Summary
Platelet-rich plasma (PRP) is a volume of plasma fraction of autologous blood having platelet concentrations above baseline whole-blood values due to processing and concentration. PRP is used in various surgical fields to enhance soft-tissue and bone healing by delivering supra-physiological concentrations of autologous platelets at the site of tissue damage. These preparations may provide a good cellular source of various growth factors and cytokines, and modulate tissue response to injury. Common clinically available materials for blood preparations combined with a two-step centrifugation protocol at 280g each, to ensure cellular component integrity, provided platelet preparations which were concentrated 2-3 fold over total blood values. Costs were shown to be lower than those of other methods which require specific equipment and high-cost disposables, while safety and traceability can be increased. PRP can be used for the treatment of wounds of all types including burns and also of split-thickness skin graft donor sites, which are frequently used in burn management. The procedure can be standardized and is easy to adapt in clinical settings with minimal infrastructure, thus enabling large numbers of patients to benefit from a form of cellular therapy.
Keywords: platelets, PRP, cell therapy, tissue repair, health economics
Abstract
Le plasma riche en plaquettes (PRP) est un volume de la fraction plasmatique du sang autologue ayant des concentrations au-dessus des valeurs de base des plaquettes sang total en raison de l’élaboration et de la concentration. Le PRP est utilisé dans différents domaines de la chirurgie pour améliorer la guérison des tissus mous et des os en délivrant des concentrations supraphysiologiques de plaquettes autologues au niveau du site de lésion tissulaire. Ces préparations peuvent fournir une bonne source cellulaire de divers facteurs de croissance et, de cytokines et moduler la réponse tissulaire à une lésion. Des matériaux communs cliniquement disponibles pour des préparations hématiques en association avec un protocole de centrifugation en deux étapes à 280g chacun, afin d’assurer l’intégrité des composants cellulaires, ont fourni des préparations de plaquettes qui ont été concentrées 2-3 fois par rapport aux valeurs de sang total. Les coûts se sont avérés inférieurs à ceux des autres méthodes qui nécessitent un équipement spécifique et des jetables ayant un coût élevé, tandis que la sécurité et la traçabilité peuvent être augmentées. Le PRP peut être utilisé pour le traitement de tous les types de lésions, y compris les brûlures comme aussi pour le traitement des sites donateurs de greffes cutanées d’épaisseur variable, qui sont fréquemment utilisées dans la gestion des brûlures. La procédure peut être standardisée et facilement adaptée dans les milieux cliniques avec une infrastructure minimale, permettant ainsi à un grand nombre de patients de bénéficier de cette forme de thérapie cellulaire.
Introduction
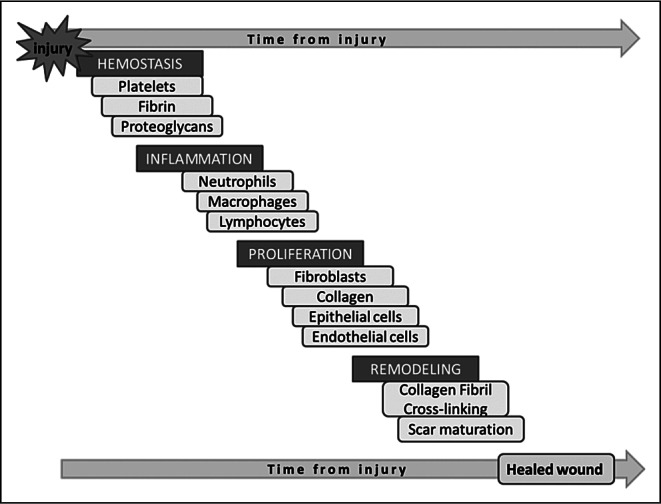
Platelet-rich plasma (PRP) is a volume of plasma of autologous blood having platelet levels above peripheral blood concentration.1 The concentration of platelets may provide a higher amount of several bioactive growth factors reported to promote healing, but the methods used vary considerably, affecting the overall quality of the platelets, outcome, and costs.2,3 Humans have an average baseline blood platelet count of 200,000 ± 75,000/μL and platelets have an in vivo half-life of ∼7-10 days. Activated platelets, upon contact with exposed endothelium within wounds or damaged tissues, are known to release key wound healing factors including platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and epidermal growth factor (EGF).4,5 All of these factors set the stage for tissue healing (Fig. 1) which involves intricate overlapping processes that are often categorized into haemostasis, inflammation, proliferation, and remodelling (Fig. 2). Once tissue injury occurs, a haematoma forms at the site of tissue damage, platelets adhere to exposed collagen creating a clot, and the inflammatory phase begins with activation of platelets resulting in release of growth, bioactive, and haemostatic factors.5 Each factor plays a unique but important integrating role in the early stages of the intrinsic and extrinsic pathways of the clotting cascade. Access to the wound site by neutrophils and macrophages occurs within hours of injury thereby initiating the phagocytosis of tissue debris. Within a few days of injury, the proliferative phase begins and this is characterized by angiogenesis, collagen deposition, granulation tissue formation, epithelialization, and wound contraction. Finally, the remodelling phase involves collagen maturation and apoptosis of excess cells which can take from several weeks to months after an injury depending of the degree of damage. Based on the above described model, acceleration of wound healing by addition of PRP is based on various platelet growth factors (PGFs) that stimulate different stages of the wound cascade. Compared to application of single, recombinant growth factors, which are applied in supraphysiological concentrations, PRP has the advantage of offering multiple, synergistically working growth factors at the wound site and in concentrations that are biologically and physiologically more pertinent.
Fig. 1. Growth factors secreted by platelets including platelet-derived growth factor, fibroblast growth factor, transforming growth factor beta, epithelial growth factor, and vascular endothelial growth factor.
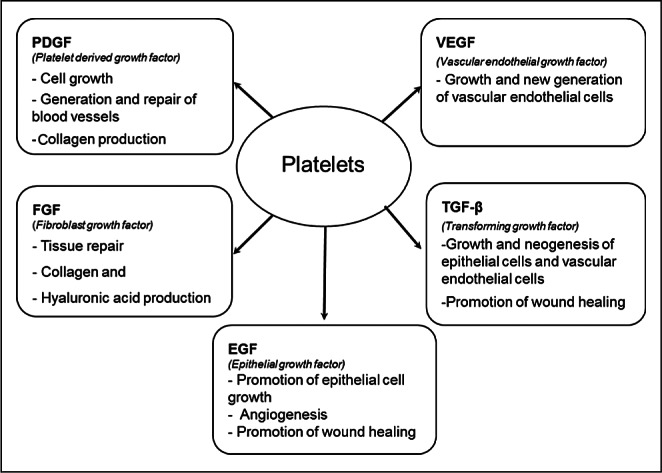
Fig. 2. Overlapping stages following tissue damage until wound healing. Phases and mechanisms of action throughout haemostasis, inflammation, proliferation, and remodelling are illustrated.
Although PRP seems to be used more frequently and in routine medical practice, there are few robust and controlled clinical trials which would establish precise clinical protocols.6-8 Parameters that need to be included for comparative optimization of PRP preparation protocols include: the absolute number and concentration of platelets over baseline, the choice between exogenous pre-activation of platelets or not, the number of injections or applications (topical), and the delay between injections or applications. It is the intention of this report to show that a simple protocol and common laboratory materials can be adapted to standardize the preparation of PRP with the emphasis on overall preservation of platelets’ physiological properties. The protocol described can be reproduced in any clinical setting, permitting a cost-effective delivery of growth factors, which may be instrumental in burn management.
Material and methods
Kit preparation
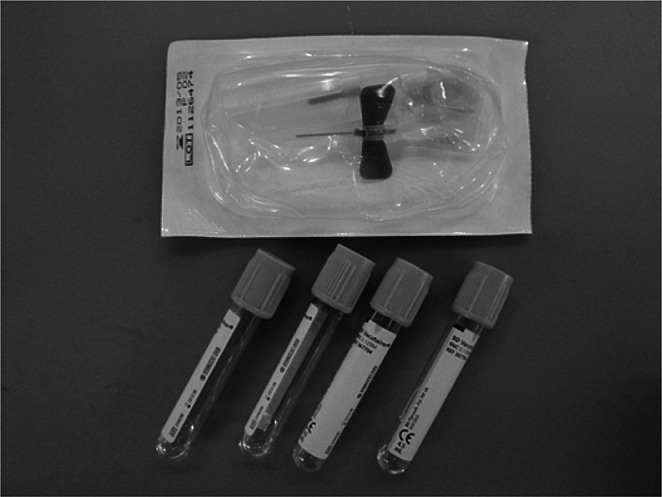
Kits were prepared by the hospital pharmacy for the blood harvest. These sterile kits contain 4.5 mL Vacutainer tubes with sodium citrate solution and a final concentration of 3.8% sodium citrate (0.129 M). Alternatively, a butterfly valve fitted to a 20 ml syringe with Cambi caps for closure of the syringe end following blood harvesting along with a 19 mm adapter can be used. When the syringe and butterfly are used for blood collection, a 20 g needle is first attached to the syringe for collection of sodium citrate solution (pharmacy preparation) and then transferred to the butterfly valve for patient use. These materials are sealed inside a pouch (235 x 340 mm) (Fig. 3). Minimal additional disposable laboratory tubes (50 ml centrifuge tubes) and pipettes are necessary for processing.
Fig. 3. Prepared PRP kit containing 4.5 mL Vacutainer tubes (0.5 ml 3.2% sodium citrate), Vacutainer unit for blood harvesting with 19 mm adapter sealed inside pouch (235 x 340 mm). Blood processing materials include syringes, needles, centrifuge tubes, pipettes, and surgical gloves.
Blood harvest and centripetal PRP preparation method
Harvesting of whole blood from the patient vein directly can be accomplished into four 4.5 mL citrate (3.8% sodium citrate)-containing Vacutainer tubes (clinical grade borosilicate glass of non-pyrogenic, non-cytotoxic and nuclease free) or, alternatively, a syringe can be used to take 3.8% citrate solution sterilely from a pharmaceutical preparation, and then attached to the butterfly valve just before drawing blood from the patient.
In Lausanne, this is done in the out-patient clinic situated next to the hospital preparation laboratory for rapid transfer and preparation. Under a laminar flow hood, the blood from the tubes or syringe is transferred gently to a 50 ml centrifuge tube.
Centrifugation is accomplished with a Megafuge centrifuge at 280 g for 15 min at room temperature. After the first centrifugation, the PRP is carefully removed with a pipette inserted above the buffy coat and transferred to a new, sterile centrifuge tube. A second centrifugation process is then made under the same conditions. Following the second centrifugation, the concentrated PRP is taken with a sterile syringe for clinical application. The volume obtained depends on the required concentration yielding of 2-4 ml and is dependent on the clinical application. In general, 1 ml is used for approximately 100-150 cm2 for burns and 10 cm2 for chronic wounds. The step-bystep protocol is described in Table I.
Table I. Step-by-step PRP preparation.
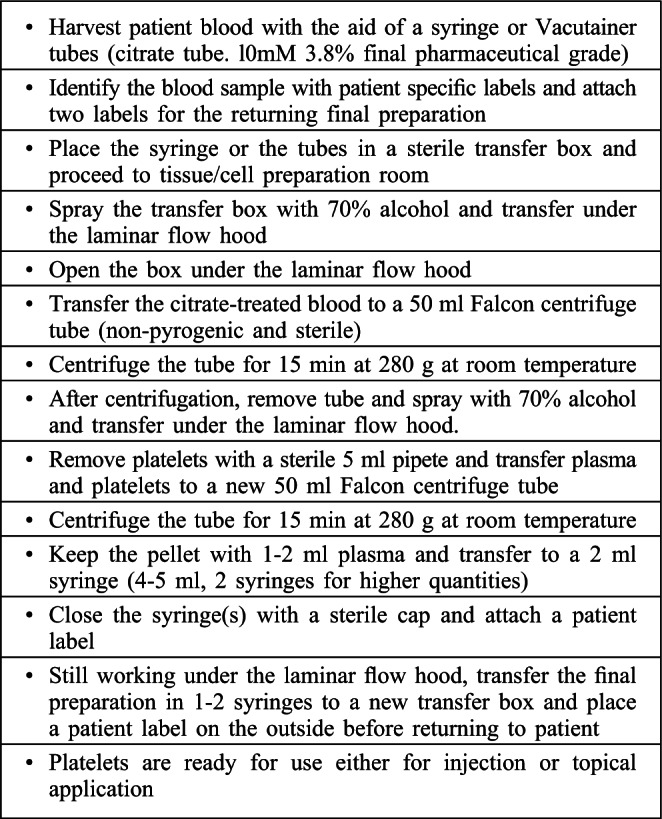
Certified laboratory technicians, cellular biologists, or other health care professionals who have had PRP training and are familiar with proper aseptic technique can safely carry out the PRP preparation following the protocol in Table I. After the blood withdrawal, the PRP can be prepared in a laboratory, operating room or out-patient clinic requiring only a table-top centrifuge for preparation, although a laminar flow hood can increase security (Fig. 4). Any physician using PRP techniques in therapy needs to be familiar with specific guidelines in the use of blood products and also have a clear understanding of possible complications. The International Cellular Medical Society stresses the need to establish standards for PRP preparation protocols, techniques, and traceability.9
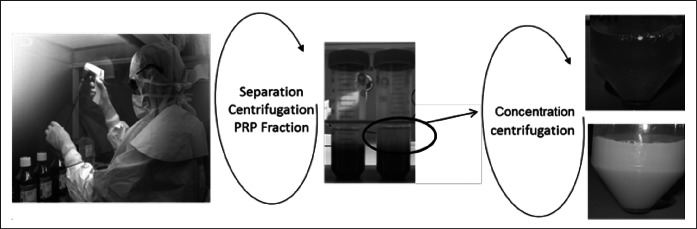
Fig. 4. Description of procedure for two-step centrifugation preparation and heterogeneity of PRP preparations from two different patients. Blood separation and manipulation under a laminar flow hood. Following first centrifugation (280 g at 15 min) in a centrifugation tube (50 ml), the three phases can be readily attained with a visible buffy coat (circled) which includes the platelet phase. This phase is isolated (5 ml sterile pipette), placed in a second sterile centrifugation tube, and centrifuged (280 g for 15 min) to obtain a platelet concentrate. Platelet pellet is suspended in a minimum quantity of plasma (2-4 ml).
Results
When anti-coagulated blood is centrifuged, three layers appear. This is due to differences in the density of the blood components: the deep layer consists of red blood cells, the middle layer contains platelets and leukocytes, and the top layer is made up of platelet-poor plasma. The bottom part of the middle layer is also called buffy coat (bottom of circled region), which is rich in leukocytes. The heterogeneity of blood samples from patients is variable in the overall physical properties such as seen in the samples illustrated (Fig. 5) with clear to opaque plasma obtained following several centrifugation protocols. The main variables considered when optimizing the technique and which play significant roles in PRP preparation included the collecting agent, the choice whether or not to use an activating agent (such as thrombin), and the concentration protocols. These have to be adapted bearing in mind both the quality and stability of the cellular elements and the importance of choosing an anticoagulant capable of preserving the platelets’ best possible functionality, integrity, and morphology. When no anticoagulant is used, platelets can be activated by the mechanical stress of centrifugation. Citrate is a suitable anticoagulant for PRP preparation if the appropriate (i.e. sterile, non-pyrogenic) tubes are available. It has been recently shown that thrombinactivated PRP releases all growth factors at the same time in a bolus, while non-activated PRP uses the platelets as a sustained drug delivery system,10 exhibiting the best wound healing effects. The centrifugation steps should be as delicate as possible but allowing a clean separation of the blood components. Performing two successive low force centrifugations can contribute to platelet preservation.
Fig. 5. PRP preparations and heterogeneity between patients.
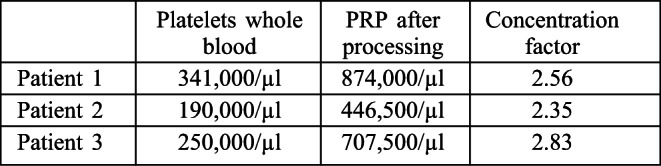
Variability can be seen in the total platelet count from one patient to another and the overall goal was to achieve a concentrating factor of 2-3 times in whole blood for any given patient as indicated by the results from three representative patients (Table II). The centrifugation steps accomplished at low centrifugation forces (280 g) limit mechanical stress to the cellular elements and are capable of producing the concentration factor desired for clinical use. These centrifugation forces are routinely used in our hospital laboratory for preparation of multiple cellular therapies including autologous skin cell separation, culture and grafting and allogenic skin cell separation and have therefore been optimized for clinically used cell therapies with particular emphasis on overall cell quality.
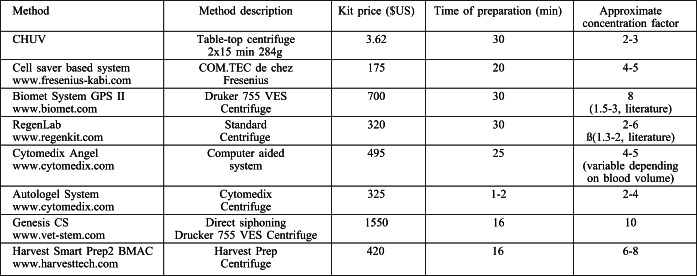
Table II. Results for platelet concentration following centrifugation.
The method of PRP preparation described uses components available in most clinics, hospitals, and pharmacies and the overall cost is relatively low, taking all the required elements together. This should be fairly comparable and therefore affordable in any clinical setting worldwide. We have compared the cost of our current hospitalprepared kit with various other PRP kits available commercially (Table III). Many of the kits are used with specialized equipment and centrifuges which augment the overall cost of PRP use, unlike most disposables associated with our preparation which can be adapted to standard centrifuge systems that are readily available in most clinic and hospital settings. Small table-top centrifuges are capable of the lower centrifugation forces that assure overall cell quality. The preparation times are directly associated with the speed of centrifugation, and kits report from several min up to as many as 30 min of centrifugation time required with one or two-step processing. One important variable reported is the approximate overall concentration factor with some kits describing fairly high concentration possibilities, but data from the literature sometimes indicate otherwise.11 There are many ready-to-use commercially available disposable kits in the price range from $US 175-1150 per kit (price quotations given to our hospital), well above the cost of the necessary materials we describe to produce reliable PRP preparations (less than 4 USD).
Table III. Comparison between PRP centrifugation methods.
Discussion
PRP is an easily accessible cell therapy that helps treat chronic wounds and severe burns. PRP is an autologous cell therapy containing many bioactive factors that are involved in wound healing and tissue repair, requiring only minimal manipulation to obtain the final product. However, understanding the differences in PRP preparations is essential when interpreting clinical study results. Therefore, since little standardization has been done to date, there is still controversy as to the clinical benefits of PRP. Large prospective and randomized studies are necessary to investigate the efficacy of standardized preparations of PRP and post-injection rehabilitation protocols.
When cell therapies are involved in the clinic, it is of utmost importance to assure the quality of the cellular component. In addition, high costs and the need to have specialized equipment to prepare PRP have critically reduced the use of autologous platelets. In our opinion, more simplified PRP preparation methods which do not require ad hoc and costly equipment would help in accumulating clinical data and in introducing the method more routinely in clinical practice as also in the operating room. An important variable that can have a critical impact on PRP quality is the centrifugation protocols, which until now have been highly variable with respect to the force, time and number of centrifugations.3,12 High centrifugation forces can lead to activation of platelets during the preparation and thus impair or diminish platelet function and activity on the wound directly.6 In addition, using higher centrifugation forces than normal in order to have higher concentrations of PRP do not necessarily mean a more effective therapy. On the contrary, Graziani et al. demonstrated in an in vitro study that the biological stimulation of cell growth varies with the dose. Comparison of different concentrations showed that moderate concentration rates of 2.5 fold gave better in vitro results on fibroblast and osteoblast proliferation and function than concentrations of PRP that were less or more concentrated.13
Rappl et al.13 noted that studies with one to three times concentrated PRP showed better results in wound healing than those with higher concentrations. Studies within a meta-analysis by Martinez-Martines-Zapata et al.14 revealed that the works with beneficial PRP treatment did indeed use protocols with lower platelet concentrations.
According to the Cochrane Report,8 there is currently no evidence to suggest that autologous PRP is of value for treating chronic wounds. The reports analysed were based on small numbers of randomized controlled studies for the treatment of chronic wounds including 325 patients, most of whom were at either high or unclear risk of bias. The technical specifications for PRP preparation were varied as were the clinical applications of prepared material. However, there are other promising indications for PRP applications, and these include:
accelerating the healing of bone fracture sites and/or bone grafts; particularly for maxillofacial indications13,15,16
treating tendinopathies especially in Achilles tendon problems and rotator cuff tissue repair17,18
application in aesthetic surgery as a filler and for skin rejuvenation in order to enhance the take of adipose tissue grafts19
treating chronic non-healing wounds such as those of diabetic patients who are at risk of impaired wound healing,8
treating acute and chronic wounds, severe burns, and associated skin graft donor sites which are necessary in patient management
taken all together, the most promising areas for using PRP use seem to be for tendinopathies.7,17,18
Large prospective and randomized studies will be necessary to investigate the efficacy of standardized preparations of PRP in wound healing.
It is proposed that the first step towards this standardization should be the production of a more cost effective and therefore more accessible PRP preparation method which would make PRP more readily available to a higher number of patients and treatment centres and facilitate the development and design of adequate powered clinical studies.
Overall, the PRP preparation technique we describe in this report can be implemented in most clinical settings and can be properly implemented by trained medical staffs, with no additional specialized training, as they are already aware of all risks related to harvesting, transportation, processing, and injection or application of the PRP to the patient.
Conclusion
We report a cost-effective method of PRP preparation aiming to obtain maximal protection for the cellular elements. PRP can be used for treatment and stimulation of healing of wounds of all types including burns and also split-thickness skin graft donor sites used frequently for burn management. The procedure can be easily adapted to most clinical settings with minimal infrastructure so that many patients can benefit from this cellular therapy and so that robust, clinical studies can be accomplished without the high economic outlay starting material costs.
Acknowledgments
This work was written, in part, for the clinical training of one of the authors for his FMH certification (KA). The authors would like to thank the Foundations of the Sandoz Family and S.A.N.T.E for funding some of the laboratory studies.
References
- 1.Panjeshahin J, Thompson M, Hulley P, et al. The biology of plateletrich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987–96. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 2.Lee KS, Wilson JJ, Rabago DP, et al. Musculoskeletal applications of platelet-rich plasma: fad or future? AJR Am J Roentgenol. 2011;196:628–636. doi: 10.2214/AJR.10.5975. [DOI] [PubMed] [Google Scholar]
- 3.Staudenmaier R, Froelich K, Birner M, et al. Optimization of platelet isolation and extraction of autogenous TGF-beta in cartilage tissue engineering. Artif Cells Blood Substit Immobil Biotechnol. 2009;37:265–72. doi: 10.3109/10731190903356446. [DOI] [PubMed] [Google Scholar]
- 4.Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38:174–187. [PMC free article] [PubMed] [Google Scholar]
- 5.Scott J, Pawson T. Cell communication: The inside story. Sci Am. 2000;282:72. doi: 10.1038/scientificamerican0600-72. [DOI] [PubMed] [Google Scholar]
- 6.Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147e–59e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 7.Ziltener J-L, Allet L, Grosclaude M, et al. Traitement des tendinopathies chroniques: intérêt des injections de plasma riche en plaquettes (PRP). Revue Med Suisse. 2011;7:1533–7. [PubMed] [Google Scholar]
- 8.Martinez-Zapata MJ, Marti-Carvajal A, Solà I, Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev, 17. 2012. CD006899. [DOI] [PubMed]
- 9.Harmon K., Hanson R., Bowen J., Section VIII: Platelet Rich Plasma (PRP) Guidelines. International Cellular Medical Society.; 2011. www.cellmedicinesociety.org. [Google Scholar]
- 10.Scherer ss, Tobalem m, Vigato e, et al. Non-activated versus thrombin-activated platelets on wound healing and fibroblast-tomyofibroblast differentiation in vivo and in vitro. Plast Reconstr Surg. 2012;129:46e–54e. doi: 10.1097/PRS.0b013e3182362010. [DOI] [PubMed] [Google Scholar]
- 11.Kaux J-F, Le Goff C, Seidel L, et al. Etude comparative de cinq techniques de préparation plaquettaire (platelet-rich-plasma). Path Biol. 2011;59:157–60. doi: 10.1016/j.patbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Zapata MJ, Martí-Carvajal A, Solà I, et al. Efficacy and safety of the use of autologous plasma rich in platelets for tissue regeneration: a systematic review. Transfusion. 2009;49:44–56. doi: 10.1111/j.1537-2995.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 13.Graziani F, Ivanovski S, Cei S, et al. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17:212–9. doi: 10.1111/j.1600-0501.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 14.Rappl LM. Effect of platelet rich plasma gel in a physiologically relevant platelet concentration on wounds in persons with spinal cord injury. International Wound Journal. 2011;8:187–95. doi: 10.1111/j.1742-481X.2011.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katagiri T, Lee T, Takeshima H, et al. Transforming growth factor-beta modulates proliferation and differentiation of mouse clonal osteoblastic MC3T3-E1 cells depending on their maturation stages. Bone Miner. 1990;11:285–93. doi: 10.1016/0169-6009(90)90025-b. [DOI] [PubMed] [Google Scholar]
- 16.Kasperk CK, Wergedal JE, Mohan S, et al. Interaction of growth factors present in bone matrix with bone cells: effects on DNA synthesis and alkaline phosphatase. Growth Factors. 1990;3:147–58. doi: 10.3109/08977199009108277. [DOI] [PubMed] [Google Scholar]
- 17.Randelli P, Arrigoni P, Ragone V, et al. Platelet rich plasma in arthroscopic rotator cuff repair: A prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20:518–28. doi: 10.1016/j.jse.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Bosch G, van Schie HT, de Groot MW, et al. Effects of platelet-rich plasma on the quality of repair of mechanical induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J Orthop Res. 2010;28:211–7. doi: 10.1002/jor.20980. [DOI] [PubMed] [Google Scholar]
- 19.Oh DS, Cheon YW, Jeon YR, et al. Activated platelet-rich plasma improves fat graft survival in nude mice: A pilot study. Dermatol Surg. 2011;37:619–25. doi: 10.1111/j.1524-4725.2011.01953.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.