Pierron G, di Stefano D, Rizzuto R, Szabadkai G, Kroemer G. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14(5):1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 51.Cardenas C, Miller R A, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung K H, Yang J, Parker I, Thompson C B, Birnbaum M J, Hallows K R, Foskett J K. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142(2):270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan MT, and Joseph SK. Role of inositol trisphosphate receptors in autophagy in DT40 cells. J. Biol. Chem. 2010;285(22):16912–16920. doi: 10.1074/jbc.M110.114207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harr MW, McColl K S, Zhong F, Molitoris J K, Distelhorst C W. l Glucocorticoids downregulate Fyn and inhibit IP(3)-mediated calcium signaling to promote autophagy in T lymphocytes. Autophagy. 2010;6(7):912–921. doi: 10.4161/auto.6.7.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Decuypere J P, Welkenhuyzen K, Luyten T, Ponsaerts R, Dewaele M, Molgó J, Agostinis P, Missiaen L, De Smedt H, Parys J B, Bultynck G. IP 3 receptor-mediated Ca ( 2+) signaling and autophagy induction are interrelated. Autophagy. 2011;7(12):1472–1489. doi: 10.4161/auto.7.12.17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vicencio J M, Ortiz C, Criollo A, Jones A W, Kepp O, Galluzzi L, Joza N, Vitale I, Morselli E, Tailler M, Castedo M, Maiuri M C, Molgó J, Szabadkai G, Lavandero S, Kroemer G. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 2009;16(7):1006–1007. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- 56.Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, Eisenstein M, Sabanay H, Pinkas-Kramarski R, Kimchi A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10(3):285–292. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zalckvar E, Berissi H, Eisenstein M, Kimchi A. Phosphorylation of Beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-XL. Autophagy. 2009;5(5):720–722. doi: 10.4161/auto.5.5.8625. [DOI] [PubMed] [Google Scholar]
- 58.Chang NC, Nguyen M, Germain M, Shore G C. Antagonism of Beclin 1-dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1. Embo J. 2010;29(3):606–618. doi: 10.1038/emboj.2009.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oberstein A, Jeffrey PD, and Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J. Biol. Chem. 2007;282(17):13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 60.Feng W, Huang S, Wu H, Zhang M. Molecular basis of Bcl-xL's target recognition versatility revealed by the structure of Bcl-xL in complex with the BH3 domain of Beclin-1. J. Mol. Biol. 2007;372(1):223–235. doi: 10.1016/j.jmb.2007.06.069. [DOI] [PubMed] [Google Scholar]
- 61.Chen R, Valencia I, Zhong F, McColl K S, Roderick H L, Bootman M D, Berridge M J, Conway S J, Holmes A B, Mignery G A, Velez P, Distelhorst C W. Bcl-2 functionally interacts with inositol 1.;4.;5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1.;4.;5-trisphosphate. J. Cell Biol. 2004;166(2):193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanson C J, Bootman M D, Distelhorst C W, Wojcikiewicz R J, Roderick H L. Bcl-2 suppresses Ca2+ release through inositol 1.;4.;5-trisphosphate receptors and inhibits Ca2+ uptake by mitochondria without affecting ER calcium store content. Cell Calcium. 2008;44(3):324–338. doi: 10.1016/j.ceca.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Palmer AE, Jin C, Reed J C, Tsien R Y. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. USA. 2004;101(50):17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rong Y P, Aromolaranm A S, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick H L, Bootman M D, Mignery G A, Parys J B, De Smedt H, Distelhorst C W. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2's inhibition of apoptotic calcium signals. Mol. Cell. 2008;31(2):255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang S H, Shih Y L, Ko W C, Wei Y H, Shih C M. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell Mol. Life Sci. 2008;65(22):3640–3652. doi: 10.1007/s00018-008-8383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Missiaen L, Callewaert G, De Smedt H, Parys J B. 2-Aminoethoxydiphenyl borate affects the inositol 1.;4.;5-trisphosphate receptor.; the intracellular Ca2+ pump and the non-specific Ca2+ leak from the non-mitochondrial Ca2+ stores in permeabilized A7r5 cells. Cell Calcium. 2001;29(2):111–116. doi: 10.1054/ceca.2000.0163. [DOI] [PubMed] [Google Scholar]
- 67.Bilmen J G, Wootton L L, Godfrey R E, Smart O S, Michelangeli F. Inhibition of SERCA Ca2+ pumps by 2-aminoethoxydiphenyl borate (2-APB). 2-APB reduces both Ca2+ binding and phosphoryl transfer from ATP.; by interfering with the pathway leading to the Ca2+-binding sites. Eur. J. Biochem. 2002;269(15):3678–3687. doi: 10.1046/j.1432-1033.2002.03060.x. [DOI] [PubMed] [Google Scholar]
- 68.Sugden M C, and Holness M J. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284(5):E855–E862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 69.Balaban R S. The role of Ca(2+) signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta. 2009;1787(11):1334–41. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szabadkai G, and Duchen M R. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23(2):84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 71.Duchen M R. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol. Aspects Med. 2004;25(4):365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Spat A, Szanda G, Csordás G, Hajnóczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium. 2008;44(1):51–63. doi: 10.1016/j.ceca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Decuypere J P, Monaco G, Bultynck G, Missiaen L, De Smedt H, Parys JB. The IP(3) receptor-mitochondria connection in apoptosis and autophagy. Biochim. Biophys. Acta. 2010;1813(5):1003–1013. doi: 10.1016/j.bbamcr.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 74.Rasola A, and Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12(5):815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 75.Elmore S P, Qian T, Grissom S F, Lemasters J J. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15(12):2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 76.Twig G, Elorza A, Molina A J, Mohamed H, Wikstrom J D, Walzer G, Stiles L, Haigh S E, Katz S, Las G, Alroy J, Wu M, Py B F, Yuan J, Deeney J T, Corkey B E, Shirihai O S. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J, and Ney PA. Role of BNIP3 and NIX in cell death.; autophagy.; and mitophagy. Cell Death Differ. 2009;16(7):939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diwan A, Matkovich S J, Yuan Q, Zhao W, Yatani A, Brown J H, Molkentin J D, Kranias E G, Dorn G W., 2nd Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J. Clin. Invest. 2009;119(1):203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cereghetti G M, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl. Acad. Sci. USA. 2008;105(41):15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bultynck G, Stangherlin A, Martins de Brito O, Chang C R, Blackstone C, Bernardi P, Scorrano L. Calcineurin and intracellular Ca2+-release channels: regulation or association? Biochem. Biophys. Res. Commun. 2003;311(4):1181–1193. doi: 10.1016/j.bbrc.2003.08.084. [DOI] [PubMed] [Google Scholar]
- 81.Kanno T, and Siebenlist U. Activation of nuclear factor-kappaB via T cell receptor requires a Raf kinase and Ca2+ influx. Functional synergy between Raf and calcineurin. J. Immunol. 1996;157(12):5277–5283. [PubMed] [Google Scholar]
- 82.Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C. p65/RelA modulates BECN1 transcription and autophagy. Mol. Cell Biol. 2009;29(10):2594–2608. doi: 10.1128/MCB.01396-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luzio J P, Bright N A, and Pryor P R. The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem. Soc. Trans. 2007;35(Pt 5):1088–1091. doi: 10.1042/BST0351088. [DOI] [PubMed] [Google Scholar]
- 84.Pryor P R, Mullock B M, Bright N A, Gray S R, Luzio J P. The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 2000;149(5):1053–1062. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malsam J, Kreye S, and Sollner TH. Membrane fusion: SNAREs and regulation. Cell Mol. Life Sci. 2008;65(18):2814–2832. doi: 10.1007/s00018-008-8352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martens S, and McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat. Rev. Mol. Cell Biol. 2008;9(7):543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 87.Jahn R, and Scheller RH. SNAREs--engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7(9):631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 88.Pryor P R, Mullock B M, Bright N A, Lindsay M R, Gray S R, Richardson S C, Stewart A, James D E, Piper R C, Luzio J P. Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep. 2004;5(6):590–595. doi: 10.1038/sj.embor.7400150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roth M G. Phosphoinositides in constitutive membrane traffic. Physiol. Rev. 2004;84(3):699–730. doi: 10.1152/physrev.00033.2003. [DOI] [PubMed] [Google Scholar]
- 90.Hay J C. Calcium: a fundamental regulator of intracellular membrane fusion? EMBO Rep. 2007;8(3):236–240. doi: 10.1038/sj.embor.7400921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luzio JP, Pryor PR, and Bright NA. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 2007;8(8):622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 92.Mills I G, Urbe S, and Clague M J. Relationships between EEA1 binding partners and their role in endosome fusion. J. Cell Sci. 2001;114(Pt 10):1959–1965. doi: 10.1242/jcs.114.10.1959. [DOI] [PubMed] [Google Scholar]
- 93.Peters C, and Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396(6711):575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 94.Adler E M, Augustine G J, Duffy S N, Charlton M P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J. Neurosci. 1991;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burgoyne R D, and Clague M J. Calcium and calmodulin in membrane fusion. Biochim. Biophys. Acta. 2003;1641(2-3):137–143. doi: 10.1016/s0167-4889(03)00089-2. [DOI] [PubMed] [Google Scholar]
- 96.Holroyd C, Kistner U, Annaert W, Jahn R. Fusion of endosomes involved in synaptic vesicle recycling. Mol. Biol. Cell. 1999;10(9):3035–3044. doi: 10.1091/mbc.10.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colombo M I, Beron W, and Stahl P D. Calmodulin regulates endosome fusion. J. Biol. Chem. 1997;272(12):7707–7712. doi: 10.1074/jbc.272.12.7707. [DOI] [PubMed] [Google Scholar]
- 98.McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98(3):377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- 99.Vergarajauregui S, Martina J A, and Puertollano R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J. Biol. Chem. 2009;284(52):36357–36366. doi: 10.1074/jbc.M109.047241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LaPlante J M, Ye C P, Quinn S J, Goldin E, Brown E M, Slaugenhaupt S A, Vassilev P M. Functional links between mucolipin-1 and Ca2+-dependent membrane trafficking in mucolipidosis IV. Biochem. Biophys. Res. Commun. 2004;322(4):1384–1391. doi: 10.1016/j.bbrc.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 101.LaPlante J M, Falardeau J, Sun M, Kanazirska M, Brown EM, Slaugenhaupt S A, Vassilev P M. Identification and characterization of the single channel function of human mucolipin-1 implicated in mucolipidosis type IV.; a disorder affecting the lysosomal pathway. FEBS Lett. 2002;532(1-2):183–187. doi: 10.1016/s0014-5793(02)03670-0. [DOI] [PubMed] [Google Scholar]
- 102.Xu H, Delling M, Li L, Dong X, Clapham D E. Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc. Natl. Acad. Sci. USA. 2007;104(46):18321–18326. doi: 10.1073/pnas.0709096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Treusch S, Knuth S, Slaugenhaupt S A, Goldin E, Grant B D, Fares H. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc. Natl. Acad. Sci. USA. 2004;101(13):4483–4488. doi: 10.1073/pnas.0400709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong X P, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman L S, Delling M, Xu H. PI(3.;5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat. Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeevi D A, Lev S, Frumkin A, Minke B, Bach G. Heteromultimeric TRPML channel assemblies play a crucial role in the regulation of cell viability models and starvation-induced autophagy. J. Cell Sci. 2010;123(Pt 18):3112–3124. doi: 10.1242/jcs.067330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grimm C, Hassan S, Wahl-Schott C, Biel M. Role of TRPML and Two-Pore Channels in Endolysosomal Cation Homeostasis. J. Pharmacol. Exp. Ther. 2012;342(2):236–244. doi: 10.1124/jpet.112.192880. [DOI] [PubMed] [Google Scholar]
- 107.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen E L. Role for Rab7 in maturation of late autophagic vacuoles. J. Cell Sci. 2004;117(Pt 20):4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 108.Nara A, Mizushima N, Yamamoto A, Kabeya Y, Ohsumi Y, Yoshimori T. SKD1 AAA ATPase-dependent endosomal transport is involved in autolysosome formation. Cell Struct. Funct. 2002;27(1):29–37. doi: 10.1247/csf.27.29. [DOI] [PubMed] [Google Scholar]
- 109.Puertollano R, and Kiselyov K. TRPMLs: in sickness and in health. Am. J. Physiol. Renal Physiol. 2009;296(6):F1245–F1254. doi: 10.1152/ajprenal.90522.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bargal R, Cormier-Daire V, Ben-Neriah Z, Le Merrer M, Sosna J, Melki J, Zangen D H, Smithson S F, Borochowitz Z, Belostotsky R, Raas-Rothschild A. Mutations in DDR2 gene cause SMED with short limbs and abnormal calcifications. Am. J. Hum. Genet. 2009;84(1):80–84. doi: 10.1016/j.ajhg.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miedel M T, Rbaibi Y, Guerriero C J, Colletti G, Weixel K M, Weisz O A, Kiselyov K. Membrane traffic and turnover in TRP-ML1-deficient cells: a revised model for mucolipidosis type IV pathogenesis. J. Exp. Med. 2008;205(6):1477–1490. doi: 10.1084/jem.20072194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soyombo A A, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J. Biol. Chem. 2006;281(11):7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- 113.Cantiello H F, Montalbetti N, Goldmann W H, Raychowdhury M K, González-Perrett S, Timpanaro G A, Chasan B. Cation channel activity of mucolipin-1: the effect of calcium. Pflugers Arch. 2005;451(1):304–312. doi: 10.1007/s00424-005-1448-9. [DOI] [PubMed] [Google Scholar]
- 114.Kim H J, Li Q, Tjon-Kon-Sang S, So I, Kiselyov K, Muallem S. Gain-of-function mutation in TRPML3 causes the mouse Varitint-Waddler phenotype. J. Biol. Chem. 2007;282(50):36138–36142. doi: 10.1074/jbc.C700190200. [DOI] [PubMed] [Google Scholar]
- 115.Nagata K, Zheng L, Madathany T, Castiglioni A J, Bartles J R, García-Añoveros J. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive.; inward rectifying currents and causes cell degeneration. Proc. Natl. Acad. Sci. USA. 2008;105(1):353–358. doi: 10.1073/pnas.0707963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grimm C, Cuajungco M P, van Aken A F, Schnee M, Jörs S, Kros C J, Ricci A J, Heller S. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc. Natl. Acad. Sci. USA. 2007;104(49):19583–19588. doi: 10.1073/pnas.0709846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Venkatachalam K, Hofmann T, and Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J. Biol. Chem. 2006;281(25):17517–17527. doi: 10.1074/jbc.M600807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim H J, Soyombo A A, Tjon-Kon-Sang S, So I, Muallem S. The Ca(2+) channel TRPML3 regulates membrane trafficking and autophagy. Traffic. 2009;10(8):1157–1167. doi: 10.1111/j.1600-0854.2009.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martina J A, Lelouvier B, and Puertollano R. The calcium channel mucolipin-3 is a novel regulator of trafficking along the endosomal pathway. Traffic. 2009;10(8):1143–1156. doi: 10.1111/j.1600-0854.2009.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci. Signal. 2009;2(71):ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brailoiu E, Churamani D, Cai X, Schrlau M G, Brailoiu G C, Gao X, Hooper R, Boulware M J, Dun N J, Marchant J S, Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009;186(2):201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Calcraft P J, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang K T, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt C N, Parrington J, Ma J, Evans A M, Galione A, Zhu M X. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459(7246):596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galione A, Morgan A J, Arredouani A, Davis L C, Rietdorf K, Ruas M, Parrington J. NAADP as an intracellular messenger regulating lysosomal calcium-release channels. Biochem. Soc. Trans. 2010;38(6):1424–1431. doi: 10.1042/BST0381424. [DOI] [PubMed] [Google Scholar]
- 124.Beck A, Kolisek M, Bagley L A, Fleig A, Penner R. Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. FASEB J. 2006;20(7):962–964. doi: 10.1096/fj.05-5538fje. [DOI] [PubMed] [Google Scholar]
- 125.Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores. Pflugers Arch. 2009;458(5):891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Churchill G C, Okada Y, Thomas J M, Genazzani A A, Patel S, Galione A. NAADP mobilizes Ca(2+) from reserve granules.; lysosome-related organelles.; in sea urchin eggs. Cell. 2002;111(5):703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 127.Koga H, Kaushik S, and Cuervo AM. Altered lipid content inhibits autophagic vesicular fusion. FASEB J. 2010;24(8):3052–3065. doi: 10.1096/fj.09-144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Geng D, Chura J, and Roberts M F. Activation of phospholipase D by phosphatidic acid. Enhanced vesicle binding.; phosphatidic acid-Ca2+ interaction.; or an allosteric effect? J. Biol. Chem. 1998;273(20):12195–12202. doi: 10.1074/jbc.273.20.12195. [DOI] [PubMed] [Google Scholar]
- 129.Swairjo M A, Roberts M F, Campos M B, Dedman J R, Seaton B A. Annexin V binding to the outer leaflet of small unilamellar vesicles leads to altered inner-leaflet properties: 31P- and 1H-NMR studies. Biochemistry. 1994;33(36):10944–10950. doi: 10.1021/bi00202a013. [DOI] [PubMed] [Google Scholar]
- 130.Buser C A, Kim J, McLaughlin S, Peitzsch R M. Does the binding of clusters of basic residues to acidic lipids induce domain formation in membranes? Mol. Membr. Biol. 1995;12(1):69–75. doi: 10.3109/09687689509038498. [DOI] [PubMed] [Google Scholar]
- 131.Rytomaa M, and Kinnunen PK. Dissociation of cytochrome c from liposomes by histone H1. Comparison with basic peptides. Biochemistry. 1996;35(14):4529–4539. doi: 10.1021/bi952413w. [DOI] [PubMed] [Google Scholar]
- 132.Denisov G, Wanaski S, Luan P, Glaser M, McLaughlin S. Binding of basic peptides to membranes produces lateral domains enriched in the acidic lipids phosphatidylserine and phosphatidylinositol 4.;5-bisphosphate: an electrostatic model and experimental results. Biophys. J. 1998;74(2 Pt 1):731–744. doi: 10.1016/S0006-3495(98)73998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun W, Yan Q, Vida T A, Bean A J. Hrs regulates early endosome fusion by inhibiting formation of an endosomal SNARE complex. J. Cell Biol. 2003;162(1):125–137. doi: 10.1083/jcb.200302083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yan Q, Sun W, McNew J A, Vida T A, Bean A J. Ca2+ and N-ethylmaleimide-sensitive factor differentially regulate disassembly of SNARE complexes on early endosomes. J. Biol. Chem. 2004;279(18):18270–18276. doi: 10.1074/jbc.M400093200. [DOI] [PubMed] [Google Scholar]
- 135.Tamai K, Tanaka N, Nara A, Yamamoto A, Nakagawa I, Yoshimori T, Ueno Y, Shimosegawa T, Sugamura K. Role of Hrs in maturation of autophagosomes in mammalian cells. Biochem. Biophys. Res. Commun. 2007;360(4):721–7. doi: 10.1016/j.bbrc.2007.06.105. [DOI] [PubMed] [Google Scholar]
- 136.Ghislat G, Aguado C, Knecht E. Annexin A5 stimulates autophagy and inhibits endocytosis. J. Cell Sci. 2012;125(Pt 1):92–107. doi: 10.1242/jcs.086728. [DOI] [PubMed] [Google Scholar]
- 137.Ghislat G, and Knecht E. New Ca2+-dependent regulators of autophagosome maturation. Commun. Integr. Biol. 2012;5(4) doi: 10.4161/cib.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peters C, Andrews P D, Stark M J, Cesaro-Tadic S, Glatz A, Podtelejnikov A, Mann M, Mayer A. Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science. 1999;285(5430):1084–1087. doi: 10.1126/science.285.5430.1084. [DOI] [PubMed] [Google Scholar]
- 139.Huber LA, Fialka I, Paiha K, Hunziker W, Sacks D B, Bähler M, Way M, Gagescu R, Gruenberg J. Both calmodulin and the unconventional myosin Myr4 regulate membrane trafficking along the recycling pathway of MDCK cells. Traffic. 2000;1(6):494–503. doi: 10.1034/j.1600-0854.2000.010607.x. [DOI] [PubMed] [Google Scholar]
- 140.De Haro L, Quetglas S, Iborra C, Lévêque C, Seagar M. Calmodulin-dependent regulation of a lipid binding domain in the v-SNARE synaptobrevin and its role in vesicular fusion. Biol. Cell. 2003;95(7):459–464. doi: 10.1016/s0248-4900(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 141.Raynal P, and Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta. 1994;1197(1):63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 142.Emans N, Gorvel J P, Walter C, Gerke V, Kellner R, Griffiths G, Gruenberg J. Annexin II is a major component of fusogenic endosomal vesicles. J. Cell Biol. 1993;120(6):1357–1369. doi: 10.1083/jcb.120.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Futter CE, and White IJ. Annexins and endocytosis. Traffic. 2007;8(8):951–8. doi: 10.1111/j.1600-0854.2007.00590.x. [DOI] [PubMed] [Google Scholar]