Abstract
Objective
To compare patients with autopsy-confirmed Alzheimer’s disease (AD, #14) and Dementia with Lewy bodies (DLB) on the frequency of behaviors related to frontal systems dysfunction and the association of these behaviors with dementia severity.
Design
Cross-sectional survey of longitudinal cohort.
Setting
University Alzheimer’s disease research center.
Participants
Volunteer sample of 19 DLB and 38 AD participants with autopsy-confirmed diagnoses, similar in age (DLB: 77.3, AD: 77.5), education (15.2, 14.7), and Mini-Mental State Examination (MMSE) score (20.6, 20.5), with impairment ranging from mild deficits to moderate dementia.
Measurements
The Frontal Systems Behavior Scale (FrSBe)-Family Rating Form assessing patient apathy, disinhibition, and executive dysfunction by a knowledgeable informant.
Results
A two-way analysis of variance with the FrSBe total as the dependent variable revealed a significant MMSE by diagnosis interaction (F(1,53)=9.34, p=.004). Mean FrSBe total for AD patients showed significant impairment across the range of dementia severity, while it was relatively preserved for DLB patients in early stage of disease. The interaction term showed the same pattern for the executive dysfunction (F(1,53)=7.62, p=.008), disinhibition (F(1,53)=4.90, p=.031), and apathy (F(1,53)=9.77, p=.003) subscales.
Conclusions
While frontal behavioral symptoms in AD patients were present regardless of stage of dementia, DLB patients showed significant frontal dysfunction only in later stages. Results suggest that frontal subcortical circuits associated with behaviors assessed by the FrSBe are affected early in AD but not until later stages in DLB. Assessing specific behaviors related to frontal systems, coupled with stage of cognitive decline, may aid in clinical differentiation of AD and DLB.
Keywords: Dementia with Lewy bodies, Alzheimer’s disease, Frontal systems, Behavioral symptoms
OBJECTIVE
Dementia with Lewy bodies (DLB) is a neurodegenerative disorder accounting for roughly 10 to 15% of cases of dementia in the elderly (1). The neuropathological hallmarks of the disease are the presence of Lewy bodies predominantly in the temporal, parietal, and frontal cortices accompanied by degeneration of the nigrostriatal dopaminergic pathway. The disease is characterized clinically by progressive cognitive decline, often with visual hallucinations, parkinsonism, and fluctuating alertness. Efforts to refine diagnostic criteria for DLB have been made (2). Nevertheless, diagnostic sensitivity has remained stubbornly low, because DLB shares features with other neurodegenerative conditions, including Alzheimer’s disease (3). The discriminative value of behavioral symptoms of dementia for diagnosis of common neurodegenerative disorders, however, has been stressed (4).
Previous studies have supported differences in the behavioral characteristics of DLB and AD. Compared to AD, DLB has been characterized by a relatively high prevalence of hallucinations, delusions, anxiety, anhedonia, and loss of energy occurring in earlier stages of disease (5) based on the Mini-Mental State Examination (MMSE) (6). The retrospective nature of these findings limited the inferences that could be made; however, similar results were obtained from prospective studies of neuropsychiatric changes in personality traits (7, 8) arising from DLB. On the Blessed Dementia Scale (9) (BDS), caregivers of DLB patients were more likely than AD caregivers to endorse behaviors such as diminished emotional responsiveness, apathy, purposeless hyperactivity, and relinquished hobbies subsumed under a factor of “passivity” despite noting similar levels of irritability and disinhibition (8). The design of the study could not address the timeline associated with the development of passivity in the DLB patients because the BDS scoring system does not allow for quantifiable ratings of behavioral change.
A series of studies (7, 10) examined the relationship between DLB and behaviors common to frontal lobe damage. Using a measure previously shown to differentiate frontotemporal dementia from AD [Middelheim Frontality Score (MFS) (11)], investigators found no difference in clinically diagnosed DLB and AD patients in the frequency of frontal lobe behaviors when divided by dementia severity.
Engelborghs et al. (10) found that for both the DLB and AD groups, there was a negative correlation between the MFS and MMSE total score. Aries et al. (7) found that for both diagnostic groups with dementia stage based on the Global Deterioration Scale (12), the group with severe dementia had more frontal lobe behaviors on the 10-item MFS than the group with mild dementia; however, they found no significant differences between mild and moderate stages. The MFS samples a variety of behaviors that include disinhibition, as well as aspontaneity and emotional bluntness, characteristics often associated with apathy. The Aries et al. study (7), however, included only four DLB patients classified as mildly demented, limiting assessment of behavioral change.
Comparing AD and DLB patients with relatively mildly impaired cognition on symptoms on the Neuropsychiatric Inventory (NPI) (13), a more recent study (14) found higher apathy subscores in the DLB group. However, only a small percentage of DLB patients had an autopsy-confirmed diagnosis and most were recruited from psychiatric clinics.
Despite limitations, several studies (5, 8, 14) have identified characteristics associated with apathy more often in DLB than in AD. This association is compelling because apathy is among the behavioral changes associated with damage to frontostriatal circuitry (15). The earlier disruption of striatal functioning in DLB compared to AD may result in prominent behavioral change associated with frontal systems.
Attaining a better understanding of behavioral change associated with frontal systems in mild cases of DLB is consequential, since neuropsychiatric complications are among the most prominent features of initial DLB presentation (5, 8, 16). This assessment may have diagnostic implications (4); however, of equal importance, it may better characterize the behavioral dysregulation common to DLB that burdens caregivers and complicates treatment (17). Previous studies of behavioral symptoms have been limited by 1) inclusion of clinically-diagnosed patients, potentially cross-contaminating groups due to misdiagnosis; 2) use of measures not specifically designed to assess behavioral change associated with frontal systems; and 3) use of instruments that are limited in their ability to provide comprehensive measures of global cognition to stage dementia.
Addressing these issues, the present study examined behavioral dysfunction associated with frontal systems across the spectrum of disease in patients with autopsy-proven DLB or AD using the Frontal Systems Behavior Scale (FrSBe) (18). With subscales composed of multiple items, the FrSBe was designed to address three behavioral syndromes associated with distinct frontostriatal circuits: apathy/akinesia associated with an anterior cingulate circuit, disinhibition/emotional dysregulation associated with an orbitofrontal circuit, and executive dysfunction associated with a dorsolateral prefrontal circuit (15, 19). Finally, while wide use of the MMSE can be helpful when comparing results across studies, its major limitation when used to divide subjects on dementia severity is the absence of items measuring the cognitive aspects of executive function. To address this issue, the present study used the total score provided by the Mattis Dementia Rating Scale (DRS) (20) in addition to the MMSE to measure stage of cognitive decline. Therefore, based on results from previous studies and greater involvement of frontostriatal circuits in DLB than in AD, we predicted that behavioral symptoms of apathy and executive dysfunction as measured by the FrSBe would be observed more frequently and would emerge at an earlier stage of dementia in DLB than in AD.
METHODS
Participants
Participants included 19 DLB (4 female, 15 male) and 38 AD (16 female, 22 male) patients with autopsy confirmation. The majority of DLB cases (n = 17) had concomitant AD pathology. All participants were clinically demented except one participant with autopsy-confirmed DLB who had mild cognitive deficits that did not reach threshold for dementia. The DLB participants represented all individuals from the University of California, San Diego (UCSD) Shiley-Marcos Alzheimer’s Disease Research Center (ADRC) autopsy series whose clinico-pathologic findings satisfied criteria for DLB, for whom FrSBe data were available, and who scored a 10 or greater on the MMSE at the time of the FrSBe. The AD (i.e., “pure” AD) participants were drawn from individuals who satisfied clinico-pathologic criteria for AD and had FrSBe data available.
General Procedure
All participants received annual neuropsychological, medical, neurological, and neuropsychiatric evaluations through the UCSD ADRC longitudinal cohort study. Between 1998 and 2003, subjects in the cohort were assessed for frontal-behavioral symptoms using the informant-based Family Form of the FrSBe. Only subjects with autopsy confirmed AD or DLB were included in the analyses. After hearing a detailed description of the study, both participant and informant signed separate written consents approved by the UCSD Human Research Protections Program. Informed consent for autopsy was obtained from participants prior to death or from the next of kin at the time of death.
Frontal Systems Behavior Scale (FrSBe)
The FrSBe – Family Rating Form (21) is a 46-item paper-and-pencil rating scale designed specifically to measure the frequency of behaviors clinically and theoretically linked to three frontal-behavioral domains: Apathy/Akinesia (14 items) (e.g., has lost interest in things that used to be fun or important to him/her), Disinhibition/Emotional Dysregulation (15 items) (e.g., talks out of turn, interrupts others in conversations), and Executive Dysfunction (17 items) (e.g., shows poor judgment, is a poor problem solver). An informant who has knowledge of the participant’s daily behavior rates the behaviors on the FrSBe during the past two weeks. Ratings are based on a 5-point Likert scale: 1=Almost Never, 2=Seldom, 3=Sometimes, 4=Frequently, and 5=Almost Always. The scale provides a total score and subscores for the three behavioral domains. The FrSBe has been shown to have good reliability and validity (18, 21-27) and normative data controlling for age, gender, and education (18) are available. The Family Rating Form was used because the reliability of self-report from participants with cognitive deficits is questionable.
Neuropathology
Autopsy was performed within 12 hours of death using a standard protocol (28). The brain was divided sagittally, then the left hemibrain fixed by immersion in 10% formalin for 5-7 days. Paraffin-embedded blocks from midfrontal, rostral superior temporal and inferior parietal neocortex, anterior cingulate gyrus, posterior cingulate gyrus, hippocampus, entorhinal cortex, basal ganglia/substantia innominata, mesencephalon, and pons were cut at 7 μm thickness for hematoxylin-eosin (H & E) and thioflavin-S counts. The midfrontal block is primarily from Brodmann area 46, the middle frontal area roughly corresponding to the dorsolateral prefrontal cortex. Depending on the cut, portions of Brodmann areas 9 and 45 may be included. The same examiner (29) using the same criteria determined total plaques, neuritic plaques, and neurofibrillary tangles (NFT). A modified Braak stage was obtained for each case. It involves counting the number of NFT in at least five neuron clusters in layer two of the entorhinal cortex and then averaging the results. Cases with Braak stage I to IV have fewer than 18 tangles on average in layer two of the entorhinal cortex and sparse neocortical tangles. Cases assigned to Braak stage V have moderate numbers of tangles in at least two neocortical sections, and in Braak stage VI, all neocortical areas assessed have at least moderate numbers of tangles.
The DLB cases met consensus criteria for the pathologic diagnosis of DLB based on hematoxylin-eosin (H & E) staining, antiubiquitin immunostaining, and anti-α-synuclein immunostaining. Cases were only construed as DLB if Lewy bodies were found in the locus coeruleus, substantia nigra, and/or nucleus basalis of Meynert, as well as in the neocortex. Because all cases categorized as DLB had neocortical as well as brainstem Lewy bodies, they all fell into either the limbic (transitional) or neocortical categories proposed in the 1996 consensus guidelines for the pathologic diagnosis of DLB. Furthermore, all DLB cases were neocortical stage 5 or 6 according to the proposed Lewy-body based staging of brain pathology related to sporadic Parkinson’s disease. Cases were not classified as DLB if Lewy bodies were found only in the amygdala. Lewy bodies were absent in cases of “pure” AD. The neuropathologic diagnosis of AD required sufficient senile plaques to meet National Institute on Aging criteria (30). All AD cases and all but two DLB cases also met CERAD neuropathology criteria for AD (31).
Statistical Analyses
FrSBe scores were converted to z-scores using published normative data correcting for age, gender, and education (18). Negative z-scores indicated greater behavioral dysfunction. DLB and AD participants were compared on normed FrSBe scores with t-tests for normally distributed continuous data, and the Mann-Whitney U test for abnormally distributed continuous data and for samples with unequal variances. Two-way analysis of variance (ANOVA) was used to characterize the effects of diagnosis (AD versus DLB) and dementia severity (MMSE ≤ 21 or MMSE ≥ 22) on FrSBe scores. The division of MMSE scores was determined by median split in order to maximize the number of subjects in each severity group. Regression analyses, with the FrSBe z-scores as dependent variables and diagnostic group membership, MMSE total score, and the interaction between group and the MMSE score as independent variables, were used to assess the impact of dementia severity on behavioral characteristics in the two diagnostic groups. These regressions were repeated substituting the DRS total score for the MMSE total. Statistical significance was set at p<.05.
RESULTS
Demographic and Cognitive Variables
Using t-tests, we confirmed that there were no significant differences between AD and DLB participants in the matching variables age (AD: mean=77.5, SD=4.1; DLB: mean=77.3, SD=4.7), education [14.7 (3.2); 15.2 (3.6)], and global cognition, with the latter measured by the MMSE [20.5 (4.7); 20.6 (5.4)] and Mattis DRS [107.9 (17.3); 105.4 (20.9)]. The AD group was 42.1 percent female; the DLB group, 21.1 percent female.
When the diagnostic groups were divided by dementia severity on the MMSE, the moderately impaired AD subjects were slightly older than those classified as mildly impaired (Table 1). There were no differences between groups in education, and, as expected for both diagnostic groups, the moderately impaired subjects had significantly lower scores on the MMSE and DRS than those mildly impaired. There were no significant differences between the DLB and AD patients within the mildly impaired group or within the moderately impaired group on any of the demographic or cognitive variables.
Table 1.
Means (SD) for demographic and cognitive measures and percent gender for groups divided by diagnosis (AD, DLB) and stage of cognitive impairment (mild, moderate) on the Mini-Mental State Examination (MMSE).
| AD | DLB | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mild N=15 |
Moderate N=23 |
Mild N=11 |
Moderate N=8 |
|||||
| Age (years) | 75.9 | (3.6) | 78.6• | (4.2) | 76.0 | (4.6) | 79.1 | (4.4) |
| Education (years) | 14.5 | (2.9) | 14.8 | (3.5) | 15.2 | (3.9) | 15.3 | (3.5) |
| MMSE total score | 25.0 | (1.6) | 17.6 * | (3.6) | 24.3 | (2.6) | 15.5 # | (3.6) |
| DRS total score | 120.9 | (9.0) | 99.4 * | (16.3) | 117.9 | (11.9) | 89.9 ° | (19.5) |
|
| ||||||||
| Percent Female | 39.1 | 46.7 | 0.0 | 50.0 | ||||
SD = Standard Deviation
AD = Alzheimer’s disease
DLB = Dementia with Lewy bodies
DRS = Mattis Dementia Rating Scale (range: 0-144)
T-tests
AD: Mild versus Moderate Age: p<0.05, t=2.05, df=36
DLB: Mild versus Moderate MMSE: p<0.001, t=−6.11, df=17
Mann-Whitney U-tests
AD: Mild versus moderate
MMSE: p<0.001, U=0.00
DRS: p<0.001, U=36.50
DLB: Mild versus Moderate DRS: p<0.01, U=4.50
FrSBe Scores
The AD and DLB groups did not differ significantly on the standardized FrSBe total score (AD: mean=−3.2, SD=2.2; DLB: mean=−2.1, SD=2.3) or subscale scores for apathy [−2.6 (1.6); −2.3 (2.2)] and executive dysfunction [−3.6 (2.1); −2.6 (2.5)]. The mean standard scores for both groups fell within the impaired range on each of these measures. The standardized disinhibition subscale scores revealed significantly greater disinhibition for the AD patients [−1.2 (2.2)] than for the DLB patients [0.21 (1.2)] (U=196.50; p=.005). For both groups, however, the mean disinhibition score was within normal limits based on normative data. One AD case with a score indicating extreme impairment on the disinhibition subscale was considered an outlier; however, testing the difference between the means of the diagnostic groups on disinhibition with this subject removed yielded essentially the same results (U=196.50; p=.007).
The means and standard deviations of the FrSBe scores for the four groups defined by diagnosis and level of cognitive impairment on the MMSE are shown in Table 2 and graphically in Figure 1. Two-way ANOVAs found significant diagnosis by severity interaction for the FrSBe total (F(1,53)=9.34, p=.004), and the subscale scores including Executive Dysfunction (F(1,53)=7.62, p=.008), Disinhibition (F(1,53)=4.90, p=.031), and Apathy (F(1,53)=9.77, p=.003). The general pattern to emerge was that dementia severity predicted FrSBe scores within the DLB group, with mildly demented DLB subjects scoring, on average, within the normal range and moderately demented DLB subjects scoring in the impaired range, while AD patients consistently performed, on average, outside the normal range regardless of stage of dementia. In general, abnormal behaviors associated with frontal systems dysfunction represented by the total FrSBe score were not attributed to the mildly demented DLB patients, but moderately demented DLB patients were rated as showing problematic behaviors (i.e., > 4.0 SD below the mean). In contrast, mildly and moderately demented AD patients exhibited a similar degree of behavioral problems associated with frontal systems dysfunction (i.e., means for both were approximately 3.0 SD below the mean).
Table 2.
Means (SD) for the Frontal Systems Behavior Scale (FrSBe) norm-based z-scores for groups divided by diagnosis (AD, DLB) and stage of cognitive impairment (mild, moderate) on the Mini-Mental State Examination (MMSE).
| AD | DLB | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| FrSBe Ratings | Mild N=15 |
Moderate N=23 |
Mild N=11 |
Moderate N=8 |
||||
| Apathy | −2.6 | (1.7) | −2.7 | (1.6) | −1.1 # | (1.8) | −4.1 *° | (1.3) |
| Disinhibition | −1.6 | (2.0) | −.83 | (2.2) | 0.86 ∞ | (0.47) | −.68 + | (1.4) |
| Executive Dysfunction | −3.5 | (2.0) | −3.7 | (2.2) | −1.2 # | (1.8) | −4.6 ° | (1.9) |
| Total | −3.3 | (2.3) | −3.2 | (2.2) | −.68 # | (1.6) | −4.1 ° | (1.6) |
SD = Standard Deviation
AD = Alzheimer’s disease
DLB = Dementia with Lewy bodies
T-tests
DLB: Mild versus Moderate
Apathy: p<0.01, t=−4.07, df=17
Executive Dysfunction: p<0.01, t=−3.97, df=17
Total: p<0.001, t=−4.56, df=17
Mild: AD versus DLB
Apathy: p<0.05, t=−2.18, df=24
Executive Dysfunction: p<0.01, t=−3.02, df=24
Total: p<0.01, t=−3.25, df=24
Moderate: AD versus DLB Apathy: p<0.05, t=2.23, df=29
Mann Whitney U-test
Mild: AD versus DLB Disinhibition: p<0.001, U=6.50
DLB: Mild versus Moderate Disinhibition: p<0.01, U=11.00
Figure 1. Bar Graphs.
The mean total FrSBe score (a) and mean FrSBe subscale scores for Executive Dysfunction (b), Disinhibition (c), and Apathy (d), with Dementia with Lewy Bodies (DLB) and Alzheimer’s Disease (AD, #14) cases divided into Mild (MMSE scores ≥ 22; dark bars) and Moderate (MMSE scores ≤ 21; light bars) dementia. Error bars represent the standard error of the mean.
Linear Regression Analyses
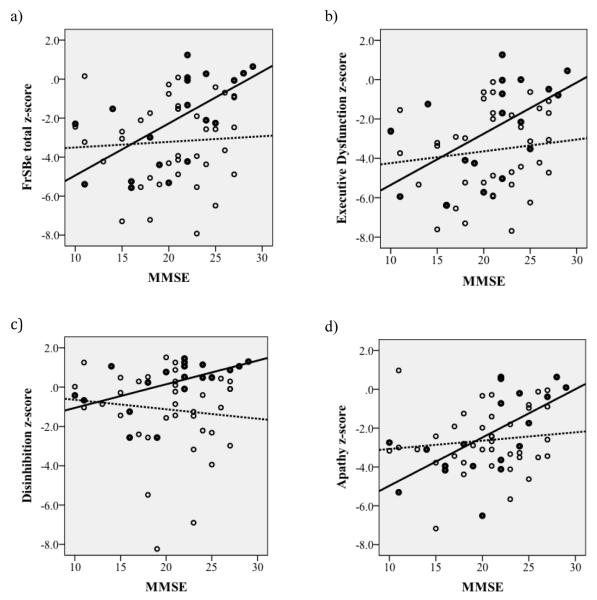
To further determine the robustness of this finding, we used linear regression analysis to examine these relationship using two continuous measures of severity of dementia, the MMSE and the Mattis DRS. Consistent with the two-way ANOVA, diagnostic group by MMSE interactions accounted for significant variance in the total FrSBe score (t=2.91, df=54, p=.005, Figure 2a), the Executive Dysfunction subscale (t=2.78, df=54, p=.007, 2b), and the Disinhibition subscale (t=2.90, df=55, p=.005, 2c). The interaction effect was less dramatic for the Apathy subscale, and did not reach statistical significance (Figure 2d).
Figure 2. Scatterplots.
FrSBe total score (a) and FrSBe subscale scores for Executive Dysfunction (b), Disinhibition (c), and Apathy (d) as a function of severity of dementia measured by the Mini-Mental State Examination (MMSE). Dementia with Lewy Bodies (DLB; dark circles) and Alzheimer’s disease (AD; open circles) cases are separately indicated.
The results of the regression analyses using the Mattis DRS to measure level of cognitive dysfunction were similar to those obtained using the MMSE (Figure 3). That is, the overall regression models for the FrSBe total and three subscale scores were significant and the group × DRS interaction term explained a significant portion of variance in the FrSBe total score (t=2.32, df=53, p=.024, Figure 3a) and the executive dysfunction (t=2.12, df=53, p=.039, 3b) and disinhibition (t=2.70, df=54, p=.009, 3c) subscale scores. As in the analysis using the MMSE as a measure of cognitive functioning, the interaction effect for the Apathy subscale did not reach statistical significance (Figure 3d).
Figure 3. Scatterplots.
FrSBe total score (a) and FrSBe subscale scores for Executive Dysfunction (b), Disinhibition (c), and Apathy (d) as a function of severity of dementia as measured by the Mattis Dementia Rating Scale (DRS). Dementia with Lewy Bodies (DLB; dark circles) and Alzheimer’s disease (AD; open circles) cases are separately indicated.
CONCLUSIONS
The study results indicate that ratings of behavioral dysfunction associated with frontal systems in patients with DLB and with “pure” AD differ as a function of dementia severity. Moderately demented patients with DLB were rated as exhibiting more apathy, behavioral executive dysfunction and disinhibition than mildly demented DLB patients, whereas behavioral ratings did not diverge based on dementia severity in AD. Mildly demented DLB patients received essentially normal behavioral ratings on the FrSBe total and subscale scores, but moderately demented DLB patients exhibited levels of behavioral dysfunction that were at least 4 SD below normal for the total score and for scores on the apathy and executive dysfunction subscales. The total score and the apathy and executive dysfunction subscale scores for AD patients, regardless of dementia severity, were below normal and similar to those of moderately demented DLB patients. Patients with AD had slightly worse disinhibition ratings than DLB patients, but mean ratings of both groups were within normal limits regardless of dementia severity. These data are in partial agreement with previous results identifying similar occurrences of behaviors ascribed to frontal lobe dysfunction in clinically diagnosed DLB and AD patients (7). Consistent with Aries and colleagues, we found no overall difference in behavioral dysfunction associated with frontal systems (i.e., total FrSBe score) in the DLB and AD groups. However, unlike the results from Aries et al., group differences were observed in the present study when mild and moderate stages of dementia were compared. The disparate results between the two studies may be due to the small number of mild DLB patients and use of a different dementia staging measure in the earlier study. A slightly larger number of mild DLB patients in our study may have allowed the relationship between disease severity and behavioral symptoms to become apparent. In addition, autopsy confirmation of diagnosis in our study strengthens our conclusions by eliminating cross-contamination of groups that could result from clinical misdiagnosis.
Contrary to our hypothesis, frontal behavioral dysfunction was not a salient feature of early DLB. Apathy and executive dysfunction became prominent in DLB only with significant disease progression. This finding suggests that neuropathology affecting frontal systems that underlie behavioral disturbances is a late occurring feature of the disease. This stands in contrast, however, to reports that cognitive executive dysfunction is an early and prominent feature of DLB (32) and suggests that cognitive and behavioral functions thought to be mediated by frontal systems are influenced by different aspects of pathology. The multi-factorial nature of DLB neuropathology (33) and persistent questions concerning the differential impact of that pathology on behavior must be more fully explored. On the other hand, the finding of frontal behavioral dysfunction in early AD is consistent with previous results (34) and suggests prominent frontal lobe dysfunction relatively early in the disease course. Furthermore, the pathology that occurs in early disease is broad enough to affect both cognitive and behavioral aspects of frontal systems. The AD pathology that co-exists with LB pathology in all but two of the DLB subjects is of note. When the groups divided by MMSE and diagnosis were compared on one aspect of AD pathology, Braak stage, there was a significant interaction of diagnosis × MMSE (two-way ANOVA, F(1,56)=19.93; p<.001) with Braak stage in the mildly impaired DLB group significantly lower (fewer tangles) than Braak stage of the other three groups. This interaction is similar to the interaction that occurs with the FrSBe behavioral constructs, suggesting that AD neuropathology is an important factor contributing to frontal behavioral dysfunction. Since the majority of DLB patients in the population have AD pathology in addition to Lewy bodies (35), the study samples are representative of DLB patients in general, and the results remain useful in differentiating the diagnostic categories when level of cognitive functioning is known.
Several limitations in the current study should be considered. The cross-sectional data do not directly capture the evolution of behaviors that seems to occur with disease progression. A longitudinal design might better identify clinical features related to the emergence of frontal behavioral dysfunction in DLB. It is also impossible to determine the relationship between the development of behavioral dysfunction and the evolution of brain pathology in DLB, since the nature and severity of pathology is not determined until the time of death. It may be the case, for example, that Lewy body pathology precedes the development of AD pathology in DLB, and it is only when AD pathology becomes prominent that behavioral dysfunction arises (36). It may be possible to use indirect measures of pathology such as metabolic activity (37-41) or diffusivity (42) to examine the temporal relationship between pathological changes and behavioral dysfunction, but these measures will not differentiate between DLB and AD pathology. Future studies that are able to image the severity and distribution of DLB (e.g., α-synuclein) and AD (e.g., β-amyloid) pathology during life and correlate it with the development of behavioral dysfunction are needed to address this issue. Other limitations include the small sample size and the use of the MMSE as a measure of cognitive functioning. In order to address the latter, we repeated the analyses with a more comprehensive measure, the Mattis DRS, and obtained the same results.
Despite limitations, the present results identify a pattern of behavioral symptoms that could help to differentiate DLB from AD and other neurodegenerative diseases. First, in mildly demented patients the presence of significant apathy and behavioral executive dysfunction is more likely in AD than in DLB. Second, cognitive dysfunction and behavioral symptoms worsen in parallel in DLB but show little correspondence in rate of decline from early to late stages in AD. Finally, significant behavioral disinhibition is not characteristic of DLB or AD and may indicate another neurodegenerative disease [i.e., frontotemporal dementia (43)]. These findings suggest that performing a valid and reliable behavioral assessment of apathy and executive dysfunction, particularly in early stages of dementia, might improve our ability to accurately diagnose DLB, develop a prognosis, and assess potential caregiver burden.
ACKNOWLEDGMENTS
We appreciate the generous contributions of the participants who made this study possible. The study was funded by grants NS049298 and AG12963 from the National Institutes of Health. The UCSD Shiley-Marcos Alzheimer’s Disease Research Center (P50 AG005131; National Institute on Aging) aided in participant recruitment and the collection and management of data.
Sources of Support: Grants NS049298, AG12963 and P50 AG005131 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No Disclosures to Report
A poster (with abstract) using data in this manuscript was presented at the International Conference on Alzheimer’s Disease in Paris, France by the second author Dr. David Salmon in July, 2010.
Contributor Information
Guerry M. Peavy, Department of Neurosciences, University of California, San Diego.
David P. Salmon, Department of Neurosciences, University of California, San Diego.
Steven D. Edland, Departments of Family and Preventive Medicine and Neurosciences.
Steven Tam, Department of Neurology, University of California, Irvine.
Lawrence A. Hansen, Departments of Pathology and Neurosciences, University of California, San Diego.
Eliezer Masliah, Departments of Pathology and Neurosciences, University of California, San Diego.
Douglas Galasko, Neurology Service, Veterans Affairs San Diego Healthcare System Department of Neurosciences, University of California, San Diego.
Joanne M. Hamilton, Department of Neurosciences, University of California, San Diego.
REFERENCES
- 1.McKeith I, Mintzer J, Aarsland D, et al. Dementia with Lewy bodies. Lancet Neurol. 2004;3:19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 3.Alves G, Muller B, Herlofson K, et al. Incidence of Parkinson’s disease in Norway: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2009;80:851–857. doi: 10.1136/jnnp.2008.168211. [DOI] [PubMed] [Google Scholar]
- 4.McKeith I, Cummings J. Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurol. 2005;4:735–742. doi: 10.1016/S1474-4422(05)70219-2. [DOI] [PubMed] [Google Scholar]
- 5.Rockwell E, Choure J, Galasko D, et al. Psychopathology at initial diagnosis in dementia with Lewy bodies versus Alzheimer disease: comparison of matched groups with autopsy-confirmed diagnoses. Int J Geriatr Psychiatry. 2000;15:819–823. doi: 10.1002/1099-1166(200009)15:9<819::aid-gps206>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 7.Aries MJ, Le Bastard N, Debruyne H, et al. Relation between frontal lobe symptoms and dementia severity within and across diagnostic dementia categories. Int J Geriatr Psychiatry. 2010;25:1186–1195. doi: 10.1002/gps.2481. [DOI] [PubMed] [Google Scholar]
- 8.Galvin JE, Malcom H, Johnson D, et al. Personality traits distinguishing dementia with Lewy bodies from Alzheimer disease. Neurology. 2007;68:1895–1901. doi: 10.1212/01.wnl.0000263131.80945.ad. [DOI] [PubMed] [Google Scholar]
- 9.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 10.Engelborghs S, Maertens K, Marien P, et al. Behavioural and neuropsychological correlates of frontal lobe features in dementia. Psychol Med. 2006;36:1173–1182. doi: 10.1017/S003329170600777X. [DOI] [PubMed] [Google Scholar]
- 11.De Deyn PP, Engelborghs S, Saerens J, et al. The Middelheim Frontality Score: a behavioural assessment scale that discriminates frontotemporal dementia from Alzheimer’s disease. Int J Geriatr Psychiatry. 2005;20:70–79. doi: 10.1002/gps.1249. [DOI] [PubMed] [Google Scholar]
- 12.Reisberg B, Ferris SH, de Leon MJ, et al. The Global Deterioration Scale for assessment of primary degenerative dementia. The American journal of psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 13.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 14.Bjoerke-Bertheussen J, Ehrt U, Rongve A, et al. Neuropsychiatric Symptoms in Mild Dementia with Lewy Bodies and Alzheimer’s Disease. Dement Geriatr Cogn Disord. 2012;34:1–6. doi: 10.1159/000339590. [DOI] [PubMed] [Google Scholar]
- 15.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 16.Tiraboschi P, Salmon DP, Hansen LA, et al. What best differentiates Lewy body from Alzheimer’s disease in early-stage dementia? Brain. 2006;129:729–735. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- 17.Leggett AN, Zarit S, Taylor A, et al. Stress and burden among caregivers of patients with Lewy body dementia. Gerontologist. 2011;51:76–85. doi: 10.1093/geront/gnq055. [DOI] [PubMed] [Google Scholar]
- 18.Grace J, Malloy PF. Frontal Systems Behavior Scale (FrSBe): Professional Manual. Psychological Assessment Resources; Lutz, FL: 2001. [Google Scholar]
- 19.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 20.Mattis S. DRS: Dementia Rating Scale professional manual. Psychological Assessment Resources; New York: 1988. [Google Scholar]
- 21.Grace J, Stout JC, Malloy PF. Assessing frontal lobe behavioral syndromes with the frontal lobe personality scale. Assessment. 1999;6:269–284. doi: 10.1177/107319119900600307. [DOI] [PubMed] [Google Scholar]
- 22.Cahn-Weiner DA, Grace J, Ott BR, et al. Cognitive and behavioral features discriminate between Alzheimer’s and Parkinson’s disease. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:79–87. [PubMed] [Google Scholar]
- 23.Malloy P, Grace J. A review of rating scales for measuring behavior change due to frontal systems damage. Cogn Behav Neurol. 2005;18:18–27. doi: 10.1097/01.wnn.0000152232.47901.88. [DOI] [PubMed] [Google Scholar]
- 24.Malloy P, Tremont G, Grace J, et al. The Frontal Systems Behavior Scale discriminates frontotemporal dementia from Alzheimer’s disease. Alzheimers Dement. 2007;3:200–203. doi: 10.1016/j.jalz.2007.04.374. [DOI] [PubMed] [Google Scholar]
- 25.Norton LE, Malloy PF, Salloway S. The impact of behavioral symptoms on activities of daily living in patients with dementia. Am J Geriatr Psychiatry. 2001;9:41–48. [PubMed] [Google Scholar]
- 26.Ready RE, Ott BR, Grace J, et al. Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:222–228. [PubMed] [Google Scholar]
- 27.Stout JC, Ready RE, Grace J, et al. Factor analysis of the frontal systems behavior scale (FrSBe) Assessment. 2003;10:79–85. doi: 10.1177/1073191102250339. [DOI] [PubMed] [Google Scholar]
- 28.Hansen L, Salmon D, Galasko D, et al. The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Sabbagh MN, Adler CH, Lahti TJ, et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009;23:295–297. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 31.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 32.Collerton D, Burn D, McKeith I, et al. Systematic review and meta-analysis show that dementia with Lewy bodies is a visual-perceptual and attentional-executive dementia. Dement Geriatr Cogn Disord. 2003;16:229–237. doi: 10.1159/000072807. [DOI] [PubMed] [Google Scholar]
- 33.Kantarci K, Lowe VJ, Boeve BF, et al. Multimodality imaging characteristics of dementia with Lewy bodies. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2011.09.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stout JC, Wyman MF, Johnson SA, et al. Frontal behavioral syndromes and functional status in probable Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:683–686. doi: 10.1176/appi.ajgp.11.6.683. [DOI] [PubMed] [Google Scholar]
- 35.Kantarci K, Ferman TJ, Boeve BF, et al. Focal atrophy on MRI and neuropathologic classification of dementia with Lewy bodies. Neurology. 2012;79:553–560. doi: 10.1212/WNL.0b013e31826357a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Compta Y, Parkkinen L, O’Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134:1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albin RL, Minoshima S, D’Amato CJ, et al. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology. 1996;47:462–466. doi: 10.1212/wnl.47.2.462. [DOI] [PubMed] [Google Scholar]
- 38.Colloby SJ, Fenwick JD, Williams ED, et al. A comparison of (99m)Tc-HMPAO SPET changes in dementia with Lewy bodies and Alzheimer’s disease using statistical parametric mapping. Eur J Nucl Med Mol Imaging. 2002;29:615–622. doi: 10.1007/s00259-002-0778-5. [DOI] [PubMed] [Google Scholar]
- 39.Ishii K, Imamura T, Sasaki M, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease. Neurology. 1998;51:125–130. doi: 10.1212/wnl.51.1.125. [DOI] [PubMed] [Google Scholar]
- 40.Lobotesis K, Fenwick JD, Phipps A, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology. 2001;56:643–649. doi: 10.1212/wnl.56.5.643. [DOI] [PubMed] [Google Scholar]
- 41.Minoshima S, Foster NL, Petrie EC, et al. Neuroimaging in dementia with Lewy bodies: metabolism, neurochemistry, and morphology. J Geriatr Psychiatry Neurol. 2002;15:200–209. doi: 10.1177/089198870201500405. [DOI] [PubMed] [Google Scholar]
- 42.Kantarci K, Avula R, Senjem ML, et al. Dementia with Lewy bodies and Alzheimer disease: neurodegenerative patterns characterized by DTI. Neurology. 2010;74:1814–1821. doi: 10.1212/WNL.0b013e3181e0f7cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]